Abstract
Metabolic surgery is currently the most effective available treatment for obesity and diabetes. However, it cannot be practiced widely, as some potential candidate patients do not have access to this procedure, primarily because it is expensive, necessitates experience on the part of operators, and requires adequate hospital facilities. Furthermore, side effects, although rare, remain a problem. Consequently, an ideal approach would be to reproduce the mechanisms of action of metabolic surgery through a noninvasive pharmacological treatment. To accomplish this, it is necessary to determine the exact mechanisms involved. Despite numerous studies in this field, a definitive conclusion has not yet been reached. Some of the known effects of metabolic surgery on organisms are described herein. Upon in-depth examination, all can be traced back to a functional modification of the autonomic GI-brain axis, mediated by afferent vagal fibers, establishing a constant relationship with brain centers to control food intake. These mechanisms act through the postsynaptic receptors of certain neurotransmitters. A viable path for implementing a pharmacological therapy for obesity may therefore be to identify drugs that act on these receptors to achieve adequate therapeutic responses. Possible candidates include substances that modulate various subtypes of NMDA glutamate receptors or gamma-aminobutyric acid (GABA) receptors. In conclusion, autonomic modifications which have so far been shown to be activated by metabolic surgery represent the pieces of a puzzle which, when put together, allow us to identify the functional modification of the GI-brain vagal axis as the primary cause of this treatment’s positive effects. These findings suggest the plausibility of an alternative pharmacological mechanism.
Similar content being viewed by others
Introduction
Obesity and the associated type 2 diabetes are major contributors to global morbidity and mortality, are increasingly prevalent, and require prompt treatment [1]. The worldwide economic burden of obesity is substantial [2], yet the current therapeutics and their possibilities are limited. Lifestyle modifications (low-calorie diet and physical activity) yield unsatisfactory results because of the organism’s strong resistance to weight loss (see below) [3].
Glucagon-like peptide (GLP-1) analogs are Food and Drug Administration-approved drugs that have demonstrated remarkable long-term efficacy when combined with a healthy diet, physical activity, and behavioral modifications. However, they are not without potential side effects, including a low risk of pancreatitis [4, 5] and gastrointestinal (GI) disturbances such as nausea, vomiting, and diarrhea [6]. Moreover, they are expensive (reducing body weight by 1% is estimated to be $985 for tirzepatide and $1,845 for semaglutide) [7], leading to lower compliance rates. Finally, when the treatment was interrupted, most, if not all, of the lost weight was regained [8].
Currently, the most effective therapy with the longest-lasting effects is metabolic surgery, mainly sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB), which involves remodeling the GI tract [9]. This treatment is invasive and not easily accessible (less than 1% of eligible patients have access, as it varies greatly between countries [10] and may be limited by the low propensity of some patients to undergo surgery), but in certain cases, it represents a valid option [11]. It provides long-term effectiveness, achieving sustained weight loss of 20–40% with significant improvements in the metabolic profile [9].
Why is metabolic surgery so effective?
Despite substantial progress in recent years, the mechanisms underlying the effectiveness of metabolic surgery are unclear [12]. However, growing evidence suggests a fundamental role of the autonomic nervous system in connecting the enteric nervous system with the central nervous system (CNS): the “gut–brain axis” [13, 14] (Fig. 1).
What is the gut–brain axis, and how is it connected to the CNS?
The GI tract has an internal nervous system called the enteric nervous system, which is considered the second brain that evolved before the CNS. It contains 200–400 million neurons distributed in thousands of small ganglia, most of which are found in the myenteric and submucosal plexuses [15]. When activated, the plexuses generate electrical impulses. Nerve impulses are also generated by contact between nutrients and specialized mucosal cells, known as enteroendocrine cells (EECs). They secrete enterohormones that activate their respective receptors on vagal fibers [14, 16]. Vagal nerves are located throughout the GI tract and extend to the apical tips of the villi [16].
The enteric nervous system is connected to the CNS through the vagal afferent fibers (VAFs). These fibers comprise the pseudounipolar cells (consisting of a rounded cell body and a single axon that bifurcates into two extensions, one peripheral that terminates in internal organs and one directed to the CNS, forming synaptic connections with neurons in the brainstem nuclei, mainly the nucleus tractus solitarius [NTS]). Nervous signals are transmitted to several brain regions, including those regulating food intake and energy balance [17,18,19].
Completing the picture of the gut–brain axis are specialized EECs, called “neuropod cells” (owing to their long cytoplasmic process extending into the lamina propria) that communicate the presence of sugars to the enteric vagal neurons [20].
