Key Points
-
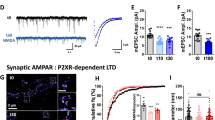
Long-term depression (LTD) encompasses a family of synaptic plasticity mechanisms that can be triggered by the synaptic or pharmacological activation of glutamate receptors — in particular NMDARs (N-methyl-D-aspartate receptors) and metabotropic glutamate receptors (mGluRs) — or receptors for other neurotransmitters.
-
LTD is expressed by a long-lasting decrease in the efficiency of synaptic transmission, in particular synaptic transmission that is mediated by the synaptic activation of AMPARs (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors). It may involve presynaptic and postsynaptic mechanisms.
-
Complex signalling cascades link the induction of LTD to its expression. The cascades involve Ca2+ sensors, protein–protein interactions, protein kinases and phosphatases, proteases and other signalling molecules.
-
Historically, a lack of specific inhibitors for LTD has hampered efforts to specify its functional role. The recent development of interference peptide inhibitors targeted at the carboxyl tail of the AMPAR subunit GluA2 subunit has proven important in specifying the functional roles of LTD.
-
LTD has diverse roles in cognition, particularly in some forms of learning and memory and in circumstances in which a flexible response is required.
-
LTD also seems to be involved in pathological states, including drug addiction, mental retardation and neurodegenerative diseases such as Alzheimer's disease.
Abstract
Long-term depression (LTD) in the CNS has been the subject of intense investigation as a process that may be involved in learning and memory and in various pathological conditions. Several mechanistically distinct forms of this type of synaptic plasticity have been identified and their molecular mechanisms are starting to be unravelled. Most studies have focused on forms of LTD that are triggered by synaptic activation of either NMDARs (N-methyl-D-aspartate receptors) or metabotropic glutamate receptors (mGluRs). Converging evidence supports a crucial role of LTD in some types of learning and memory and in situations in which cognitive demands require a flexible response. In addition, LTD may underlie the cognitive effects of acute stress, the addictive potential of some drugs of abuse and the elimination of synapses in neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bliss, T., Collingridge, G. L. & Morris, R. in The Hippocampus Book (eds. Anderson, P., Morris R., Amaral, D., Bliss, T. & O'Keefe, J.) 343–476 (Oxford Univ. Press, 2007).
Collingridge, G. L., Olsen, R. W., Peters, J. & Spedding, M. A nomenclature for ligand-gated ion channels. Neuropharmacology 56, 2–5 (2009).
Fujii, S., Saito, K., Miyakawa, H., Ito, K. & Kato, H. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Res. 555, 112–122 (1991).
Dudek, S. M. & Bear, M. F. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc. Natl Acad. Sci. USA 89, 4363–4367 (1992).
Collingridge, G. L., Kehl, S. J. & McLennan, H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J. Physiol. 334, 33–46 (1983).
Collingridge, G. L., Kehl, S. J., Loo, R. & McLennan, H. Effects of kainic and other amino acids on synaptic excitation in rat hippocampal slices: 1. Extracellular analysis. Exp. Brain Res. 52, 170–178 (1983).
Lee, H. K., Kameyama, K., Huganir, R. L. & Bear, M. F. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron 21, 1151–1162 (1998).
Kameyama, K., Lee, H. K., Bear, M. F. & Huganir, R. L. Involvement of a postsynaptic protein kinase A substrate in the expression of homosynaptic long-term depression. Neuron 21, 1163–1175 (1998).
Morishita, W. et al. Regulation of synaptic strength by protein phosphatase 1. Neuron 32, 1133–1148 (2001).
Ulbrich, M. H. & Isacoff, E. Y. Rules of engagement for NMDA receptor subunits. Proc. Natl Acad. Sci. USA 105, 14163–14168 (2008).
Collingridge, G. L., Isaac, J. T. & Wang, Y. T. Receptor trafficking and synaptic plasticity. Nature Rev. Neurosci. 5, 952–962 (2004).
Wong, T. P. et al. Hippocampal long-term depression mediates acute stress-induced spatial memory retrieval impairment. Proc. Natl Acad. Sci. USA 104, 11471–11476 (2007). This paper describes experiments that show that effects of acute stress on hippocampal LTD cause spatial memory retrieval deficits.
Duffy, S., Labrie, V. & Roder, J. C. D-serine augments NMDA-NR2B receptor-dependent hippocampal long-term depression and spatial reversal learning. Neuropsychopharmacology 33, 1004–1018 (2008).
Brigman, J. L. et al. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J. Neurosci. 30, 4590–4600 (2010). This study demonstrates the essential role of the hippocampal GluN2B subunit in LTD induction and provides strong evidence to support an important role of hippocampal LTD in spatial memory.
Bartlett, T. E. et al. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology 52, 60–70 (2007).
Morishita, W. et al. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology 52, 71–76 (2007).
