Abstract
Objective
Experiencing ostracism (i.e., social exclusion) may impact self-regulatory eating behaviors, particularly in youths with excess weight. Yet, research in pediatric patients with obesity is lacking. Hence, we examined the effect of Virtual Reality(VR)-based ostracism on motivation for food in children and adolescents with BMI ≥97th percentile.
Methods
In a randomized experimental between-subject design, forty-one patients (Mage = 13.37 years, 46% female) with a diagnosis of obesity (ICD-10: E66) were randomized to a social exclusion or inclusion condition in a VR-Cyberball-paradigm. Patients’ salivary cortisol, heart rate and heart rate variability were assessed. Furthermore, we measured patients’ motivation to consume high-calorie food, their prosocial behavior, their self-reported urge to eat and subjective stress.
Results
Results indicate that the experience of social exclusion in youths with obesity leads to a blunted salivary cortisol response; in contrast, no effects of social exclusion on the sympathetic nervous system were observed. Social exclusion was associated with an increased perceived threat to fundamental social needs. Similarly, ostracized participants demonstrated heightened self-regulatory behaviors regarding their motivation for high-calorie food intake, selecting fewer grams of sweets following social exclusion. Furthermore, ostracism tended to increase helping behavior post-exclusion, although this effect was not significant. Self-reported urge to eat and stress levels during the experiment showed no significant effect.
Conclusion
Ostracism-induced reduction of motivation for food suggests that affiliative behaviors like increasing compliance regarding eating behaviors may play a role in youths with obesity with BMI ≥97th percentile in the context of social stress. Future research should explore the broader social context, including family and friends, to better understand the dynamics between social stress, physiological reactivity, and self-regulatory behaviors in treating obesity.
Clinical trial registration
As this study does not constitute a clinical trial, the study design and analyses plans were not preregistered.
Similar content being viewed by others
Introduction
Childhood obesity is defined by the WHO as excessive fat accumulation and is classified as a BMI (kg/m²) ≥97th percentile for age and sex, and as severe obesity when the BMI is ≥99th percentile [1, 2]. One factor in the development and maintenance of childhood obesity is eating behavior, particularly the tendency to over-consume high-calorie foods [3, 4]. Research suggests that individuals with obesity are more likely to use eating as a coping mechanism for stress compared to those with an average weight [5]. Among stressors, the experience of ostracism – characterized by social exclusion, rejection, or being ignored – is considered highly impactful for all social beings [6]. Particularly social exclusion has been shown to – on the one hand – undermine fundamental social needs such as belonging and control [7, 8] and – on the other hand – to deplete attentional resources essential for self-regulation. Consequently, it has been suggested that social exclusion may also influence food intake [9]. Even brief ostracism episodes have been found to increase the likelihood of consuming unhealthy foods in average-weight adults [10, 11]. Compared to adults, adolescents may be even more vulnerable to ostracism-triggered comfort eating due to their heightened rejection sensitivity [12]. This vulnerability may be particularly pronounced among individuals with obesity, who not only face social exclusion but also experience weight-related stigmatization in their personal relationships [13].
Initial studies in adolescents show that experimental ostracism indeed increases motivation for food and food intake [5, 9, 14]. Yet, these past samples were heterogeneous and included no children and/or adolescents with more severe forms of obesity (i.e., with BMI ≥99th percentile). This is notable, as research suggests that children with severe obesity may experience higher levels of emotional eating and stress [15], as well as lower overall health-related quality of life than those with less severe obesity [16]. Additionally, no study has so far considered physiological reactivity in ostracized children and adolescents with obesity, although there is strong evidence for the impact of social exclusion on the hypothalamic–pituitary–adrenal (HPA) axis and the autonomic nervous system (ANS) [17]. For instance, the use of another type of stressor, a social-evaluative paradigm (TSST [18]), has resulted in elevated cortisol levels, which in turn increased food intake in adults [19] and children [20] with obesity.
Given the lack of research, particularly in children and adolescents with more severe forms of obesity, we aimed to explore how a brief social exclusion episode impacts self-regulation with regards to high-calorie foods in children with BMI ≥97th percentile. To replicate realistic social interactions, we used virtual reality (VR), known for its high ecological validity [21, 22]. In addition to assessing motivation for food, we evaluated one form of prosocial behavior (i.e., helping the experimenter pick up pens), as social exclusion has been shown to impact prosocial behaviors [23], particularly helping behavior [24] in average-weight individuals. Lastly, we set out to measure heart rate as an indicator of ANS activity and salivary cortisol as an indicator of HPA reactivity, both of which are understudied in individuals with obesity in the context of ostracism.
