Abstract
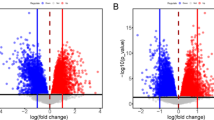
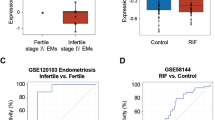
Infertility and recurrent pregnancy loss (RPL) are prevalent but distinct causes of reproductive failure that often remain unexplained despite extensive investigations1,2. Analysis of midsecretory endometrial samples revealed that SGK1, a kinase involved in epithelial ion transport and cell survival3,4,5,6, is upregulated in unexplained infertility, most prominently in the luminal epithelium, but downregulated in the endometrium of women suffering from RPL. To determine the functional importance of these observations, we first expressed a constitutively active SGK1 mutant in the luminal epithelium of the mouse uterus. This prevented expression of certain endometrial receptivity genes, perturbed uterine fluid handling and abolished embryo implantation. By contrast, implantation was unhindered in Sgk1−/− mice, but pregnancy was often complicated by bleeding at the decidual-placental interface and fetal growth retardation and subsequent demise. Compared to wild-type mice, Sgk1−/− mice had gross impairment of pregnancy-dependent induction of genes involved in oxidative stress defenses. Relative SGK1 deficiency was also a hallmark of decidualizing stromal cells from human subjects with RPL and sensitized these cells to oxidative cell death. Thus, depending on the cellular compartment, deregulated SGK1 activity in cycling endometrium interferes with embryo implantation, leading to infertility, or predisposes to pregnancy complications by rendering the feto-maternal interface vulnerable to oxidative damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Evers, J.L. Female subfertility. Lancet 360, 151–159 (2002).
Rai, R. & Regan, L. Recurrent miscarriage. Lancet 368, 601–611 (2006).
Amato, R. et al. Sgk1 activates MDM2-dependent p53 degradation and affects cell proliferation, survival, and differentiation. J. Mol. Med. 87, 1221–1239 (2009).
Brunet, A. et al. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol. Cell Biol. 21, 952–965 (2001).
Lang, F. et al. (Patho) physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol. Rev. 86, 1151–1178 (2006).
Loffing, J., Flores, S.Y. & Staub, O. Sgk kinases and their role in epithelial transport. Annu. Rev. Physiol. 68, 461–490 (2006).
Dey, S.K. et al. Molecular cues to implantation. Endocr. Rev. 25, 341–373 (2004).
Horcajadas, J.A., Pellicer, A. & Simon, C. Wide genomic analysis of human endometrial receptivity: new times, new opportunities. Hum. Reprod. Update 13, 77–86 (2007).
Wilcox, A.J., Baird, D.D. & Weinberg, C.R. Time of implantation of the conceptus and loss of pregnancy. N. Engl. J. Med. 340, 1796–1799 (1999).
Alam, S.M. et al. A uterine decidual cell cytokine ensures pregnancy-dependent adaptations to a physiological stressor. Development 134, 407–415 (2007).
Gellersen, B., Brosens, I.A. & Brosens, J.J. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin. Reprod. Med. 25, 445–453 (2007).
Leitao, B. et al. Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals. FASEB J. 24, 1541–1551 (2010).
Feroze-Zaidi, F. et al. Role and regulation of the serum- and glucocorticoid-regulated kinase 1 in fertile and infertile human endometrium. Endocrinology 148, 5020–5029 (2007).
Jeong, J.W. et al. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology 146, 3490–3505 (2005).
Talbi, S. et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 147, 1097–1121 (2006).
Campbell, E.A. et al. Temporal expression profiling of the uterine luminal epithelium of the pseudo-pregnant mouse suggests receptivity to the fertilized egg is associated with complex transcriptional changes. Hum. Reprod. 21, 2495–2513 (2006).
Satokata, I., Benson, G. & Maas, R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature 374, 460–463 (1995).
Stewart, C.L. et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359, 76–79 (1992).
Wang, H. & Dey, S.K. Roadmap to embryo implantation: clues from mouse models. Nat. Rev. Genet. 7, 185–199 (2006).
Xie, H. et al. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc. Natl. Acad. Sci. USA 104, 18315–18320 (2007).
Franco, H.L. et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 25, 1176–1187 (2011).
Lee, K. et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat. Genet. 38, 1204–1209 (2006).
Lee, K.Y. et al. Bmp2 is critical for the murine uterine decidual response. Mol. Cell Biol. 27, 5468–5478 (2007).
Yang, J.Z. et al. Abnormally enhanced cystic fibrosis transmembrane conductance regulator-mediated apoptosis in endometrial cells contributes to impaired embryo implantation in controlled ovarian hyperstimulation. Fertil. Steril. 95, 2100–2106 (2011).
Joswig, A., Gabriel, H.D., Kibschull, M. & Winterhager, E. Apoptosis in uterine epithelium and decidua in response to implantation: evidence for two different pathways. Reprod. Biol. Endocrinol. 1, 44 (2003).
