Abstract
Positron emission tomography (PET) and the high affinity D2/3 radiotracer [18F]fallypride allow the assessment of D2/3 receptor occupancy of antipsychotic drugs in striatal and extrastriatal brain regions. We measured regional occupancy attained across a range of clinical dosing by the partial D2 agonist aripiprazole using these methods. Twenty-eight PET scans were acquired on the ECAT EXACT HR+ camera in 19 patients with schizophrenia or schizoaffective disorder. Daily aripiprazole doses ranged from 2 to 40 mg, with a minimum of 10 days on steady dose. Mean regional occupancies, a model-independent estimate of aripiprazole effect on pituitary binding, and PANSS ratings changes were evaluated. Occupancy levels were high across regions of interest, ranging from 71.6±5.5% at 2 mg/day to 96.8±5.3% at 40 mg/day. Occupancy levels were higher in extrastriatal than striatal regions. Pituitary measures of aripiprazole effect correlated with doses and were unrelated to prolactin levels, which remained within the normal range under medication. PANSS positive (but not negative) symptom improvement correlated with striatal but not extrastriatal occupancies. These data show, for the first time, D2 occupancy by aripiprazole in treated patients with schizophrenia in extrastriatal as well as striatal regions, with high occupancy for all doses. We discuss possible explanations for higher extrastriatal than striatal occupancy. Correlations of ratings of clinical improvement with regional occupancy suggest that aripiprazole, as do other antipsychotics, benefits positive symptoms of schizophrenia most directly through its modulation of striatal rather than cortical or other extrastriatal dopamine activity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS et al (2000). Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA 97: 8104–8109.
Abi-Dargham A, Simpson N, Kegeles L, Parsey R, Hwang D-R, Anjilvel S et al (1999). PET studies of binding competition between endogenous dopamine and the D1 radiotracer [11C]NNC 756. Synapse 32: 93–109.
Agid O, Mamo D, Ginovart N, Vitcu I, Wilson AA, Zipursky RB et al (2007). Striatal vs extrastriatal dopamine D2 receptors in antipsychotic response—a double-blind PET study in schizophrenia. Neuropsychopharmacology 32: 1209–1215.
Bergstrom M, Muhr C, Lundberg PO, Langstrom B (1991). PET as a tool in the clinical evaluation of pituitary adenomas. J Nucl Med 32: 610–615.
Bigliani V, Mulligan RS, Acton PD, Visvikis D, Ell PJ, Stephenson C et al (1999). In vivo occupancy of striatal and temporal cortical D2/D3 dopamine receptors by typical antipsychotic drugs. [123I]epidepride single photon emission tomography (SPET) study. Br J Psychiatry 175: 231–238.
Brix G, Zaers J, Adam LE, Bellemann ME, Ostertag H, Trojan H et al (1997). Performance evaluation of a whole-body PET scanner using the NEMA protocol. National Electrical Manufacturers Association. J Nucl Med 38: 1614–1623.
Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T et al (2002). Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther 302: 381–389.
Deutch AY (1992). The regulation of subcortical dopamine systems by the prefrontal cortex: interactions of central dopamine systems and the pathogenesis of schizophrenia. J Neural Transm Suppl 36: 61–89.
DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders, 4th edn, Text Revision (2000). American Psychiatric Association: Washington, DC.
Farde L, Nordström AL, Wiesel FA, Pauli S, Halldin C, Sedvall G (1992). Positron emission tomography analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Arch Gen Psychiatry 49: 538–544.
Farde L, Suhara T, Nyberg S, Karlsson P, Nakashima Y, Hietala J et al (1997). A PET-study of [11C]FLB 457 binding to extrastriatal D2-dopamine receptors in healthy subjects and antipsychotic drug-treated patients. Psychopharmacology (Berlin) 133: 396–404.
Farde L, Wiesel FA, Halldin C, Sedvall G (1988). Central D2-dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugs. Arch Gen Psychiatry 45: 71–76.
Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frakowiak RSJ (1995). Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapping 2: 189–210.
Garris PA, Collins LB, Jones SR, Wightman RM (1993). Evoked extracellular dopamine in vivo in the medial prefrontal cortex. J Neurochem 61: 637–647.
