Abstract
Background
Obesity may affect an individual’s immune response and subsequent risk of infection, such as a SARS-CoV-2 infection. It is less clear whether overweight and long-term obesity also constitute risk factors. We investigated the association between the degree and duration of overweight and obesity and SARS-CoV-2 infection.
Methods
We analyzed data from nine prospective population-based cohorts of the Netherlands Cohorts Consortium, with a total of 99,570 participants, following a standardized procedure. Body mass index (BMI) and waist circumference (WC) were assessed two times before the pandemic, with approximately 5 years between measurements. SARS-CoV-2 infection was defined by self-report as a positive PCR or rapid-antigen test or as COVID-19 ascertained by a physician between March 2020 and January 2023. For three cohorts, information on SARS-CoV-2 infection by serology was available. Results were pooled using random-effects meta-analyses and adjusted for age, sex, educational level, and number of SARS-CoV-2 infection measurements.
Results
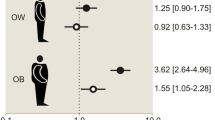
Individuals with overweight (25 ≤ BMI < 30 kg/m2) (odds ratio (OR) = 1.08, 95%-confidence interval (CI) 1.04-1.13) or obesity (BMI ≥ 30 kg/m2) (OR = 1.43, 95%-CI 1.18–1.75) were more likely to report SARS-CoV-2 infection than individuals with a healthy body weight. We observed comparable ORs for abdominal overweight (men: 94 cm≤WC < 102 cm, women: 80 cm≤WC < 88 cm) (OR = 1.09, 95%-CI 1.04–1.14, I2 = 0%) and abdominal obesity (men: WC ≥ 102 cm, women: WC ≥ 88 cm) (OR = 1.24, 95%-CI 0.999–1.55, I2 = 57%). Individuals with obesity long before the pandemic, but with a healthy body weight or overweight just before the pandemic, were not at increased risk.
Conclusion
Overweight and obesity were associated with increased risk of SARS-CoV-2 infection with stronger associations for obesity. Individuals with a healthier weight prior to the pandemic but previous obesity did not have an increased risk of SARS-CoV-2, suggesting that weight loss in those with obesity reduces infection risk. These results underline the importance of obesity prevention and weight management for public health.
Similar content being viewed by others
Introduction
Obesity is associated with both the risk and severity of respiratory infections, which was clearly observed during the Swine flu pandemic in 2009 and the more recent COVID-19 pandemic [1, 2]. The underlying mechanism relates to an impaired immune response in those with obesity [3]. Therefore, obesity may increase the risk of getting infected with SARS-CoV-2, the virus that causes COVID-19 [4]. Results from a meta-analysis including 20 studies showed that individuals with obesity have a 46% higher odds of testing positive for SARS-CoV-2 than individuals with a healthy weight [5].
The risk of obesity-related comorbidities such as type 2 diabetes may depend on the length of time an individual has obesity, possibly due to longer exposure to inflammation and metabolic dysregulation [6, 7]. To our knowledge, the association between duration of obesity and SARS-CoV-2 infection has not yet been explored. An explanation for this may be the hospital-based designs of previous studies in which information on body weight was collected only during hospital admission [5]. In contrast, population-based cohorts often have repeatedly collected information on obesity-measures during a longer period prior to the pandemic. Therefore, these studies are ideally suited to investigate SARS-CoV-2 infection risk among individuals with long-term obesity [8]. Furthermore, population-based studies contribute to identifying high-risk groups for mild SARS-CoV-2 infections for which hospitalization was not necessary, but for which public health impact could be considerable.
Besides obesity, overweight might also increase SARS-CoV-2 infection risk [9, 10]. Today, more than a third of the population in Western countries are overweight, defined as a body mass index (BMI) ≥ 25 and <30 kg/m2 [11]. Given this substantial prevalence, a higher risk of infection among those with overweight may have a large societal impact due to an increased burden of disease, and therefore warrants further study. Furthermore, one may hypothesize that the amount of abdominal fat (of which waist circumference (WC) is an indicator) is more important in relation to SARS-CoV-2 infection than general obesity (of which BMI is an indicator), because of its higher secretion rate of inflammation markers and worse immune response [12, 13]. So far, however, research on abdominal obesity and SARS-CoV-2 infection is scarce, and the few available studies into this topic have reported mixed results [14,15,16].
Therefore, the aim of this study was to investigate the association between the degree and the duration of (abdominal) overweight and obesity and the risk of SARS-CoV-2 infection among a large sample of Dutch adults by meta-analyzing data from nine population-based prospective cohort studies.
Methods
Study population and design
We conducted a meta-analysis using data from nine prospective cohorts of the Netherlands Cohorts Consortium (NCC) [17]. NCC aims to include all population-based and population-representative Dutch cohort studies that provide longitudinal clinical phenotyping and biomedical data for research into the determinants of multimorbidity. The NCC currently consists of >450,000 participants from the Netherlands originating from 11 cohort studies. Two NCC cohorts could not participate in the current study because they did not collect data on infection with SARS-CoV-2. In the other nine cohorts, all active participants (n = 8) or a random subsample of active participants (n = 1) were invited to take part in one or more follow-up rounds during the pandemic. Table 1 presents an overview of the characteristics of the participating nine NCC cohorts. A brief description of the cohorts can be found in Supplementary Text S1 and a detailed description can be found in the cohort profiles [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. All cohort studies were approved by an Institutional Review Board and written informed consent was obtained from all participants. Participating cohorts were asked to select adult participants with information on BMI and SARS-CoV-2 infection. Participants with missing information on age, sex and educational level were excluded from the study population. In total, 99,570 participants were included in the current study (see Fig. S1 for a flowchart of study participants). Per cohort, analyses were conducted by researchers of the individual cohorts following instructions from a standardized procedure.
