Abstract
Since the first studies in the early 2000s, an increasing number of articles have used biomolecular tools for studying archaeological and historical cloth materials, produced by animal and plant fibres, leather and furs. Genomic and proteomic studies have particularly contributed to prior visual and microscopic methods to broaden complex themes such as society, identity, technology, economy and trade. We have termed this new interdisciplinary field “clothomics”, as it applies diverse omics methodologies, such as genomics and proteomics, to expand the horizons of cloth research. This paper aims at providing users with a set of practical step-by-step guides for the most widely applied omics analyses of cloth, proteomics and genomics, in animal-based materials. The paper reviews current applications, provides recommendations for selecting the right analytical strategy, focusing on practical considerations like how to sample, how to choose between proteomic and genomic methodological approaches, and where we see the current limitations. We are optimistic with the field of clothomics as we see it receives more attention scientifically and from the funding bodies. Although it faces several technical challenges, we also experience attempts to overcome these by recovering and detecting more biomolecules and becoming a more inclusive field through data sharing and participatory science. With a close collaboration between scholars of different disciplines, clothomics will provide a better understanding of human-animal interactions and the use of animal products beyond subsistence.
Similar content being viewed by others
Introduction
Research of archaeological and historical textiles and skins has increased massively over the past two decades1,2. Although these carefully manufactured products, leather, fur, and textiles of animal or plant fibres, are derived from different raw materials and produced using different manufacturing methods3,4, Susanna Harris’ research has highlighted the close relationship between them and their shared functionality as they are ‘flexible, thin sheets that can be wrapped, shaped, and folded and are used to clothe, cover and contain’5,6,7.
Cloth-type materials, or cloth, were used by all societies, yet the way they were used varied according to cultural identity, preferences, and values7. The term ‘cloth cultures’ which we adopt here, signifies just that; while every society makes use of cloth-type materials, they do so in culturally specific ways. Moreover, cloth cultures include far more than just clothing. As the examples of this article will demonstrate, cloth was a fundamental aspect of human societies and was used for furnishing, storage, transportation by boat or sledge, as well as protection in the form of shields, sheats and shelters, and much more. Both raw materials and more or less finished products were traded over large distances from very early times onward and were valued greatly, which is also clearly evident from early written sources. Much later and mostly in Europe, the search for raw materials, as well as the urge to improve production techniques, were among the main drivers behind the development of the colonial enterprise, and the Industrial Revolution8,9,10. Today, cloth of natural raw materials remains fundamental in our society even after the invention of synthetic fabrics and other alternatives.
Despite cloth’s economical value and profound impact across cultures, its significance has often been overlooked. This is in part due to its poor chances of survival in the archaeological record and in part to a lack of understanding of its value, importance and original abundance in ancient societies. Lately, however, cloth-type materials have been increasingly recognised as an important means to study broad and complex themes such as society, identity, technology, economy and trade (e.g. refs. 11,12,13,14,15,16).
Central for such studies is the understanding of its entire chaîne opératoire, from the procurement of raw materials to the creation of the final product. Each step in this process varies depending on the type of cloth, and directly affects the properties of the final product17. Traditionally, animal skin and fibre have been studied using morphometric methods, most prominently visual and microscopic techniques (e.g. refs. 3,18,19). These can provide taxonomic identifications, as well as details on the composition and structure of skins and fibres like the colour, quality and preservation state20. These methods have their strength in often being non-invasive to minimally destructive and widely accessible. However, their application requires extensive training and knowledge about, amongst others, intra and interspecies variation. A recurring concern for their use in species identification has been the absence of objective, standardised methods and comprehensive modern reference collections encompassing various species and geographic regions. Efforts to address this issue, such as those undertaken by Skinner et al.21 represent notable advancements. Nevertheless, morphometric approaches face certain challenges in archaeological materials, particularly due to degradation and post-depositional processes. Manufacturing methods can also alter morphological traits, such as skin grain surfaces or hair scale patterns, thereby hindering optimal analysis.
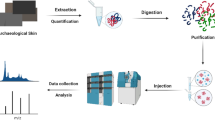
In order to fully understand the meaning and role of cloth materials, it is important to gain as much information as possible from textiles and animal skins. The range of scientific methods that contribute to the study of archaeological cloth has expanded dramatically over the past decades. Today, it encompasses a wide and increasing array of biomolecular tools, of which the analysis of ancient DNA and proteins has received particular attention (Fig. 1). We have termed this new interdisciplinary field ‘clothomics’, as it applies diverse omics methodologies, such as genomics, proteomics, lipidomics, metabolomics and/or isotopic analysis, to expand the horizons of cloth research.
A full list of the publications can be found in Table S1.
In this review, we provide a practical step-by-step guide for scholars working between cloth research, conservation and heritage science, and entering this new field. In doing so, we address common questions about the use of DNA- and protein-based techniques in cloth materials, focusing on their current applications, different methodological approaches, limitations, and practical considerations, such as preservation, sampling and storage, that should be considered in the design of a clothomic study. Finally, we provide recommendations for future research and highlight the importance of integrating results with archaeological knowledge.
The review emphasises genomics and proteomics which are the most widely used omics methods in ancient cloth research and are within our expertise, while acknowledging the potential for future exploration of other techniques. Likewise, the focus is largely on animal-based materials, wool, fur, and skin, which have been more extensively researched. While various studies have been conducted on silk (e.g. refs. 22,23) the potential for applying these techniques to plant materials in the future remains open. Additionally, we point the readers to supplementary resources, including previous reviews focusing on textiles and skins24,25, peptide mass fingerprinting (PMF) of collagen (also called ZooMS or Zooarchaeology by Mass Spectrometry)26, palaeoproteomics27 and ancient DNA analyses28.
Applications
Initial attempts to retrieve ancient DNA on cloth during the early 2000s showed the difficulties of conducting genetic analysis in historical and modern leather, due to significant degradation occurring during the tanning process29,30. This was backed in 2014 by Brandt and colleagues who showed that DNA was not present in skin garments from Danish peat bogs, whereas there were promising prospects for protein recovery31. Proteomics had already been shown to be successful in Arctic utilitarian skin objects32. Additionally, Hollemeyer et al.33,34 applied proteomics on animal hair in the pioneering study of the clothing of Ötzi, a 5000-year-old mummy preserved in the Alps. The preservation of DNA on fibres was first investigated by Brandt et al.35 and Brandt36 on modern and archaeological fibres from Denmark and Greenland, but showed that DNA is generally very degraded in fibres from archaeological environments. With these early findings of limited DNA preservation in ancient and historical cloth, interest has turned towards the application of proteomics (Fig. 1).
Since these early studies, omics analyses have contributed to answer and enrich various problematics in cloth research (Fig. 2). In particular, they have been useful for species identification of common domestic animals like sheep, goat or cattle (e.g. refs. 37,38). More recently, regional domesticated and wild species have gained more attention. For example, Solazzo and Phipps39 determined that Precolumbian textiles from coastal Peru, dating as early as 300 BCE-100 CE, were made with fibres of mountain Viscacha, providing the first archaeological evidence of its use. In addition, this animal is geographically distributed in the highlands, which raises new questions about trade and cultural interactions in the region. Azémard et al.40 conducted the first attempt to differentiate four South American camelids (guanaco, vicuña, llama, alpaca) and identified proteomically the use of llama and alpaca fibres in Peru around 950–1350 CE, shedding more light into the diversity of animal resources used for fibre production in this area.
The field examines various aspects, such as the choice of locally available natural resources, the set of animal husbandry and farming practices tailored to answer garment production demands, the contribution of hunting in economic activities, landscape use and mobility, as well as cultural exchanges and trade routes. It also sheds light on dress practices and identities of past individuals and communities, and craft technologies related to the functionality and material properties of cloth.
Clothomics can also shed light on modes of production that are usually invisible in the archaeological record, such as hunting or nomadic and semi-nomadic pastoralism and their relation to agricultural societies. Nomadic Scythians utilised species like red fox, felids and squirrels41, while during the Viking Age, seal hair38 and beaver fur were employed42. In ancient Egypt, the use of skin from gazelle has been evidenced21 and in the Norse and historical Arctic, species such as Arctic fox, Arctic hare, wolverine and various seal species have also been documented43,44.