Grunder G, Landvogt C, Vernaleken I, Buchholz HG, Ondracek J, Siessmeier T et al (2006). The striatal and extrastriatal D2/D3 receptor-binding profile of clozapine in patients with schizophrenia. Neuropsychopharmacology 31: 1027–1035.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN et al (2007). Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27: 1533–1539.
Inoue A, Seto M, Sugita S, Hide I, Hirose T, Koga N et al (1998). Differential effects on D2 dopamine receptor and prolactin gene expression by haloperidol and aripiprazole in the rat pituitary. Brain Res Mol Brain Res 55: 285–292.
Jordan S, Regardie K, Johnson JL, Chen R, Kambayashi J, McQuade R et al (2007). In vitro functional characteristics of dopamine D2 receptor partial agonists in second and third messenger-based assays of cloned human dopamine D2Long receptor signalling. J Psychopharmacol 21: 620–627.
Kapur S, Zipursky R, Jones C, Remington G, Houle S (2000). Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry 157: 514–520.
Kapur S, Zipursky RB, Remington G (1999). Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry 156: 286–293.
Kay SR, Fiszbein A, Opler LA (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schiz Bull 13: 261–276.
Kenakin T (1995). Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol Sci 16: 232–238.
Kenakin T (2007). Functional selectivity through protean and biased agonism: who steers the ship? Mol Pharmacol 72: 1393–1401.
Kerwin R, Millet B, Herman E, Banki CM, Lublin H, Pans M et al (2007). A multicentre, randomized, naturalistic, open-label study between aripiprazole and standard of care in the management of community-treated schizophrenic patients Schizophrenia Trial of Aripiprazole: (STAR) study. Eur Psychiatry 22: 433–443.
Kessler RM, Ansari MS, Riccardi P, Li R, Jayathilake K, Dawant B et al (2005). Occupancy of striatal and extrastriatal dopamine D2/D3 receptors by olanzapine and haloperidol. Neuropsychopharmacology 30: 2283–2289.
Kessler RM, Ansari MS, Riccardi P, Li R, Jayathilake K, Dawant B et al (2006). Occupancy of striatal and extrastriatal dopamine D2 receptors by clozapine and quetiapine. Neuropsychopharmacology 31: 1991–2001.
Kessler RM (2007). Aripiprazole: what is the role of dopamine D(2) receptor partial agonism? Am J Psychiatry 164: 1310–1312.
Kessler RM, Mason N, Jones C, Ansari MS, Manning RF, Price RR (2000). [18F]N-allyl-5-fluoropropylepidepride (fallypride): radiation dosimetry, quantification of striatal and extrastriatal dopamine receptors in man. NeuroImage 11: S32.
Kikuchi T, Tottori K, Uwahodo Y, Hirose T, Miwa T, Oshiro Y et al (1995). 7-(4-[4-(2, 3-Dichlorophenyl)-1-piperazinyl]butyloxy)-3, 4-dihydro-2(1H)-qui nolinone (OPC-14597), a new putative antipsychotic drug with both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity. J Pharmacol Exp Ther 274: 329–336.
Lahti RA, Roberts RC, Tamminga CA (1995). D2-family receptor distribution in human postmortem tissue: an autoradiographic study. Neuroreport 6: 2505–2512.
Lammertsma AA, Hume SP (1996). Simplified reference tissue model for PET receptor studies. Neuroimage 4: 153–158.
Laruelle M, Slifstein M, Huang Y (2003). Relationships between radiotracer properties and image quality in molecular imaging of the brain with positron emission tomography. Mol Imaging Biol 5: 363–375.
Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA et al (1999). Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology 20: 612–627.
Mailman RB (2007). GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci 28: 390–396.
Mallikaarjun S, Salazar DE, Bramer SL (2004). Pharmacokinetics, tolerability, and safety of aripiprazole following multiple oral dosing in normal healthy volunteers. J Clin Pharmacol 44: 179–187.
Mamo D, Graff A, Mizrahi R, Shammi CM, Romeyer F, Kapur S (2007). Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A) receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am J Psychiatry 164: 1411–1417.
Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y et al (2003). Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 23: 285–300.
Mukherjee J, Yang ZY, Das MK, Brown T (1995). Fluorinated benzamide neuroleptics--III. Development of (S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3-[18F]fluoropropyl)-2, 3-dimethoxybenzamide as an improved dopamine D-2 receptor tracer. Nucl Med Biol 22: 283–296.
Nordstrom AL, Farde L, Wiesel FA, Forslund K, Pauli S, Halldin C et al (1993). Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry 33: 227–235.