Exposures
Assessment of BMI and WC was conducted between 2000 and 2020, with specific periods of data collection varying between the cohorts (Table 1). Four exposure variables were used in this meta-analysis.
-
1.
Most recent BMI status before the COVID-19 pandemic was defined as the most recent BMI measurement available before March 2020 (start of the COVID-19 pandemic in the Netherlands). International cut-off points were used to classify participants’ BMI: healthy body weight (BMI < 25 kg/m2), overweight (25 ≤ BMI < 30 kg/m2) and obesity (BMI ≥ 30 kg/m2) [33].
-
2.
Long-term obesity was defined based on two BMI measurements before March 2020 and preferably with a period of about 5 years between both measurements. For all nine cohorts two measurements were available. Long-term obesity was classified into: 1) no obesity at both measurements (reference group), 2) only former obesity, i.e. obesity at the least recent measurement, but not the most recent measurement, 3) only recent obesity, i.e. obesity at the most recent measurement, but not the least recent measurement, and 4) long-term obesity, i.e. obesity at both measurements.
-
3.
Most recent WC status before the pandemic was defined as the most recent WC measurement available before March 2020. For eight cohorts information on waist circumference was available. Sex-specific cut offs were used to classify participants’ WC: healthy waist (men: WC < 94 cm, women: WC < 80 cm), abdominal overweight (men: 94 cm≤WC < 102 cm, women: 80 cm≤WC < 88 cm), and abdominal obesity (men: WC ≥ 102 cm, women: WC ≥ 88 cm) [33].
-
4.
Long-term abdominal obesity was defined based on two WC measurements before March 2020 and preferably with a period of about 5 years between both measurements. For six cohorts two WC measurements were available. Long-term abdominal obesity was classified into: 1) no abdominal obesity at both measurements (reference group), 2) only former abdominal obesity, i.e. abdominal obesity at the least recent measurement, but not the most recent measurement, 3) only recent abdominal obesity, i.e. obesity at the most recent measurement, but not the least recent measurement, and 4) long-term abdominal obesity, i.e. abdominal obesity at both measurements.
Outcomes
Information on SARS-CoV-2 infection was collected between March 2020 and January 2023. The first outcome (self-reported SARS-CoV-2 infection) was based on having at least one SARS-CoV-2 infection (yes vs. no) confirmed with a positive PCR or rapid-antigen test based on self-report or ascertained by a physician based on self-report between March 2020-January 2023 (specific period depends on the individual cohort, see Table 1). Five cohorts collected information on SARS-CoV-2 infection once (Table 1). For the other cohorts, the mean number of SARS-CoV-2 infection measurements varied between 1.8-13.7 times per participant. The second outcome (SARS-CoV-2 infection by serology) was based on having at least one positive serological test for SARS-CoV-2 (yes vs. no) between March 2020-January 2022 (Table 1). SARS-CoV-2 infection by serology was available for three cohorts based on measurements in blood samples collected within the cohorts or based on linkage to existing registration data.
Confounders
Age (in years on March 1, 2020), sex and educational level were included as confounders, because men, older adults and the lower educated are known high-risk groups for (severe) COVID-19 [34, 35]. For one cohort (HELIUS), ethnicity was additionally included, because this cohort oversampled participants with a migration background [36]. Lastly, the available number of SARS-CoV-2 infection measurements was included, because the probability of reporting SARS-CoV-2 positive at least once is greater if the number of SARS-CoV-2 infection measurements is higher.
Statistical analysis
Analyses of the individual cohorts
Analyses by the individual cohorts were performed between March-September 2023. Logistic regression analyses were conducted for all four exposures and the two outcome measures separately. The analyses were adjusted for age, sex, educational level, number of SARS-CoV-2 infection measurements, and, if applicable, ethnicity. Odds ratios (ORs) and 95% confidence intervals (95%-CI) were reported. To assess the potential for bias in each cohort specific analysis, the risk of bias for each included cohort was assessed using the ROBINS-E tool [37].
Meta-analysis
ORs and 95%-CIs of the individual cohorts were transformed into log metrics. Subsequently, random-effects meta-analyses were conducted using restricted maximum likelihood as estimation method, as previously recommended [38, 39]. Individual study effects were pooled by applying the inverse variance method. Overall ORs and 95%-CIs were reported and forest plots were constructed. The I2 statistic was evaluated to assess between-study heterogeneity. Based on the Cochrane Handbook cut-off points, an I2 of 0%–40% was considered low heterogeneity, 30%–60% moderate heterogeneity, 50%–90% substantial heterogeneity, and 75%–100% high heterogeneity [40].
As 74% of all participants included in this meta-analysis were from one cohort (Lifelines), the meta-analyses were also performed without Lifelines to examine to which extent results were driven by this cohort.
Sensitivity analysis
Sensitivity analyses were conducted on individual cohorts only for the most recent measurement of BMI and WC and results were meta-analyzed thereafter. First, we stratified for measurement before January 2021 and from January 2021 onwards. By doing so, we differentiated between the phase of the pandemic where vaccines were available, as the Dutch SARS-CoV-2 vaccination campaign started on January 6, 2021. In addition, stratification based on time point is also relevant because there were other variants of the SARS-CoV-2 virus prevalent over time [41]. Furthermore, the availability of rapid-antigen tests changed over time. All may have influenced the results.
Analyses were also stratified by sex (men vs. women) and age ( < 70 years vs. ≥70 years), because men and older adults are known high-risk groups for (severe) COVID.
Analyses of the individual cohorts were conducted using IBM SPSS Statistics (IBM Corp, NY) and STATA (College Station, TX). STATA was used to conduct the meta-analyses. A p value < 0.05 was considered statistically significant.
