Abstract
The detection of viral dynamics and localization in the context of controlled HIV infection remains a challenge and is limited to blood and biopsies. We developed a method to capture total-body simian immunodeficiency virus (SIV) replication using immunoPET (antibody-targeted positron emission tomography). The administration of a poly(ethylene glycol)-modified, 64Cu-labeled SIV Gp120–specific antibody led to readily detectable signals in the gastrointestinal and respiratory tract, lymphoid tissues and reproductive organs of viremic monkeys. Viral signals were reduced in aviremic antiretroviral-treated monkeys but detectable in colon, select lymph nodes, small bowel, nasal turbinates, the genital tract and lung. In elite controllers, virus was detected primarily in foci in the small bowel, select lymphoid areas and the male reproductive tract, as confirmed by quantitative reverse-transcription PCR (qRT-PCR) and immunohistochemistry. This real-time, in vivo viral imaging method has broad applications to the study of immunodeficiency virus pathogenesis, drug and vaccine development, and the potential for clinical translation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
11 June 2015
In the version of this article initially published, the first name of Emmeline L. Blanchard was misspelled as Emeline. The error has been corrected in the HTML and PDF versions of the article.
References
Sáez-Cirión, A. et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 9, e1003211 (2013).
Zaunders, J. & van Bockel, D. Innate and adaptive immunity in long-term non-progression in HIV disease. Front Immunol. 4, 95 (2013).
Gray, L. et al. Phenotype and envelope gene diversity of nef-deleted HIV-1 isolated from long-term survivors infected from a single source. Virol. J. 4, 75 (2007).
Visco-Comandini, U., Aleman, S., Yun, Z. & Sönnerborg, A. Human immunodeficiency virus type 1 variability and long-term non-progression. J. Biol. Regul. Homeost. Agents 15, 299–303 (2001).
Laird, G.M. et al. Rapid quantification of the latent reservoir for HIV-1 using a viral outgrowth assay. PLoS Pathog. 9, e1003398 (2013).
Di Mascio, M. et al. Noninvasive in vivo imaging of CD4 cells in simian-human immunodeficiency virus (SHIV)-infected nonhuman primates. Blood 114, 328–337 (2009).
Dadachova, E. et al. Targeted killing of virally infected cells by radiolabeled antibodies to viral proteins. PLoS Med. 3, e427 (2006).
Boerman, O.C. & Oyen, W.J. Immuno-PET of cancer: a revival of antibody imaging. J. Nucl. Med. 52, 1171–1172 (2011).
Fortin, M.A. et al. Immuno-PET of undifferentiated thyroid carcinoma with radioiodine-labelled antibody cMAb U36: application to antibody tumour uptake studies. Eur. J. Nucl. Med. Mol. Imaging 34, 1376–1387 (2007).
Lucignani, G. et al. FDG-PET imaging in HIV-infected subjects: relation with therapy and immunovirological variables. Eur. J. Nucl. Med. Mol. Imaging 36, 640–647 (2009).
Brenchley, J.M. & Paiardini, M. Immunodeficiency lentiviral infections in natural and non-natural hosts. Blood 118, 847–854 (2011).
Lackner, A.A. & Veazey, R.S. Current concepts in AIDS pathogenesis: insights from the SIV/macaque model. Annu. Rev. Med. 58, 461–476 (2007).
Edinger, A.L. et al. Characterization and epitope mapping of neutralizing monoclonal antibodies produced by immunization with oligomeric simian immunodeficiency virus envelope protein. J. Virol. 74, 7922–7935 (2000).
White, T.A. et al. Three-dimensional structures of soluble CD4-bound states of trimeric simian immunodeficiency virus envelope glycoproteins determined by using cryo-electron tomography. J. Virol. 85, 12114–12123 (2011).
White, T.A. et al. Molecular architectures of trimeric SIV and HIV-1 envelope glycoproteins on intact viruses: strain-dependent variation in quaternary structure. PLoS Pathog. 6, e1001249 (2010).
