Abstract
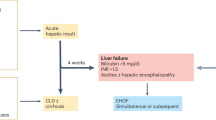
The definition of acute-on-chronic liver failure (ACLF) remains contested. In Europe and North America, the term is generally applied according to the European Association for the Study of the Liver-Chronic Liver Failure (EASL-CLIF) Consortium guidelines, which defines this condition as a syndrome that develops in patients with cirrhosis and is characterized by acute decompensation, organ failure and high short-term mortality. One-third of patients who are hospitalized for acute decompensation present with ACLF at admission or develop the syndrome during hospitalization. ACLF frequently occurs in a closed temporal relationship to a precipitating event, such as bacterial infection or acute alcoholic, drug-induced or viral hepatitis. However, no precipitating event can be identified in approximately 40% of patients. The mechanisms of ACLF involve systemic inflammation due to infections, acute liver damage and, in cases without precipitating events, probably intestinal translocation of bacteria or bacterial products. ACLF is graded into three stages (ACLF grades 1–3) on the basis of the number of organ failures, with higher grades associated with increased mortality. Liver and renal failures are the most common organ failures, followed by coagulation, brain, circulatory and respiratory failure. The 28-day mortality rate associated with ACLF is 30%. Depending on the grade, ACLF can be reversed using standard therapy in only 16–51% of patients, leaving a considerable proportion of patients with ACLF that remains steady or progresses. Liver transplantation in selected patients with ACLF grade 2 and ACLF grade 3 increases the 6-month survival from 10% to 80%.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ginés, P. et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology 7, 122–128 (1987).
Schrier, R. W. et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology 8, 1151–1157 (1998).
Fraser, C. L. & Arieff, A. I. Hepatic encephalopathy. N. Engl. J. Med. 313, 865–873 (1985).
Polio, J. & Groszmann, R. J. Hemodynamic factors involved in the development and rupture of esophageal varices: a pathophysiologic approach to treatment. Semin. Liver Dis. 6, 318–331 (1986).
Ramachandran, P., Iredale, J. P. & Fallowfield, J. A. Resolution of liver fibrosis: basic mechanisms and clinical relevance. Semin. Liver Dis. 35, 119–131 (2015).
Qin, N. et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 513, 59–64 (2014).
Bernardi, M., Moreau, R., Angeli, P., Schnabl, B. & Arroyo, V. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J. Hepatol. 63, 1272–1284 (2015).
Moreau, R. et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 144, 1426–1437.e9 (2013). This article describes the first prospective investigation assessing the diagnostic criteria (EASL-CLIF Consortium definition of ACLF), prevalence, precipitating events, grading of severity and prognosis of ACLF in a large series of European patients hospitalized with decompensated cirrhosis using a pragmatic approach.
Wlodzimirow, K. A., Eslami, S., Abu-Hanna, A., Nieuwoudt, M. & Chamuleau, R. A. F. M. A systematic review on prognostic indicators of acute on chronic liver failure and their predictive value for mortality. Liver Int. 33, 40–52 (2013).
Jalan, R. & Williams, R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif. 20, 252–261 (2002).
Sarin, S. K. et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL). Hepatol. Int. 3, 269–282 (2009). This article describes the results of a consensus conference promoted by the APASL aiming to assess the diagnostic definition of ACLF.
Sarin, S. K. et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol. Int. 8, 453–471 (2014).
Bajaj, J. S. et al. Survival in infection-related acute-on-chronic liver failure is defined by extra-hepatic organ failures. Hepatology 60, 250–256(2014). This study shows the prevalence of ACLF and associated mortality in a large series of patients with cirrhosis and bacterial infection from the United States.
Jalan, R. et al. Acute-on chronic liver failure. J. Hepatol. 57, 1336–1348 (2012).
Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Diseases and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. [Diagnostic and treatment guidelines for liver failure (2012 version)]. Zhonghua Gan Zang Bing Za Zhi 21, 177–183 (in Chinese) (2013).
Vincent, J. L. et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 22, 707–710 (1996).
Wehler, M., Kokoska, J., Reulbach, U., Hahn, E. G. & Strauss, R. Short-term prognosis in critically ill patients with cirrhosis assessed by prognostic scoring systems. Hepatology 34, 255–261 (2001).