Results
Description of the cohorts
Participant characteristics of the nine cohorts are described in Table S1. The number of participants varied between 913 and 74,049 participants, resulting in a total of 99,570 participants. In total, 60% of the participants were women (range: 49.8%–71.7%). The mean age ranged between 48.1 and 74.2 years. On average, 39.6% of the participants had overweight (range: 29.5%–46.9%) and 16.7% had obesity (range: 9.8%–37.0%) on the most recent measurement before the pandemic. The percentage of participants with a self-reported SARS-CoV-2 infection varied between 0.9% and 24.7% across the nine cohorts (Table S1), depending on the timing and number of SARS-CoV-2 infection measurements (Table 1). In three cohorts with available data on serology (i.e. Doetinchem Cohort Study, HELIUS, The Rotterdam Study), the SARS-CoV-2 infection rate varied between 9.8% and 22.2%. The results of the risk of bias assessment of the 9 included cohorts is shown in Supplementary Text S2.
Association between overweight and SARS-CoV-2 infection
Overweight status
The pooled results of the nine cohorts showed that individuals with overweight had a 1.08 times higher odds of a self-reported SARS-CoV-2 infection than individuals with a healthy body weight (OR = 1.08, 95%-CI 1.04–1.13, I2 = 0%) (Table 2, Fig. 1). The narrow confidence interval of this OR was largely due to the weight of Lifelines, and after excluding this cohort, the OR was similar, albeit no longer statistically significant (OR = 1.14, 95%-CI 0.99–1.30, I2 = 0%) (Table S2). The higher odds of having a SARS-CoV-2 infection among participants with overweight was not observed when using data from serological tests from three cohorts (OR = 1.02, 95%-CI 0.79–1.31, I2 = 54%) (Table 2, Fig. S4a).
CI confidence interval, HELIUS Healthy Life in an Urban Setting study, LASA Longitudinal Aging Study Amsterdam, NEO Netherlands Epidemiology of Obesity, NTR The Netherlands Twin Register, OR odds ratio. Odds ratios are adjusted for age, sex, educational level, and number of SARS-CoV-2 infection measurements (and ethnicity for one cohort).
Individuals with obesity were also more likely to report a SARS-CoV-2 infection than individuals with a healthy body weight (OR = 1.43, 95%-CI 1.18–1.75, I2 = 56%) (Table 2, Fig. 2). The effect estimate was hardly affected after excluding Lifelines (OR = 1.52, 95%-CI 1.29–1.79, I2 = 0) (Table S2). Higher odds for SARS-CoV-2 infection among individuals with obesity compared to individuals with a healthy weight were also observed by serological testing (OR = 1.22, 95%-CI 1.01–1.47, I2 = 0%) (Table 2, Fig. S4b).
CI confidence interval, HELIUS Healthy Life in an Urban Setting study, LASA Longitudinal Aging Study Amsterdam, NEO Netherlands Epidemiology of Obesity, NTR The Netherlands Twin Register, OR odds ratio. Odds ratios are adjusted for age, sex, educational level, and number of SARS-CoV-2 infection measurements (and ethnicity for one cohort).
For abdominal overweight, we observed similar associations with SARS-CoV-2 infection (based on both self-report and serology) as for general overweight based on BMI (Table 2, Fig. S2a). For abdominal obesity, however, slightly lower ORs were observed than for general obesity (Table 2). Individuals with abdominal obesity had a 1.24 times higher odds of a self-reported SARS-CoV-2 infection than individuals with a healthy waist (95%-CI 0.999–1.55, I2 = 57%) (Table 2, Fig. S2b). After excluding the Lifelines cohort, this OR was 1.35 (95%-CI 1.06-1.71, I2 = 28%) (Table S2).
Long-term obesity
The results stratified on duration of obesity showed that individuals with recent obesity only (i.e. obesity on the most recent, but not on the least recent measurement prior to the pandemic) had a higher odds of SARS-CoV-2 infection than individuals without obesity on both measurements (OR = 1.51, 95%-CI 1.– 01–2.26, I2 = 57% by self-report and OR = 1.61, 95%-CI 1.18–2.19, I2 = 0% by serology) (Table 2 and Fig. 3 and S6b). A similar higher odds for a self-reported SARS-CoV-2 infection was observed among individuals with long-term obesity (i.e. obesity on both measurements) (OR = 1.48, 95%-CI 1.15–1.91, I2 = 65%) (Table 2, Fig. 4), though this was not observed for SARS-CoV-2 infection by serology (OR = 1.18, 95%-CI 0.95–1.46, I2 = 0%) (Fig. S6c). No association between former obesity only (i.e. obesity on the least recent, but not on the most recent measurement) and SARS-CoV-2 infection was found (Table 2, Fig. 5 and S6a).
CI confidence interval, HELIUS Healthy Life in an Urban Setting study, LASA Longitudinal Aging Study Amsterdam, NEO Netherlands Epidemiology of Obesity, NTR The Netherlands Twin Register, OR odds ratio. Odds ratios are adjusted for age, sex, educational level, and number of SARS-CoV-2 infection measurements (and ethnicity for one cohort).
CI confidence interval, HELIUS Healthy Life in an Urban Setting study, LASA Longitudinal Aging Study Amsterdam, NEO Netherlands Epidemiology of Obesity, NTR The Netherlands Twin Register, OR odds ratio. Odds ratios are adjusted for age, sex, educational level, and number of SARS-CoV-2 infection measurements (and ethnicity for one cohort).
CI confidence interval, HELIUS Healthy Life in an Urban Setting study, LASA Longitudinal Aging Study Amsterdam, NEO Netherlands Epidemiology of Obesity, NTR The Netherlands Twin Register, OR odds ratio. Odds ratios are adjusted for age, sex, educational level, and number of SARS-CoV-2 infection measurements (and ethnicity for one cohort).