Methods
Participants
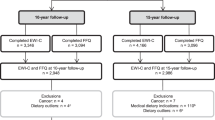
Participants were 41 children and adolescents aged 10–18 years (Mage = 13.37, SDage = 2.46 years, 46% females) with an ICD-10-diagnosis of obesity (E66.0 [2]). Participants were recruited between summer 2021 and summer 2023 at the Outpatient Clinic for Pediatric Obesity and Dyslipidemia of the Division of Pediatric Pulmonology, Allergology and Endocrinology, Department of Pediatrics and Adolescent Medicine, see Fig. 1.
Patients were eligible if they fulfilled the following inclusion criteria: (1) 10–18 years of age, (2) an ICD-10 diagnosis of obesity due to excess calories (E66.0) with a BMI ≥97th percentile [2], and (3) sufficient German language proficiency. Diagnoses were extracted a priori from the institution’s patient registry. Exclusion criteria were (1) estimated cognitive impairment (IQ < 70) and/or intellectual disability, (2) a diagnosis of schizophrenia, schizotypal or delusional disorder (F20–29), bipolar affective disorder (F31), manic episode (F30), or acute crisis (suicidality), (3) poor visual acuity, (4) a pronounced motion sickness, and (5) pregnancy or hormonal contraceptives (pill, hormonal IUD). Consistent with the inclusion and exclusion criteria, the final sample had no identified psychiatric comorbidities (also no known trauma). Additionally, there were no differences in clinical or demographic characteristics between the inclusion (n = 20) and the exclusion group (n = 21), see Table 1.
Procedure
This study adopted a single blinded randomized experimental between-subject design (following block randomization) and was approved by the local ethics committee (vote no. 2170/2019). Potential participants were approached by study staff during their outpatient appointment. In case of approval, a separate appointment was scheduled.
Experiments were conducted in a temperature-controlled environment (22 °C) between 11:30–15:30 to account for diurnal cortisol variations. Patients were asked to abstain from eating 1 h prior to their visit, and not to consume caffeine (e.g., energy drinks) or nicotine. All patients complied with these requirements. Upon arrival, a legal guardian (a parent in all cases) provided written informed consent. Additionally, written consents (patients ≥14 years of age) or assents ( < 14 years of age) were obtained from children and adolescents. A stratified allocation scheme (by age and sex) was used by unblinded study staff to randomly assign participants to either the exclusion group or the inclusion/control group. Participants were blinded to their allocation and the study’s objectives.
Five minutes prior to the stressor, heart rate (HR) measurements (baseline) were started. Then, the head mounted display (HMD, model: HTC vive, Taiwan) was donned and participants engaged in the VR-Cyberball-game (see next section for details). Post exposure, patients recovered for 5 min (HR recovery period), were exposed to two behavioral tasks (assessing motivation for food and prosocial behavior) and proceeded to complete the last questionnaires, before they were debriefed. Patients did not receive any remuneration.
VR-Cyberball paradigm
To manipulate ostracism, we used the Cyberball paradigm [25], which constitutes a ball-tossing game, typically played on a desktop PC. Participants toss the ball to two animated figures until they are unexpectedly excluded from the game. This brief exclusion task is known to elicit strong emotional and physiological responses [26, 27] but has also been criticized [22, 28] for its artificial nature. By reverting to VR, which better simulates real social interactions [17, 29], we aimed to enhance ecological validity. Our VR-Cyberball game, developed in Unreal Engine 4 and displayed via a HTC Vive-headset, features two virtual agents (one male, one female) in a gymnasium (Fig. 2). Participants experience the game from a first-person view, standing upright. They may look around but not leave their spot. Controllers vibrate to indicate when it’s their turn to toss the ball. The virtual agents do not engage in any verbal or non-verbal communication. The game involves 180 tosses, with participants being excluded after 60 tosses in the exclusion condition or receiving 33% of passes (50:50 from both co-players) in the inclusion condition. The duration is 5 min for both conditions.
Measures
Baseline measures
Alongside clinical data (diagnosis, height, weight), a sociodemographic questionnaire assessed age, sex, type of school, parents’ level of education, socioeconomic status, and parents’ marital status. Furthermore, symptom severity (e.g., emotional symptoms, conduct problems) was assessed with the 25-item proxy report Strengths and Difficulties Questionnaire (SDQ [30]; sdqinfo.org). The 26-item Emotional Eating Scale – adapted for Children and Adolescents (EES-C [31]) reflected the self-reported urge to eat in response to a range of negative emotions. Finally, children reported their chronic stress levels on the 10-item Perceived Stress Scale (PSS [32]).