In other contexts, studies have brought unexpected findings, such as the use of human skin by Scythian populations to cover some of their quivers, supporting the claim by Herodotus that these were made of human skin from their defeated enemies41.
But, certainly, one of the best-analysed ancient wardrobes belongs to the Ötzi man. Multiple proteomic and genetic analyses have been conducted over the years on the various clothing items and elements with which he was equipped33,34,45,46,47. These proteomic analyses successfully identified various animal sources, including two samples from his coat, one from his legging to sheep and one from his moccasins to cattle and in 2012 added goat, brown bear, chamois, red deer and a canid to the list of animal types used.
On the other hand, large-scale studies have shed light on animal use patterns and correlations with certain types of garment or material. A study of 109 samples of animal fibres from the Bronze Age-Iron Age in China identified a predominance of sheep and goat, followed by cattle, and the occasional use of human and camel hair48. Interestingly, the study found that textiles were made mainly of sheep wool, whereas furs or pelts were mostly goat. Another study conducted by Brandt et al.42 determined that fur clothing was produced exclusively from wild animals while leather used for accessories or grave furnishing was made of domesticated animals. In 2020, a paper by Brandt et al.49 used a protein-based method to identify 115 elements of shoes dating to the Danish Viking Age or mediaeval period. The identifications showed a particular pattern of some shoe elements consistently being made of the skin of a specific species arguing for at least some choices of material being made based on functionality of elements, and some being made by taste, fashion or affordability.
Besides species identification, clothomics also holds promise for the investigation of animal management strategies and the selection of specific types of animals for cloth production. Shirazi and colleagues50 carried out the first genetic analysis to determine sex ratios from an assemblage of archaeological leather. It demonstrated that 13th-century Indigenous populations from the American Plains preferentially used hides from female bison to produce leather moccasins. It is not clear if selection occurred during the hunt or if they were chosen among the hunted bison, but future comparisons with bone remains could provide additional insights into hunting strategies and the use of hides. Age could also be a selection criteria for leather, as the skin of young animals are softer and thinner. Exactly the use of calf skin has been demonstrated through proteomics by the recovery of foetal haemoglobin - a blood protein specific to very young animals31.
Deciding on your strategy for analysis
Which biomolecules can be analysed in archaeological cloth?
Proteins and DNA are the main biomolecules that can be studied in archaeological cloth. Proteins play a pivotal role in virtually all biological processes within a cell. Twenty different amino acids constitute the building blocks of proteins, which are synthesised through the transcription and translation of DNA sequences. This linear chain of amino acids represents the protein’s primary structure, but they can further fold into intricate 3D shapes, known as secondary and tertiary structures (Fig. 3). Within archaeological contexts, the most abundant proteins are collagen in the case of, for example, bone and skin (Box 1), keratin in tissues such as wool and hair (Box 2) and fibroin in silk (since the protein sericin is partially or completely removed during the production of threads24). For more information on the structure and characteristics of fibroin, we refer to previous articles22,24. Because amino acid sequence variations in collagen and keratin can be found between different biological species (reflecting genetic signals), proteomics has been widely used in archaeology for species determination.
Deoxyribonucleic acid (DNA) is the hereditary material of all known living organisms. It is structured as a double-stranded helix with sequences of nucleotides on each strand that store the genetic information as a ‘code’. The code is made up of a combination of four chemical bases: adenine (A), thymine (T), cytosine (C) and guanine (G). These bases pair with each other, so that A on one strand always pairs with T on the opposite strand, and C on one strand always pairs with G on the other. The pairs are often referred to as base pairs (bp). The complete DNA sequence is known as the genome and is divided into chromosomes and smaller segments called genes, which encode proteins. Nuclear DNA is located in the cell nucleus where they exist in two variants, one from each parent. A small part of the genome is found outside the nucleus in the cell’s energy-producing organelles, the mitochondria. This mitochondrial DNA (mtDNA) is inherited only through the maternal line, it is not recombined and it is highly variable, which enables reconstruction of maternal lineages (haplogroups) through time.
What factors affect molecular preservation in archaeological cloth and how does it impact analytical success?
When deciding on an analytical strategy for archaeological cloth, information on cloth biography, including its age, processing methods and burial environment, is of major importance to ensure the best results and success.
The manufacturing process is known to significantly impact molecular preservation. Previous research has proved that dyeing and mordanting of animal fibres, as well as tanning of animal skin, hamper the potential for DNA analysis29,35, whereas these have successfully yielded proteins31,39,49.
In terms of environmental factors, while certain contexts are favourable for the preservation of textiles and skins, they may still be harmful to DNA preservation. This is at least the case for waterlogged environments with low pH or acidic conditions, which, on the other hand, can still preserve proteins, as demonstrated by the successful analysis of leather from Danish peat bogs31. Similarly, animal fibres from several Danish Viking Age burials exhibited very poor endogenous DNA preservation, rendering any interpretation of DNA impossible, yet proteins were preserved in the samples42. Sinding et al.43 have, however, demonstrated that, in cold Arctic environments, it remains possible to extract mtDNA from fibres and this was also shown on the Ötzi´s clothing45. This is promising for at least some of the environments from which cloth is well-preserved such as cold and permafrozen sites of Greenland, Northern Scandinavia, and Mongolia. For other sites with well-preserved textiles, such as dry sites in North and South America and Asia, this potential has yet to be demonstrated. Theoretically, heat degrades both DNA and proteins, but studies conducted in arid or dry climates have experienced good results using proteomic methods in animal fibres from South America and Asia39,48 and also leather from Egypt51,52,53. Even mineralised fibres on copper have yielded successful peptide mass fingerprints38.
Despite these successful examples, proteins degrade over time and are also impacted by factors such as time, temperature, burial environment and manufacturing. Protein deamidation, the conversion of glutamine (Gln/Q) and asparagine (Asn/N) to glutamic (Glu/E) and aspartic (Asp/D) acids influenced by pH and temperature result in a mass shift of +0.984 Da24. Other frequent forms of degradation are hydrolysis and oxidation which can be accelerated by factors such as the above-mentioned and can lead to breakage of the protein chain and thus shorter peptides, which may be more difficult to identify54. Furthermore, it has been shown that KAPs are less abundant than ɑ-keratins in aged wool when exposed to increased microbial activity55, but can still survive under certain conditions. In leather, mainly collagen is preserved due to production processes like dehairing and tanning. Altogether, this could lead to a lower level of taxonomic identification if peptides with amino acid differences between species are completely or partially degraded37.
In the case of ancient DNA, only a fraction of the original DNA is preserved as it undergoes increasing fragmentation, and this results typically in fragments shorter than 100 bp56. Furthermore, aDNA molecules accumulate chemical modifications. The most prominent is deamination which occurs when cytosines are converted to uracils (and sequenced as thymines by the instrument) at the end of the fragments, resulting in increased C-to-T misincorporations. At the moment, nuclear DNA has not yet been successfully retrieved from ancient textiles. This may be due to the initial low amount of DNA in hair, combined with a more rapid degradation than mtDNA57. Hundreds to thousands of mtDNA copies exist in each cell which makes it easier to retrieve in archaeological materials, compared to nuclear DNA.
In conclusion, both DNA and proteins are affected by degradation, albeit to varying extents. Hence, the following section provides further guidance on when it is more appropriate to select proteins or DNA as an analytical strategy.
What are the opportunities and challenges of protein and DNA analysis respectively?
Proteins have been shown to be more resistant and survive longer than DNA. This is evidenced in remarkable findings from 6.5 and 3.8 million-year-old ostrich eggshells58,59 and a 3.4 million-year-old camel bone60. The binding of proteins to minerals has been described as one of the key factors contributing to their preservation58. While the mineral component of animal skin and fibre is minimal, the presence of metals like iron or copper in contact with textiles can result in the replacement of fibres by minerals and this has been shown to enhance protein preservation in fibre and also likely skin38. Other factors, such as the protein 3D structure, folding and cross-linking, may play a role in protein preservation in artefacts from harsh environments where DNA no longer persists, although this is still poorly understood61,62. Thus, as described above, in very old, mineralised, arid, and acidic environments and in materials that have undergone harsh manufacturing processes, the survival and, thus, analysis of proteins is superior to that of DNA.