The results regarding duration of abdominal obesity were similar to those of general obesity based on BMI, except for individuals with recent abdominal obesity only. For this group, a statistically significant higher odds of SARS-CoV-2 infection by serology was observed (OR = 1.49, 95%-CI 1.13–1.97) (Table 2, Fig. S7b), but not for SARS-CoV-2 infection by self-report (OR = 1.17, 95%-CI 0.90–1.52) (Fig. S3b). For general obesity, this association was found for both SARS-CoV-2 infection outcomes (by self-report and serology).
Sensitivity analysis
Stratification before January 2021 vs. January 2021 onwards
Table S3 and Fig. S8 show that the effect estimates of the association between overweight and self-reported SARS-CoV-2 infection were similar before 2021 and from 2021 onwards. However, for obesity, the association was stronger before 2021 (OR = 1.59, 95%-CI 1.24–2.04, I2 = 44%) than from 2021 onwards (OR = 1.17, 95%-CI 0.96–1.43, I2 = 39%). For abdominal obesity, the same pattern was observed, but the differences in the magnitude of the associations between the two time periods was smaller (Table S3, Fig. S9). Results based on serology data, showed a similar pattern for the association with overweight (based on BMI or WC) before 2021 and from 2021 onwards (Table S4). However, these results should be interpreted with caution as they mostly rely on data from only two cohorts.
Stratification by sex and age
We observed similar results for men and women (Table S5). Stratifying the results by age did not lead to different conclusions for individuals aged <70 vs. ≥70 years for (abdominal) overweight and abdominal obesity (Table S6). However, the association between obesity based on BMI and self-reported SARS-CoV-2 infection seemed to be stronger in individuals ≥70 years than in individuals <70 years (OR 2.15, 95%-CI 1.30–3.55, I2 = 28% vs. OR 1.35, 95%-CI 1.09–1.68, I2 = 0%).
Discussion
In this meta-analysis of nine population-based cohort studies, individuals with obesity were at increased risk of SARS-CoV-2 infection compared to those with a healthy weight. To a lesser extent, this risk was also increased among individuals with overweight. The higher odds of SARS-CoV-2 infection was primarily observed in individuals with obesity at the most recent measurement prior to the COVID-19 pandemic. Individuals who previously had obesity, but no longer had obesity at the most recent measurement before the pandemic did not have an increased risk of SARS-CoV-2 infection. The association between obesity and SARS-CoV-2 infection appeared to be stronger in the period before 2021 than from 2021 onwards, when vaccines became available.
Our findings are in line with the results of an earlier meta-analysis that reported an increased risk of testing positive for SARS-CoV-2 among individuals with obesity [5]. The current study extends this work by covering a longer follow-up period during the COVID-19 pandemic and including other overweight-related exposures which have been studied less frequently. Two previous studies reported an association between overweight and SARS-CoV-2 infection [9, 10], and the present study supports their findings. In the present study, the association between overweight and SARS-CoV-2 infection was weaker than that of obesity. The effects of overweight were also less consistent, as no association was found when measuring SARS-CoV-2 infection by serology. However, serology was only available among 7200 individuals from three cohorts. A study among 235,928 participants of the UK Biobank cohort did find an association based on serology, but all data for this study were obtained in the beginning of the pandemic [9]. Therefore, more studies using data on SARS-CoV-2 obtained during a longer period of the pandemic are needed to confirm whether overweight is associated with higher SARS-CoV-2 infection susceptibility.
The current meta-analysis also identified abdominal overweight/obesity as possible risk factors for SARS-CoV-2 infection, but this was also not fully supported by serology data. Two earlier studies provided evidence for a (causal) association between abdominal fat and susceptibility for SARS-CoV-2 infection [14, 16]. Body fat, in particular visceral fat, is associated with high secretion of inflammation markers such an adipokines and cytokines and probably therefore a higher infection risk [12, 13]. Therefore, one could speculate that effect estimates of associations between WC, as a marker for abdominal fat, and SARS-CoV-2 infection would be larger than effect estimates for BMI as a marker of overall body fat. However, we did not observe such differences, which is in line with the results of an earlier study that used genetic instruments for BMI and WC assessment [15]. A postulated hypothesis in literature is that abdominal obesity is related to COVID-19 disease severity rather than risk of getting infected with SARS-CoV-2, which is supported by the results of two observational studies [42, 43]. Taken together further research is needed to assess the potential added risk of abdominal obesity compared to general obesity in the development of SARS-CoV-2 infection.
Earlier work on overweight and SARS-CoV-2 infection has mostly been conducted in hospital-based studies [5]. An advantage of the population-based cohorts in the current study is that they have prospective data, with multiple exposure-measurements from before the pandemic, which allowed us to examine duration of obesity. An interesting finding is that the SARS-CoV-2 infection risk was not higher in individuals who had obesity a long time before the pandemic ( > 5 years) but not shortly before the pandemic. This implies that obesity at the time of exposure to the infectious agent is more important than long-term obesity and that weight loss resulting in overweight or a healthy body weight leads to a lower risk. This is in line with a previous Korean study that found weight loss to be associated with a reduced SARS-CoV-2 infection rate [44]. Also supportive of our findings is a large prospective study that identified weight gain as a risk factor for pneumonia [45]. From a public health perspective, the indication that losing weight to obtain a healthier body weight may be associated with reduced infection risk underlines the importance of obesity prevention and management.