Experimental measures
Stress
A single item on a visual analog scale (VAS, 100 mm, 0 = not at all – 100 = very much) assessed stress (“How stressed do you feel now?”) at 4 time points: (1) upon arrival (baseline), (2) during the waiting phase (anticipation), (3) after the VR-Cyberball game (stressor), (4) during the recovery phase (habituation).
Hunger
One item (“How strong is your urge to eat something now?”) assessed hunger (VAS, 100 mm, 0 = very small − 100 = very large; see [33]) at 4 time points: (1) baseline, (2) anticipation, (3) stressor, (4) habituation.
Manipulation check (MC)
The 20-item Fundamental Social Needs Scale, FSNS [8, 34] assessed (1) belonging, (2) self-esteem, (3) meaningful existence, and (4) control on a 5-point Likert scale (1 = not at all – 5 = extremely). Also, the two items “I was ignored”, “I was excluded” (1 = not at all – 5 = extremely) served as a MC, alongside a third item asking participants to estimate the percentage of in-game passes they received. Internal consistency (Cronbach’s α) of the sub-scales was satisfactory, ranging from Cronbach’s α = 0.70 for the sub-scale control to α = 0.85 for the sub-scale belonging. Furthermore, the 6-item Children’s Rejection Sensitivity Questionnaire (CRSQ [35, 36]) on a 6-point Likert scale (1 = not at all – 6 = very) served as a baseline measure to check for pre-existing differences between the two groups regarding their rejection sensitivity. The sum score reflects children’s expectancy to be socially rejected by others (rejection expectancy, Cronbach’s α = 0.70 in this study).
Physiological measures
Salivary cortisol
Cortisol levels [37] were quantified from commercial cotton swabs (Salivette®, Sarstedt, Nuembrecht, Germany) using Elecsys Cortisol II electrochemiluminescence (ECLIA) assays (Roche, Rotkreuz, Switzerland) on cobas®e801 analyzers (Roche). The measuring interval of this test ranges from 0.054 µg/dL–634 µg/dL (conversion to nmol/L = µg/dLx27.586) and the manufacturer reports inter-assay precision in human saliva to be between 1.3–4.9% CV (coefficient of variation) and intermediate precision to be between 1.9–7.8% CV. Participants put the swab into their cheek pouch for 120 s at a resting-state-phase and post-exposure to VR-Cyberball. Analyses were conducted at the certified (ISO 9001) and accredited (ISO 15189) Department of Laboratory Medicine, Medical University of Vienna.
Heart rate (HR)
A chest belt with a wireless sensor (Polar V800, Polar Electro, Finland) measured HR for 15 min as an indicator of ANS activity (5 min baseline, 5 min VR-Cyberball, 5 min habituation). Data were pre-processed via visual inspection of artefacts. Subsequently, mean HR and heart rate variability (HRV, root mean square of successive difference, rMSSD) were calculated based on the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology recommendations [38]. Patients were standing up during baseline and stressor but sat down for the habituation phase.
Behavioral measures
Self-regulatory behaviors
Similar to past experiments [5, 10, 11], we used the reinforcing value of unhealthy snacks as an indicator of self-regulation. Like in Baumeister et al. [10], a bowl containing a large serving (1728 g) of bite-size high-calorie sweets (e.g., Twix, Maoam, PEZ; see supplement) was placed in front of participants post VR-Cyberball. Yet, contrary to past research [5, 10, 11], participants did not actually consume (or taste-test [10, 11]) these high-calorie foods. After consulting with the medical obesity team, we concluded that this approach was inappropriate due to our participants’ health conditions and the potential to interfere with their ongoing medical care. Participants were instructed to take as many or as few sweets as they wished to consume at this point in time and place them in a plastic bag (instead of eating them on the spot). The bag was then collected by study-staff and weighed. The serving’s weight (in grams) served as an indicator of motivation for high-calorie food consumption, with a higher weight reflecting greater motivation for sweets and, consequently, poorer self-regulation. At the end of the experiment, participants were debriefed about this study’s measures.
Helping behaviors
To assess one form of prosocial behavior post-exclusion, the experimenter accidentally dropped a cup of pens in the participant’s imminent vicinity [21]. Helping the experimenter to pick up the pens (y/n) and the time to pick them up, both served as indicators of prosocial behavior.