Yashiro, K. & Philpot, B. D. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology 55, 1081–1094 (2008).
Vasuta, C. et al. Effects of exercise on NMDA receptor subunit contributions to bidirectional synaptic plasticity in the mouse dentate gyrus. Hippocampus 17, 1201–1208 (2007).
Woo, N. H. et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nature Neurosci. 8, 1069–1077 (2005).
Kim, J. J., Foy, M. R. & Thompson, R. F. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proc. Natl Acad. Sci. USA 93, 4750–4753 (1996).
Yang, C. H., Huang, C. C. & Hsu, K. S. Behavioral stress enhances hippocampal CA1 long-term depression through the blockade of the glutamate uptake. J. Neurosci. 25, 4288–4293 (2005).
Xu, L., Anwyl, R. & Rowan, M. J. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature 387, 497–500 (1997).
Staubli, U. & Scafidi, J. Studies on long-term depression in area CA1 of the anesthetized and freely moving rat. J. Neurosci. 17, 4820–4828 (1997).
Kemp, A. & Manahan-Vaughan, D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 30, 111–118 (2007).
Manahan-Vaughan, D., Kulla, A. & Frey, J. U. Requirement of translation but not transcription for the maintenance of long-term depression in the CA1 region of freely moving rats. J. Neurosci. 20, 8572–8576 (2000).
Manahan-Vaughan, D. Long-term depression in freely moving rats is dependent upon strain variation, induction protocol and behavioral state. Cereb. Cortex 10, 482–487 (2000).
Massey, P. V. & Bashir, Z. I. Long-term depression: multiple forms and implications for brain function. Trends Neurosci. 30, 176–184 (2007).
Palmer, M. J., Irving, A. J., Seabrook, G. R., Jane, D. E. & Collingridge, G. L. The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology 36, 1517–1532 (1997).
Stanton, P. K., Chattarji, S. & Sejnowski, T. J. 2-Amino-3-phosphonopropionic acid, an inhibitor of glutamate-stimulated phosphoinositide turnover, blocks induction of homosynaptic long-term depression, but not potentiation, in rat hippocampus. Neurosci. Lett. 127, 61–66 (1991).
Bashir, Z. I., Jane, D. E., Sunter, D. C., Watkins, J. C. & Collingridge, G. L. Metabotropic glutamate receptors contribute to the induction of long-term depression in the CA1 region of the hippocampus. Eur. J. Pharmacol. 239, 265–266 (1993).
Bolshakov, V. Y. & Siegelbaum, S. A. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science 264, 1148–1152 (1994).
Conquet, F. et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature 372, 237–243 (1994).
Yokoi, M. et al. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science 273, 645–647 (1996).
Cho, K. et al. A new form of long-term depression in the perirhinal cortex. Nature Neurosci. 3, 150–156 (2000).
Laezza, F., Doherty, J. J. & Dingledine, R. Long-term depression in hippocampal interneurons: joint requirement for pre- and postsynaptic events. Science 285, 1411–1414 (1999).
Park, Y., Jo, J., Isaac, J. T. & Cho, K. Long-term depression of kainate receptor-mediated synaptic transmission. Neuron 49, 95–106 (2006).
Berretta, N. & Cherubini, E. A novel form of long-term depression in the CA1 area of the adult rat hippocampus independent of glutamate receptors activation. Eur. J. Neurosci. 10, 2957–2963 (1998).
Volk, L. J., Pfeiffer, B. E., Gibson, J. R. & Huber, K. M. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J. Neurosci. 27, 11624–11634 (2007).
Harney, S. C., Rowan, M. & Anwyl, R. Long-term depression of NMDA receptor-mediated synaptic transmission is dependent on activation of metabotropic glutamate receptors and is altered to long-term potentiation by low intracellular calcium buffering. J. Neurosci. 26, 1128–1132 (2006).
Jin, Y., Kim, S. J., Kim, J., Worley, P. F. & Linden, D. J. Long-term depression of mGluR1 signaling. Neuron 55, 277–287 (2007). The first demonstration that synaptic responses mediated by the activation of mGlu receptors can undergo mGluR-LTD.
Morishita, W., Marie, H. & Malenka, R. C. Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. Nature Neurosci. 8, 1043–1050 (2005).
Nagerl, U. V., Eberhorn, N., Cambridge, S. B. & Bonhoeffer, T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron 44, 759–767 (2004).
Zhou, Q., Homma, K. J. & Poo, M. M. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 44, 749–757 (2004).
Becker, N., Wierenga, C. J., Fonseca, R., Bonhoeffer, T. & Nagerl, U. V. LTD induction causes morphological changes of presynaptic boutons and reduces their contacts with spines. Neuron 60, 590–597 (2008).