How does metabolic surgery affect the gut–brain connection?
Many studies have indicated that metabolic surgery modifies vagal signaling from the GI tract to the CNS, thus altering its anatomical integrity and functional properties [21,22,23].
However, to answer this question more precisely, it is useful to assemble a puzzle with a certain number of pieces (corresponding to the known effects) currently available (Fig. 2).
Piece no. 1: Resetting the body weight
Why is it so hard to lose weight?
The body maintains a stable weight to preserve its energy reserves. According to the set-point theory, this occurs at a pre-established level with a feedback mechanism controlled by the hypothalamus [24].
Changes in the gut–brain axis restore the normal body weight set point (lowering it by approximately 30%) [25].
RYGB modifies several hypothalamic neurotransmitters [25]. In animal models, the expression of the AgRP gene in neurons that produce orexigenic (appetite-stimulating) neuropeptide Y decreases to levels similar to those in lean animals [26]. The expression of this gene was significantly lower in animals that underwent SG than in those that underwent gastric banding [27]. In contrast, the expression of genes produced by neurons secreting the anorexic polypeptide pro- opiomelanocortin is significantly upregulated after metabolic surgery [27]. Finally, RYGB leads to the normalization of brain m-opioid receptors, which are significantly reduced in various limbic and cortical regions in obesity [28].
These changes have not been observed in people with overweight or obesity who lost the same weight through diet alone [29].
Piece no. 2: Failure of the counter-regulatory response to weight loss
According to the set-point theory, mammalian physiology has evolved to prevent body weight loss under variable environmental conditions [30]. This evolutionary response safeguards energy reserves in the event of food shortage (metabolic adaptation) [12, 31]. Normally, in the face of weight loss, the CNS reacts by increasing hunger and attraction to high-calorie foods, reducing energy consumption, and activating genes that govern orexigenic stimuli [32]. This reaction is absent in animals and humans who have lost weight after undergoing metabolic surgery [12], suggesting that surgery suppresses counter-regulation [33]. Findings from several studies suggest an inappropriately high energy expenditure in patients who undergo RYGB compared to the reduction in body weight achieved. Therefore, metabolic surgery, particularly RYGB, can override strong biological signals that cause hypometabolism and increase hunger due to weight loss [19].
This may be related to the restoration of the hypothalamic weight set point due to the alteration of the gut–brain vagal axis (see above) [12]. Orexigenic genes are not activated after weight loss caused by RYGB [32].
Piece no. 3: Effect of high-calorie diet on VAFs
What are the harmful effects of a diet that is too high in calories on the neurovegetative system?
In experimental animals, prolonged intake of diets rich in fats or sugars (hypercaloric) alters vagal afferent signaling and the biophysical properties of the gut–brain vagal axis. This includes reduced excitability of afferent vagal neurons (reduced vagal activation), reduced sensitivity of their receptors [33], and impairment of the vagovagal reflexes [34].
Plasticity changes (i.e., the ability of neurons to change their shape and function in response to environmental alterations) also occur [35]. Morphological alterations in neurons, such as increased size and dendritic arborization, are associated with these changes [34].
Animals with obesity experience a decrease in the excitability of vagal afferent neurons, leading to reduced activation in response to hormonal metabolic signals from the gut [36]. This reduced sensitivity requires a higher stimulus (hunger) to elicit an appropriate action potential [36]. The threshold required to activate the gastric mechanoreceptors also increases [35].
This leads to excessive food consumption owing to the reduced suppressive effects of intestinal nutrients [37, 38] and reduced satiety, hyperphagia, and weight gain [39, 40]. This also applies to people with obesity, in whom food signaling from the GI tract is altered because of the reduced sensitivity of the intestinal vagal afferents [41].
RYGB corrects many of these effects [35]. This reversibility suggests that vagovagal neurocircuits are open to modulation and adaptation and may represent targets for obesity therapy (see below) [35]. After SG, the NTS sprouts new vagal afferents (an increase in axonal collaterals) and forms new synapses [22].
Piece no. 4: Effect of a high-calorie diet on brain activity
Prolonged exposure to high-calorie foods can also desensitize the ventral tegmental dopaminergic area (VTA) of the brain, which is part of the reward system [42, 43]. Positron emission tomography has demonstrated reduced availability of dopaminergic D2/3 receptors in individuals with obesity compared with controls, due to a primary receptor deficiency or secondary downregulation of the receptor [44]. According to the “reward deficiency” theory, in cases of obesity, a greater desire for palatable foods is not accompanied by a sufficient reward, thus promoting excessive consumption [45]. This can lead to a form of “addiction” to calorie-rich food [46, 47]. However, dopamine’s role in the control of food intake is probably more complicated than this hypothesis. It is possible that dopamine level alterations in the neurons in the subcortical basal ganglia of the forebrain, known as the “striatum,” may influence the control of eating and, therefore, contribute to excess adiposity in people with obesity [48].