Statistical methods
Analyses were conducted using IBM SPSS 29, and R 4.4.2. Linear mixed-effects models were used to analyze primary outcomes – including salivary cortisol, heart rate (HR), heart rate variability (HRV), stress, and urge to eat – while accounting for repeated measures nested within participants. These models included fixed effects for time, group, and their interaction, with random intercepts to capture individual variability. T-tests (or Welch’s t-tests when the assumption of homogeneity of variances was violated), as well as χ²-tests, were used to analyze the secondary outcomes of fundamental social needs, self-regulatory behavior, and helping behavior. Tukey-corrected simple effects analyses were applied whenever interactions reached significance. The sample size needed was established a priori. Power analysis using G*Power [39] with α = 0.05 and 1-β = 0.80 indicated that our study was sufficiently powered; that is, we expected small to moderate effect sizes (Cohen’s d = 0.40) for group comparisons and the time*group interaction, which requires an N of 28–42 to be considered clinically significant. In our study context, a Cohen’s d of 0.4 corresponds to a difference of approximately 5 beats per minute (bpm) in HR or about 9 rating points on the urge-to-eat scale between the two conditions at a specific time point. While these differences may appear modest, a 5 bpm change in HR can be physiologically significant and a 9-point difference on a 0–100 scale likely indicates a meaningful behavioral shift, providing a practical benchmark for clinical relevance.
Results
Manipulation check
There were no differences in clinical and demographic characteristics between conditions (Table 1). Furthermore, there was no significant difference regarding self-reported rejection expectancy (CRSQ) between the groups (see Table 2 for the statistical results for each outcome). Included participants reported receiving significantly more ball tosses than excluded participants (32% vs. 13%). Moreover, as shown in Fig. 3A, excluded participants reported significantly lower satisfaction of fundamental social needs – specifically, lower levels of belonging, self-esteem, meaningful existence, and control – compared to those in the inclusion condition.
A FSNS Fundamental Social Needs Scale [8, 34] with four sub-scales; B Salivary cortisol in micrograms (µg) per deciliter (dl). C Heart rate in beats per minute (bmp); y-axis capped at 80 bpm for brevity D HRV heart rate variability, rMSSD root mean square of successive difference, y-axis capped at 4 rMSSD for brevity; E Weight of sweets in gram (g).
Self-reported urge to eat and stress
Additionally, we analyzed the urge to eat and stress across four time points. For urge to eat, linear mixed-effects models showed no significant main effect of time, group, as well as no time*group interaction. Similarly, for self-reported stress, we found no significant effect of time, no between-group effect, and no time*group interaction. See Table 2 for statistical results.
Physiological reactivity
Results indicate a significant increase in salivary cortisol after social exclusion compared to the resting-state phase. Additionally, a significant time*group interaction effect demonstrated that the excluded group exhibited significantly lower salivary cortisol reactivity compared to the included group. No significant between-group effect for salivary cortisol was observed. Additionally, a significant increase in HR was observed during the task across groups; however, no significant time*group interaction was found for HR, and no between-group differences were detected. Similar results were found for HRV represented by rMSSD values, with a decrease in HRV during the task in all participants, neither significant between-group effects nor a significant time*group interaction were observed; see Fig. 3B–D and Table 2.
Behavioral measures
A significant group difference was observed regarding self-regulatory behavior after VR-Cyberball. As illustrated in Fig. 3E, excluded participants picked significantly fewer grams of sweets than included participants. Helping behavior, as measured by the pen task, tended to be higher in excluded compared to included participants, though this difference did not reach statistical significance (p = 0.054). This effect was limited to the decision to help rather than the latency to help.
Discussion
Consistent with prior studies [5, 8, 17, 21], being ostracized in VR led to a significant depletion of fundamental social needs like belonging, self-esteem, meaningful existence, and control in children and adolescents with obesity (BMI ≥97th [2]). However, we did not find that experiencing social exclusion deteriorated self-regulatory eating behaviors [5, 9, 14]. In our study, excluded participants showed less motivation for high-calorie foods than those in the inclusion/control condition.
While it is commonly assumed that ostracism draws attentional resources away from self-monitoring and impairs one’s control over food intake [5], our study suggests the opposite – at least for an experimental manipulation which did not entail actually eating high-calorie foods [5, 10, 11]. Due to the health condition of our participants (BMI ≥97th percentile [2]), including those with more severe obesity (BMI ≥99th percentile), we decided to measure motivation for food, rather than actual intake. Unlike previous studies [10, 11] which masked food intake by framing it as a taste-test to obtain an incidental measure of self-regulatory behaviors, we offered our participants a large serving of sweets and allowed them to take as many or as few as they wished to consume at a given time. Hence, our participants were aware of collecting unhealthy foods, and this awareness may explain the differences in results between our study and previous research. In circumstances in which the self, or parts of the self, contribute to the exclusion, an adaptive response may entail altering this part of the self to regain social acceptance. Potential responses to ostracism may thus include increased conformity [34], such as reducing food intake or engaging in more prosocial behavior [40]. In addition to the lesser servings of sweets, we also observed that excluded participants tended to help the experimenter more frequently. Although this was only a trend, together with the smaller servings, it may be indicative of participants’ adaptive response to ostracism. Future research should establish whether a heightened attentional focus on restoring social acceptance could help explain differences in self-regulation.