Wang, X. B., Yang, Y. & Zhou, Q. Independent expression of synaptic and morphological plasticity associated with long-term depression. J. Neurosci. 27, 12419–12429 (2007).
Enoki, R., Hu, Y. L., Hamilton, D. & Fine, A. Expression of long-term plasticity at individual synapses in hippocampus is graded, bidirectional, and mainly presynaptic: optical quantal analysis. Neuron 62, 242–253 (2009).
Stanton, P. K. et al. Long-term depression of presynaptic release from the readily releasable vesicle pool induced by NMDA receptor-dependent retrograde nitric oxide. J. Neurosci. 23, 5936–5944 (2003).
Foy, M. R., Stanton, M. E., Levine, S. & Thompson, R. F. Behavioral stress impairs long-term potentiation in rodent hippocampus. Behav. Neural Biol. 48, 138–149 (1987).
Feinmark, S. J. et al. 12-lipoxygenase metabolites of arachidonic acid mediate metabotropic glutamate receptor-dependent long-term depression at hippocampal CA3–CA1 synapses. J. Neurosci. 23, 11427–11435 (2003).
Gerdeman, G. L., Ronesi, J. & Lovinger, D. M. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nature Neurosci. 5, 446–451 (2002).
Nevian, T. & Sakmann, B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J. Neurosci. 26, 11001–11013 (2006).
Qiu, D. L. & Knopfel, T. Presynaptically expressed long-term depression at cerebellar parallel fiber synapses. Pflugers Arch. 457, 865–875 (2009).
Yasuda, H., Huang, Y. & Tsumoto, T. Regulation of excitability and plasticity by endocannabinoids and PKA in developing hippocampus. Proc. Natl Acad. Sci. USA 105, 3106–3111 (2008).
Chevaleyre, V. & Castillo, P. E. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron 43, 871–881 (2004).
Kandler, K., Katz, L. C. & Kauer, J. A. Focal photolysis of caged glutamate produces long-term depression of hippocampal glutamate receptors. Nature Neurosci. 1, 119–123 (1998).
Rammes, G. et al. Activation of mGlu receptors induces LTD without affecting postsynaptic sensitivity of CA1 neurons in rat hippocampal slices. J. Physiol. 546, 455–460 (2003).
Luthi, A. et al. Bi-directional modulation of AMPA receptor unitary conductance by synaptic activity. BMC Neurosci. 5, 44 (2004).
Ito, M. Experimental verification of Marr–Albus' plasticity assumption for the cerebellum. Acta Biol. Acad. Sci. Hung. 33, 189–199 (1982).
Snyder, E. M. et al. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nature Neurosci. 4, 1079–1085 (2001).
Mulkey, R. M., Herron, C. E. & Malenka, R. C. An essential role for protein phosphatases in hippocampal long-term depression. Science 261, 1051–1055 (1993).
Nishiyama, M., Hong, K., Mikoshiba, K., Poo, M. M. & Kato, K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature 408, 584–588 (2000).
Palmer, C. L. et al. Hippocalcin functions as a calcium sensor in hippocampal LTD. Neuron 47, 487–494 (2005). This paper identified a role for the high-affinity Ca2+ sensor hippocalcin (a member of the NCS family) in NMDAR-LTD.
Han, K. et al. Regulated RalBP1 binding to RalA and PSD-95 controls AMPA receptor endocytosis and LTD. PLoS. Biol. 7, e1000187 (2009).
Brown, T. C., Tran, I. C., Backos, D. S. & Esteban, J. A. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron 45, 81–94 (2005).
Davidson, H. T., Xiao, J., Dai, R. & Bergson, C. Calcyon is necessary for activity-dependent AMPA receptor internalization and LTD in CA1 neurons of hippocampus. Eur. J. Neurosci. 29, 42–54 (2009).
Hanley, J. G. & Henley, J. M. PICK1 is a calcium-sensor for NMDA-induced AMPA receptor trafficking. EMBO J. 24, 3266–3278 (2005).
Lin, D. T. & Huganir, R. L. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. J. Neurosci. 27, 13903–13908 (2007).
Peineau, S. et al. A systematic investigation of the protein kinases involved in NMDA receptor-dependent LTD: evidence for a role of GSK-3 but not other serine/threonine kinases. Mol. Brain 2, 22 (2009).
Oliet, S. H., Malenka, R. C. & Nicoll, R. A. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron 18, 969–982 (1997).
Thorsen, T. S. et al. Identification of a small-molecule inhibitor of the PICK1 PDZ domain that inhibits hippocampal LTP and LTD. Proc. Natl Acad. Sci. USA 107, 413–418 (2010).
Terashima, A. et al. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron 57, 872–882 (2008).
Emond, M. R. et al. AMPA receptor subunits define properties of state-dependent synaptic plasticity. J. Physiol. 588, 1929–1946 (2010).