Yue, L. et al. Cyclin G1 and cyclin G2 are expressed in the periimplantation mouse uterus in a cell-specific and progesterone-dependent manner: evidence for aberrant regulation with Hoxa-10 deficiency. Endocrinology 146, 2424–2433 (2005).
Aghajanova, L. et al. The protein kinase A pathway–regulated transcriptome of endometrial stromal fibroblasts reveals compromised differentiation and persistent proliferative potential in endometriosis. Endocrinology 151, 1341–1355 (2010).
Salker, M. et al. Natural selection of human embryos: impaired decidualization of the endometrium disables embryo-maternal interactions and causes recurrent pregnant loss. PLoS ONE 5, e10287 (2010).
Al-Sabbagh, M. et al. NADPH oxidase–derived reactive oxygen species mediate decidualization of human endometrial stromal cells in response to cyclic AMP signaling. Endocrinology 152, 730–740 (2011).
Cloke, B. et al. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology 149, 4462–4474 (2008).
Higuchi, T. et al. Induction of tissue inhibitor of metalloproteinase 3 gene expression during in vitro decidualization of human endometrial stromal cells. Endocrinology 136, 4973–4981 (1995).
Takano, M. et al. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol. Endocrinol. 21, 2334–2349 (2007).
Tang, M., Naidu, D., Hearing, P., Handwerger, S. & Tabibzadeh, S. LEFTY, a member of the transforming growth factor-β superfamily, inhibits uterine stromal cell differentiation: a novel autocrine role. Endocrinology 151, 1320–1330 (2010).
Fluhr, H., Krenzer, S. & Zygmunt, M. Different regulation of tissue inhibitors of metalloproteinases-1, -2 and -3 in human endometrial stromal cells during decidualization in vitro. Reprod. Med. Biol. 7, 169–175 (2008).
Crossey, P.A., Pillai, C.C. & Miell, J.P. Altered placental development and intrauterine growth restriction in IGF binding protein-1 transgenic mice. J. Clin. Invest. 110, 411–418 (2002).
Sakuma, R. et al. Inhibition of Nodal signalling by Lefty mediated through interaction with common receptors and efficient diffusion. Genes Cells 7, 401–412 (2002).
Tabibzadeh, S. et al. Dysregulated expression of ebaf, a novel molecular defect in the endometria of patients with infertility. J. Clin. Endocrinol. Metab. 85, 2526–2536 (2000).
Tang, M., Taylor, H.S. & Tabibzadeh, S. In vivo gene transfer of lefty leads to implantation failure in mice. Hum. Reprod. 20, 1772–1778 (2005).
Jauniaux, E., Poston, L. & Burton, G.J. Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum. Reprod. Update 12, 747–755 (2006).
Jauniaux, E. et al. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 157, 2111–2122 (2000).
Acknowledgements
We are grateful to all the women who participated in this study. This work was further supported by funds to J.J.B. and M.C. from the Contraceptive Research and Development Program Consortium for Industrial Collaboration in Contraceptive Research (CIG-08-122), the UK National Institute for Health Research Biomedical Research Centre funding scheme and from the Genesis Research Trust (M.S.S.) We are grateful to M. Parker for his insightful suggestions.
Author information
Authors and Affiliations
Contributions
M.C., F.L. and J.J.B. designed the research; M.S.S., J.H.S., J.N., Z.W., M.A.-S., G.P., M.F. and C.L. carried out the research; S.L., G.T., S.Q., L.R. and J.J.B. phenotyped the subjects and provided samples; M.S.S., A.M.S., J.D.A., M.C., F.L. and J.J.B. analyzed the data; and J.J.B. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Supplementary Figures
Supplementary Figures 1–14, Supplementary Table 1 and Supplementary Methods (PDF 963 kb)
Rights and permissions
About this article
Cite this article
Salker, M., Christian, M., Steel, J. et al. Deregulation of the serum- and glucocorticoid-inducible kinase SGK1 in the endometrium causes reproductive failure. Nat Med 17, 1509–1513 (2011). https://doi.org/10.1038/nm.2498
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nm.2498
This article is cited by
-
Role of Immunological Testing in Infertility
Current Obstetrics and Gynecology Reports (2024)
-
Estrogen-sensitive activation of SGK1 induces M2 macrophages with anti-inflammatory properties and a Th2 response at the maternal–fetal interface
Reproductive Biology and Endocrinology (2023)
-
Uterine epithelial Gp130 orchestrates hormone response and epithelial remodeling for successful embryo attachment in mice
Scientific Reports (2023)
-
Serum/glucocorticoid-inducible kinase 1 deficiency induces NLRP3 inflammasome activation and autoinflammation of macrophages in a murine endolymphatic hydrops model
Nature Communications (2023)
-
Activation of SGK1/ENaC Signaling Pathway Improves the Level of Decidualization in Unexplained Recurrent Spontaneous Abortion
Reproductive Sciences (2023)