Nyberg S, Dencker SJ, Malm U, Dahl ML, Svenson JO, Halldin C et al (1998). D(2)- and 5-HT(2) receptor occupancy in high-dose neuroleptic-treated patients. Int J Neuropsychopharmacol 1: 95–101.
Olsson H, Farde L (2001). Potentials and pitfalls using high affinity radioligands in PET and SPET determinations on regional drug induced D2 receptor occupancy—a simulation study based on experimental data. Neuroimage 14: 936–945.
Ozdemir V, Fourie J, Ozdener F (2002). Aripiprazole (Otsuka Pharmaceutical Co). Curr Opin Investig Drugs 3: 113–120.
Pilowsky LS, Mulligan RS, Acton PD, Ell PJ, Costa DC, Kerwin RW (1997). Limbic selectivity of clozapine. Lancet 350: 490–491.
Rieck RW, Ansari MS, Whetsell Jr WO, Deutch AY, Kessler RM (2004). Distribution of dopamine D2-like receptors in the human thalamus: autoradiographic and PET studies. Neuropsychopharmacology 29: 362–372.
Robertson GS, Matsumura H, Fibiger HC (1994). Induction patterns of Fos-like immunoreactivity in the forebrain as predictors of atypical antipsychotic activity. J Pharmacol Exp Ther 271: 1058–1066.
Samama P, Cotecchia S, Costa T, Lefkowitz RJ (1993). A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem 268: 4625–4636.
Seamans JK, Yang CR (2004). The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 74: 1–58.
Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR et al (2003). Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology 28: 1400–1411.
Shim JC, Shin JG, Kelly DL, Jung DU, Seo YS, Liu KH et al (2007). Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebo-controlled trial. Am J Psychiatry 164: 1404–1410.
Talvik M, Nordstrom AL, Nyberg S, Olsson H, Halldin C, Farde L (2001). No support for regional selectivity in clozapine-treated patients: a PET study with [(11)C]raclopride and [(11)C]FLB 457. Am J Psychiatry 158: 926–930.
Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H et al (2007a). Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320: 1–13.
Urban JD, Vargas GA, von Zastrow M, Mailman RB (2007b). Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signaling pathways. Neuropsychopharmacology 32: 67–77.
Woods RP, Mazziotta JC, Cherry SR (1993). MRI-PET registration with automated algorithm. J Comput Assist Tomogr 17: 536–546.
Xiberas X, Martinot JL, Mallet L, Artiges E, Canal M, Loc’h C et al (2001). In vivo extrastriatal and striatal D2 dopamine receptor blockade by amisulpride in schizophrenia. J Clin Psychopharmacol 21: 207–214.
Yokoi F, Grunder G, Biziere K, Stephane M, Dogan AS, Dannals RF et al (2002). Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology 27: 248–259.
Acknowledgements
We thank Bristol-Myers Squibb for financial support; Erica Scher, Elisa Reich, and Erica Meyers for technical support; and Jonathan Javitch for fruitful scientific discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE/CONFLICTS OF INTEREST
Dr Kegeles has received research support from Pfizer. Dr Slifstein has received research support from GSK and is a consultant for GSK and Amgen. Dr Frankle has received research support from GSK and Sepracor, is a consultant or advisory board member for Sepracor, BMS, Transcept, Eli Lilly, and member of the Speaker's Bureau for BMS and Otsuka pharmaceuticals. Dr Abi-Dargham has received research support from Eli Lilly, BMS, and GSK, is a consultant or advisory board member for Sanofi-Aventis, BMS, Wanda, Eli Lilly, Intracellular Therapeutics, and member of the Speaker's Bureau for Sanofi-Aventis, BMS and, Otsuka pharmaceuticals.
Appendix
Appendix
Throughout the Appendix, the nomenclature of Innis et al (2007) is used: CS (specifically bound compartment concentration), CND (nondisplaceable compartment concentration), and fND (tissue free fraction) are as defined in that reference and BPP and BPND are as defined in the reference and in the Methods section above.
A.1: DERIVATION OF DOPAMINE OCCUPANCY RATIOS FROM REGIONAL DIFFERENCES IN ED80
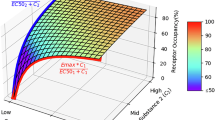
The assumptions in this estimating procedure are that (i) aripiprazole has the same affinity for all D2-like receptors in all regions, (ii) the concentration of dopamine in the proximity of the receptors is not substantially changed following aripiprazole treatment compared to baseline, and (iii) ED80 ratios between regions are equivalent to EC80 ratios between regions.
Let RA,S, RA,E, RDA,S, and RDA,E be the ratio of concentration to inhibition constant for aripiprazole (A) and dopamine (DA) in striatum (S) and extrastriatal regions (E). Then, occupancy in both region types is equal to