While our results provide further support for an association of overweight with SARS-CoV-2 infection, it remains to be seen whether this points to a causal mechanism or if there are other factors explaining this association. Differences in self-reported SARS-CoV-2 infection by weight status could for example result from differences in testing behavior. Individuals with obesity were already targeted as medical/high risk group for a more severe COVID-19 prognosis early in the pandemic [46]. Therefore, they may have been more likely to do a PCR or rapid-antigen test or visit a physician and be diagnosed with a SARS-CoV-2 infection than individuals with a healthy body weight. However, our results based on serology generally support the existence of a biological mechanism between obesity and acquiring a SARS-CoV-2 infection. Evidence from literature supports such a causal link. First of all, SARS-CoV-2 binds to the angiotensin-converting enzyme 2 receptor (ACE2), which is highly expressed in adipose tissue [3]. In addition, ACE2 is overexpressed among those having obesity which may determine greater viral entry and replication [13]. This suggests a role for adipose tissue as a virus reservoir, enhancing viral spread which is also known from other infectious diseases [47]. Furthermore, ACE2 expression is enhanced by several proinflammatory cytokines [12], the levels of which are already elevated in individuals with obesity. The state of chronic low-grade inflammation and associated impaired immune response in individuals with obesity contributes to being more susceptible to infections like SARS-CoV-2 [4]. Furthermore, our results also indicate that weight loss may reduce infection risk, which also supports a biological mechanism. Still, it needs to be confirmed whether weight loss itself or an associated healthier lifestyle, such as increased physical activity might be the primary protective factor against SARS-CoV-2 infection. However, Yoon et al. [44] observed an association between weight loss and reduced infection risk even after adjusting for physical activity [44]. This supports the hypothesis of weight loss to be directly responsible for the observed lower risk.
Interestingly, the observed increased SARS-CoV-2 infection risk among individuals with obesity, was higher in the period before 2021 than from 2021 onwards. Several differences between these periods exists. First, SARS-CoV-2 vaccines became available in the Netherlands in the beginning of 2021. In the Netherlands, individuals with severe obesity were considered a risk group for severe COVID-19 and had earlier access to the vaccine than the general population. In addition, individuals with obesity may have been more willing to receive the vaccine, which was shown in England, where vaccine uptake in 2021 has been found to be higher among those with overweight and obesity compared to those with a healthy weight [48]. This may have reduced the SARS-CoV-2 infection risk and/or symptoms in individuals with obesity from 2021 onwards, which may have resulted in a weaker association between obesity and SARS-CoV-2 infection in this period. Second, rapid-antigen tests also became available in the beginning of 2021. This may have led to an increase in the identification of a-symptomatic or mild SARS-CoV-2 cases, particularly among low-risk individuals with a healthy weight who might not have visited a testing facility or physician otherwise. This could also partly explain the weaker association between obesity and SARS-CoV-2 infection observed from 2021 onwards. Furthermore, subjective self-report is dependent on recall and testing behavior [49]. Therefore, serology is possibly a more objective measure to assess SARS-CoV-2 infections, especially early in the pandemic. Unfortunately, serology data in the present study did not allow the stratification according to period (before 2021 and from 2021 onwards) due to limited data. Lastly, dominant SARS-CoV-2 variants changed over time. While before 2021 almost all infections in The Netherlands were caused by the original strain, from 2021 onwards new variants emerged, which had higher transmissibility but resulted in less severe disease [41]. When more patients became asymptomatic, this may have resulted in more misclassification of cases and therefore weaker associations with overweight and obesity.
Methodological considerations
We pooled the results from protocolized analyses in nine individual cohort studies, resulting in a large study population covering a large share of the geographical and cultural variation within the Netherlands. Although we have no reason to assume that our results would not be generalizable to other high-income countries with similar or higher obesity rates, more research is needed to confirm this. Compared to most meta-analyses that combine results from published studies, we used a different approach in which individual studies conducted the same analyses based on a standardized protocol. Therefore, these analyses are more comparable in terms of the classification of the exposures, the adjustments to the models that were performed, and the sensitivity analyses that were carried out. This is also visible in the results of these particular domains of the risk of bias assessment (Text S2). Together, this might have contributed to the relatively low heterogeneity (I2) in most analyses. Nevertheless, the risk of bias assessment did reveal a high risk of bias due to missing data, which may have caused selection bias. Both non-response bias and attrition bias are common and inevitable in prospective cohorts such as those included in the current meta-analysis, though this does not necessarily cause biased results [50,51,52].
Despite the standardized protocol aiming to increase comparability between cohorts and analyses, the cohorts still varied in the timing and assessment of exposure and outcome and in the characteristics and numbers of included participants. The timing and method (measured or self-reported) of the BMI and WC assessment varied between cohorts, though in the majority of individuals the most recent BMI and WC status was objectively measured within 5 years before the start of the pandemic. Some cohorts only had one SARS-CoV-2 measurement in the first months of the pandemic (when testing opportunities were more limited), whereas others had multiple measurements throughout the pandemic. As a result, the number of self-reported SARS-CoV-2 infections also strongly differed between the cohorts. To take this into account, analyses were adjusted for the number of SARS-CoV-2 infection measurements. Besides assessment time, the cohorts also varied in size. Lifelines contained almost three quarters of all participants included in the current meta-analysis, and may as a consequence have dominated the results. After excluding Lifelines from the meta-analysis, effect estimates were in general slightly higher and heterogeneity was somewhat lower. However, the overall conclusion remained similar with and without Lifelines.
Conclusion
This study showed that individuals with (abdominal) obesity, and to a lesser extent individuals with (abdominal) overweight, were at increased risk of SARS-CoV-2 infection. Individuals with obesity long before the pandemic, but a healthier body weight just before the pandemic were not at increased risk. This indication that weight loss may be associated with reduced infection risk suggests that targeting overweight and obesity may not only contribute to improving population health by lowering the risk of chronic diseases, but also the risk of infectious diseases. Since it is likely that infectious diseases will continue to emerge and re-emerge in the future, these results therefore underline once more the importance of overweight prevention and weight reduction for public health.