Wattendorf, U. & Merkle, H.P. PEGylation as a tool for the biomedical engineering of surface modified microparticles. J. Pharm. Sci. 97, 4655–4669 (2008).
Mortimer, J.E. et al. Functional imaging of human epidermal growth factor receptor 2-positive metastatic breast cancer using 64Cu-DOTA-trastuzumab PET. J. Nucl. Med. 55, 23–29 (2014).
Tamura, K. et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J. Nucl. Med. 54, 1869–1875 (2013).
Picker, L.J. Immunopathogenesis of acute AIDS virus infection. Curr. Opin. Immunol. 18, 399–405 (2006).
Heise, C., Vogel, P., Miller, C.J., Halsted, C.H. & Dandekar, S. Simian immunodeficiency virus infection of the gastrointestinal tract of rhesus macaques. Functional, pathological, and morphological changes. Am. J. Pathol. 142, 1759–1771 (1993).
Veazey, R.S. et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280, 427–431 (1998).
Barber, S.A. et al. Longitudinal analysis of simian immunodeficiency virus (SIV) replication in the lungs: compartmentalized regulation of SIV. J. Infect. Dis. 194, 931–938 (2006).
Le Tortorec, A. & Dejucq-Rainsford, N. HIV infection of the male genital tract–consequences for sexual transmission and reproduction. Int. J. Androl. 33, e98–e108 (2010).
Le Tortorec, A. et al. Human prostate supports more efficient replication of HIV-1 R5 than X4 strains ex vivo. Retrovirology 5, 119 (2008).
Shehu-Xhilaga, M. et al. The testis and epididymis are productively infected by SIV and SHIV in juvenile macaques during the post-acute stage of infection. Retrovirology 4, 7 (2007).
Miller, C.J. Localization of simian immunodeficiency virus-infected cells in the genital tract of male and female rhesus macaques. J. Reprod. Immunol. 41, 331–339 (1998).
Chacko, A.M. et al. Targeted delivery of antibody-based therapeutic and imaging agents to CNS tumors: crossing the blood-brain barrier divide. Expert Opin. Drug Deliv. 10, 907–926 (2013).
Barton, K.M., Burch, B.D., Soriano-Sarabia, N. & Margolis, D.M. Prospects for treatment of latent HIV. Clin. Pharmacol. Ther. 93, 46–56 (2013).
Chun, T.W. & Fauci, A.S. HIV reservoirs: pathogenesis and obstacles to viral eradication and cure. AIDS 26, 1261–1268 (2012).
Hill, A. Optimizing HIV treatment. Curr. Opin. HIV AIDS 8, 34–40 (2013).
Zhou, T. et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329, 811–817 (2010).
Andrieux-Meyer, I. et al. Preferred antiretroviral drugs for the next decade of scale up. J. Int. AIDS Soc. 15, 17986 (2012).
Haase, A.T. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu. Rev. Med. 62, 127–139 (2011).
Lairmore, M.D., Post, A.A., Goldsmith, C.S. & Folks, T.M. Cytokine enhancement of simian immunodeficiency virus (SIV/mac) from a chronically infected cloned T-cell line (HuT-78). Arch. Virol. 121, 43–53 (1991).
Daniel, M.D. et al. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science 228, 1201–1204 (1985).
Hong, J.J., Amancha, P.K., Rogers, K., Ansari, A.A. & Villinger, F. Spatial alterations between CD4+ T follicular helper, B, and CD8+ T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J. Immunol. 188, 3247–3256 (2012).
Hong, J.J., Amancha, P.K., Rogers, K., Ansari, A.A. & Villinger, F. Re-evaluation of PD-1 expression by T cells as a marker for immune exhaustion during SIV infection. PLoS ONE 8, e60186 (2013).
Torriani, M. et al. Increased FDG uptake in association with reduced extremity fat in HIV patients. Antivir. Ther. 18, 243–248 (2013).
Yant, L.J. et al. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 80, 5074–5077 (2006).