In addition to this, proteins have other unique characteristics that render them particularly useful compared with DNA. Unlike the genome, the proteome (all proteins in an organism) is a dynamic entity comprising over 10,000 proteins that are differentially expressed over time and space. As a result, proteins can vary across different biological tissues, life developmental stages and health conditions. Moreover, once formed, proteins undergo post-translational modifications (PTMs) that alter their properties and function. Thus, while the genome remains static, the proteome is more complex and continually changes under different conditions, providing additional layers of information.
For example, different components like collagens and keratins can be discerned in skin and hair samples, and the presence of haemoglobin in animal skin has been used to identify the young age of an archaeological calfskin31. Studies of modern sheep wool have also demonstrated the ability to distinguish modern sheep breeds and detect differences associated with fibre characteristics, like curvature and colour, and nutrition (e.g. refs. 63,64). So far, tissue-specificity and stage-specific expression still need to be further studied in archaeological skin and textiles before their full potential can be evaluated.
Unlike the analysis of the structural protein collagen that has not evolved much over time, DNA offers greater variation in its sequence27 and thus also precision in terms of species determination. For example, sometimes it is not possible to determine the species with protein analysis, regardless of protein sequencing or PMF, but only the genus or family due to phylogenetic proximity as in the case of various African Bovidae65. Beyond identifying species, DNA can offer insights into the sex of the animal and fine-scale population structure. For example, it can be used to distinguish between domestic and wild variants, as well as infer relatedness between different populations or breeds. This may prove invaluable for understanding preferences for certain types of animals as a source of leather or fibre, and how these changed across various periods and regions. A notable example is the study of the York Gospels, where each parchment bifolium was identified with protein analysis, but further characterised with genetics in terms of sex and population diversity66. Thus, DNA analysis can help to shed light on demographic history or geographic provenance. Finally, examining genetic variants associated with wool traits and selection may reveal shifts in wool properties, such as quality or colour (e.g. ref. 67). Even in samples where nuclear DNA is poorly preserved, it might still be possible to retrieve mtDNA sequences due to its abundance in cells and, based on this, reconstruct maternal lines.
Overall, DNA provides additional information about population history and sex attribution. The greatest challenge is however its preservation. Proteins can offer opportunities for species identification in these cases and also age determination, among others.
How can you determine the preservation status of an archaeological sample?
Several screening methods have been employed in archaeology to assess molecular preservation prior to palaeo-genomic and -proteomic analysis, a process that is destructive, costly and time-consuming. Visual assessment of the material appearance, including parameters like flexibility or dryness/brittleness, can give an approximate idea of the degradation state, but are limited by their subjective nature and may not accurately reflect molecular preservation.
Collagen preservation can be tested using Fourier transform infrared spectroscopy in attenuated total reflectance mode (FTIR-ATR). For bones from arid environments, it has been shown that bones with a collagen content of less than 3% of the total weight, did not yield any peptides68. Another study presented different thresholds to distinguish between well and poorly preserved bone samples for DNA and protein analysis69. Portable FTIR-ATR devices have also the advantage that can been deployed in the field to predict ZooMS success70. Archaeological leather degradation has also been examined with FTIR-ATR71 whereas FTIR microspectroscopy in reflectance mode has been favoured for fragile excavated textiles due to its non-invasive and non-destructive nature72. Nevertheless, to our knowledge, fibre and leather degradation have not been compared directly to palaeo-genomic or -proteomic results. Other pre-screening methods for bones include portable near-infrared (NIR) spectroscopy73 and handheld Raman spectroscopy74,75, which can assess collagen preservation in the field and museums and could be applied to textiles or leather as well.
Thus, depending on the ‘value’ or rarity of the sample, its size, the opportunity to destructively sample it, and the questions asked, a strategy can be decided in a collaboration between heritage scientists, cloth researchers and conservators (Fig. 4).
a Collagen structure after26. b Keratin structure. Graphics: Signe Bjerregaard.
A guideline for proteomic and genomic users
What are the best practices to obtain, handle and store archaeological and historical samples?
Sampling and storage
Different sampling protocols are available for palaeoproteomics, from minimally destructive to non-invasive techniques. A non-invasive eraser method, initially developed for the analysis of parchment76, has, since its introduction, been tested on leather as well. However, its application to leather presents some challenges, as initial attempts have yielded varied results due to factors such as dirt accumulation and the presence of colourant coatings on the grain surface21,77. In addition, the flesh side may not be accessible and if the leather surface is degraded and friable, it risks damage during the sampling. It has been shown, for instance, that the use of the eraser can leave traces on bone surfaces78. The most reliable results have been obtained from leather fibres extracted from the edges or cross-sections within the dermis layer21,77. Other non-destructive methods used in bone artefacts include the analysis of the plastic storage bags or membrane boxes which have contained the objects in question79,80, but they usually provide lower taxonomic resolution and quality spectra compared to destructive analysis. Within the cultural heritage field, various films or gels have been applied to object surfaces81,82,83,84 but non-invasive methods remain largely untested on leather and wool substrates.
Generally, destructive sampling requires only a small sample size (<10 mg), with recent methods allowing to work with 1 mg or less of sample85. However, while minimal destruction should be attempted and is often required when the sample is fragile, small or rare, it is important to consider that this could result in a decreased protein recovery. Thus, it should be ensured that enough sample material has been taken to successfully retrieve proteins. In the case of wool, estimating the necessary material amount may be challenging as the weight can be highly variable across samples of similar dimensions, especially in field or museum settings, where precise analytical balances may not be available. Similarly, the weight of leather samples may vary depending if the sample is dry or wet. The weight can increase by at least 50% when wet, as usually happens in Northern European contexts, compared to dry samples that have been stored in museums for a long period86. It is preferable to sample leather when it is dry, to avoid the development of mould, especially during transportation.
Considering whether a single sample could be used for multiple analyses is also important. For example, the same protein extract employed for ZooMS can be reused for shotgun proteomics, without the need to take a new sample. Combined protein and dye extraction methods have also been tested, and offer great potential to minimise the number of samples87. However, enough subsamples should be taken to carry out other potential analyses, such as DNA analysis, C14 dating, microscopy or isotope analysis, in order to insert proteomics in the multi-proxy analysis of a single artefact. For DNA analysis, for example, around 10–200 mg of ancient wool is needed, which for most samples equals a thread of 2–3 cm in length35.
Regarding wool textiles, either fragments of fabric or individual threads can be taken. It is particularly important to note whether the sample is from the warp (vertical system) or weft (horizontal system) and why it was decided to sample only one or both systems. Ideally, samples should be retrieved from one thread and one weave direction, as the warp may be composed of different fibres than the weft. Also, wool could be sourced from different individuals or species and mixed during the processing of raw wool or the spinning, so taking multiple samples is recommended if possible. It is also recommended to avoid areas with dyes or colourants as these treatments could impact the results. To ensure traceability, marking the precise location on a photograph of the whole object and/or fragment and thoroughly documenting the whole sampling process is very important. Photographs taken before sampling are the most important, if one has to prioritise, as the sample will show the after-state. Collaboration between archaeologists, physical anthropologists and experts in cloth research, familiar with the material, burial, site, region or period is highly recommended, as they can assist in developing an appropriate sample strategy, selecting the best fragments, providing basic documentation and interpreting the results. Thus, their insights can significantly enhance the quality and relevance of the proteomic or genomic analysis.
Contamination
When determining the exact location for sampling, several aspects should be taken into consideration. Conservators often favour edges or loose pieces for sampling, but these areas may be more degraded or contaminated. In addition, sampling at places that show evidence of previous conservation treatments should be avoided. This is particularly relevant for samples excavated many years ago or preserved with treatments that are no longer used, because we know they are harmful to the specimens and also, in some cases, the individuals handling them. Here, access to records of previous treatment processes, cleaning methods or storage conditions is very useful. For example, the conservation of leather with polyethylene glycol (PEG) may be problematic for subsequent ZooMS analysis if its molecular weight is the same as the molecular weight of identifying peptide markers (see section 7). These peptides may be impossible to detect on the mass spectrum of the sample (Fig. 5). Thus, careful consideration of the sampling location is essential to ensure good results.