These changes in the dopaminergic system do not regress after weight loss is achieved with a simple diet [47]. However, RYGB and SG reverse this trend [49] by inducing the intake of low- calorie foods, such as fruits and vegetables (see below) [50].
VAF signaling modified by surgery plays an important role in these changes [50,51,52,53]. Through the vagus nerve, the GI tract can control dopamine release in the brain reward area and modulate its neuroplasticity [51, 54]. Thus, the vagal gut–brain axis is an integral component of the neuronal reward pathway. Moreover, there is a neuronal population of “reward neurons” among the sensory cells of the right (not the left) vagus nerve [53].
Accordingly, functional magnetic resonance imaging (fMRI) revealed greater decreases in reactivity to food cues (in the cerebral reward systems, fusiform and parahippocampal gyrus) post- RYGB and post-SG in individuals with overweight/obesity compared with those without any interventions or non-surgical/non-pharmacological interventions, such as after low-calorie diet or very low-calorie diet, even when weight loss was similar [55].
fMRI has also shown that after RYGB, caloric food-induced activation is reduced in the VTA and dorsal striatal areas [53]. However, this lower activation was not evident in SG, indicating a possible difference between the effects of the two surgical techniques [43].
Piece no. 5: Changing eating habits
Do patients who have undergone surgery modify their eating approach?
Changes in eating behavior after obesity surgery include decreased hunger; increased satiety and fullness; decreased food hedonism, reward, and motivation; modified taste and food preferences; and potential food aversion or avoidance resulting from adverse post-ingressive symptoms [26, 51]. Effectively, the diet changes spontaneously from “hedonic” (eating to obtain pleasure in the absence of an energy deficit) to “energetic” (nutrition-based attitude toward food) [53]. This “healthier” diet is characterized by a preference for less caloric foods (increased consumption of fruits and vegetables [56] and a reduction in meal size because of an earlier onset of satiety [50]. This does not occur as a result of weight loss achieved through other means [11, 46, 57]. This reduction in meal size is followed by a gradual increase within the first postoperative year with progressive adaptation [58]. Interestingly, as reported above, activation of the right, but not the left, vagus sensory ganglion affects the flavor and natural preferences of food by influencing dopamine release from the substantia nigra [53].
The reason for these positive changes is thought to be modified VAF signaling to the brain’s VTA [42, 52] and the restoration of the correct sensitivity of the dopaminergic area to nutrients [53, 59] with changes in the hedonic processing of food intake [19]. Thus, the GI tract can control dopamine release through the vagus in the reward sectors by modulating neuroplasticity [51, 54, 60].
RYGB and SG effectively induce this new eating behavior [61]. This finding suggests a common biological mechanism [50]; however, this effect is more pronounced after RYGB than SG [59].
Piece no. 6: Survival of afferent vagal branches after RYGB
Are all VAFs involved in metabolic surgery?
In RYGB, only the dorsal and ventral gastric vagal branches are severed during the surgery; the celiac branches traveling with the gastroduodenal and superior mesenteric arteries remain intact [62].
The signals transmitted by the spared VAFs (contained in the celiac branches) originating from the middle and lower intestines and from the Roux branch (the first intestinal segment that food reaches after surgery) contribute to the efficacy of RYGB [63]. Indeed, RYGB, accompanied by dissecting the celiac and dorsal gastric vagal branches, is less efficient in suppressing food intake and reducing body weight [63]. In a rat model, it was found that when the dorsal neurovascular bundle (near the division of the dorsal gastric and celiac branches) was transected, RYGB produced less suppression of food intake and weight loss without affecting GLP-1 and peptide YY (PYY) levels [64]. The important role of the spared VAFs in RYGB has been confirmed by the observation that rats, in which the para-esophageal neurovascular bundle (which contains the celiac vagal trunk) is simultaneously sectioned, recover all the weight they lose and ingest the same amount of food as sham-operated animals [64].
These results are consistent with the hypothesis that, as mentioned above, after RYGB, at least during the early postoperative period, overstimulation of chemosensors and mechanosensors due to the uncontrolled influx of undigested nutrients into the surviving intestinal segments leads to intense vagal signaling that activates satiety and weight loss [20, 63].