Rocca, D. L., Martin, S., Jenkins, E. L. & Hanley, J. G. Inhibition of Arp2/3-mediated actin polymerization by PICK1 regulates neuronal morphology and AMPA receptor endocytosis. Nat. Cell Biol. 10, 259–271 (2008).
Brandon, E. P. et al. Hippocampal long-term depression and depotentiation are defective in mice carrying a targeted disruption of the gene encoding the RIβ subunit of cAMP-dependent protein kinase. Proc. Natl Acad. Sci. USA 92, 8851–8855 (1995).
Ohshima, T. et al. Impairment of hippocampal long-term depression and defective spatial learning and memory in p35 mice. J. Neurochem. 94, 917–925 (2005).
Zhu, Y. et al. Rap2-JNK removes synaptic AMPA receptors during depotentiation. Neuron 46, 905–916 (2005).
Peineau, S. et al. LTP inhibits LTD in the hippocampus via regulation of GSK3β. Neuron 53, 703–717 (2007). The first evidence of the involvement of glycogen synthase kinase-3 in NMDAR-LTD, providing a molecular link between NMDAR-LTD and various pathologies, including Alzheimer's disease.
Li, Z. et al. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell 141, 859–871 (2010). This paper directly demonstrates that a cascade involving cytochrome c , caspase-9 and caspase-3 is involved in NMDAR-LTD.
Ahmadian, G. et al. Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD. EMBO J. 23, 1040–1050 (2004).
Bhattacharyya, S., Biou, V., Xu, W., Schluter, O. & Malenka, R. C. A critical role for PSD-95/AKAP interactions in endocytosis of synaptic AMPA receptors. Nature Neurosci. 12, 172–181 (2009).
Kim, M. J. et al. Synaptic accumulation of PSD-95 and synaptic function regulated by phosphorylation of serine-295 of PSD-95. Neuron 56, 488–502 (2007).
Wu, L. J. et al. DREAM (Downstream Regulatory Element Antagonist Modulator) contributes to synaptic depression and contextual fear memory. Mol. Brain 3, 3 (2010).
Gladding, C. M., Fitzjohn, S. M. & Molnar, E. Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacol. Rev. 61, 395–412 (2009).
Luscher, C. & Huber, K. M. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron 65, 445–459 (2010).
Linden, D. J. & Connor, J. A. Participation of postsynaptic PKC in cerebellar long-term depression in culture. Science 254, 1656–1659 (1991).
Jo, J. et al. Metabotropic glutamate receptor-mediated LTD involves two interacting Ca(2+) sensors, NCS-1 and PICK1. Neuron 60, 1095–1111 (2008).
Fitzjohn, S. M. et al. A characterisation of long-term depression induced by metabotropic glutamate receptor activation in the rat hippocampus in vitro. J. Physiol. 537, 421–430 (2001).
Schnabel, R., Kilpatrick, I. C. & Collingridge, G. L. An investigation into signal transduction mechanisms involved in DHPG-induced LTD in the CA1 region of the hippocampus. Neuropharmacology 38, 1585–1596 (1999).
Bellone, C. & Luscher, C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nature Neurosci. 9, 636–641 (2006). A paper demonstrating that a form of mGluR-LTD induced by the activation of group I mGluRs can reverse the effects of cocaine on synaptic transmission.
Moult, P. R., Correa, S. A., Collingridge, G. L., Fitzjohn, S. M. & Bashir, Z. I. Co-activation of p38 mitogen-activated protein kinase and protein tyrosine phosphatase underlies metabotropic glutamate receptor-dependent long-term depression. J. Physiol. 586, 2499–2510 (2008).
Bolshakov, V. Y., Carboni, L., Cobb, M. H., Siegelbaum, S. A. & Belardetti, F. Dual MAP kinase pathways mediate opposing forms of long-term plasticity at CA3–CA1 synapses. Nature Neurosci. 3, 1107–1112 (2000).
Rush, A. M., Wu, J., Rowan, M. J. & Anwyl, R. Group I metabotropic glutamate receptor (mGluR)-dependent long-term depression mediated via p38 mitogen-activated protein kinase is inhibited by previous high-frequency stimulation and activation of mGluRs and protein kinase C in the rat dentate gyrus in vitro. J. Neurosci. 22, 6121–6128 (2002).
Gallagher, S. M., Daly, C. A., Bear, M. F. & Huber, K. M. Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J. Neurosci. 24, 4859–4864 (2004).
Hou, L. & Klann, E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J. Neurosci. 24, 6352–6361 (2004).
Moult, P. R. et al. Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated long-term depression. J. Neurosci. 26, 2544–2554 (2006).
Gladding, C. M. et al. Tyrosine dephosphorylation regulates AMPAR internalisation in mGluR-LTD. Mol. Cell Neurosci. 40, 267–279 (2009).