To retrieve the samples, it is advisable to follow specific precautions to avoid contamination, since skin or other keratin proteins could mask proteins of interest. Nitrile gloves (not latex) should be worn during sampling and, if possible, accompanied by long sleeves and hairnets. In some cases where objects have been handled a lot, this may, however, have less impact on the total amount of contamination. The use of wool clothing should be avoided to prevent keratin contamination. Regarding the equipment, scissors or scalpels are suitable to cut the material, while tweezers can be employed to gently pull out and place the sample into individual plastic tubes or bags. If the sample is fragile or gets loose, it can be placed on a folded paper or aluminium foil beforehand. Depending on the object history, these may have been extensively handled over the years, preserved in situ on the body or bone remains or stored with other samples, so there is a possible risk of cross-contamination. Despite this, sterile tools should be utilised for sampling and thoroughly cleaned between each sample to avoid further cross-contamination. For this reason, samples should be taken as early as possible during the excavation, to avoid minimal handling. In the case of DNA, these precautions should be followed even more carefully as the risk of contamination is higher due to the involved Polymerase Chain Reaction (PCR) step, which can amplify even a few contaminant sequences to large amounts that will mask endogenous DNA signals.
Management of data and samples
The archaeological context is very important for future interpretation and traceability and samples should be given a unique and consistent identifier. The artefact number and provenance information should be included in the label so that it can be linked to the original artefact, museum catalogues, reports of the sites and burials, and excavation numbers.
Last but not least, it is important to establish agreements about how and what material (e.g. unused sample material or extracts) should be returned to museums or researchers, and how, where and how long they should be stored. Moreover, data management procedures should be outlined. This includes how data is kept, shared and published, including negative results to prevent future unnecessary destruction of samples88.
What are the most commonly employed genomic methods?
Most studies of archaeological cloth so far have used PCR-based approaches to retrieve target regions of interest, such as short mtDNA fragments. The advent of next-generation sequencing (NGS) has allowed the analysis of whole ancient genomes (mitochondrial and nuclear) by sequencing millions of untargeted DNA sequences or reads at the same time, particularly suitable for short damaged fragments, while reducing time and cost. During the last years NGS has also been applied to archaeological textiles or fur42,43,44,45,50.
NGS approaches involve several steps: sampling, extraction, library preparation, PCR amplification and sequencing (Fig. 6). This is by far the main route and although other ways are possible, optional steps are not covered here (see ref. 28).
The peak (m/z 1201) is within the range of where one should expect to find the collagen peptide marker COL1α2 978–990, which for instance is important for distinguishing cattle (m/z 1192+1208) from sheep and goat (m/z 1180+1196) and horse (m/z 1182+1198), and could easily overlay and thus hinder their identification.
What are the differences between the different proteomic methods and how to choose between them?
There are two main routes for protein analysis on cloth and archaeological materials in general: peptide mass fingerprinting (PMF) and shotgun proteomics (Table 1). PMF focuses on one protein (the most dominant in a sample, i.e. collagen in skin and keratin in wool textiles and fur), while shotgun proteomics analyses all proteins contained in a sample. Although the extraction, digestion and purification of the sample can be similar, the following mass spectrometry (MS) differs. The first method employs single MS, such as MALDI-TOF (matrix-assisted laser desorption/ionisation–time-of-flight), which generates spectra indicating the intensity (abundance) of peptides of a certain mass/charge ratio (m/z). These spectra are then searched for peptide ‘markers’ or fingerprints, which are known peptides with differing m/z between species (see flow chart in e.g. ref. 26).
The effectiveness of species determination using PMF relies ultimately on the existence of markers unique to each species. For instance, sheep and goats can be distinguished by a single peptide marker (the mass of the peptide located at the position COL1ɑ2 757–789 is 3077.4 for goat and 3017.4 for sheep89). Similarly, in keratin PMF, separation of sheep and goats can be achieved based on the keratin K33a marker90. However, some species are so closely related to each other that they do not present any discernible amino acid differences in current PMF markers and often require further differentiation by liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis.
In contrast to PMF, with this method peptides are further separated into fragments, enabling the determination of the actual amino acid sequences (Fig. 7). This approach can provide evidence of degradation patterns and allows the analysis of complex mixtures of proteins. The resolution of shotgun proteomics analysis is thus higher (see examples in refs. 41,42), but the downstream analysis is also more complex and time-consuming. Whereas PMF can be conducted using open source software like mMass91 and comparing manually with reference lists (e.g. for collagen, a dataset is available through the University of York (https://docs.google.com/spreadsheets/d/1ipm9fFFyha8IEzRO2F5zVXIk0ldwYiWgX5pGqETzBco/edit)), shotgun proteomics requires more advanced software such as MaxQuant or PEAKS that conducts protein sequence searches against large publicly available databases such as NCBI or UniProt. The price of MS/MS analyses is higher due to the need for more advanced instrumentation, which may be done at another institution, and the longer analysis time26,27. Therefore, single MS is more often used for larger sample sets, although the final decision will ultimately depend on the specific research question(s).
After samples are obtained, the cells are broken down and DNA is released. Extraction is followed by purification, where DNA is isolated. Next-generation sequencing requires DNA libraries to be built. Compared to PCR methods which target specific regions, libraries contain all the extracted DNA in a sample. Double-stranded library preparation is commonly used and involves the repair and fill-in of damaged DNA fragments, together with the addition of adaptors (short fragments of DNA) and “indexes”, which are unique short sequences that act like barcodes to differentiate samples when these are sequenced in parallel. Following library preparation, PCR amplification is necessary prior to sequencing, given that the amount of endogenous DNA from archaeological samples is typically small. PCR involves multiple cycles of temperature changes which causes strands of DNA to be replicated. Subsequently, the amplified libraries are pooled and sequenced and data is analysed with bioinformatic tools. The first step is to authenticate ancient DNA sequences and assess the degree of contamination. The potential of the data depends upon how well the genome is covered—the higher the coverage, the larger the analytical potential. Graphics: Signe Bjerregaard.
Proteins are extracted from the samples using various solutions, based on their chemical properties. Hair which contains cysteines requires a reduction and alkylation step to break disulfide bonds. Digestion is then performed with an enzyme, normally trypsin, which cleaves the protein into peptides. Before the peptides enter the mass spectrometer, they are separated by their hydrophobicity using liquid chromatography. These are then ionised and introduced successively to the mass spectrometer using electrospray ionisation (ESI). In tandem MS (or MS/MS), a first mass analyser is used to record peptide masses in a particular retention time (MS1). In data dependent acquisition (DDA), only the top intensity peptides from the first MS round are selected with a very narrow window, while lower abundance peptides are left out. This is the main acquisition method used in palaeoproteomics. The individual peptides (called precursor ions) are isolated and passed on to a collision cell (CID/HCD) for fragmentation. This process breaks the precursor ion into smaller fragment ions that are further analysed by a second mass analyser to generate m/z values of the fragments (MS2). The whole amino acid sequence of the peptide can be reconstructed by analysing the differences between masses, which correspond to the mass of one amino acid. Protein identification involves a database search that compares the MS2 spectrum against theoretically predicted spectra found on a chosen database to obtain the best match. Graphics: Signe Bjerregaard.
What do I need to take into account when budgeting this type of study?
Proteomic and genomic analyses can be undertaken in different ways: commercial analysis, research collaboration or hiring of trained personnel. Prices may vary between institutions based on their infrastructure, setups and how sample cost is calculated, i.e. including or not including salary. Currently, only a few institutions offer commercial analyses of ancient cloth materials. While facilities analysing modern materials are more abundant, these may, however, not be set up to handle specific challenges relating to ancient samples such as contamination. Using a commercial service is an easy way to have samples analysed, but if choosing this route, it is important to ensure that the results come with an interpretation. Otherwise, they may be hard to use without the necessary background.
In a research collaboration, an agreement on the economic contribution to the analysis and possibly salary has to be agreed upon. While this approach can foster extensive discussion on samples, context, results and interpretations, there are risks involved. People who are funded mainly by other projects may not be able to prioritise side projects, potentially leading to delays in established timelines. Finally, an alternative is to hire dedicated staff to carry out analyses. This ensures more security that results are delivered on time, but it is also a more expensive solution.
What are their current limitations?