Das, V. et al. Cirrhotic patients in the medical intensive care unit: early prognosis and long-term survival. Crit. Care Med. 38, 2108–2116 (2010).
Levesque, E. et al. Prospective evaluation of the prognostic scores for cirrhotic patients admitted to an intensive care unit. J. Hepatol. 56, 95–102 (2012).
Jalan, R. et al. Development and validation of a prognostic score to predict mortality in patients with acute on chronic liver failure. J. Hepatol. 61, 1038–1047 (2014). This study describes a prognostic score specifically designed for patients with ACLF. The accuracy of this score (CLIF-C ACLF score) is significantly higher than that of all scores currently used in clinical practice.
Bernal, W. et al. Acute-on-chronic liver failure. Lancet 386, 1576–1587 (2015).
Bernsmeier, C. et al. Patients with acute-on-chronic liver failure have increased numbers of regulatory immune cells expressing the receptor tyrosine kinase MERTK. Gastroenterology 148, 603–615.e14 (2015).
Zhang, Q. et al. Comparison of current diagnostic criteria for acute-on-chronic liver failure. PLoS ONE 10, e0122158 (2015).
Li, H. et al. Characteristics, diagnosis and prognosis of acute-on-chronic liver failure in cirrhosis associated to hepatitis B. Sci. Rep. 6, 25487 (2016). This study indicates that the EASL-CLIF Consortium definition of ACLF designed for European patients can also be used in Chinese patients with cirrhosis due to HBV infection with no major differences in prevalence, severity and prognosis.
Dirchwolf, M. et al. Immune dysfunction in cirrhosis: distinct cytokines phenotypes according to cirrhosis severity. Cytokine 77, 14–25 (2015).
Amarapurkar, D. et al. Acute-on-chronic liver failure: a prospective study to determine the clinical profile, outcome, and factors predicting mortality. Indian J. Gastroenterol. 34, 216–224 (2015).
Li, H. et al. Submassive hepatic necrosis distinguishes HBV-associated acute on chronic liver failure from cirrhotic patients with acute decompensation. J. Hepatol. 63, 50–59 (2015).
Singh, H. & Pai, C. G. Defining acute-on-chronic liver failure: east, west or middle ground? World J. Hepatol. 7, 2571–2577 (2015).
Agrawal, S., Duseja, A., Gupta, T., Dhiman, R. K. & Chawla, Y. Simple organ failure count versus CANONIC grading system for predicting mortality in acute-on-chronic liver failure. J. Gastroenterol. Hepatol. 30, 575–581 (2015).
Lee, M. et al. CLIF-SOFA scoring system accurately predicts short-term mortality in acutely decompensated patients with alcoholic cirrhosis: a retrospective analysis. Liver Int. 35, 46–57 (2015).
Dhiman, R. K., Agrawal, S., Gupta, T., Duseja, A. & Chawla, Y. Chronic Liver Failure-Sequential Organ Failure Assessment is better than the Asia-Pacific Association for the Study of Liver criteria for defining acute-on-chronic liver failure and predicting outcome. World J. Gastroenterol. 20, 14934–14941 (2014).
Kim, H. Y. et al. Characterization of acute-on-chronic liver failure and prediction of mortality in Asian patients with active alcoholism. J. Gastroenterol. Hepatol. 31, 427–433 (2016).
Kim, T. Y. & Kim, D. J. Acute-on-chronic liver failure. Clin. Mol. Hepatol. 19, 349–359 (2013).
Jalan, R. et al. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology 147, 4–10 (2014).
Kim, T. Y. et al. Characteristics and discrepancies in acute-on-chronic liver failure: need for a unified definition. PLoS ONE 11, e0146745 (2016). This study is the first to show that the EASL-CLIF Consortium definition and the APASL definition of ACLF include different populations of patients.
Sarin, S. K. & Choudhury, A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 13, 131–149 (2016).
Olson, J. C. & Kamath, P. S. Acute-on-chronic liver failure: concept, natural history, and prognosis. Curr. Opin. Crit. Care 17, 165–169 (2011).