Piece no. 7: Block of the ascending vagus and vagotomy
What are the consequences of this?
Applying high-frequency electrical pulses to the gastric vagal trunk blocks nerve transmission, reducing food intake and inducing weight loss [65]. Similarly, blocking VAF signaling through continuous electrical stimulation of the vagus at the level of the gastroesophageal junction increases satiety in people with obesity, resulting in significantly greater excess weight loss at 1 year than in sham controls (24.4% vs. 15.9%) [66]. This effect remained fairly stable at the 2-year follow-up with associated metabolic improvements [65, 67].
Piece no. 8: Gastric pouch
What could be the role of the gastric pouch formed by the surgery?
The amount of regained weight after metabolic surgery is significantly correlated with the dilation of the small pouch that is occasionally left in the upper part of the stomach [68]. This is suggested by the effectiveness, regarding weight loss and glucose reduction, of a silicone band placed around the gastric pouch below the gastroesophageal junction [69,70,71].
Such an effect is not due to the reduced absorption of nutrients by a smaller stomach but to the activity of the gut–brain axis [72]. This is demonstrated by the lack of a physiological counter- regulatory reaction to weight loss (previously discussed) and because satiety is maintained during interprandial periods and fasting [69]. VAFs are likely involved: blocking afferent vagal activity with capsaicin abolishes this effect [73].
Piece no. 9: Food interaction with the duodenal wall
Does the duodenum play a physiopathological role?
The interaction between ingested food and the first part of the small intestine informs the CNS about the size and composition of the meal to optimize digestion and absorption; nutrient sensing plays a vital role in the context of obesity and type 2 diabetes [74].
The duodenum may be considered a new target for treating obesity-related type 2 diabetes. Contact between ingested nutrients and the duodenal wall can be prevented using a duodenal- jejunal sleeve that extends from the duodenal bulb along the entire length of the proximal small intestine [75]. The same effect can be achieved with a paste called “Luminal Coating of the Intestine” (already used to cover gastric ulcers), which, when ingested, sticks to the mucosa [76]. These procedures promote weight loss and improve diabetes. Duodenal mucosal resurfacing, an endoscopic procedure in which the duodenal mucosa is removed by infusing warm water, is equally effective in reducing blood glucose and improving insulin sensitivity and secretion within a few months. In addition, the incretin system is not responsible for this effect [77].
Such isolation of the duodenal wall from food could be considered a functional duodenal vagotomy as duodenal VAFs cease to be stimulated by nutrients postprandially, with effects comparable to those of RYGB [76].
The same therapeutic effect can be achieved with SG, wherein the passage of food through the duodenum is not eliminated. How can this happen?
Conceivably, there is a lack of vagal regulation of food flow between the stomach and duodenum [78]. For example, anthropyloric phasic contractions control transpyloric pulsatile flow and the mechanism of gastric emptying conditions the absorptive capacity of the duodenum [79]. In their absence, the rapid duodenal transit of food and its lack of absorption may modify VAFs signaling [79, 80].
Piece no. 10: Electroacupuncture of the ear’s auricle
Besides, significant variations in gastric volume caused by SG can cause profound alterations in vagal reflexes due to entry and exit of food, highlighting the potential efficacy of targeting gastric afferent pathways to achieve weight loss [81].
Many studies have illustrated the use of acupuncture (traditional Chinese auriculotherapy) and electroacupuncture in the external ear for the treatment of obesity. Electrostimulation of the cymba conchae (upper cavity of the pinna) activates the central projections of the auricular branch of the vagus nerve (part of the vagal afferent pathways) toward the brain [82]. In particular, they activate different regions involved in controlling food intake, including the ipsilateral NTS, bilateral spinal trigeminal nucleus, dorsal raphe, locus coeruleus and contralateral parabrachial area, amygdala, and nucleus accumbens [82]. Acupuncture has proven to be effective in treating obesity [83,84,85]. The mechanisms underlying this effect remain unclear, but neuroendocrine regulation of the aforementioned brain areas may play an important role [86].
Piece no. 11: VAFs, liver, and endocrine pancreas
The liver plays a crucial role in regulating the effects of the autonomic nervous system on energy homeostasis [87]. Hepatic glucose production (HGP) is regulated by sympathetic (activation) and parasympathetic (inhibition) activities. VAFs mediate the ability of nutrients to regulate glycemia through the gut–brain–liver axis [88]. For example, the interaction of cholecystokinin (one of the enteric hormones produced by EECs) with VAFs inhibits HGP via this axis [89].