The preservation of DNA is the main limitation of aDNA analysis. This may be overcome in the future by focusing on bone materials rather than cloth for evolutionary questions, method development and selection of samples from very favourable environments. Because of these challenging preservation conditions, here, we will focus on the current limitations of palaeoproteomic methods. While the application of palaeoproteomics to cloth materials has become more widespread, several challenges persist in both PMF and LC-MS/MS analyses regarding the recovery, detection and analysis of proteins26,27.
Recovery and detection methods
One area of particular importance is the recovery and detection of proteins. Various factors, including preservation conditions and chemical alterations occurring during manufacturing and post-depositional processes, have a profound impact on the efficacy of analytical protocols and mass spectrometry instrumentation. Keratins are structurally rigid and stable proteins and have numerous cystine disulfide linkages which can be hard to break down. In addition, KAPs tend to be underrepresented due to degradation processes such as dyeing and mordanting, which would require further investigations87. This is significant because KAPs present a broader diversity of protein types and sequences compared to keratins, characterised by repetitive regions92.
In the forthcoming years, we expect, for instance, to see the development and optimisation of extraction protocols tailored for keratins which will be able to increase the yield and characterisation of wool and fur42,85. Furthermore, the use of alternative enzymes for digestion or their combination has been shown to increase the number of peptides and proteins recovered, obtaining a larger proportion of the proteome and coverage93,94,95,96. For example, until recently, horses and donkeys remained indistinguishable, but the use of a different enzyme, chymotrypsin, enabled the identification of a new ZooMS marker96.
Finally, to overcome some of the limitations of PMF (lower confidence in protein identification) and LC-MS/MS (cost, time, complex data analysis) new methods such as species by proteome investigation (SPIN) have been developed for archaeological bones97. Contrary to the standard palaeoproteomic methods, SPIN uses data-independent acquisition (DIA). In DIA, peptides within a wide, moving m/z window are systematically selected for further fragmentation. This allows the identification of lower abundance peptides and a higher sample throughput, at the expense of more challenging data analysis and the inability to use database search. However, these methods remain untested on fibre and skin-based materials and warrant further research.
Analysis and interpretation of data
Following developments in protein extraction, we expect improvements within the data analysis for both PMF and LC-MS/MS. The success of the identification of proteins depends partly on having good reference databases. For shotgun proteomics, the process typically relies on comparisons with databases such as UniProt and NCBI, except when using de novo sequencing, which does not rely on existing databases. A major issue with these publicly available databases is that the representation of proteins varies massively between taxonomic groups. For example, a search on Uniprot (10/2024) shows 456 keratin protein entries for sheep (Ovis aries), 499 for goat (Capra hircus), 250 for cattle (Bos taurus) while only 153 for Bactrian camel (Camelus bactrianus) or 132 for alpaca (Vicugna Pacos). This can lead to a database-dependent identification bias as better-covered species may receive more hits than worse-covered ones. Thus, sequences from species that are absent in the database will give a closest hit to the most closely related species present in the database, which are not, in fact, the correct species. For instance, Brandt et al.42 demonstrated how a fur sample from the Danish Viking Age was almost misidentified due to an incomplete database. The sample initially matched to alpine marmot (Marmota marmota), until genetic data from NCBI was translated and identified it as Sciuridae, which was not present in the protein database. Another problem may be the specificity of proteins. Most keratin regions are conserved across different species, while only a few ones contain specific sequence variations. Thus, many peptides will be shared between multiple species. In the coming years, we, therefore, hope and expect protein databases to grow in terms of species and protein coverage through de novo sequencing or translation of genetic data.
For keratins, a specific challenge is an inconsistency in the keratin gene and protein names98 and PMF markers, which is hindering comparison between studies, although this is currently being addressed in the field. We are hoping in the future to see a common nomenclature as the one that has been suggested for collagen99 and also a centralised open-access database for PMF keratin marker references, as the one that is currently being curated by the University of York for collagen. This database is constantly growing and covers all domesticated animals commonly used for skin, as well as many wild species. However, no such central reference database exists for PMF of keratin, which poses a significant barrier to the study of archaeological fibres. Each group has their own internal dataset with keratin markers compiled from individual papers, which need to be constantly updated38,90,100. In addition, at present, keratin PMF markers have only been established for a limited number of mammalian species, highlighting the need for additional characterisation and expansion. For shotgun proteomic approaches, a targeted database for keratin-dominated tissues with compiled criteria for which specific proteins and taxa to include and a unified gene list would be extremely useful for the community.
The feasibility of big data studies depends on time. At the moment, PMF identifications require the manual analysis of spectra and is thus very time-consuming. The development of automated data analysis tools could help increase the number of samples that can be analysed. Some examples include the Bacollite package101 and SpecieScan102. Bacollite, initially developed for identifying parchment, currently contains only four species: sheep, goat, cattle and deer, but more species are currently being added. Conversely, SpecieScan includes all taxa from the online ZooMS database. Both tools are currently limited to bone collagen materials.
Palaeoproteomic approaches have focused mostly on determining the animal species by looking at the presence or absence of proteins in a sample, rather than comparing protein abundance between samples as in quantitative proteomic approaches103. This provides a ‘snapshot in time’ and could be useful for determining the animal age (proteins that are expressed differently during life) and different wool qualities, as observed in modern samples63,64. Nevertheless, these proteins (like KAPs or minor proteins) are not always detected in archaeological samples due to degradation and thus, recovered proteins may not accurately reflect the sample’s original protein composition and abundance.
Sharing of data
Finally, the field of palaeoproteomics has not had any agreed way of publishing spectra and uploading raw data according to FAIR principles. In fact, many early articles did not present this opportunity at all. Much data has not been made available and there is no common repository for data. This is changing and we are seeing platforms such as Zenodo increasing its content of PMF data. Protocols are shared through protocols.io and LC-MS/MS data is being uploaded on a variety of platforms including proteomeXchange, enhancing data accessibility and collaboration within the scientific community. We expect that this is a trend that will continue in the future and we hope and encourage that data will be uploaded in public data repositories with standardised metadata and databases used for LC-MS/MS, which are currently often lacking.
How do proteomics and genomics relate to other methods, approaches and findings in archaeology?
Being able to characterise textiles, fur and leather together with evidence from animal bone remains, textile tools, palaeoenvironmental studies and textual or visual sources is obviously of great importance. Ideally, these analyses will be combined in order to altogether give the strongest possible witness of the biography of cloth. Therefore, it is important to insert clothomics in the multi-proxy analysis of objects and phenomena, to answer not only the what, but also the when, where and how. We are observing with pleasure that several such studies are coming out and that the field seems to be moving in the direction of using species IDs as a starting point for larger discussions.
A good example of a study which integrates several approaches was done by Oras et al.104 on two child mummies. Here, the analysis of the burial textiles was combined with osteological analysis of the bodies, AMS radiocarbon dating, CT scans and ancient DNA analysis, among others, allowing multiple aspects of the bodies, the embalming and the death rituals to come together.
Proteomic analyses on textiles and animal skins from ancient Sudan are tracking the use of raw resources through time and space and how garments impacted economic production and different subsistence modes like hunting, nomadic pastoralism or agriculture105. Combined with microscopy, ancient DNA or isotope analysis, it will shed light on past Sudanese ecology, revealing interactions between humans, animals and their natural environment, and like this better understand dress practices and the building of past identities.
The same approach is used in Brandt’s continuing studies of fur in Viking Age graves42. Ancient protein and DNA analyses will be combined with isotope analyses to aim at provenancing non-local animal furs and, thus also, Viking Age trading networks. Together with evidence from literary sources and iconography, these will expand our understanding of whether exclusive and traded furs were worn by only a limited part of the population as a marker of status.
Conclusions and future perspectives
As cross-disciplinary researchers on cloth, we experience an increasing number of requests for analyses of samples and help in understanding the potentials of biomolecular analysis. Within the last years in Denmark, we have also seen several projects of archaeological cloth integrating biomolecular and/or other natural scientific aspects receive funding. Past problematics with visual species identification in cloth research have been answered or enriched by proteomics especially, which has been improved as a tool by the inclusion of more species in databases.