Singh, K. K., Panda, S. K., & Shalimar Acharya, S. K. Patients with diabetes mellitus are prone to develop severe hepatitis and liver failure due to hepatitis virus infection. J. Clin. Exp. Hepatol. 3, 275–280 (2013).
Verbeke, L., Nevens, F. & Laleman, W. Bench-to-beside review: acute-on-chronic liver failure — linking the gut, liver and systemic circulation. Crit. Care 15, 233 (2011).
Suntharalingam, G. et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355, 1018–1028 (2006).
Medzhitov, R., Schneider, D. S. & Soares, M. P. Disease tolerance as a defense strategy. Science 335, 936–941 (2012).
Medzhitov, R. Origin and physiological roles of inflammation. Nature 454, 428–435 (2008).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Iwasaki, A. & Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 16, 343–353 (2015).
Wu, J. & Chen, Z. J. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 32, 461–488 (2014).
Xu, H. et al. Innate immune sensing of bacterial modifications of Rho GTPases by the pyrin inflammasome. Nature 513, 237–241 (2014).
Zhao, Y. et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600 (2011).
Martinon, F., Mayor, A. & Tschopp, J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 (2009).
Kono, H. & Rock, K. L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 8, 279–289 (2008).
Rickard, J. A. et al. RIPK1 regulates RIPK3–MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 157, 1175–1188 (2014).
Angus, D. C. & van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 369, 840–851 (2013).
Medzhitov, R. Inflammation 2010: new adventures of an old flame. Cell 140, 771–776 (2010).
Iwasaki, A. & Medzhitov, R. Regulation of adaptive immunity by the innate immune system. Science 327, 291–295 (2010).
Chovatiya, R. & Medzhitov, R. Stress, inflammation, and defense of homeostasis. Mol. Cell 54, 281–288 (2014).
Jalan, R. et al. Bacterial Infections in cirrhosis. A position statement based on the EASL Special Conference 2013. J. Hepatol. 60, 1310–1324(2014).
Wiest, R., Lawson, M. & Geuking, M. Pathological bacterial translocation in liver cirrhosis. J. Hepatol. 60, 197–209 (2014).
Byl, B., Roucloux, I., Crusiaux, A., Dupont, E. & Devière, J. Tumor necrosis factor alpha and interleukin 6 plasma levels in infected cirrhotic patients. Gastroenterology 104, 1492–1497 (1993).
Navasa, M. et al. Tumor necrosis factor and interleukin-6 in spontaneous bacterial peritonitis in cirrhosis: relationship with the development of renal impairment and mortality. Hepatology 27, 1227–1232 (1998). References 57 and 58 show for the first time an in vivo overproduction of pro-inflammatory cytokines in patients with cirrhosis.
Gustot, T., Durand, F., Lebrec, D., Vincent, J.-L. & Moreau, R. Severe sepsis in cirrhosis. Hepatology 50, 2022–2033 (2009).
Devière, J. et al. Excessive in vitro bacterial lipopolysaccharide-induced production of monokines in cirrhosis. Hepatology 11, 628–634 (1990). This paper shows for the first time that the ex vivo innate immune response to LPS is deregulated in monocytes from patients with alcoholic cirrhosis.
Le Moine, O. et al. Role of defective monocyte interleukin-10 release in tumor necrosis factor-alpha overproduction in alcoholics cirrhosis. Hepatology 22, 1436–1439 (1995).
Tazi, K. A. et al. Upregulation of TNF-alpha production signaling pathways in monocytes from patients with advanced cirrhosis: possible role of Akt and IRAK-M. J. Hepatol. 45, 280–289 (2006). This paper describes the intracellular mechanisms involved in the excessive innate immune response to LPS in monocytes from patients with decompensated cirrhosis.
Tazi, K. A. et al. Protein array technology to investigate cytokine production by monocytes from patients with advanced alcoholic cirrhosis: an ex vivo pilot study. Hepatol. Res. 39, 706–715 (2009).
Galbois, A. et al. Ex vivo effects of high-density lipoprotein exposure on the lipopolysaccharide-induced inflammatory response in patients with severe cirrhosis. Hepatology 49, 175–184 (2009).
Coant, N. et al. Glycogen synthase kinase 3 involvement in the excessive proinflammatory response to LPS in patients with decompensated cirrhosis. J. Hepatol. 55, 784–793 (2011).