If malfunctioning, this link can play a critical pathophysiological role in the diabetes that accompanies obesity. One of the hallmarks of this disease is the hepatic hyperproduction of glucose through gluconeogenesis [87]. High postprandial concentrations of glucagon (a potent activator of HGP) caused by an inappropriate vagal stimulus also play a significant role [87].
Metabolic surgery contributes to treating diabetes through HGP reduction, which is linked to an early improvement in hepatic insulin sensitivity [90]. Interruption of the vagal connection between the intestine and liver may play a determining role in this effect [12]. Inactivation of the vagus nerve induces HGP suppression [91]. Therefore, we can hypothesize a reduction in HGP through selective denervation of the hepatic branches of the vagus.
The early improvement in glycemic control after RYGB (which would be too early to be due to weight loss) can be explained by the restoration of the first (cephalic) phase of insulin secretion in response to oral glucose (which is absent or severely impaired in type 2 diabetes) [92]. This effect may also depend on the post-surgical modification of vagal control over pancreatic insulin secretion. To confirm this hypothesis, in a rat model with diabetes (but without obesity), surgical diversion of the proximal intestine caused a rapid improvement in glycemia without a reduction in food intake or weight change [93].
Piece no. 12: Incretins and importance of paracrine processes
Do incretins play a role in metabolic surgery?
Surgery results in a marked post-prandial increase in incretins, particularly GLP-1 (up to 10 times), reaching levels similar to those in patients without obesity), which play an essential role in weight loss and diabetes reduction [94]. This increase is principally caused by the rapid transit of partially digested nutrients to the distal intestine, which contains the highest concentration of L cells that secrete GLP-1 [95].
In dealing with mechanisms of feeding and energy control, an additional focus is deserved to paracrine processes. Paracrine signaling consists of the release of factors into the adjacent extracellular space that modifies the function of cells located a short distance away. In this, it differs from endocrine signaling, in which hormones are transported to distant targets by the bloodstream.
GLP-1 is a typical example. In fact, paracrine GLP-1 signaling, activating adjacent receptors expressed on vagal terminals of gastrointestinal origin, plays an important role in eating behavior and metabolic homeostasis [96]. Actually, although a small percentage of GLP-1 can enter the circulation and act directly on cerebral neurons, its action is mainly paracrine because of its short half-life (2–3 min); only 10– 15% of GLP-1 is found in the circulation in its intact form [97].
A recent extensive discussion details the importance of the vagus nerve in mediating the regulatory effects of GLP-1 on feeding behavior and energy balance [97]. As proof of this importance, the GLP-1 efficacy is almost completely lost after bilateral subdiaphragmatic vagotomy [98, 99] or the destruction or damage of VAFs by capsaicin (a chemical neurotoxin) [36]. Thus, in human and animal models, EECs profoundly affect vagal signaling through paracrine incretin signaling [100].
Are GLP-1 receptors on VAFs uniformly distributed throughout the GI tract?
It has been shown that most vagal sensory neurons expressing the GLP-1 receptor are concentrated in the stomach and tend to become fewer along the intestine [97]. Interestingly, they are predominantly composed of neuronal populations that transmit signals of distension of the corpus or fundus of the stomach (vagal mechanosensory neurons) [97]. This may contribute to the explanation for the effectiveness of SG, in which these parts are drastically reduced.
Finally, the function of GLP-1 that is synthesized and released directly in the CNS (and strongly implicated in the control of eating behavior by the CNS) is partially regulated by inputs from vagal sensory neurons as well [97].
Among the main candidate mechanisms, the intestinal hormones GLP-1 and PYY likely play a critical role in metabolic surgery because they can influence energy balance (by controlling food intake and expenditure) and glucose homeostasis. However, this role appears not to be fundamental because their genetic absence or blocking of receptors does not alter the effects of surgery (as demonstrated in rodents and humans) [101,102,103]. Differences exist between RYGB and SG in their effects on entero-hormone levels. SG lowers GLP-1 concentrations and has a slightly smaller reduction in body mass index than RYGB while maintaining a positive effect on diabetes [104]. Furthermore, secretion of the gastric inhibitory polypeptide (GIP) hormone is relatively unaffected after RYGB but may be markedly elevated after SG [105]. After SG, the level of circulating ghrelin (which is mainly secreted from the fundus and upper part of the gastric body) is reduced to a greater extent than that after RYGB [106], contributing to decreased appetite [107]. Finally, in contrast to RYGB, fasting in SG was not associated with an increase in ghrelin concentration [107].