Haralick, R.M., Shanmugam, K. & Dinstein, I. Textural features for image classification. IEEE Trans. Syst. Man Cybern. 3, 610–621 (1973).
Acknowledgements
We thank the animal and care staff of the Yerkes National Primate Research Center; B. Lawson, E. Strobert, J. Wood, S. Jean and M. Thompson; and D. Votaw and the Emory Clinic imaging team for their help in the conduct of these studies. Funding for these studies was provided by a developmental grant from the Yerkes National Primate Research Center, the Georgia Research Alliance, US National Institute of Allergy and Infectious Diseases grants R21-AI095129 to P.J.S. and F.V., R01 AI078775 and 8R24 OD010947 to F.V. and R01 AI078773 to A.A.A., the Center for AIDS Research P30 AI050409 and the base grant P51 OD11132 in support of Yerkes. E.H. is supported by a Georgia Research Alliance Eminent scholarship. We also thank M. Miller (Gilead Inc.) for the PMPA and FTC and D. Hazuda (Merck) for the integrase inhibitor. We gratefully acknowledge the help, support, encouragement and advice of O. Sharma (NIAID) given during the course of these studies.
Author information
Authors and Affiliations
Contributions
P.J.S., E.H. and F.V. conceived of the project. C.Z. produced the imaging agent, and P.J.S. and F.V. performed the radioactive labeling. P.J.S., F.V., C.Z., E.L.B., K.A.R., S.G., K.S., F.C.-S., P.K.A., J.J.H. and S.N.B. performed experiments. A.A.A. contributed animals and reagents. E.H. and J.A.H. provided reagents and advice. D.M.S. assisted with PET imaging. B.V. performed the statistical analyses. P.J.S., F.V., A.A.A. and E.H. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
P.J.S. and F.V. have filed a patent for the detection of HIV using immunoPET.
Integrated supplementary information
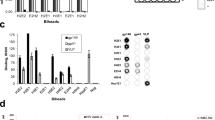
Supplementary Figure 1 In vitro binding of modified mAb clone 7D3 to SIV gp120, interrogated by scintillation counter, fluorescence microscopy and flow cytometry.
a. Counts per minute from 7D3-PEG-64Cu-DOTA labeled SIV-1C serially diluted with Hut78 (non-infected) cells, demonstrating a linear response in samples with equal cell numbers. b. Counts per minute from cryopreserved and thawed splenocytes and lymph node cells, originating from uninfected and SIV infected animals collected at necropsy. The data demonstrates that even after a freeze-thaw cycle the 7D3-PEG- 64Cu-DOTA probe binds specifically to infected cells. Shown are means and standard deviations from lymph node and spleen cells from 2 and 1 uninfected controls and 3 and 5 infected monkeys, respectively. c. Competition assay where unlabeled “cold”-7D3 was used to compete 200 ng bound “hot” 7D3-PEG-64Cu-DOTA from SIV-1C and Hut78 cells, demonstrating epitope-specific binding of the probe. d. Probe bound virus pelleted from infected (Viremic 1 and Viremic 2) versus non-infected (Control 1 and Control 2) animal plasmas, 24 hrs post contrast agent injection, measured in counts per minute normalized to the dose given to each animal. This demonstrates that at 24 hrs post injection, the probe is still stable, and can bind to its target specifically. e. Representative images of 7D3-PEG-DOTA (modified 7D3) and 7D3-PEG-Dylight 650 binding to SIV-1C cells, demonstrating specific binding. No statistical difference was found between the two probes. No signal was detected for either probe in Hut78 control cells (data not shown). This experiment demonstrates that the 650 labeled fluorescent probe binds with the same efficacy than the 7D3-PEG-DOTA (cold) probe. f. Flow cytometry profiles of 7D3-PEG-Dylight 650 binding to control cells, SIV-1C cells, and SIV-1C cells in the presence of pre-infection and post-infection serum. While both pre and post infection serum appear to compete with 7D3 binding to the infected cells, specific signal is still clearly seen relative to binding to uninfected cells.