Gandoura, S. et al. Gene- and exon-expression profiling reveals an extensive LPS-induced response in immune cells in patients with cirrhosis. J. Hepatol. 58, 936–948 (2013).
Heller, J. et al. Effects of lipopolysaccharide on TNFα production, hepatic NOS2 activity, and hepatic toxicity in rats with cirrhosis. J. Hepatol. 33, 376–381 (2000).
Moreau, R. et al. Terlipressin inhibits in vivo aortic iNOS expression induced by lipopolysaccharide in rats with biliary cirrhosis. Hepatology 36, 1070–1078 (2002).
Urbanowicz, W. et al. Tezosentan, an endothelin receptor antagonist, limits liver injury in endotoxin challenged cirrhotic rats. Gut 53, 1844–1849 (2004).
Tazi, K. A. et al. In vivo altered unfolded protein response and apoptosis in livers from lipopolysaccharide-challenged cirrhotic rats. J. Hepatol. 46, 1075–1088 (2007). This paper shows for the first time that LPS-induced hepatic endoplasmic reticulum stress inhibits the accumulation of NF-κB-dependent anti-apoptotic proteins in livers from rats with cirrhosis.
Thabut, D. et al. High-density lipoprotein administration attenuates liver proinflammatory response, restores liver endothelial nitric oxide synthase activity, and lowers portal pressure in cirrhotic rats. Hepatology 46, 1893–1906 (2007).
Malhi, H. & Kaufman, R. J. Endoplasmic reticulum stress in liver disease. J. Hepatol. 54, 795–809 (2011).
Chaisson, M. L., Brooling, J. T., Ladiges, W., Tsai, S. & Fausto, N. Hepatocyte-specific inhibition of NF-κB leads to apoptosis after TNF treatment, but not after partial hepatectomy. J. Clin. Invest. 110, 193–202 (2002).
Louvet, A. et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology 137, 541–548 (2009).
Lucey, M. R., Mathurin, P. & Morgan, T. R. Alcoholic hepatitis. N. Engl. J. Med. 360, 2758–2769 (2009).
Úbeda, M. et al. Obeticholic acid reduces bacterial translocation and inhibits intestinal inflammation in cirrhotic rats. J. Hepatol. 64, 1049–1057 (2015).
Du Plessis, J. et al. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J. Hepatol. 58, 1125–1132 (2013).
Francés, R. et al. Bacterial translocation is downregulated by anti-TNFα monoclonal antibody administration in rats with cirrhosis and ascites. J. Hepatol. 46, 797–803 (2007).
Dominguez, M. et al. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology 136, 1639–1650 (2009). This paper shows that neutrophil-attracting chemokines are overexpressed in livers from patients with severe alcoholic cirrhosis.
Charo, I. F. & Ransohoff, R. M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621 (2006).
Kubes, P. & Mehal, W. Z. Sterile inflammation in the liver. Gastroenterology 143, 1158–1172 (2012).
Larosche, I. et al. Prolonged ethanol administration depletes mitochondrial DNA in MnSOD-overexpressing transgenic mice, but not in their wild type littermates. Toxicol. Appl. Pharmacol. 234, 326–338 (2009).
Choumar, A. et al. Lipopolysaccharide-induced mitochondrial DNA depletion. Antioxid. Redox Signal. 15, 2837–2854 (2011).
West, A. P. et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520, 553–557 (2015).
Dubuquoy, L. et al. Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut 64, 1949–1960 (2015).
Brenner, D. A., Paik, Y.-H. & Schnabl, B. Role of gut microbiota in liver disease. J. Clin. Gastroenterol. 49, S25–S27 (2015).
Bajaj, J. S. et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J. Hepatol. 60, 940–947 (2014).
Bajaj, J. S. et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G168–G175 (2012).
Chen, Y. et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 54, 562–572 (2011).
Chen, Y. et al. Gut dysbiosis in acute-on-chronic liver failure and its predictive value for mortality. J. Gastroenterol. Hepatol. 30, 1429–1437 (2015).
Pan, C. et al. Dynamic changes of lipopolysaccharide levels in different phases of acute on chronic hepatitis B liver failure. PLoS ONE 7, e49460 (2012).