Data availability
The data that support the findings of this study are available from the nine individual cohorts but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the individual cohorts.
References
Van Kerkhove MD, Vandemaele KA, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA, et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8:e1001053.
Wadman M. Why obesity worsens COVID-19. Science. 2020;369:1280–1.
Aghili SMM, Ebrahimpur M, Arjmand B, Shadman Z, Pejman Sani M, Qorbani M, et al. Obesity in COVID-19 era, implications for mechanisms, comorbidities, and prognosis: a review and meta-analysis. Int J Obes (Lond). 2021;45:998–1016.
Dalamaga M, Christodoulatos GS, Karampela I, Vallianou N, Apovian CM. Understanding the co-epidemic of obesity and COVID-19: current evidence, comparison with previous epidemics, mechanisms, and preventive and therapeutic perspectives. Curr Obes Rep. 2021;10:214–43.
Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21:e13128.
Abdullah A, Wolfe R, Mannan H, Stoelwinder JU, Stevenson C, Peeters A. Epidemiologic merit of obese-years, the combination of degree and duration of obesity. Am J Epidemiol. 2012;176:99–107.
Abdullah A, Wolfe R, Stoelwinder JU, de Courten M, Stevenson C, Walls HL, et al. The number of years lived with obesity and the risk of all-cause and cause-specific mortality. Int J Epidemiol. 2011;40:985–96.
Sorlie P, Wei GS. Population-based cohort studies: still relevant? J Am Coll Cardiol. 2011;58:2010–3.
Ho FK, Celis-Morales CA, Gray SR, Katikireddi SV, Niedzwiedz CL, Hastie C, et al. Modifiable and non-modifiable risk factors for COVID-19, and comparison to risk factors for influenza and pneumonia: results from a UK Biobank prospective cohort study. BMJ Open. 2020;10:e040402.
Holt H, Talaei M, Greenig M, Zenner D, Symons J, Relton C, et al. Risk factors for developing COVID-19: a population-based longitudinal study (COVIDENCE UK). Thorax. 2022;77:900–12.
NCD. Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016;387:1377–96.
Christen T, Trompet S, Rensen PCN, Willems van Dijk K, Lamb HJ, Jukema JW, et al. The role of inflammation in the association between overall and visceral adiposity and subclinical atherosclerosis. Nutr Metab Cardiovasc Dis. 2019;29:728–35.
Nigro E, D’Agnano V, Quarcio G, Mariniello DF, Bianco A, Daniele A, et al. Exploring the Network between Adipocytokines and Inflammatory Response in SARS-CoV-2 Infection: A Scoping Review. Nutrients. 2023;15.
Chen L, Sun X, Han D, Zhong J, Zhang H, Zheng L. Visceral adipose tissue and risk of COVID-19 susceptibility, hospitalization, and severity: A Mendelian randomization study. Front Public Health. 2022;10:1023935.
Freuer D, Linseisen J, Meisinger C. Impact of body composition on COVID-19 susceptibility and severity: A two-sample multivariable Mendelian randomization study. Metabolism. 2021;118:154732.
Yates T, Razieh C, Zaccardi F, Davies MJ, Khunti K. Obesity and risk of COVID-19: analysis of UK biobank. Prim Care Diabetes. 2020;14:566–7.
Netherlands Cohorts Consortium (NCC). Netherlands Cohort Consortium (NCC): a nationwide infrastructure for population health sciences. https://www.demaastrichtstudie.nl/netherlands-cohorts-consortium-ncc.
de Mutsert R, den Heijer M, Rabelink TJ, Smit JW, Romijn JA, Jukema JW, et al. The Netherlands Epidemiology of Obesity (NEO) study: study design and data collection. Eur J Epidemiol. 2013;28:513–23.
Hoogendijk EO, Deeg DJH, de Breij S, Klokgieters SS, Kok AAL, Stringa N, et al. The Longitudinal Aging Study Amsterdam: cohort update 2019 and additional data collections. Eur J Epidemiol. 2020;35:61–74.
Hoogendijk EO, van der Horst MHL, Poppelaars J, van Vliet M, Huisman M. Multiple domains of functioning in older adults during the pandemic: design and basic characteristics of the Longitudinal Aging Study Amsterdam COVID-19 questionnaire. Aging Clin Exp Res. 2021;33:1423–8.
Ikram MA, Brusselle G, Ghanbari M, Goedegebure A, Ikram MK, Kavousi M, et al. Objectives, design and main findings until 2020 from the Rotterdam Study. Eur J Epidemiol. 2020;35:483–517.
Kuijpers Y, Picavet HSJ, de Rond L, de Zeeuw-Brouwer ML, Rutkens R, Gijsbers E, et al. Potential determinants of antibody responses after vaccination against SARS-CoV-2 in older persons: the Doetinchem Cohort Study. Immun Ageing. 2023;20:57.
Licher S, Terzikhan N, Splinter MJ, Velek P, van Rooij FJA, Heemst JV, et al. Design, implementation and initial findings of COVID-19 research in the Rotterdam Study: leveraging existing infrastructure for population-based investigations on an emerging disease. Eur J Epidemiol. 2021;36:649–54.
Ligthart L, van Beijsterveldt CEM, Kevenaar ST, de Zeeuw E, van Bergen E, Bruins S, et al. The Netherlands twin register: longitudinal research based on twin and twin-family designs. Twin Res Hum Genet. 2019;22:623–36.