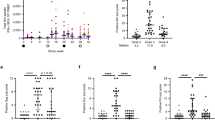
Supplementary Figure 2 Additional PET and CT results from chronically SIV-infected viremic and control macaques.
a, b, c. Frontal, sagittal and axial views are presented, as well as magnified views (marked by a colored box and associated image denoted by the same color outline) of regions of the frontal and sagittal sections. The site corresponding to the axial section is identified within the sagittal view by a yellow line, while the sagittal view location, if not centerline, is denoted by a blue line in the frontal view. Single plane PET/CT cross-sections from macaques, Viremic 2-4, (for plasma viral loads see Supplementary Table 1) scanned at 24 hrs post injection of the contrast agent. Both animals demonstrated PET signals within the GI tract, including the ileum, jejunum, and colon, as well as within axillary and inguinal lymph nodes, and lungs. SIVgag p27 specific IHC for select organs of macaques Viremic 2-4, are also presented. Infected mononuclear cells (black arrows) were detected in the ileum, jejunum, and colon, as well as within lymph nodes and the spleen, verifying the PET results. d. Single plane images of modified 7D3 within non-infected macaque, Control 2. The sagittal image is taken from the centerline and the axial view is denoted by the yellow line within the sagittal view. The 7D3 probe did not yield significant uptake within the gut, axillary, mesenchymal, or inguinal lymph nodes. Background uptake was highest within the liver, heart, kidneys and spleen, as expected, though the signals measured in these organs were markedly lower than those quantified from the same organs in SIV infected monkeys, suggesting specific uptake above background in the infected animals. e. Control monkey number 1 given a modified isotype control IgG antibody; background signal detected was significantly lower than in the infected animals, even within the liver, heart, and kidneys, sites for which background was expected. f. Single plane images of modified mouse IgG within chronically-infected macaque, Viremic 5. Uptake was generally similar to the modified mouse IgG in the non-infected animals. g. SUVmax quantification for the chronically infected and non-infected animals, with the isotype control probe (modified mouse IgG) data in a chronically infected animal was added, demonstrating similar uptake to the non-infected animal.
Supplementary Figure 3 Cross-sectional views of representative animals.
a. Cross-sections of the frontal torso and abdomen of SIV infected viremic monkey Viremic 2. Each slice is 1.37 mm thick; 28 slices are displayed, amounting to a frontal view every 2.74 mm. A SIV positive signal is apparent throughout the gastrointestinal tract, especially within the ileum, jejunum and colon, and within the lung. Background signal, as expected, was high within the liver, heart, and kidneys. b. Cross-sections of the frontal torso and abdomen of SIV infected viremic monkey Viremic 1. Each slice is 1.37 mm thick; 24 slices are displayed, amounting to a frontal view every 2.74 mm. A SIV positive signal is apparent throughout the GI tract and within the lung. Background signal, as expected, was high within the liver, heart, and kidneys. c. Cross-sections of the frontal torso and abdomen of uninfected monkey Control 1 given modified 7D3. In the frontal views, each slice is 0.98 mm thick, 24 slices are displayed, amounting to a frontal view every 1.96 mm. Very little signal above muscle background within the gastrointestinal tract, and lymph nodes. Background signal, as expected, was high within the liver, heart, and kidneys, and measurable within the lung.
Supplementary Figure 4 PET-CT results from two additional chronically infected macaques, before and at 5 weeks of ART treatment.
PET/CT images of two SIV chronically infected macaques, ART 2 and 3, prior to and at 5 weeks of ART. a. Standard uptake value (SUV) maps of GI tract, lymph nodes, genital tract, spleen and small bowel, demonstrating decreased probe uptake after 5 weeks of ART. b. SUVmax values before and after 5 weeks of ART, compared with background uptake in non-infected animals. c. qRT-PCR verification of residual virus compared with SUVmax PET data.
Supplementary Figure 5 Kinetics of plasma viral loads in the SIV-infected monkeys ART 1, 2 and 3, before and during ART.