This is the first study to consider both HPA and ANS reactivity to ostracism in pediatric patients with BMI ≥97th percentile. While HR levels were increased during the Cyberball-game but showed no differences between the inclusion and exclusion condition, salivary cortisol levels were significantly higher in included individuals than in excluded ones. The fact that HPA axis and ANS reactivity remain understudied in the context of obesity [3, 41], particularly in children, complicates the contextualization of our results. Similar to our findings, adult studies show increased HR during social exclusion [17, 42] and blunted cortisol responses in ostracized women [43, 44]. Furthermore, research suggests that the presence of trauma (i.e., adverse childhood events, post traumatic stress disorder, PTSD) may be associated with a blunted cortisol response towards social stress, leading to a diminished capacity of the body to regain homeostasis and hence, to prolonged periods of stress [45, 46]. In the context of our study, this may help further explain the lowered motivation for food in excluded individuals. However, similar to trauma, excess fat tissue has also been linked to chronically downregulated HPA and ANS in overweight children, causing lower overall ANS activity and blunted cortisol responses compared to average-weight peers [3, 41]. These complex interactions highlight the need for more social stress research in children and adolescents with obesity, all the more given the pivotal role of HPA in the disease’s etiology [47].
Limitations
Our results must be interpreted in relation to several limitations. First, we classified participants based on the ICD-10 E-Diagnoses (e.g., E66.0 [1, 2]), commonly used in Europe, particularly in Germany and Austria. However, as of 2024, the U.S. has adopted the ICD-10-CM Z-codes [48, 49], which provide a more detailed classification. For instance, class 3 obesity (Z68.56) is defined as a BMI ≥ 140% of the 95th percentile [48, 49]. Comparisons between studies using these different classification systems should therefore be interpreted with caution. Furthermore, our sample was small but ensured adequate statistical power. However, replicating the study in larger samples, particularly with a more balanced distribution of sex, as well as focusing exclusively on children and adolescents with severe obesity, who may face different challenges to those with less severe obesity [15, 16], would be beneficial. Second, the experimental design may to some degree limit the ecological validity of our results. In everyday life there are more alternatives to coping with social stress than just food [5]. The context in which stress is experienced, as well as available social resources like significant others may also have an impact on the ability to self-regulate under stress [50]. Collecting data in real-life circumstances with Ecological Momentary Assessment [51] may better be suited to reflect the complex association between social stress and depleted self-regulatory behaviors regarding high-calorie food consumption. Finally, we measured acute, short-term physiological stress responses. Yet, as chronically elevated cortisol levels may increase appetite, insulin secretion, and fat accumulation in the long-term [3], collecting scalp hair samples as indicators of systemic cortisol secretion [52] may be an additional route of action for this field of research.
Conclusion
In conclusion, our study is the first to examine ostracism in relation to salivary cortisol and heart rate in patients with obesity (BMI ≥97th percentile). Using self-report, physiological and behavioral measures to cover a broad range of responses, we found that the painful experience of being ostracized led to a diminished motivation for food in children and adolescents with excess weight, a possible motive being the need to replenish social resources. Based on these results, we may hypothesize that affiliative behaviors might play a role in dietary decisions, especially in the face of social stress. Clinical interventions could thus incorporate social support strategies, such as peer groups, to help mitigate the emotional impact of social exclusion experiences on dietary behaviors. Additionally, psychological therapies like cognitive behavioral therapy (CBT), can foster resilience against social stress and promote healthier eating patterns. Furthermore, future research should take a broader social context into account, also probing intimate relationships with family and friends, to learn more about the dynamics between depleted fundamental needs, physiological reactivity, and self-regulatory behaviors in the hope of contributing to the notoriously difficult treatment of pediatric patients with obesity.
Data availability
Research data are not in the public domain as they contain sensitive patient data. Data may be requested by contacting the corresponding author.
References
World Health Organization. BMI-for-age (5-19 years). WHO-Website. 2025. https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/bmi-for-age.
Wabitsch M, Moss A. S3-Leitlinie Therapie und Prävention der Adipositas im Kindes- und Jugendalter [S3 guideline therapy and prevention of obesity in childhood and adolescence]. Arbeitsgemeinschaft Adipositas im Kindes und Jugendalter (AGA). 2019. https://register.awmf.org/de/leitlinien/detail/050-002.