Bauer, T. M. et al. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am. J. Gastroenterol. 97, 2364–2370 (2002). This paper shows an association between microbiota alterations and systemic endotoxaemia in patients with cirrhosis.
Zapater, P. et al. Serum and ascitic fluid bacterial DNA: a new independent prognostic factor in noninfected patients with cirrhosis. Hepatology 48, 1924–1931 (2008).
McPhail, M. J. W. et al. Increased survival for patients with cirrhosis and organ failure in liver intensive care and validation of the Chronic Liver Failure-Sequential Organ Failure Scoring System. Clin. Gastroenterol. Hepatol. 13, 1353–1360.e8 (2015).
Silva, P. E. S. E. et al. Single-centre validation of the EASL-CLIF Consortium definition of acute-on-chronic liver failure and CLIF-SOFA for prediction of mortality in cirrhosis. Liver Int. 35, 1516–1523 (2015).
Gustot, T. et al. Clinical course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology 62, 243–252 (2015). This study is the first investigation defining the clinical course of ACLF within 28 days following diagnosis. It shows that ACLF is an extremely dynamic syndrome that may improve, worsen or follow a steady course. Prognosis is highly dependent on the clinical course within the first week after diagnosis.
Shi, Y. et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology 62, 232–242 (2015). This study confirms the results of the CANONIC study in European patients, showing that short-term (28-day and 90-day) mortality in Asian patients with ACLF depends on the number of organ failures and not on the aetiology (the type of precipitating events) of the syndrome.
Jalan, R. et al. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J. Hepatol. 62, 831–840 (2015).
Kamath, P. S. & Kim, W. R. The model for end-stage liver disease (MELD). Hepatology 45, 797–805 (2007).
Durand, F. & Valla, D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J. Hepatol. 42, S100–S107 (2005).
Biggins, S. W. Use of serum sodium for liver transplant graft allocation: a decade in the making, now is it ready for primetime? Liver Transpl. 21, 279–281 (2015).
Fernandez, J. & Arroyo, V. Bacterial infections in cirrhosis: a growing problem with significant implications. Clin. Liver Dis. 2, 102–105 (2013).
Sarin, S. K. et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol. Int. 10, 1–98 (2016).
Sort, P. et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N. Engl. J. Med. 341, 403–409 (1999). This randomized controlled trial was the first to show that intravenous albumin administration (1.5 g per kg of body weight at infection diagnosis and 1 g per kg of body weight at the third day) is highly effective in preventing type 1 HRS and mortality in patients with cirrhosis and spontaneous bacterial peritonitis.
Arroyo, V., García-Martinez, R. & Salvatella, X. Human serum albumin, systemic inflammation, and cirrhosis. J. Hepatol. 61, 396–407 (2014).
Guevara, M. et al. Albumin for bacterial infections other than spontaneous bacterial peritonitis in cirrhosis. A randomized, controlled study. J. Hepatol. 57, 759–765 (2012).
Thévenot, T. et al. Effect of albumin in cirrhotic patients with infection other than spontaneous bacterial peritonitis. A randomized trial. J. Hepatol. 62, 822–830 (2015).
Ginés, P. et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: results of a double-blind, placebo-controlled trial. Hepatology 12, 716–724 (1990).
Soriano, G. et al. Norfloxacin prevents bacterial infection in cirrhotics with gastrointestinal hemorrhage. Gastroenterology 103, 1267–1272 (1992).
Fernández, J. et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology 133, 818–824 (2007). This randomized controlled trial was the first to show that long-term oral administration of norfloxacin prevents the development of spontaneous bacterial peritonitis and type 1 HRS and improves survival in patients with cirrhosis and severe liver and renal dysfunction.
Zapater, P. et al. Norfloxacin modulates the inflammatory response and directly affects neutrophils in patients with decompensated cirrhosis. Gastroenterology 137, 1669–1679.e1 (2009).
Gómez-Hurtado, I. et al. Interleukin-10-mediated heme oxygenase 1-induced underlying mechanism in inflammatory down-regulation by norfloxacin in cirrhosis. Hepatology 53, 935–944 (2011).