All 3 monkeys were subjected to PET/CT analysis prior to ART initiation and again after 34 days on ART, before being euthanized on days 38/39 for tissue collections and analyses. By 3-4 weeks of ART, plasma viral loads were below the 60 copies/ml detection limit of the qRT-PCR assay.
Supplementary Figure 6 Tissue viral loads in SIV-infected, ART-treated monkeys.
a. The average tissue viral loads (SIV vRNA copies/ng total RNA) were generated from 6 separate spatial samples for the colon, 4 for the jejunum and 4 for the ileum. The average viral loads were derived from the results of the 4-6 different sites collected and analyzed for each GI section. While average values for the colon were comparable for the 3 monkeys, there was far more variability (up to 2 orders of magnitude) in values obtained from the small bowel segments, suggesting potential differences in viral clearance during ART. In contrast, measuring gp120 via PET, an approximately 10-fold difference was observed between monkeys. b. These figures illustrate the differences in qRT-PCR signals obtained from various collection sites from the same GI segment of a given animal on ART. It is obvious from the data that the variation in viral RNA levels can range up to 2 orders of magnitude within the same tissue, especially for the colon. This clearly has implications for the interpretation of data obtained from biopsies relative to an entire organ. All qRT-PCR data is presented also in Supplementary Table 3.
Supplementary Figure 7 Additional PET-CT results from an EC SIV-infected macaque.
a,b. Frontal, sagittal and axial views are presented, as well as magnified views (marked by a colored box and associated image denoted by the same color outline) of regions of the frontal and sagittal sections. Single plane PET/CT cross-sections from macaque EC 2 and 3, 36 hrs post injection of the contrast agent. EC 2 and 3 demonstrated PET signal foci in the GI tract, including the ileum, jejunum, and colon, as well as within axillary, mesenteric, and inguinal lymph nodes, and male genital tract. IHC for p27 Gag results from biopsy samples for each monkey are also presented. Infected mononuclear cells (black arrows) were detected in the rectum, epididymis and jejunum, verifying the PET results. b. Cross-sections of frontal and axial views of macaque EC 3, an elite controller. For the frontal views, each slice is 1.37 mm thick, 24 slices are displayed, amounting to a frontal view every 5.46 mm. The axial view thickness is 5 mm and images are displayed every 3 mm. SIV positive signal is apparent throughout the GI tract, and within the lung. Background signal, as expected, was high within the liver, heart, and kidneys.
Supplementary Figure 8 Comparison of PET signal distribution in viremic and EC macaques.
Representative axial cross sections of chronically infected macaques (Viremic 2) and controller macaques (EC3). The uptake in the chronically viremic infected animals (white arrows) tend to follow the length of the GI organs, while in the controllers (white arrows), the signal localizes to specific foci, some of which are mesenteric lymph nodes (from CT), while in other cases they correspond to specific regions of the small intestines or colon. The red arrows indicate uptake within the liver.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Tables 1–3 and Supplementary Note (PDF 1318 kb)
Rights and permissions
About this article
Cite this article
Santangelo, P., Rogers, K., Zurla, C. et al. Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy–treated macaques. Nat Methods 12, 427–432 (2015). https://doi.org/10.1038/nmeth.3320
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nmeth.3320
This article is cited by
-
Rebound HIV-1 in cerebrospinal fluid after antiviral therapy interruption is mainly clonally amplified R5 T cell-tropic virus
Nature Microbiology (2023)
-
First-in-human immunoPET imaging of HIV-1 infection using 89Zr-labeled VRC01 broadly neutralizing antibody
Nature Communications (2022)
-
HIV induces airway basal progenitor cells to adopt an inflammatory phenotype
Scientific Reports (2021)
-
T cell-tropic HIV efficiently infects alveolar macrophages through contact with infected CD4+ T cells
Scientific Reports (2021)
-
Persistence of viral RNA in lymph nodes in ART-suppressed SIV/SHIV-infected Rhesus Macaques
Nature Communications (2021)