Kappes C, Stein R, Körner A, Merkenschlager A, Kiess W. Stress, stress reduction and obesity in childhood and adolescence. Horm Res Paediatr. 2023;96:88–96. https://doi.org/10.1159/000519284.
Roy SK, Jahan K, Alam N, Rois R, Ferdaus A, Israt S, et al. Perceived stress, eating behavior, and overweight and obesity among urban adolescents. J Health Popul Nutr. 2021;40:54. https://doi.org/10.1186/s41043-021-00279-2.
Salvy SJ, Bowker JC, Nitecki LA, Kluczynski MA, Germeroth LJ, Roemmich JN. Impact of simulated ostracism on overweight and normal-weight youths’ motivation to eat and food intake. Appetite. 2011;56:39–45. https://doi.org/10.1016/j.appet.2010.11.140.
Williams KD, Nida SA. Ostracism and social exclusion: implications for separation, social isolation, and loss. Curr Opin Psychol. 2022;47:101353. https://doi.org/10.1016/j.copsyc.2022.101353.
Williams KD. Ostracism: the kiss of social death. Soc Personal Psychol Compass. 2007;1:236–47. https://doi.org/10.1111/j.1751-9004.2007.00004.x.
Zadro L, Williams KD, Richardson R. How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-esteem, and meaningful existence. J Exp Soc Psychol. 2004;40:560–7. https://doi.org/10.1016/j.jesp.2003.11.006.
Salvy SJ, Bowker JC, Nitecki LA, Kluczynski MA, Germeroth LJ, Roemmich JN. Effects of ostracism and social connection-related activities on adolescents’ motivation to eat and energy intake. J Pediatr Psychol. 2012;37:23–32. https://doi.org/10.1093/jpepsy/jsr066.
Baumeister RF, DeWall CN, Ciarocco NJ, Twenge JM. Social exclusion impairs self-regulation. J Pers Soc Psychol. 2005;88:589–604. https://doi.org/10.1037/0022-3514.88.4.589.
Oaten M, Williams KD, Jones A, Zadro L. The effects of ostracism on self–regulation in the socially anxious. J Soc Clin Psychol. 2008;27:471–504. https://doi.org/10.1521/jscp.2008.27.5.471.
Sisk LM, Gee DG. Stress and adolescence: vulnerability and opportunity during a sensitive window of development. Curr Opin Psychol. 2022;44:286–92. https://doi.org/10.1016/j.copsyc.2021.10.005.
Puhl RM, Moss-Racusin CA, Schwartz MB, Brownell KD. Weight stigmatization and bias reduction: perspectives of overweight and obese adults. Health Educ Res. 2008;23:347–58. https://doi.org/10.1093/her/cym052.
Senese VP, Pezzella M, Pasquariello L, Ali S. Effects of social exclusion and maternal rejection on Children’s high-caloric food consumption. Appetite. 2020;145:104494. https://doi.org/10.1016/j.appet.2019.104494.
Thaker VV, Osganian SK, deFerranti SD, Sonneville KR, Cheng JK, Feldman HA, et al. Psychosocial, behavioral and clinical correlates of children with overweight and obesity. BMC Pediatr. 2020;20:291. https://doi.org/10.1186/s12887-020-02145-2.
Black WR, Borner KB, Beauchamp MT, Davis AM, Dreyer Gillette ML, Sweeney B, et al. Health-related quality of life across recent pediatric obesity classification recommendations. Children. 2021;8:303. https://doi.org/10.3390/children8040303.
Kothgassner OD, Goreis A, Glenk LM, Kafka JX, Beutl L, Kryspin-Exner I, et al. Virtual and real-life ostracism and its impact on a subsequent acute stressor. Phys Behav. 2021;228:113205. https://doi.org/10.1016/j.physbeh.2020.113205.
Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. https://doi.org/10.1159/000119004.
Herhaus B, Ullmann E, Chrousos G, Petrowski K. High/low cortisol reactivity and food intake in people with obesity and healthy weight. Transl Psychiatry. 2020;10:40. https://doi.org/10.1038/s41398-020-0729-6.
Wijnant K, Klosowska J, Braet C, Verbeken S, De Henauw S, Vanhaecke L, et al. Stress responsiveness and emotional eating depend on youngsters’ chronic stress level and overweight. Nutrients. 2021;13:3654. https://doi.org/10.3390/nu13103654.
Kothgassner OD, Felnhofer A. Does virtual reality help to cut the Gordian knot between ecological validity and experimental control? Ann Int Commun Assoc. 2020. https://doi.org/10.1080/23808985.2020.1792790.