The field, therefore, seems optimistic, and we are so, too. The technical challenges outlined above are being addressed and improved by the communities; for proteomics, we are seeing initiatives for automatic identification of MS1 spectra which will enable working with larger datasets and in turn reveal more diversity than small-scale studies where specific objects are selected for analysis106. We have also seen the optimisation of protein recovery and MS2 analysis to ensure the identification of as many peptides as possible. Both of these will help provide more resolution of protein data.
So far, palaeoproteomics has been applied to specific case studies but not on a large scale. We see a new trend with, for instance, the Citizen science initiative Next Generation Lab (Denmark), where high school students conduct a large-scale analysis of mediaeval and Renaissance leather fragments from Copenhagen, that would otherwise be discarded107. Democratisation of such large datasets along with metadata, will also allow for a better understanding of protein and DNA degradation in various contexts and the impact of different manufacturing processes.
Ancient DNA analyses of textiles and skins have laid low for some years, probably based on discomforting results as presented by Brandt36. Nevertheless, we are experiencing a growing interest in the development and refining of sheep’s wool—also from an ancient DNA point of view. With improved technologies and favourable samples, we may see a positive development in this area of clothomics as well.
As the field expands, the data will need to be further integrated and contextualised with zooarchaeological data, burial context, environmental and landscape records, technical analysis of textiles and skins, historical texts, and visual representations. Archaeologists will undoubtedly become a key part of these future developments. If we succeed in establishing true interdisciplinary collaborations, we believe that clothomics will continue to provide a better understanding of human-animal interactions and the use of animal products beyond subsistence. This will put cloth production back at the heart of archaeological discussions on past societies and economies and answer complex themes such as identity, technology, economy and trade. We hope this article takes a step towards this goal.
Data availability
Data were not generated during the current study, but a list of publications used for Fig. 1 is provided within the paper and its Supplementary Information files.
References
Strand, E. A. et al. Old textiles – new possibilities. Eur. J. Archaeol. 13, 149–173 (2010).
Strand, E. A., Mannering, U. & Nosch, M.-L. Old textiles – new possibilities. Ten years on. In Ancient Textile Production from an Interdisciplinary Perspective (eds Ulanowska, A., Grömer, K., Vanden Berghe, I. & Öhrman, M.) 19–35 (Springer, Cham, 2022).
Kite, M. & Thomson, R. Conservation of Leather and Related Materials (Routledge, 2006).
Emery, I. The Primary Structures of Fabrics : An Illustrated Classification (The Textile Museum, 1966).
Harris, S. Textiles, cloth, and skins: the problem of terminology and relationship. Textile 6, 222–237 (2008).
Harris, S. M. Cloth cultures in prehistoric Europe; project concept and approach. Archaeol. Text. Newsl. 50, 30–31 (2010).
Harris, S. From the Parochial to the universal: comparing cloth cultures in the Bronze age. Eur. J. Archaeol. 15, 61–97 (2012).
Hobsbawm, E. J. Industry and Empire: The Birth of the Industrial Revolution (The New Press, 1999).
Styles, J. Fashion, textiles and the origins of Industrial Revolution. East Asian J. Br. History 5, 161–189 (2016).
Beckert, S. Empire of Cotton: A Global History (Alfred A Knopf, 2014).
Gleba, M. You are what you wear: Scythian costume as identity. In Dressing the Past (eds Gleba, M. Munkholt, C. & Nosch, M.-L.) 13–28 (Oxbow Books, 2008).
Frei, K. M. et al. Provenance of ancient textiles-a pilot study evaluating the strontium isotope system in wool. Archaeometry 51, 252–276 (2009).
Nosch, M.-L., Koefoed, H. & Strand, E. A. Textile Production and Consumption in the Ancient Near East: Archaeology, Epigraphy, Iconography (Oxbow Books, 2013).
Hansen, K. T. The world in dress: anthropological perspectives on clothing, fashion, and culture. Annu. Rev. Anthropol. 33, 369–392 (2004).
Harris, S. & Veldmeijer, A. J. Why Leather? The Material and Cultural Dimensions of Leather (Sidestone Press, Leiden, 2014).
Breniquet, C. & Michel, C. Wool Economy in the Ancient Near East and the Aegean (Oxbow Books, 2014).
Strand, E. A. The textile chaîne opératoire: using a multidisciplinary approach to textile archaeology with a focus on the Ancient Near East. Paléorient 38, 21–40 (2012).
Rast-Eicher, A. Fibres: Microscopy of Archaeological Textiles and Furs (Archaeolingua Alapítvány, 2016).
Reed, R. Ancient Skins, Parchments and Leathers (Seminar Press, 1972).
Ryder, M. L. A re-assessment of bronze age wool. J. Archaeol. Sci. 10, 327–331 (1983).
Skinner, L. et al. Modified methods for species identification of archaeological skin-based objects: dealing with degradation and improving standards. In Proc. 11th Interim Meeting of the ICOM-CC Leather and Related Materials Working Group (eds Robinet, L., Dignard, C. & Sturge, T.) 14–25 (International Council of Museums–Committee for Conservation, 2020).
Lee, B., Pires, E., Pollard, A. M. & McCullagh, J. S. O. Species identification of silks by protein mass spectrometry reveals evidence of wild silk use in antiquity. Sci. Rep. 12, 4579 (2022).
Yu, X. et al. Mass spectrometry analysis of textile used in decorating the coronet of Empress Xiao of the Sui Dynasty (581–618 A.D.). J. Cult. Herit. 25, 185–188 (2017).
Solazzo, C. Characterizing historical textiles and clothing with proteomics. Conserv. Patrim. 31, 97–114 (2019).
Gianvincenzo, F. D., Granzotto, C. & Cappellini, E. Skin, furs, and textiles: Mass spectrometry-based analysis of ancient protein residues. In The Textile Revolution in Bronze Age Europe: Production, Specialisation, Consumption (eds Sabatini, S. & Bergerbrant, S.) 304–316 (Cambridge University Press, 2019).
Richter, K. K., Codlin, M. C., Seabrook, M. & Warinner, C. A primer for ZooMS applications in archaeology. Proc. Natl Acad. Sci. USA 119, e2109323119 (2022).
Warinner, C., Korzow Richter, K. & Collins, M. J. Paleoproteomics. Chem. Rev. 122, 13401–13446 (2022).
Orlando, L. et al. Ancient DNA analysis. Nat. Rev. Methods Prime. 1 (2021).
Vuissoz, A. et al. The survival of PCR-amplifiable DNA in cow leather. J. Archaeol. Sci. 34, 823–829 (2007).
Burger, J., Pfeiffer, I., Hummel, S., Fuchs, R. & Brenig, B. Mitochondrial and nuclear DNA from (pre) historic hide-derived material. Anc. Biomol. 3, 227–238 (2001).
Brandt, L. Ø. et al. Species identification of archaeological skin objects from Danish bogs: comparison between mass spectrometry-based peptide sequencing and microscopy-based methods. PLoS ONE 9, e106875 (2014).
Kirby, D. P., Buckley, M., Promise, E., Trauger, S. A. & Holdcraft, T. R. Identification of collagen-based materials in cultural heritage. Analyst 138, 4849–4858 (2013).
Hollemeyer, K., Altmeyer, W., Heinzle, E. & Pitra, C. Species identification of Oetzi’s clothing with matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry based on peptide pattern similarities of hair digests. Rapid Commun. Mass Spectrom. 22, 2751–2767 (2008).
Hollemeyer, K., Altmeyer, W., Heinzle, E. & Pitra, C. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry combined with multidimensional scaling, binary hierarchical cluster tree and selected diagnostic masses improves species identification of Neolithic keratin sequences from furs of the Tyrolean Iceman Oetzi. Rapid Commun. Mass Spectrom. 26, 1735–1745 (2012).
Brandt, L. Ø. et al. Characterising the potential of sheep wool for ancient DNA analyses. Archaeol. Anthropol. Sci. 3, 209–221 (2011).
Brandt, L. Ø. Species Identification of Skins and Development of Sheep Wool: An Interdisciplinary Study Combining Textile Research, Archaeology, and Biomolecular Methods. PhD thesis, University of Copenhagen (2015).
Ebsen, J. A., Haase, K., Larsen, R., Sommer, D. V. P. & Brandt, L. Ø. Identifying archaeological leather – discussing the potential of grain pattern analysis and zooarchaeology by mass spectrometry (ZooMS) through a case study involving medieval shoe parts from Denmark. J. Cult. Herit. 39, 21–31 (2019).