Mc Intyre K, Lanting P, Deelen P, Wiersma HH, Vonk JM, Ori APS, et al. Lifelines COVID-19 cohort: investigating COVID-19 infection and its health and societal impacts in a Dutch population-based cohort. BMJ Open. 2021;11:e044474.
Picavet HSJ, Blokstra A, Spijkerman AMW, Verschuren WMM. Cohort Profile Update: The Doetinchem Cohort Study 1987-2017: lifestyle, health and chronic diseases in a life course and ageing perspective. Int J Epidemiol. 2017;46:1751–g.
Scholtens S, Smidt N, Swertz MA, Bakker SJ, Dotinga A, Vonk JM, et al. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int J Epidemiol. 2015;44:1172–80.
Schram MT, Sep SJ, van der Kallen CJ, Dagnelie PC, Koster A, Schaper N, et al. The Maastricht Study: an extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. Eur J Epidemiol. 2014;29:439–51.
Snijder MB, Galenkamp H, Prins M, Derks EM, Peters RJG, Zwinderman AH, et al. Cohort profile: the Healthy Life in an Urban Setting (HELIUS) study in Amsterdam, The Netherlands. BMJ Open. 2017;7:e017873.
van Dongen J, Willemsen G, Heijmans BT, Neuteboom J, Kluft C, Jansen R, et al. Longitudinal weight differences, gene expression and blood biomarkers in BMI-discordant identical twins. Int J Obes (Lond). 2015;39:899–909.
Verschuren WM, Blokstra A, Picavet HS, Smit HA. Cohort profile: the Doetinchem Cohort Study. Int J Epidemiol. 2008;37:1236–41.
Westendorp RG, van Heemst D, Rozing MP, Frölich M, Mooijaart SP, Blauw GJ, et al. Nonagenarian siblings and their offspring display lower risk of mortality and morbidity than sporadic nonagenarians: The Leiden Longevity Study. J Am Geriatr Soc. 2009;57:1634–7.
Han TS, Sattar N, Lean M. ABC of obesity. Assessment of obesity and its clinical implications. Bmj. 2006;333:695–8.
Singu S, Acharya A, Challagundla K, Byrareddy SN. Impact of social determinants of health on the emerging COVID-19 pandemic in the United States. Front Public Health. 2020;8:406.
Zhang JJ, Dong X, Liu GH, Gao YD. Risk and protective factors for COVID-19 morbidity, severity, and mortality. Clin Rev Allergy Immunol. 2023;64:90–107.
Coyer L, Boyd A, Schinkel J, Agyemang C, Galenkamp H, Koopman ADM, et al. Differences in SARS-CoV-2 infections during the first and second wave of SARS-CoV-2 between six ethnic groups in Amsterdam, the Netherlands: A population-based longitudinal serological study. Lancet Reg Health Eur. 2022;13:100284.
Higgins JPT, Morgan RL, Rooney AA, Taylor KW, Thayer KA, Silva RA, et al. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ Int. 2024;186:108602.
Langan D, Higgins JPT, Jackson D, Bowden J, Veroniki AA, Kontopantelis E, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019;10:83–98.
Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat. 2005;30:261–93.
Deeks JJ, Higgins JPT, Altman DG Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. www.training.cochrane.org/handbook.
National Institute for Public Health and the Environment. Variants of the coronavirus SARS-CoV-2. https://www.rivm.nl/en/coronavirus-covid-19/current/variants.
Chandarana H, Dane B, Mikheev A, Taffel MT, Feng Y, Rusinek H. Visceral adipose tissue in patients with COVID-19: risk stratification for severity. Abdom Radio (NY). 2021;46:818–25.
Petersen A, Bressem K, Albrecht J, Thieß HM, Vahldiek J, Hamm B, et al. The role of visceral adiposity in the severity of COVID-19: Highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110:154317.
Yoon SS, Lim Y, Jeong S, Han HW. Association of weight changes with SARS-CoV-2 infection and severe COVID-19 outcomes: A nationwide retrospective cohort study. J Infect Public Health. 2023;16:1918–24.
Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med. 2000;160:3082–8.
Frühbeck G, Baker JL, Busetto L, Dicker D, Goossens GH, Halford JCG, et al. European association for the study of obesity position statement on the global COVID-19 pandemic. Obes Facts. 2020;13:292–6.
Ryan PM, Caplice NM. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obes (Silver Spring). 2020;28:1191–4.
Piernas C, Patone M, Astbury NM, Gao M, Sheikh A, Khunti K, et al. Associations of BMI with COVID-19 vaccine uptake, vaccine effectiveness, and risk of severe COVID-19 outcomes after vaccination in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2022;10:571–80.
Kim Y, Donnelly CA, Nouvellet P. Drivers of SARS-CoV-2 testing behaviour: a modelling study using nationwide testing data in England. Nat Commun. 2023;14:2148.
Kondal D, Awasthi A, Patel SA, Chang HH, Ali MK, Deepa M, et al. Evaluating bias with loss to follow-up in a community-based cohort: empirical investigation from the CARRS Study. J Epidemiol Community Health. 2024;78:220–7.
Lacey RJ, Jordan KP, Croft PR. Does attrition during follow-up of a population cohort study inevitably lead to biased estimates of health status? PLoS One. 2013;8:e83948.
Saiepour N, Ware R, Najman J, Baker P, Clavarino A, Williams G. Do participants with different patterns of loss to follow-up have different characteristics? A multi-wave longitudinal study. J Epidemiol. 2016;26:45–9.