Let ED80(ROI) be the estimated ED80 for a given ROI type, striatal (STR) or extrastriatal (EXT). Then, at the ED80 concentrations, RDA,E is given by the affine function (line with non-zero intercept) of RDA,S

Figure 8 is a plot of RDA,E vs RDA,S for a range of RDA,S values using the ED80 ratio value of .693 observed in this data set generated from BPND values from 2TCM (Figure 4).
A.2: DRUG OCCUPANCY MEASURED BY PET WHEN DRUG CONCENTRATION IS CHANGING DURING THE SCAN
Theory
An underlying assumption used to derive aripiprazole occupancy from the fractional change in BP is that the bound drug concentration is constant during the scanning period. If this quantity is changing during the course of the scan, the apparent occupancy will then be a weighted sum of the dynamically changing occupancy over the course of the scan, and the weights will be influenced by time-varying free concentration of the radioligand. Free radioligand concentration will be different across brain regions, due to the ability of the receptor pool to act like a capacitance, removing ligand from the free compartment by specific binding in the early scan phase when free ligand exceeds the equilibrium binding point, and returning ligand to the free compartment in the later phase when specifically bound ligand exceeds the equilibrium point. The phenomenon will be more pronounced in high receptor density regions (striatum) than in low receptor density regions (cortex and limbic regions), leading to different weights in the estimated occupancies. In particular, if receptor-bound drug was in an approximately exponentially decreasing phase during the course of the scan, the following analysis and simulation show that this phenomenon could cause apparent differences in occupancy in the absence of true differences.
In addition to the standard modeling assumptions regarding rapid equilibration of radioligand free fractions and constant VND (nonspecific distribution volume) across regions, we also assume here that arterial plasma clearance of the radioligand is the same across conditions. This is only for computational simplification, and does not materially affect the result.
In this study, occupancy is measured as