Solazzo, C. et al. Species identification by peptide mass fingerprinting (PMF) in fibre products preserved by association with copper-alloy artefacts. J. Archaeol. Sci. 49, 524–535 (2014).
Solazzo, C. & Phipps, E. Chasing the elusive viscacha in Precolumbian textiles at the intersection of art and science. J. Archaeol. Sci. 140, 105575 (2022).
Azémard, C. et al. Untangling the fibre ball: Proteomic characterization of South American camelid hair fibres by untargeted multivariate analysis and molecular networking. J. Proteomics 231, 104040 (2021).
Brandt, L. Ø., Mackie, M., Daragan, M., Collins, M. J. & Gleba, M. Human and animal skin identified by palaeoproteomics in Scythian leather objects from Ukraine. PLoS ONE 18, e0294129 (2023).
Brandt, L. Ø. et al. Palaeoproteomics identifies beaver fur in Danish high-status Viking Age burials - direct evidence of fur trade. PLoS ONE 17, e0270040 (2022).
Sinding, M.-H. S., Vieira, F. G. & Smith, M. H. Unmatched DNA preservation prove arctic hare and sheep wool in Norse Greenlandic textile from ‘The Farm Beneath the Sand’. J. Archaeol. Sci. Rep. 14, 603–608 (2017).
Schmidt, A. L., Brandt, L. Ø., Sinding, M.-H. S., Stenderup, J. & Vieira, F. G. Species Identification of inuit skin and fur clothing: analyses of DNA, hair microscopy, and macroscopical identification. Arctic 76, 382–399 (2023).
O’Sullivan, N. J. et al. A whole mitochondria analysis of the Tyrolean Iceman’s leather provides insights into the animal sources of Copper Age clothing. Sci. Rep. 6, 31279 (2016).
Olivieri, C. et al. Phylogenetic position of a copper age sheep (Ovis aries) mitochondrial DNA. PLoS ONE 7, e33792 (2012).
Olivieri, C. et al. Positioning the red deer (Cervus elaphus) hunted by the Tyrolean Iceman into a mitochondrial DNA phylogeny. PLoS ONE 9, e100136 (2014).
Azémard, C. et al. Animal fibre use in the Keriya valley (Xinjiang, China) during the Bronze and Iron Ages: a proteomic approach. J. Archaeol. Sci. 110, 104996 (2019).
Brandt, L. Ø., Ebsen, J. A. & Haase, K. Leather shoes in early Danish cities: choices of animal resources and specialization of crafts in viking and medieval Denmark. Eur. J. Archaeol. 23, 428–450 (2020).
Shirazi, S. et al. Ancient DNA-based sex determination of bison hide moccasins indicates Promontory cave occupants selected female hides for footwear. J. Archaeol. Sci. 137, 105533 (2022).
Elnaggar, A. et al. Paleoproteomic profiling for identification of animal skin species in ancient Egyptian archaeological leather using liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). Herit. Sci. 10, 182 (2022).
Tarasov, P. E. et al. New results of radiocarbon dating and identification of plant and animal remains from the Oglakhty cemetery provide an insight into the life of the population of southern Siberia in the early 1st millennium CE. Quat. Int. 623, 169–183 (2022).
Daragan, M. et al. The perishable material culture of the pontic steppe scythians: scientific investigation of a fourth‐century BC kurgan burial at bulhakovo, Ukraine. Oxf. J. Archaeol. 41, 397–422 (2022).
Solazzo, C. et al. Application of redox proteomics to the study of oxidative degradation products in archaeological wool. J. Cult. Herit. 16, 896–903 (2015).
Solazzo, C. et al. Proteomic evaluation of the biodegradation of wool fabrics in experimental burials. Int. Biodeter. Biodegr. 80, 48–59 (2013).
Sawyer, S., Krause, J., Guschanski, K., Savolainen, V. & Pääbo, S. Temporal patterns of nucleotide misincorporations and DNA fragmentation in ancient DNA. PLoS ONE 7, e34131 (2012).
Bengtsson, C. F. et al. DNA from keratinous tissue. Part I: hair and nail. Ann. Anat. 194, 17–25 (2012).
Demarchi, B. et al. Protein sequences bound to mineral surfaces persist into deep time. eLife 5, e17092 (2016).
Demarchi, B. et al. Survival of mineral-bound peptides into the Miocene. eLife 11, e82849 (2022).
Rybczynski, N. et al. Mid-Pliocene warm-period deposits in the High Arctic yield insight into camel evolution. Nat. Commun. 4, 1550 (2013).
Solazzo, C. et al. Modeling deamidation in sheep α-keratin peptides and application to archeological wool textiles. Anal. Chem. 86, 567–575 (2014).
Schweitzer, M. H., Schroeter, E. R. & Goshe, M. B. Protein molecular data from ancient (>1 million years old) fossil material: pitfalls, possibilities and grand challenges. Anal. Chem. 86, 6731–6740 (2014).
Plowman, J., Thomas, A., Perloiro, T., Clerens, S. & de Almeida, A. M. Characterisation of white and black merino wools: a proteomics study. Animal 13, 659–665 (2019).
Plowman, J. E. et al. The wool proteome and fibre characteristics of three distinct genetic ovine breeds from Portugal. J. Proteomics 225, 103853 (2020).
Le Meillour, L. et al. The name of the game: palaeoproteomics and radiocarbon dates further refine the presence and dispersal of caprines in eastern and southern Africa. R. Soc. Open Sci. 10, 231002 (2023).
Teasdale, M. D. et al. The York Gospels: a 1000-year biological palimpsest. R. Soc. Open Sci. 4, 170988 (2017).
Svensson, E. M. et al. Coat colour and sex identification in horses from Iron Age Sweden. Ann. Anat. 194, 82–87 (2012).
Le Meillour, L. et al. Identification of degraded bone and tooth splinters from arid environments using palaeoproteomics. Palaeogeogr. Palaeoclimatol. Palaeoecol. 511, 472–482 (2018).
Kontopoulos, I. et al. Screening archaeological bone for palaeogenetic and palaeoproteomic studies. PLoS ONE 15, e0235146 (2020).
Pothier Bouchard, G. et al. Portable FTIR for on-site screening of archaeological bone intended for ZooMS collagen fingerprint analysis. J. Archaeol. Sci. Rep. 26, 101862 (2019).
Halldórsdóttir, H. H., Williams, R., Greene, E. M. & Taylor, G. Rapid deterioration in buried leather: archaeological implications. RSC Adv. 14, 3762–3770 (2024).
Margariti, C. The application of FTIR microspectroscopy in a non-invasive and non-destructive way to the study and conservation of mineralised excavated textiles. Herit. Sci. 7, 63 (2019).
Sponheimer, M. et al. Saving old bones: a non-destructive method for bone collagen prescreening. Sci. Rep. 9, 13928 (2019).
Pestle, W. J. et al. Hand-held Raman spectroscopy as a pre-screening tool for archaeological bone. J. Archaeol. Sci. 58, 113–120 (2015).
Madden, O., Chan, D. M. W., Dundon, M. & France, C. A. M. Quantifying collagen quality in archaeological bone: improving data accuracy with benchtop and handheld Raman spectrometers. J. Archaeol. Sci. Rep. 18, 596–605 (2018).
Fiddyment, S. et al. Animal origin of 13th-century uterine vellum revealed using noninvasive peptide fingerprinting. Proc. Natl Acad. Sci. USA 112, 15066–15071 (2015).
Skinner, L. Characterisation of ancient leather hide and skin selection in the Nile Valley, from the third millennium BC until the first millennium AD. (University of Northampton, 2023).
Sinet-Mathiot, V. et al. The effect of eraser sampling for proteomic analysis on Palaeolithic bone surface microtopography. Sci. Rep. 11, 23611 (2021).
Martisius, N. L. et al. Non-destructive ZooMS identification reveals strategic bone tool raw material selection by Neandertals. Sci. Rep. 10, 7746 (2020).
McGrath, K. et al. Identifying archaeological bone via non-destructive ZooMS and the materiality of symbolic expression: examples from Iroquoian bone points. Sci. Rep. 9, 11027 (2019).