Acknowledgements
The authors wish to acknowledge the services of the nine cohorts of the NCC participating in the current study (Doetinchem Cohort Study, Healthy Life in an Urban Setting study (HELIUS), Longitudinal Aging Study Amsterdam (LASA), Leiden Longevity Study, Lifelines, The Netherlands Twin Register (NTR), The Netherlands Epidemiology of Obesity (NEO) study, The Maastricht Study, and The Rotterdam Study) and all the study participants. The authors thank Lannie Ligthart and Meike Bartels for their contribution to the COVID-19 data collection of NTR. With regards to the NEO study, the authors thank P. van Beelen for collecting data, P. Noordijk and her team for sample handling, and I. de Jonge for data management of the NEO study. With regards to The Rotterdam Study, the authors acknowledge Frank van Rooij as data manager and thank Jolande van Heemst and Natalie Terzikhan for their invaluable contribution to the collection of the data and are grateful to the contribution of patient representatives from the Dutch Cancer Society and Harteraad to the design of the questionnaire.
Funding
The current study was funded by the Dutch Ministry of Health, Welfare and Sports as part of the COVID-19 program, subtheme Health Impact. The Doetinchem Cohort Study is supported by the Dutch Ministry of Health, Welfare and Sport and the National Institute for Public Health and the Environment. The HELIUS study is also funded by the Dutch Heart Foundation (2010 T084), ZonMw (200500003), the European Union (FP-7) (278901), and the European Fund for the Integration of non-EU immigrants (EIF) (2013EIF013). The HELIUS COVID-19 substudy was also supported by ZonMw (10430022010002) and the Public Health Service of Amsterdam (Research and Development 2021 75722692, Public Health Laboratory grant 2022). The HELIUS study is conducted by Amsterdam UMC, location Academic Medical Center and the Public Health Service of Amsterdam. Both organizations provided core support for HELIUS. The Longitudinal Aging Study Amsterdam (LASA) is largely supported by a grant from the Netherlands Ministry of Health Welfare and Sports, Directorate of Long-Term Care. The Leiden Longevity Study has received funding from the European Union’s Seventh Framework Programme (FP7/2007-2011: grant agreementnr 259679). This study was further supported by BBMRI-NL, a Research Infrastructure financed by the Dutch government (NWO 184.021.007 and 184.033.111), and the VOILA consortium (ZonMw; 457001001). The Lifelines initiative has been made possible by subsidy from the Dutch Ministry of Health, Welfare and Sport, the Dutch Ministry of Economic Affairs, the University Medical Center Groningen (UMCG), Groningen University and the Provinces in the North of the Netherlands (Drenthe, Friesland, Groningen). NTR is supported by the Corona Fast track grant: Extended twin-family study of COVID-19 and its impact (NWO-440-20-022). The NEO study is supported by the participating Departments, Division, and Board of Directors of the Leiden University Medical Center, and by the Leiden University, Research Profile Area Vascular and Regenerative Medicine. The Maastricht Study is supported by the European Regional Development Fund via OP-Zuid, the Province of Limburg, the Dutch Ministry of Economic Affairs (grant 31 O.041), Stichting De Weijerhorst (Maastricht, the Netherlands), the Pearl String Initiative Diabetes (Amsterdam, the Netherlands), the Cardiovascular Center (CVC, Maastricht, the Netherlands), CARIM School for Cardiovascular Diseases (Maastricht, the Netherlands), CAPHRI Care and Public Health Research Institute (Maastricht, the Netherlands), NUTRIM School for Nutrition and Translational Research in Metabolism (Maastricht, the Netherlands), Stichting Annadal (Maastricht, the Netherlands), Health Foundation Limburg (Maastricht, the Netherlands), and by unrestricted grants from Janssen-Cilag BV (Tilburg, the Netherlands), Novo Nordisk Farma BV (Alphen aan den Rijn, the Netherlands), and Sanofi-Aventis Netherlands BV (Gouda, the Netherlands). The Rotterdam Study is funded by the Erasmus University Medical Centre and Erasmus University, Rotterdam; the Netherlands Organisation for Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly (RIDE); the Ministry of Education, Culture and Science; the Ministry of Health, Welfare and Sport; the European Commission (DG XII); and the Municipality of Rotterdam.
Author information
Authors and Affiliations
Consortia
Contributions
BL, JMAB, and SWB were responsible for designing the study protocol and writing the manuscript. BL, MB, SLC, EOH, FH, DMEP, MJRS, JHPMV, and SWB were responsible for analyzing data. All authors contributed to designing the study protocol, writing the manuscript, and interpreting results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All cohort studies were approved by an Institutional Review Board (Doetinchem Cohort Study: Medical Ethical Committee of Utrecht and the Netherlands Organization for Applied Scientific Research; Healthy Life in an Urban Setting study: Medical Ethical Committee of the Academic Medical Center Amsterdam; Longitudinal Aging Study Amsterdam: Medical Ethical Committee of the VU University Medical Center; Leiden Longevity Study: Medical Ethical Committee of the Leiden University Medical Center; Lifelines: Medical Ethical Committee of the University Medical Center Groningen; The Netherlands Twin Register: Medical Ethical Committee of the VU University Medical Center; The Maastricht Study: Medical Ethical Committee Maastricht University Medical Center and the Dutch Ministry of Health, Welfare and Sport; The Netherlands Epidemiology of Obesity: Medical Ethical Committee of the Leiden University Medical Center; The Rotterdam Study: Medical Ethics Committee of the Erasmus MC and the Dutch Ministry of Health, Welfare, and Sport) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Loef, B., Boer, J.M.A., Beekman, M. et al. The association of overweight, obesity, and long-term obesity with SARS-CoV-2 infection: a meta-analysis of 9 population-based cohorts from the Netherlands Cohorts Consortium. Int J Obes 49, 586–595 (2025). https://doi.org/10.1038/s41366-024-01660-x
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41366-024-01660-x
This article is cited by
-
The Longitudinal Aging Study Amsterdam: design and cohort update 2025
European Journal of Epidemiology (2025)