Huber, K. M., Kayser, M. S. & Bear, M. F. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288, 1254–1257 (2000).
Nosyreva, E. D. & Huber, K. M. Developmental switch in synaptic mechanisms of hippocampal metabotropic glutamate receptor-dependent long-term depression. J. Neurosci. 25, 2992–3001 (2005).
Zhang, Y. et al. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J. Neurosci. 28, 10561–10566 (2008).
Davidkova, G. & Carroll, R. C. Characterization of the role of microtubule-associated protein 1B in metabotropic glutamate receptor-mediated endocytosis of AMPA receptors in hippocampus. J. Neurosci. 27, 13273–13278 (2007).
Waung, M. W., Pfeiffer, B. E., Nosyreva, E. D., Ronesi, J. A. & Huber, K. M. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron 59, 84–97 (2008).
Huber, K. M., Gallagher, S. M., Warren, S. T. & Bear, M. F. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl Acad. Sci. USA 99, 7746–7750 (2002).
Hou, L. et al. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron 51, 441–454 (2006).
Dickinson, B. A. et al. A novel mechanism of hippocampal LTD involving muscarinic receptor-triggered interactions between AMPARs, GRIP and liprin-α. Mol. Brain 2, 18 (2009).
Kamsler, A., McHugh, T. J., Gerber, D., Huang, S. Y. & Tonegawa, S. Presynaptic m1 muscarinic receptors are necessary for mGluR long-term depression in the hippocampus. Proc. Natl Acad. Sci. USA 107, 1618–1623 (2010).
Piccinin, S. et al. Interaction between Ephrins and mGlu5 metabotropic glutamate receptors in the induction of long-term synaptic depression in the hippocampus. J. Neurosci. 30, 2835–2843 (2010).
Diamond, D. M., Park, C. R., Campbell, A. M. & Woodson, J. C. Competitive interactions between endogenous LTD and LTP in the hippocampus underlie the storage of emotional memories and stress-induced amnesia. Hippocampus 15, 1006–1025 (2005).
Martin, S. J., Grimwood, P. D. & Morris, R. G. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 23, 649–711 (2000).
O'Keefe, J. & Nadel, L. The Hippocampus as a Cognitive Map. (Clarendon Press, Oxford, 1978).
Squire, L. R., Stark, C. E. & Clark, R. E. The medial temporal lobe. Annu. Rev. Neurosci. 27, 279–306 (2004).
Manns, J. R. & Eichenbaum, H. Evolution of declarative memory. Hippocampus 16, 795–808 (2006).
Zeng, H. et al. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell 107, 617–629 (2001).
Nicholls, R. E. et al. Transgenic mice lacking NMDAR-dependent LTD exhibit deficits in behavioral flexibility. Neuron 58, 104–117 (2008).
Morice, E. et al. Parallel loss of hippocampal LTD and cognitive flexibility in a genetic model of hyperdopaminergia. Neuropsychopharmacology 32, 2108–2116 (2007).
Kemp, A. & Manahan-Vaughan, D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc. Natl Acad. Sci. USA 101, 8192–8197 (2004).
Manahan-Vaughan, D. & Braunewell, K. H. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc. Natl Acad. Sci. USA 96, 8739–8744 (1999).
Li, S., Cullen, W. K., Anwyl, R. & Rowan, M. J. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nature Neurosci. 6, 526–531 (2003).
Lemon, N. & Manahan-Vaughan, D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J. Neurosci. 26, 7723–7729 (2006).
Xu, L., Anwyl, R. & Rowan, M. J. Spatial exploration induces a persistent reversal of long-term potentiation in rat hippocampus. Nature 394, 891–894 (1998).
Abraham, W. C., Logan, B., Greenwood, J. M. & Dragunow, M. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus. J. Neurosci. 22, 9626–9634 (2002).
Yu, S. Y., Wu, D. C., Liu, L., Ge, Y. & Wang, Y. T. Role of AMPA receptor trafficking in NMDA receptor-dependent synaptic plasticity in the rat lateral amygdala. J. Neurochem. 106, 889–899 (2008).
Quirk, G. J. & Mueller, D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33, 56–72 (2008).
Dalton, G. L., Wang, Y. T., Floresco, S. B. & Phillips, A. G. Disruption of AMPA receptor endocytosis impairs the extinction, but not acquisition of learned fear. Neuropsychopharmacology 33, 2416–2426 (2008).
Kim, J. et al. Amygdala depotentiation and fear extinction. Proc. Natl Acad. Sci. USA 104, 20955–20960 (2007). One of a series of experiments that implicate AMPAR endocytosis in amygdala depotentiation and fear extinction.
Migues, P. V. et al. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nature Neurosci. 13, 630–634 (2010). This work provided the first evidence that the ZIP peptide impairs amygdala LTP and hence fear memory maintenance by facilitating GluA2-dependent AMPAR endocytosis.