Cicatiello, P. et al. Minimally invasive and portable method for the identification of proteins in ancient paintings. Anal. Chem. 90, 10128–10133 (2018).
Kirby, D. P., Manick, A. & Newman, R. Minimally invasive sampling of surface coatings for protein identification by peptide mass fingerprinting: a case study with photographs. J. Amer. Inst. Conserv. 59, 235–245 (2020).
Zilberstein, G., Zilberstein, S., Maor, U. & Righetti, P. G. Surface analysis of ancient parchments via the EVA film: the Aleppo codex. Anal. Biochem. 604, 113824 (2020).
Calvano, C. D., Rigante, E., Picca, R. A., Cataldi, T. R. I. & Sabbatini, L. An easily transferable protocol for in-situ quasi-non-invasive analysis of protein binders in works of art. Talanta 215, 120882 (2020).
Solazzo, C. & Niepold, T. A simplified sample preparation for hair and skin proteins towards the application of archaeological fur and leather. J. Proteomics 274, 104821 (2023).
Ebsen, J. A. Experiments with Uncontrolled Drying of Archaeological Leather from the Next Generation Lab Project (Odense City Museum, 2024).
Serafini, I., Favero, G., Curini, R., Kavich, G. M. & Cleland, T. P. Development of a combined protein and dye extraction approach for the analysis of keratin-based textiles. J. Proteome Res. 23, 3890–3903 (2024).
Pálsdóttir, A. H., Bläuer, A., Rannamäe, E., Boessenkool, S. & Hallsson, J. H. Not a limitless resource: ethics and guidelines for destructive sampling of archaeofaunal remains. R. Soc. Open Sci. 6, 191059 (2019).
Buckley, M. et al. Distinguishing between archaeological sheep and goat bones using a single collagen peptide. J. Archaeol. Sci. 37, 13–20 (2010).
Solazzo, C. et al. Characterisation of novel α-keratin peptide markers for species identification in keratinous tissues using mass spectrometry. Rapid Commun. Mass Spectrom. 27, 2685–2698 (2013).
Strohalm, M., Hassman, M., Kosata, B. & Kodícek, M. mMass data miner: an open source alternative for mass spectrometric data analysis. Rapid Commun. Mass Spectrom. 22, 905–908 (2008).
Flanagan, L. M., Plowman, J. E. & Bryson, W. G. The high sulphur proteins of wool: towards an understanding of sheep breed diversity. Proteomics 2, 1240–1246 (2002).
Lanigan, L. T. et al. Multi-protease analysis of Pleistocene bone proteomes. J. Proteomics 228, 103889 (2020).
Fagernäs, Z., Troché, G., Olsen, J. V. & Welker, F. Digging deeper into ancient skeletal proteomes through consecutive digestion with multiple proteases. J. Proteomics 298, 105143 (2024).
Wilkin, S. et al. Sequential trypsin and ProAlanase digestions unearth immunological protein biomarkers shrouded by skeletal collagen. iScience 27, 109663 (2024).
Paladugu, R. et al. Your horse is a donkey! Identifying domesticated equids from Western Iberia using collagen fingerprinting. J. Archaeol. Sci. 149, 105696 (2023).
Rüther, P. L. et al. SPIN enables high throughput species identification of archaeological bone by proteomics. Nat. Commun. 13, 2458 (2022).
Schweizer, J. et al. New consensus nomenclature for mammalian keratins. J. Cell Biol. 174, 169–174 (2006).
Brown, S., Douka, K., Collins, M. J. & Richter, K. K. On the standardization of ZooMS nomenclature. J. Proteomics 235, 104041 (2021).
Solazzo, C. Follow‐up on the characterization of peptidic markers in hair and fur for the identification of common North American species. Rapid Commun. Mass Spectrom. 31, 1375–1384 (2017).
Hickinbotham, S., Fiddyment, S., Stinson, T. L. & Collins, M. J. How to get your goat: automated identification of species from MALDI-ToF spectra. Bioinformatics 36, 3719–3725 (2020).
Végh, E. I. & Douka, K. SpecieScan: semi-automated taxonomic identification of bone collagen peptides from MALDI-ToF-MS. Bioinformatics 40, btae054 (2024).
Jersie-Christensen, R. R. et al. Quantitative metaproteomics of medieval dental calculus reveals individual oral health status. Nat. Commun. 9, 4744 (2018).
Oras, E. et al. Multidisciplinary investigation of two Egyptian child mummies curated at the University of Tartu Art Museum, Estonia (Late/Graeco-Roman Periods). PLoS ONE 15, e0227446 (2020).
Yvanez, E. Archaeology of dress along the middle Nile. Project Repos. J. 16, 12–15 (2023).
Brandt, L. Ø., Lillemark, M. R., Toftdal, M., Andersen, V. L. & Tøttrup, A. P. Are we betting on the wrong horse? Insignificant archaeological leather fragments provide the first evidence for the exploitation of horsehide in renaissance Denmark. Heritage 5, 972–990 (2022).
Brandt, L. Ø., Lillemark, M. R., Rytter, M., Collins, M. J. & Tøttrup, A. P. Next generation lab puts Denmark’s past in the hands of its future. Antiquity 96, 221–228 (2022).
Brandt, L. Ø. & Allentoft, M. E. Archaeological wool textiles—a window into ancient sheep genetics. In The Textile Revolution in Bronze Age Europe: Production, Specialisation, Consumption (eds Sabatini, S. & Bergerbrant, S.) 274–303 (Cambridge Univ. Press, Cambridge, 2019).
Fiddyment, S. et al. So you want to do biocodicology? A field guide to the biological analysis of parchment. Herit. Sci. 7, 35 (2019).
Kijas, J. W. et al. Genome-wide analysis of the world’s sheep breeds reveals high levels of historic mixture and strong recent selection. PLoS Biol. 10, e1001258 (2012).
Uitto, J. Connective tissue biochemistry of the aging dermis. Age-related alterations in collagen and elastin. Dermatol. Clin. 4, 433–446 (1986).
Reilly, D. M. & Lozano, J. Skin collagen through the lifestages: importance for skin health and beauty. Plast. Aesthet. Res. 8, 2 (2021).
Henriksen, K. & Karsdal, M. A. Chapter 1 - Type I collagen. In Biochemistry of Collagens, Laminins and Elastin (ed. Karsdal, M. A.) 1–12 (Academic Press, 2019).
Shoulders, M. D. & Raines, R. T. Collagen structure and stability. Annu. Rev. Biochem. 78, 929–958 (2009).
Banasaz, S. & Ferraro, V. Keratin from animal by-products: structure, characterization, extraction and application-A review. Polymers 16, 1999 (2024).
Wang, B., Yang, W., McKittrick, J. & Meyers, M. A. Keratin: structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Prog. Mater. Sci. 76, 229–318 (2016).
Plowman, J. E. Proteomics in Wool and Fibre Research. In Proteomics in Domestic Animals: from Farm to Systems Biology (eds de Almeida, A. M., Eckersall, D. & Miller, I.) (Springer, Cham, 2018).
Acknowledgements
We are grateful to Elsa Yvanez, Helene Forum Winther, Ismael Rodríguez Palomo and Lucy Anne Skinner, from the University of Copenhagen, Denmark, for useful conversations and suggestions for the text. We thank Kristine Korzow Richter from Texas A&M University for allowing us to use a previously published figure and Signe Bjerregaard for the graphics design of the figures. Also, we would like to thank the reviewers for their crucial feedback. This research was funded by the Novo Nordisk Foundation for the grant Next Generation Lab (NNF20OC0062346 and NNF23OC0084958) and the European Research Council for the project Fashioning Sudan (ERC Starting Grant 101039416).
Funding
Open access funding provided by Copenhagen University.
Author information
Authors and Affiliations
Contributions
L.C.V.-C. and L.Ø.B. wrote and edited the manuscript together and both prepared figures and tables. Both authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Viñas-Caron, L.C., Brandt, L.Ø. Clothomics: a practical guide to understand the opportunities and challenges of omics-based methods in archaeological cloth research. npj Herit. Sci. 13, 80 (2025). https://doi.org/10.1038/s40494-025-01623-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s40494-025-01623-z