where drug and baseline refer to the scan conditions. Based on the assumptions above and the fact that compartmental distribution volumes are equivalent to the ratio of the AUC to infinity of the compartment to the AUC of the arterial plasma, and denoting the specifically bound compartment CS, the right-hand side of this expression simplifies to

This is an identity when the modeling assumptions are met exactly. If receptor availability is changing over the course of the scan during the drug condition, the equation is an approximation in the sense that the fitting procedure will find the closest curve to the data from the set of all curves that fit the model equations by least squares minimization, and AUC(CS) of this curve will be influenced by, although not necessarily identical to, AUC(CS) of the data. The AUCs can be derived from the linearized mass action laws for the conditions. Denoting the nondisplaceable compartment as CND,

The parameter k3 equals the constant fND kon Bmax during the baseline condition. Its explicit time dependence applies during the drug occupancy condition. The effect of the drug is represented as a multiplicative factor (k3(t)) due to tracer conditions for the radioligand, so that the drug concentration is not perturbed by the presence of the tracer, and tracer ‘sees’ a time-varying receptor availability. The solution to this differential equation is

for the baseline condition and

for the drug condition, where ⊗ is the convolution operator and D(t) is 1−the fractional occupancy of receptors by the drug at time t. Using the stated assumptions and the properties of convolutions, the occupancy simplifies to

For the case that the bound drug is decreasing exponentially,

where occ0 is the occupancy at the beginning of the scan and the rate constant is λ=ln(2)/τ where τ is the half-life of the drug washout from the receptors. Substituting for D and simplifying leads to

Finally, noting AUC(CND(baseline)) is equal across regions, it can be seen that it is possible for occ0 to be the same across regions, but still have regional differences in estimated occupancy due to differences in AUC(e−λtCND(drug)). Apparent occupancy will therefore be affected by three factors: occ0 and the regional difference in Bmax, each of which influences the shape of CND during the drug condition, and τ, the half-life of the drug.
Simulations
To test the predictive power of this analysis, simulations were performed. Time activity curves were generated using a plasma input function and kinetic parameters similar to human [18F]fallypride parameters in anterior putamen and hippocampus for baseline conditions and for drug occupancy with exponential washout as above. Initial occupancy was tested at 60, 75, and 90%. Based on Figure 2 in Mallikaarjun et al (2004) (also see discussion section), drug off-rates were tested in the range of 10–20 h. For each initial occupancy and washout rate combination, 1000 pairs of curves were generated in each region with Gaussian noise according to the formula C(t) × (1+0.05z), where z was sampled from a standard normal distribution and C(t) was the noise-free time activity curve. Data were then fitted and occupancy computed according to the methods used in the study. Results (Table A1) show that both for the AUC-based prediction and the simulations, striatum occupancy was slightly but consistently less than hippocampus. The results also show that the AUC analysis is numerically closer to striatum fits than hippocampus; in hippocampus, the estimated occupancies are closer to the initial values than the AUC equation predicts. The differences are greatest at high (90%) initial occupancy, where the simulated regional differences are comparable to those seen in the aripiprazole study.
A.3: EFFECT OF D2/3 RECEPTORS IN THE REFERENCE REGION
This section of the Appendix shows that the presence of D2/3 receptors in the reference region would not differentially affect ROIs of low compared with high D2/3 receptor density. Let us assume the null hypothesis that there are a small amount of D2/3 receptors in the reference region, and that after aripiprizole, all regions have the same fractional occupancy by aripiprizole. Let α=1−occupancy, that is, if the baseline binding potential is BP, then after aripiprizole, it becomes αBP. Then at baseline,

and after drug,

and the apparent occupancy is

so that there is a bias (true occupancy=1−α) but it affects all regions to the same extent.
Rights and permissions
About this article
Cite this article
Kegeles, L., Slifstein, M., Frankle, W. et al. Dose–Occupancy Study of Striatal and Extrastriatal Dopamine D2 Receptors by Aripiprazole in Schizophrenia with PET and [18F]Fallypride. Neuropsychopharmacol 33, 3111–3125 (2008). https://doi.org/10.1038/npp.2008.33
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2008.33
Keywords
This article is cited by
-
Antipsychotic dose, dopamine D2 receptor occupancy and extrapyramidal side-effects: a systematic review and dose-response meta-analysis
Molecular Psychiatry (2023)
-
Therapeutic Reference Range for Aripiprazole in Schizophrenia Revised: a Systematic Review and Metaanalysis
Psychopharmacology (2022)
-
In vivo absolute quantification of striatal and extrastriatal D2/3 receptors with [123I]epidepride SPECT
EJNMMI Research (2020)
-
A positron emission tomography occupancy study of brexpiprazole at dopamine D2 and D3 and serotonin 5-HT1A and 5-HT2A receptors, and serotonin reuptake transporters in subjects with schizophrenia
Neuropsychopharmacology (2020)
-
Switching strategies for antipsychotic monotherapy in schizophrenia: a multi-center cohort study of aripiprazole
Psychopharmacology (2020)