Gómez-Hurtado, I. et al. Role of interleukin 10 in norfloxacin prevention of luminal free endotoxin translocation in mice with cirrhosis. J. Hepatol. 61, 799–808 (2014).
Akriviadis, E. et al. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 119, 1637–1648 (2000).
Nguyen-Khac, E. et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N. Engl. J. Med. 365, 1781–1789 (2011).
Thursz, M. R. et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N. Engl. J. Med. 372, 1619–1628 (2015).
Kedarisetty, C. K. et al. Combination of granulocyte colony-stimulating factor and erythropoietin improves outcomes of patients with decompensated cirrhosis. Gastroenterology 148, 1362–1370.e7 (2015).
Arroyo, V., Moreau, R., Jalan, R. & Ginès, P. Acute-on-chronic liver failure: a new syndrome that will re-classify cirrhosis. J. Hepatol. 62, S131–S143 (2015).
Seto, W.-K., Lai, C.-L. & Yuen, M.-F. Acute-on-chronic liver failure in chronic hepatitis B. J. Gastroenterol. Hepatol. 27, 662–669 (2012).
Garg, H. et al. Tenofovir improves the outcome in patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. Hepatology 53, 774–780 (2011).
Philips, C. A. & Sarin, S. K. Potent antiviral therapy improves survival in acute on chronic liver failure due to hepatitis B virus reactivation. World J. Gastroenterol. 20, 16037–16052 (2014). The results of this study are a clear indication that potent antiviral therapy significantly improves the clinical course and survival of patients with ACLF due to reactivation of HBV.
Arabi, Y. M. et al. Antimicrobial therapeutic determinants of outcomes from septic shock among patients with cirrhosis. Hepatology 56, 2305–2315 (2012).
Tandon, P. & Garcia-Tsao, G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin. Liver Dis. 28, 26–42 (2008).
Fernández, J., Tandon, P., Mensa, J. & Garcia-Tsao, G. Antibiotic prophylaxis in cirrhosis: good and bad. Hepatology 63, 2019–2031(2016).
Chen, T. et al. Nucleoside analogues improve the short-term and long-term prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure. Clin. Exp. Med. 12, 159–164 (2012).
Xie, F. et al. Effects of nucleoside analogue on patients with chronic hepatitis B-associated liver failure: meta-analysis. PLoS ONE 8, e54773 (2013).
Yang, J. et al. Initial combination anti-viral therapy with lamivudine and adefovir dipivoxil decreases short-term fatality rate of hepatitis-B-virus-related acute-on-chronic liver failure. Virol. J. 12, 97 (2015).
Garg, V. et al. Granulocyte colony-stimulating factor mobilizes CD34+ cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology 142, 505–512.e1 (2012).
Angeli, P. et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J. Hepatol. 62, 968–974 (2015). This article provides the most modern clinical guidelines on the diagnosis and treatment of AKI in cirrhosis.
Belcher, J. M. et al. Urinary biomarkers and progression of AKI in patients with cirrhosis. Clin. J. Am. Soc. Nephrol. 9, 1857–1867 (2014).
Rivers, E. et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 345, 1368–1377 (2001).
Fede, G. et al. Adrenocortical dysfunction in liver disease: a systematic review. Hepatology 55, 1282–1291 (2012).
Vilstrup, H. et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 60, 715–735 (2014).
Bianchini, M., De Pietri, L. & Villa, E. Coagulopathy in liver diseases: complication or therapy? Dig. Dis. 32, 609–614 (2014).
de Franchis, R. & Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J. Hepatol. 63, 743–752 (2015). This article provides the most modern clinical guidelines on the diagnosis management of portal hypertension and gastrointestinal haemorrhage in cirrhosis. There is a detailed description on the management of coagulopathy in patients with portal vein thrombosis.
Lee, S. Y., Kim, H. J. & Choi, D. Cell sources, liver support systems and liver tissue engineering: alternatives to liver transplantation. Int. J. Stem Cells 8, 36–47 (2015).
Struecker, B., Raschzok, N. & Sauer, I. M. Liver support strategies: cutting-edge technologies. Nat. Rev. Gastroenterol. Hepatol. 11, 166–176 (2014). This article contains a comprehensive review of the artificial liver support strategies in patients with acute liver failure and ACLF.