These inequalities can indicate differences in the mechanisms of action between the two surgical techniques (Table 1).
What can we discern by putting the pieces of the puzzle together?
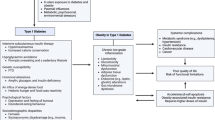
VAFs and the gut–brain axis have been confirmed to play important roles in mediating the therapeutic efficacy of metabolic surgery, as proposed in other articles and studies [14, 20, 22, 106, 108]. Alterations in vagal signaling between the GI tract and brain regions caused by surgical modifications are directly involved in the modified control of food intake (homeostatic and hedonic) and the correction of carbohydrate metabolism disorders [12, 18, 92, 109] (Fig. 3).
As previously illustrated, the effects of the two surgical techniques differ slightly. Therefore, although widely shared, the mechanisms present differences that can help reveal their nature [41, 43, 51, 110].
Based on this functional interpretation, what are the underlying molecular/cellular mechanisms that represent possible pharmacological targets?
Impulse transmission from one neuron to another occurs through the release of neurotransmitters in the synaptic cleft. Glutamate is the primary neurotransmitter involved in the vagovagal transmission. The synaptic contacts of VAFs in various vagal nuclei along their pathways are predominantly mediated by the N-methyl-d-aspartate (NMDA) glutamate receptor [111].
Human genome-wide association studies (GWAS) of differences in genes associated with obesity within species have shown that those related to glutamatergic signaling and postsynaptic plasticity of NMDA receptors are increased [112].
NMDA receptors are responsible for synaptic plasticity (long-term potentiation and depression of signal transmission) [113]. That induced by the vagus in NTS neurons is extensive and allows adaptation to different pathophysiological conditions [114]. Neuroplasticity plays an important role in the regulation of energy balance, body weight homeostasis, and is a potential therapeutic approach to obesity [115].
NMDA signaling contributes to food intake suppression at multiple sites where vagal afferents transmit peripheral information, such as the NTS [116, 117].
Thus, NMDA modulation may be used for the pharmacological treatment of obesity. The pharmacological potential of NMDA receptor blockers for treating obesity has been demonstrated experimentally [118]. For example, administration of the NMDA receptor antagonist memantine induces anorexia and weight loss in rodents and modifies their food preferences [119].
However, its therapeutic use is hampered by the fact that, as a widespread receptor in the nervous system, pharmacological alterations in its function are associated with significant side effects [120].
This drawback was circumvented experimentally in two ways. Subcutaneous injections of the potent receptor antagonist MK-801, combined with a GLP-1 analog produced potent weight loss without adverse effects in mice with diet-induced obesity [118]. Furthermore, intracellular protein complexes related to postsynaptic glutamate receptor signaling [121], that are responsible for the stability of the complexes, were successfully targeted, representing a promising therapy for obesity [122].
NMDA receptors are composed of subunits that enclose the opening through which ions flow. The subunits are two GluN1 e two GluN2 or one GluN3. Each subunit exhibits different functional properties [123].
Specific agonists or antagonists of the subunits (especially those belonging to the GluN2 subclass such as GluN2B, GluN2C, and GluN2D) can influence food intake by acting on VAFs at the NTS level [116, 124, 125].
NMDA glutamate receptors have functions that make them effective drug targets [126]. The objective of various studies has been to obtain the same effects as that of metabolic surgery in a noninvasive manner and to identify therapies aimed at specific receptor subunits capable of modulating the action of VAFs [12, 14, 127, 128]. Experimental studies have led to the development of small molecules capable of identifying those with useful functional and pharmacological properties among the various subunits of GluN2 [129]. Hopefully, this will also apply to drugs of interest for the treatment of obesity.
Furthermore, allosteric modulators (substances that bind to different receptor sites to change their response to stimuli) have been shown to be potential therapeutic agents for treating various addiction-dependent pathologies (to which, as described above, hyperphagia apparently belongs) [130, 131] (Fig. 4). For example, neurosteroids (steroids synthesized in the CNS) act as allosteric agonists of NMDA receptors. These were among the first agents identified as capable of modulating the glutamate receptor response [132]. Consequently, they can prove effective in controlling food intake and body weight, as they appear to be effective, for example, on neuroplasticity [133].
Identifying positive or negative allosteric modulators of NMDA that can affect energy homeostasis (as has already been done for pain, epilepsy, cognitive impairment, and schizophrenia) is necessary to identify the specific structure-activity relationship underlying their actions [127]. Although this task is challenging, it has a considerable potential.