Hannesson, D. K., Howland, J. G. & Phillips, A. G. Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats. J. Neurosci. 24, 4596–4604 (2004).
Winters, B. D. & Bussey, T. J. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J. Neurosci. 25, 52–61 (2005).
Brown, M. W. & Bashir, Z. I. Evidence concerning how neurons of the perirhinal cortex may effect familiarity discrimination. Phil. Trans. R. Soc. Lond. B 357, 1083–1095 (2002).
Warburton, E. C. et al. Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron 38, 987–996 (2003).
Lee, S. H., Liu, L., Wang, Y. T. & Sheng, M. Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron 36, 661–674 (2002).
Griffiths, S. et al. Expression of long-term depression underlies visual recognition memory. Neuron 58, 186–194 (2008). This paper describes a study which showed, using the interference peptide, that in the perirhinal cortex AMPAR endocytosis has a crucial role for visual recognition memory.
Ito, M. & Kano, M. Long-lasting depression of parallel fiber–Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci. Lett. 33, 253–258 (1982).
Chen, C. & Thompson, R. F. Temporal specificity of long-term depression in parallel fiber–Purkinje synapses in rat cerebellar slice. Learn. Mem. 2, 185–198 (1995).
Welsh, J. P. et al. Normal motor learning during pharmacological prevention of Purkinje cell long-term depression. Proc. Natl Acad. Sci. USA 102, 17166–17171 (2005).
Kakegawa, W. et al. Differential regulation of synaptic plasticity and cerebellar motor learning by the C-terminal PDZ-binding motif of GluRdelta2. J. Neurosci. 28, 1460–1468 (2008).
McConnell, M. J., Huang, Y. H., Datwani, A. & Shatz, C. J. H2-K(b) and H2-D(b) regulate cerebellar long-term depression and limit motor learning. Proc. Natl Acad. Sci. USA 106, 6784–6789 (2009).
De Zeeuw, C. I. et al. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo–ocular reflex. Neuron 20, 495–508 (1998).
Burguiere, E. et al. Spatial navigation impairment in mice lacking cerebellar LTD: a motor adaptation deficit? Nature Neurosci. 8, 1292–1294 (2005).
Ho, N. et al. Impaired synaptic plasticity and cAMP response element-binding protein activation in Ca2+/calmodulin-dependent protein kinase type IV/Gr-deficient mice. J. Neurosci. 20, 6459–6472 (2000).
Boyden, E. S. et al. Selective engagement of plasticity mechanisms for motor memory storage. Neuron 51, 823–834 (2006).
Wang, Y. T. & Linden, D. J. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron 25, 635–647 (2000).
Matsuda, S., Launey, T., Mikawa, S. & Hirai, H. Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. EMBO J. 19, 2765–2774 (2000).
Chung, H. J., Steinberg, J. P., Huganir, R. L. & Linden, D. J. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science 300, 1751–1755 (2003).
Smith, G. B., Heynen, A. J. & Bear, M. F. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Phil. Trans. R. Soc. Lond. B 364, 357–367 (2009).
Bear, M. F., Cooper, L. N. & Ebner, F. F. A physiological basis for a theory of synapse modification. Science 237, 42–48 (1987).
Heynen, A. J. et al. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nature Neurosci. 6, 854–862 (2003).
Crozier, R. A., Wang, Y., Liu, C. H. & Bear, M. F. Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proc. Natl Acad. Sci. USA 104, 1383–1388 (2007).
Yoon, B. J., Smith, G. B., Heynen, A. J., Neve, R. L. & Bear, M. F. Essential role for a long-term depression mechanism in ocular dominance plasticity. Proc. Natl Acad. Sci. USA 106, 9860–9865 (2009). A crucial demonstration of the specific role of LTD in visual cortex development.
Howland, J. G. & Wang, Y. T. Synaptic plasticity in learning and memory: stress effects in the hippocampus. Prog. Brain Res. 169, 145–158 (2008).
Sapolsky, R. M. Stress, the Aging Brain, and the Mechanisms of Neuron Death (MIT Press, Cambridge, Massachusetts, 1992).
McEwen, B. S. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 22, 105–122 (1999).
Chaouloff, F., Hemar, A. & Manzoni, O. Acute stress facilitates hippocampal CA1 metabotropic glutamate receptor-dependent long-term depression. J. Neurosci. 27, 7130–7135 (2007).
de Quervain, D. J., Roozendaal, B. & McGaugh, J. L. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 394, 787–790 (1998).
Karst, H. et al. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc. Natl Acad. Sci. USA 102, 19204–19207 (2005).
Brebner, K. et al. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science 310, 1340–1343 (2005). The first study that used the GluA2 3Y peptide to examine the role of AMPAR LTD in behaviour.
Van den Oever, M. C. et al. Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nature Neurosci. 11, 1053–1058 (2008).