Bañares, R. et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology 57, 1153–1162 (2013).
Kribben, A. et al. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology 142, 782–789.e3 (2012).
Lee, K. C. L. et al. Extracorporeal liver assist device to exchange albumin and remove endotoxin in acute liver failure: results of a pivotal pre-clinical study. J. Hepatol. 63, 634–642 (2015).
Larsen, F. S. et al. High-volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. J. Hepatol. 64, 69–78 (2016).
Bahirwani, R., Shaked, O., Bewtra, M., Forde, K. & Reddy, K. R. Acute-on-chronic liver failure before liver transplantation: impact on posttransplant outcomes. Transplantation 92, 952–957 (2011).
Duan, B.-W. et al. Liver transplantation in acute-on-chronic liver failure patients with high model for end-stage liver disease (MELD) scores: a single center experience of 100 consecutive cases. J. Surg. Res. 183, 936–943 (2013).
Finkenstedt, A. et al. Acute-on-chronic liver failure: excellent outcomes after liver transplantation but high mortality on the wait list. Liver Transpl. 19, 879–886 (2013).
Sharma, P., Schaubel, D. E., Gong, Q., Guidinger, M. & Merion, R. M. End-stage liver disease candidates at the highest model for end-stage liver disease scores have higher wait-list mortality than status-1A candidates. Hepatology 55, 192–198 (2012).
Chan, A. C. et al. Liver transplantation for acute-on-chronic liver failure. Hepatol. Int. 3, 571–581 (2009).
Chan, A. C. Y. & Fan, S. T. Criteria for liver transplantation in ACLF and outcome. Hepatol. Int. 9, 355–359 (2015).
Reddy, M. S., Rajalingam, R. & Rela, M. Liver transplantation in acute-on-chronic liver failure: lessons learnt from acute liver failure setting. Hepatol. Int. 9, 508–513 (2015).
Khanam, A. et al. Altered frequencies of dendritic cells and IFN-γ-secreting T cells with granulocyte colony-stimulating factor (G-CSF) therapy in acute-on- chronic liver failure. Liver Int. 34, 505–513 (2014).
Duan, X.-Z. et al. Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic liver failure. World J. Gastroenterol. 19, 1104–1110 (2013).
Ma, X.-R. et al. Transplantation of autologous mesenchymal stem cells for end-stage liver cirrhosis: a meta-analysis based on seven controlled trials. Gastroenterol. Res. Pract. 2015, 908275 (2015).
Volk, M. L., Tocco, R. S., Bazick, J., Rakoski, M. O. & Lok, A. S. Hospital readmissions among patients with decompensated cirrhosis. Am. J. Gastroenterol. 107, 247–252 (2012).
Reddy, K. R. et al. High risk of delisting or death in liver transplant candidates following infections: results from the North American Consortium for the Study of End-Stage Liver Disease. Liver Transpl. 21, 881–888 (2015).
O'Leary, J. G. et al. Long-term use of antibiotics and proton pump inhibitors predict development of infections in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 13, 753–759.e2 (2015).
Battle, C. E., Davies, G. & Evans, P. A. Long term health-related quality of life in survivors of sepsis in south west Wales: an epidemiological study. PLoS ONE 9, e116304 (2014).
Vanwijngaerden, Y.-M. et al. Critical illness evokes elevated circulating bile acids related to altered hepatic transporter and nuclear receptor expression. Hepatology 54, 1741–1752 (2011).
Katoonizadeh, A. et al. Early features of acute-on-chronic alcoholic liver failure: a prospective cohort study. Gut 59, 1561–1569 (2010).
Hotchkiss, R. S., Monneret, G. & Payen, D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 13, 862–874 (2013).
Gomez, H. et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 41, 3–11 (2014). This article contains a comprehensive review of the mechanisms of AKI associated with systemic inflammation in sepsis. Concepts might be extended to AKI and other types of organ failure in ACLF.
Jiménez, W., Clária, J., Arroyo, V. & Rodés, J. Carbon tetrachloride induced cirrhosis in rats: a useful tool for investigating the pathogenesis of ascites in chronic liver disease. J. Gastroenterol. Hepatol. 7, 90–97 (1992).