Parsons TD. Virtual reality for enhanced ecological validity and experimental control in the clinical, affective and social neurosciences. Front Hum Neurosci. 2015;9. https://doi.org/10.3389/fnhum.2015.00660.
Twenge JM, Baumeister RF, DeWall CN, Ciarocco NJ, Bartels JM. Social exclusion decreases prosocial behavior. J Pers Soc Psychol. 2007;92:56–66. https://doi.org/10.1037/0022-3514.92.1.56.
Kothgassner OD, Griesinger M, Kettner K, Wayan K, Völkl-Kernstock S, Hlavacs H, et al. Real-life prosocial behavior decreases after being socially excluded by avatars, not agents. Comput Hum Behav. 2017;70:261–9. https://doi.org/10.1016/j.chb.2016.12.059.
Williams KD, Jarvis B. Cyberball: a program for use in research on interpersonal ostracism and acceptance. Behav Res Methods. 2006;38:174–80. https://doi.org/10.3758/BF03192765.
Cacioppo S, Frum C, Asp E, Weiss RM, Lewis JW, Cacioppo JT, et al. A quantitative meta-analysis of functional imaging studies of social rejection. Sci Rep. 2013;3:2027. https://doi.org/10.1038/srep02027.
Vijayakumar N, Cheng TW, Pfeifer JH. Neural correlates of social exclusion across ages: a coordinate-based meta-analysis of functional MRI studies. NeuroImage. 2017;153:359–68. https://doi.org/10.1016/j.neuroimage.2017.02.050.
Kothgassner OD, Hlavacs H, Beutl L, Glenk LM, Palme R, Felnhofer A, et al. Two experimental virtual paradigms for stress research: developing avatar-based approaches for interpersonal and evaluative stressors. In: Entertainment computing-ICEC 2016: 15th IFIP TC 14 international conference. Vienna, Austria: Springer International Publishing; 2016. https://doi.org/10.1007/978-3-319-46100-7_5.
Kassner MP, Wesselmann ED, Law AT, Williams KD, et al. Virtually ostracized: studying ostracism in immersive virtual environments. Cyberpsychol Behav Soc Netw. 2012;15:399–403. https://doi.org/10.1089/cyber.2012.0113.
Goodman R, Ford T, Simmons H, Gatward R, Meltzer H. Using the Strengths and Difficulties Questionnaire (SDQ) to screen for child psychiatric disorders in a community sample. Br J Psychiatry. 2000;177:534–9. https://doi.org/10.1192/bjp.177.6.534.
Tanofsky-Kraff M, Theim KR, Yanovski SZ, Bassett AM, Burns NP, Ranzenhofer LM, et al. Validation of the emotional eating scale adapted for use in children and adolescents (EES-C). Int J Eat Disord. 2007;40:232–40. https://doi.org/10.1002/eat.20362.
Klein EM, Brähler E, Dreier M, Reinecke L, Müller KW, Schmutzer G, et al. The German version of the Perceived Stress Scale—psychometric characteristics in a representative German community sample. BMC Psychiatry. 2016;16:159. https://doi.org/10.1186/s12888-016-0875-9.
Rouach V, Bloch M, Rosenberg N, Gilad S, Limor R, Stern N, et al. The acute ghrelin response to a psychological stress challenge does not predict the post-stress urge to eat. Psychoneuroendocrinology. 2007;32:693–702. https://doi.org/10.1016/j.psyneuen.2007.04.010.
Williams KD. Ostracism: a temporal need-threat model. Adv Exp Soc Psychol. 2009. https://doi.org/10.1016/S0065-2601(08)00406-1.
Downey G, Lebolt A, Rincón C, Freitas AL. Rejection sensitivity and children’s interpersonal difficulties. Child Dev. 1998;69:1074–91. https://doi.org/10.1111/j.1467-8624.1998.tb06161.x.
London B, Downey G, Bonica C, Paltin I. Social causes and consequences of rejection sensitivity. J Res Adolesc. 2007;17:481–506. https://doi.org/10.1111/j.1532-7795.2007.00531.x.
Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15:669–74. https://doi.org/10.1038/nn.3086.
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Task Force of the European Society of Cardiology, Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996. https://doi.org/10.1161/01.CIR.93.5.1043.
Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G* Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–60. https://doi.org/10.3758/BRM.41.4.1149.
Balliet D, Ferris DL. Ostracism and prosocial behavior: a social dilemma perspective. Organ Behav Hum Decis Process. 2013;120:298–308. https://doi.org/10.1016/j.obhdp.2012.04.004.