Howland, J. G. & Cazakoff, B. N. Effects of acute stress and GluN2B-containing NMDA receptor antagonism on object and object-place recognition memory. Neurobiol. Learn. Mem. 93, 261–267 (2010).
Dolen, G. et al. Correction of fragile X syndrome in mice. Neuron 56, 955–962 (2007).
Dolen, G. & Bear, M. F. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J. Physiol. 586, 1503–1508 (2008).
Coyle, J. T. & Tsai, G. NMDA receptor function, neuroplasticity, and the pathophysiology of schizophrenia. Int. Rev. Neurobiol. 59, 491–515 (2004).
Frey, B. N. et al. The role of hippocampus in the pathophysiology of bipolar disorder. Behav. Pharmacol. 18, 419–430 (2007).
Pittenger, C. & Duman, R. S. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33, 88–109 (2008).
Holderbach, R., Clark, K., Moreau, J. L., Bischofberger, J. & Normann, C. Enhanced long-term synaptic depression in an animal model of depression. Biol. Psychiatry 62, 92–100 (2007).
Miyakawa, T. et al. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc. Natl Acad. Sci. USA 100, 8987–8992 (2003).
Polter, A. et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology 31 Mar 2010 (doi: 10.1038/npp.2010.43).
Thomas, M. J., Kalivas, P. W. & Shaham, Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br. J. Pharmacol. 154, 327–342 (2008).
Wolf, M. E., Sun, X., Mangiavacchi, S. & Chao, S. Z. Psychomotor stimulants and neuronal plasticity. Neuropharmacology 47 (Suppl 1.), 61–79 (2004).
Martin, M., Chen, B. T., Hopf, F. W., Bowers, M. S. & Bonci, A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nature Neurosci. 9, 868–869 (2006).
Centonze, D. et al. Chronic cocaine prevents depotentiation at corticostriatal synapses. Biol. Psychiatry 60, 436–443 (2006).
Kauer, J. A. & Malenka, R. C. Synaptic plasticity and addiction. Nature Rev. Neurosci. 8, 844–858 (2007).
Conrad, K. L. et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454, 118–121 (2008).
Mameli, M. et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nature Neurosci. 12, 1036–1041 (2009).
Hooper, C., Killick, R. & Lovestone, S. The GSK3 hypothesis of Alzheimer's disease. J. Neurochem. 104, 1433–1439 (2008).
Li, S. et al. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 62, 788–801 (2009).
Herron, C. E., Lester, R. A., Coan, E. J. & Collingridge, G. L. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature 322, 265–268 (1986).
Rumpel, S., LeDoux, J., Zador, A. & Malinow, R. Postsynaptic receptor trafficking underlying a form of associative learning. Science 308, 83–88 (2005).
Wang, Y. T. Probing the role of AMPAR endocytosis and long-term depression in behavioural sensitization: relevance to treatment of brain disorders, including drug addiction. Br. J. Pharmacol. 153, S389–S395 (2008).
Fox, C. J., Russell, K., Titterness, A. K., Wang, Y. T. & Christie, B. R. Tyrosine phosphorylation of the GluR2 subunit is required for long-term depression of synaptic efficacy in young animals in vivo. Hippocampus 17, 600–605 (2007).
Hayashi, T. & Huganir, R. L. Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J. Neurosci. 24, 6152–6160 (2004).
Famous, K. R. et al. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J. Neurosci. 28, 11061–11070 (2008).
Kim, C. H., Chung, H. J., Lee, H. K. & Huganir, R. L. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc. Natl Acad. Sci. USA 98, 11725–11730 (2001).
Schwarze, S. R., Ho, A., Vocero-Akbani, A. & Dowdy, S. F. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285, 1569–1572 (1999).
Robinson, T. E. & Berridge, K. C. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 95 (Suppl 2.), S91–S117 (2000).
Epstein, D. H., Preston, K. L., Stewart, J. & Shaham, Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berlin) 189, 1–16 (2006).
Thomas, M. J., Beurrier, C., Bonci, A. & Malenka, R. C. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nature Neurosci. 4, 1217–1223 (2001).
Robinson, T. E. & Kolb, B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47 (Suppl 1.), 33–46 (2004).
Boudreau, A. C. & Wolf, M. E. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J. Neurosci. 25, 9144–9151 (2005).
Nelson, C. L., Milovanovic, M., Wetter, J. B., Ford, K. A. & Wolf, M. E. Behavioral sensitization to amphetamine is not accompanied by changes in glutamate receptor surface expression in the rat nucleus accumbens. J. Neurochem. 109, 35–51 (2010).
Kourrich, S., Rothwell, P. E., Klug, J. R. & Thomas, M. J. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J. Neurosci. 27, 7921–7928 (2007).