Harry, D. et al. Increased sensitivity to endotoxemia in the bile duct-ligated cirrhotic rat. Hepatology 30, 1198–1205 (1999).
Bass, N. M. et al. Rifaximin treatment in hepatic encephalopathy. N. Engl. J. Med. 362, 1071–1081 (2010). This article reports a randomized controlled trial showing that oral rifaximin is highly effective in preventing the recurrence of hepatic encephalopathy in cirrhosis.
Bernardi, M. et al. Long-term use of human albumin for the treatment of ascites in patients with hepatic cirrhosis: the interim analysis of the ANSWER study. Dig. Liv. Dis. 47 (Suppl. 1), e6 (2015).
Neuschwander-Tetri, B. A. et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–965 (2015).
Verbeke, L. et al. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology 59, 2286–2298 (2014).
Verbeke, L. et al. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. Am. J. Pathol. 185, 409–419 (2015). This article reports a study in experimental cirrhosis showing that oral obeticholic acid improves gut permeability, intestinal inflammation and bacterial translocation.
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Broz, P. Immunology: caspase target drives pyroptosis. Nature 526, 642–643 (2015).
Sargenti, K., Prytz, H., Nilsson, E. & Kalaitzakis, E. Predictors of mortality among patients with compensated and decompensated liver cirrhosis: the role of bacterial infections and infection-related acute-on-chronic liver failure. Scand. J. Gastroenterol. 50, 875–883 (2015).
Blei, A. T. & Córdoba, J. Hepatic encephalopathy. Am. J. Gastroenterol. 96, 1968–1976 (2001).
Acknowledgements
The European Association for the Study of the Liver-Chronic Liver Failure (EASL-CLIF) Consortium is endorsed by the European Association for the Study of the Liver and supported by an unrestricted grant from Grifols. The authors thank D. J. Kim for the supply of the published Korean data and the Data Management Centre of the EASL-CLIF Consortium for providing the unpublished European data used in Figure 2.
Author information
Authors and Affiliations
Contributions
Introduction (V.A.); Epidemiology (P.S.K.); Mechanisms/pathophysiology (R.M. and B.S.); Diagnosis, screening and prevention (P.G., V.A. and J.F.); Management (R.J., G.G.-T., U.T. and J.F.); Quality of life (P.S.K.); Outlook (F.N. and V.A.); Overview of Primer (V.A.). V.A. and R.M. contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
V.A. has received research funding from Grifols and has served on the scientific advisory board for Takeda. P.G. has received research funding from Grifols, served on the scientific advisory board for Ferring and Squana Medical and received research funding from Sequana Medical. R.J. has received research funding from Vital Therapies, has served on the scientific advisory board for Conatus Pharma and Takeda, has ongoing research collaborations with Gambro and Grifiols and is the principal investigator of an industry sponsored study (Sequana Medical). F.N. has served on the scientific advisory board of Center Fract, Croix Rouge Belgium, Intercept, Gore, Bristol-Myers Squibb, AbbVie, Novartis, MSD, Janssen-Cilag, Promethera Biosciences and Gilead, and has received grants from Roche, Astellas, Ferring, Novartis, Janssen-Cilag and AbbVie. All other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Arroyo, V., Moreau, R., Kamath, P. et al. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers 2, 16041 (2016). https://doi.org/10.1038/nrdp.2016.41
Published:
DOI: https://doi.org/10.1038/nrdp.2016.41
This article is cited by
-
Lebererkrankungen auf der Intensivstation
Medizinische Klinik - Intensivmedizin und Notfallmedizin (2024)
-
A novel prognostic model to predict mortality in patients with acute-on-chronic liver failure in intensive care unit
Internal and Emergency Medicine (2024)
-
Cell therapy in end-stage liver disease: replace and remodel
Stem Cell Research & Therapy (2023)
-
Partial splenic embolization as a rescue and emergency treatment for portal hypertension and gastroesophageal variceal hemorrhage
BMC Gastroenterology (2023)
-
Risk factors for the mortality of hepatitis B virus-associated acute-on-chronic liver failure: a systematic review and meta-analysis
BMC Gastroenterology (2023)