Doom JR, Lumeng JC, Sturza J, Kaciroti N, Vazquez DM, Miller AL. Longitudinal associations between overweight/obesity and stress biology in low-income children. Int J Obes. 2020;44:646–55. https://doi.org/10.1038/s41366-019-0447-4.
Williamson TJ, Thomas KS, Eisenberger NI, Stanton AL. Effects of social exclusion on cardiovascular and affective reactivity to a socially evaluative stressor. Int J Behav Med. 2018;25:410–20. https://doi.org/10.1007/s12529-018-9720-5.
Weik U, Kuepper Y, Hennig J, Deinzer R. Effects of pre-experience of social exclusion on hypothalamus-pituitary-adrenal axis and catecholaminergic responsiveness to public speaking stress. PLoS ONE. 2013;8:e60433. https://doi.org/10.1371/journal.pone.0060433.
Zöller C, Maroof P, Weik U, Deinzer R. No effect of social exclusion on salivary cortisol secretion in women in a randomized controlled study. Psychoneuroendocrinology. 2010;35:1294–8. https://doi.org/10.1016/j.psyneuen.2010.02.019.
Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events: a study among healthy young subjects. Psychoneuroendocrinology. 2008;33:227–37. https://doi.org/10.1016/j.psyneuen.2007.11.004.
Metz S, Duesenberg M, Hellmann-Regen J, Wolf OT, Roepke S, Otte C, et al. Blunted salivary cortisol response to psychosocial stress in women with posttraumatic stress disorder. J Psychiatry Res. 2020;130:112–9. https://doi.org/10.1016/j.jpsychires.2020.07.014.
Hewagalamulage SD, Lee TK, Clarke IJ, Henry BA. Stress, cortisol, and obesity: a role for cortisol responsiveness in identifying individuals prone to obesity. Domest Anim Endocrinol. 2016;56:S112–20. https://doi.org/10.1016/j.domaniend.2016.03.004.
Centers for Disease Control and Prevention. International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM). National Center for Health Statistics. 2024. https://www.cdc.gov/nchs/icd/icd-10-cm/?CDC_AAref_Val=https://www.cd.
Obesity Medicine Association. New ICD-10 codes for obesity treatment: advancements in accurate diagnosis and care. 2024. https://obesitymedicine.org/blog/new-icd-10-codes-for-obesity-treatment-advancements-in-accurate-diagnosis-and-care/.c.gov/nchs/icd/icd-10-cm.htm.
Altheimer G, Urry HL. Do emotions cause eating? The role of previous experiences and social context in emotional eating. Curr Dir Psychol Sci. 2019;28:234–40. https://doi.org/10.1177/0963721419837685.
Goldschmidt AB, Smith KE, Crosby RD, Boyd HK, Dougherty E, Engel SG, et al. Ecological momentary assessment of maladaptive eating in children and adolescents with overweight or obesity. Int J Eat Disord. 2018;51:549–57. https://doi.org/10.1002/eat.22864.
Kitani RA, Letsou K, Kokka I, Kanaka-Gantenbein C, Bacopoulou F. Difference in hair cortisol concentrations between obese and non-obese children and adolescents: a systematic review. Children. 2022;9:715. https://doi.org/10.3390/children9050715.
Acknowledgements
We would like to thank Jeremias Büker, Katharina Goinska, Kathrin Kertesz and Nina-Katharina Walleczek for their assistance in recruiting patients and collecting parts of the data. Furthermore, we thank Robimo for programming and Stephan Mayrhofer for his assistance in pre-processing the heart rate data.
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Contributions
All authors fulfill the criteria for authorship. Conceptualization: AF and ODK. Data curation: AF, ODK, HH, and RM. Formal analysis: AG, AF, and ODK. Funding acquisition: n.a. Investigation: AF and LW. Methodology: AF and ODK. Project administration: AF, HH, LW, and CN. Resources: PLP, SGP, GS, CN, HH, and RM. Software: PLP, AF, and ODK. Supervision: AF, PLP, SGP, and RM. Writing—original draft: AF, AG, and ODK. Writing—review and editing: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest with regards to this study. In addition, PLP declares that he is an advisor for Boehringer-Ingelheim and received speaker honoraria from GLK, Janssen, InfectoPharm and Oral B.
Ethical approval
All procedures were performed in compliance with relevant laws and institutional guidelines and have been approved by the ethics committee of the Medical University of Vienna (vote no. 2170/2019).
Informed consent
Written informed consent was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Felnhofer, A., Goreis, A., Weiss, L. et al. Ostracism, cortisol reactivity, and motivation for high-calorie food in children and adolescents with obesity. Int J Obes 49, 2117–2124 (2025). https://doi.org/10.1038/s41366-025-01824-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-025-01824-3