Boudreau, A. C., Reimers, J. M., Milovanovic, M. & Wolf, M. E. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J. Neurosci. 27, 10621–10635 (2007).
Scholz, R., Berberich, S., Rathgeber, L., Kolleker, A., Köhr, G. & Kornau, H.-C. AMPA receptor signaling through BRAG2 and Arf6 critical for long-term synaptic depression. Neuron 66, 768–780 (2010).
Park, S. et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron 59, 70–83 (2008).
Acknowledgements
We would like to thank C. A. Thai for editorial assistance with this manuscript. G.L.C. and S.P. are supported by the Medical Research Council. G.L.C. is a Royal Society–Wolfson Merit Award Holder and a World Class University International Scholar. J.G.H. is supported by the Natural Sciences and Engineering Research Council of Canada, National Alliance for Research on Schizophrenia and Depression, Saskatchewan Health Research Foundation, and Canada Foundation for Innovation. Y.T.W. is supported by the Canadian Institutes for Health Research and is also a Howard Hughes Medical Institute International Research Scholar and the Heart and Stroke Foundation of British Columbia and Yukon Chair in Stroke Research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Involvement of NMDA and mGlu receptors in homosynaptic AMPA-LTD induction at various synapses of mammalian CNS (selected references are indicated) (PDF 363 kb)
Related links
Related links
FURTHER INFORMATION
MRC Centre for Synaptic Plasticity
Glossary
- Long-term potentiation (LTP)
-
A long-lasting (hours or days) increase in the synaptic response of neurons to stimulation of their afferents following a brief patterned stimulus (for example, 1 second stimulation at 100 Hz).
- Long-term depression (LTD)
-
A long-lasting decrease in the synaptic response of neurons to stimulation of their afferents following a long patterned stimulus (for example, 15 minutes of stimulation at 1 Hz).
- Low-frequency stimulation (LFS)
-
Stimulation of synapses by a train of electric pulses delivered by an electrode at a frequency that usually never exceeds 10 Hz on the afferent fibres.
- Single-shock low-frequency stimulation
-
A version of low-frequency stimulation in which the train of pulses used in the stimulation protocol is constituted of single pulses.
- Paired-pulse low-frequency stimulation
-
A version of low-frequency stimulation in which the train of pulses used in the stimulation protocol is constituted of a pair of pulses (usually at 20 Hz).
- Retrograde messenger
-
A biological signal released by the postsynaptic side of the synapse to induce a modification on the presynaptic side of the synapse. The most common retrograde messengers are nitric oxide and endocannabinoids.
- Clathrin-mediated endocytosis
-
A process in which vesicles are formed using a complex of proteins that are mainly associated with the cytosolic protein clathrin in order to internalize molecules from the extracellular space and regulate membrane composition by removing specific membrane proteins such as AMPARs.
- Scaffolding proteins
-
Proteins that can link with several other proteins and lipids and thus position proteins that are involved in signalling pathways close to their targets.
- Reversal learning
-
A task in which participants are trained to respond differentially to two stimuli under conditions of reward and punishment (or non-reward). The participants therefore learn to change their behaviour when the reward values are reversed.
- Behavioural flexibility
-
Alterations in well-established behaviours in response to changes in the environment.
- Haloperidol
-
An antipsychotic medication with dopamine receptor antagonist properties, particularly in relation to the dopamine receptor 2 subtype.
- Extinction
-
Reduced responses to a previously conditioned cue when the cue is presented repeatedly in the absence of the previously paired aversive or appetitive stimulus.
- ZIP peptide
-
A cell-permeable inhibitor of the persistently active kinase protein kinase C isoform M ζ (PKMζ).
- Vestibulo-ocular reflex
-
Reflex movements of the eyes that are elicited by vestibular stimulation. These movements keep the retinal image stable, preventing degradation of visual processing.
Rights and permissions
About this article
Cite this article
Collingridge, G., Peineau, S., Howland, J. et al. Long-term depression in the CNS. Nat Rev Neurosci 11, 459–473 (2010). https://doi.org/10.1038/nrn2867
Issue date:
DOI: https://doi.org/10.1038/nrn2867
This article is cited by
-
The multifaceted functions of β-arrestins and their therapeutic potential in neurodegenerative diseases
Experimental & Molecular Medicine (2024)
-
Intracellular magnesium optimizes transmission efficiency and plasticity of hippocampal synapses by reconfiguring their connectivity
Nature Communications (2024)
-
Targeting metaplasticity mechanisms to promote sustained antidepressant actions
Molecular Psychiatry (2024)
-
Systematic characterization of a non-transgenic Aβ1–42 amyloidosis model: synaptic plasticity and memory deficits in female and male mice
Biology of Sex Differences (2023)
-
Mitochondrial impairment and synaptic dysfunction are associated with neurological defects in iPSCs-derived cortical neurons of MERRF patients
Journal of Biomedical Science (2023)