Finally, the neurotransmitter gamma-aminobutyric acid (GABA) also plays an important role in modulating energy homeostasis. It is the most important inhibitory neurotransmitter in the CNS. GABAergic neurons in the hypothalamus play key roles in controlling feeding (particularly hedonic) and energy homeostasis [134]. Its receptors, particularly GABA-A, are subjected to effective pharmacological modulation in experimental models of obesity. Stimulation with benzodiazepines resulted in reduced weight gain in fa/fa rats with genetic obesity, and their daily food intake patterns were comparable to those in lean rats [135]. However, further studies are needed to establish whether this modulation is an effective tool for pharmacologically regulating feeding and fight obesity [134].
Conclusion
The pieces of the puzzle illustrated here, which represent distinct effects of metabolic surgery and are characterized by overlapping pathophysiological pathways, suggest that, based on current knowledge, future research should focus on functional changes in the gut-brain axis and, in particular, on those of the VAFs.
Therefore, the goal of achieving the same effects as metabolic surgery in a non-invasive way should be, with sufficient certainty, the identification of therapies targeting specific NMDA subunits able to modulate the action of VAFs [136]. This interpretation could pave the way for promising alternative approaches to obesity therapy [137].
References
Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, et al. American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:e984–e1010.
Nagi MA, Ahmed H, Rezq MAA, Sangroongruangsri S, Chaikledkaew U, Almalki Z, et al. Economic costs of obesity: a systematic review. Int J Obes ((Lond)). 2024;48:33–43.
Look AHEAD Research Group, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–54.
Sodhi M, Rezaeianzadeh R, Kezouh A, Etminan M. Risk of gastrointestinal adverse events associated with glucagon-likepeptide-1 receptor agonists for weight loss. JAMA. 2023;330:1795–97.
Cohen D. Reports of pancreatitis are 20-30 times more likely with GLP-1 drugs, analysis finds. BMJ. 2013;346:f2607.
Lebovitz HE. Incretin-based therapies: facing the realities of benefits versus side effects. Diab Technol Ther. 2013;15:909–13.
Azuri J, Hammerman A, Aboalhasan E, Sluckis B, Arbel R. Tirzepatide versus semaglutide for weight loss in patients with type 2 diabetes mellitus: a value for money analysis. Diab Obes Metab. 2023;25:961–4.
Wilding JPH, Batterham RL, Davies M, Van Gaal LF, Kandler K, Konakli K, et al. STEP 1 Study Group. Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diab Obes Metab. 2022;24:1553–64.
Kirwan JP, Courcoulas AP, Cummings DE, Goldfine AB, Kashyap SR, Simonson DC, et al. Diabetes remission in the alliance of randomized trials of medicine versus metabolic surgery in type 2 diabetes (ARMMS-T2D). Diab Care. 2022;45:1574–83.
Koliaki C, Tzeravini E, Papachristoforou E, Severi I, El Deik E, Karaolia M, et al. Eligibility and awareness regarding metabolic surgery in patients with type 2 diabetes mellitus in the real-world clinical setting; estimate of possible diabetes remission. Front Endocrinol ((Lausanne)). 2020;11:383.
Cresci B, Cosentino C, Monami M, Mannucci E. Metabolic surgery for the treatment of type 2 diabetes: a network meta-analysis of randomized controlled trials. Diab Obes Metab. 2020;22:1378–87.
Albaugh VL, He Y, Münzberg H, Morrison CD, Yu S, Berthoud HR. Regulation of body weight: lessons learned from bariatric surgery. Mol Metab. 2023;68:101517.
Zsombok A. Autonomic control and bariatric procedures. Auton Neurosci. 2013;177:81–86.
Wachsmuth HR, Weninger SN, Duca FA. Role of the gut-brain axis in energy and glucose metabolism. Exp Mol Med. 2022;54:377–92.
Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–94.
Powley TL. Vagal input to the enteric nervous system. Gut. 2000;47:30–32.
Holt MK. The ins and outs of the caudal nucleus of the solitary tract: an overview of cellular populations and anatomical connections. J Neuroendocrinol. 2022;34:e13132.
Blasi C. The role of the vagal nucleus tractus solitarius in the therapeutic effects of obesity surgery and other interventional therapies on type 2 diabetes. Obes Surg. 2016;26:3045–57.
Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17.
Kaelberer MM, Rupprecht LE, Liu WW, Weng P, Bohórquez DV. Neuropod cells: the emerging biology of gut-brain sensory transduction. Annu Rev Neurosci. 2020;43:337–53.
Richards P, Thornberry NA, Pinto S. The gut-brain axis: Identifying new therapeutic approaches for type 2 diabetes, obesity, and related disorders. Mol Metab. 2021;46:101175.
Berthoud HR, Shin AC, Zheng H. Obesity surgery and gut-brain communication. Physiol Behav. 2011;105:106–19.
Ballsmider LA, Vaughn AC, David M, Hajnal A, Di Lorenzo PM, Czaja K. Sleeve gastrectomy and Roux-en-Y gastric bypass alter the gut-brain communication. Neural Plast. 2015;2015:601985.
Harris RB. Role of set-point theory in regulation of body weight. FASEB J. 1990;4:3310–8.
Hankir MK, Seyfried F, Miras AD, Cowley MA. Brain feeding circuits after roux-en-y gastric bypass. Trends Endocrinol Metab. 2018;29:218–37.
Akalestou E, Miras AD, Rutter GA, le Roux CW. Mechanisms of weight loss after obesity surgery. Endocr Rev. 2022;43:19–34.
Kawasaki T, Ohta M, Kawano Y, Masuda T, Gotoh K, Inomata M, et al. Effects of sleeve gastrectomy and gastric banding on the hypothalamic feeding center in an obese rat model. Surg Today. 2015;45:1560–6.
Karlsson HK, Tuominen L, Tuulari JJ, Hirvonen J, Parkkola R, Helin S, et al. Obesity is associated with decreased μ-opioid but unaltered dopamine D2 receptor availability in the brain. J Neurosci. 2015;35:3959–65.
Burghardt PR, Rothberg AE, Dykhuis KE, Burant CF, Zubieta JK. Endogenous opioid mechanisms are implicated in obesity and weight loss in humans. J Clin Endocrinol Metab. 2015;100:3193–201.
Woodie LN, Melink LC, Midha M, de Araújo AM, Geisler CE, Alberto AJ, et al. Hepatic vagal afferents convey clock-dependent signals to regulate circadian food intake. Science. 2024;386:673–77.
Doucet E, Imbeault P, St-Pierre S, Alméras N, Mauriège P, Després JP, et al. Greater than predicted decrease in energy expenditure during exercise after body weight loss in obese men. Clin Sci ((Lond)). 2003;105:89–95.
Stefater MA, Pérez-Tilve D, Chambers AP, Wilson-Pérez HE, Sandoval DA, Berger J, et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology. 2010;138:2426–36.
Patkar PP, Hao Z, Mumphrey MB, Townsend RL, Berthoud HR, Shin AC. Unlike calorie restriction, Roux-en-Y gastric bypass surgery does not increase hypothalamic AgRP and NPY in mice on a high-fat diet. Int J Obes ((Lond)). 2019;43:2143–50.
Paulino G, Barbier de la Serre C, Knotts TA, Oort PJ, Newman JW, Adams SH, et al. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am J Physiol Endocrinol Metab. 2009;296:E898–903.
Browning KN, Fortna SR, Hajnal A. Roux-en-Y gastric bypass reverses the effects of diet-induced obesity to inhibit the responsiveness of central vagal motoneurones. J Physiol. 2013;591:2357–72.
Cork SC. The role of the vagus nerve in appetite control: Implications for the pathogenesis of obesity. J Neuroendocrinol. 2018;30:e126439.
de Lartigue G, Xu C. Mechanisms of vagal plasticity influencing feeding behavior. Brain Res. 2018;1693:146–50.
Covasa M, Ritter RC. Adaptation to high-fat diet reduces inhibition of gastric emptying by CCK and intestinal oleate. Am J Physiol Regul Integr Comp Physiol. 2000;278:R166–170.
Little TJ, Feinle-Bisset C. Oral and gastrointestinal sensing of dietary fat and appetite regulation in humans: modification by diet and obesity. Front Neurosci. 2010;4:178.
Loper H, Leinen M, Bassoff L, Sample J, Romero-Ortega M, Gustafson KJ, et al. Both high fat and high carbohydrate diets impair vagus nerve signaling of satiety. Sci Rep. 2021;11:10394.
de Lartigue G, Diepenbroek C. Novel developments in vagal afferent nutrient sensing and its role in energy homeostasis. Curr Opin Pharm. 2016;31:38–43.
Page AJ. Vagal afferent dysfunction in obesity: cause or effect. J Physiol. 2016;594:5–6.
Faulconbridge LF, Ruparel K, Loughead J, Allison KC, Hesson LA, Fabricatore AN, et al. Changes in neural responsivity to highly palatable foods following roux-en-Y gastric bypass, sleeve gastrectomy, or weight stability: An fMRI study. Obes (Silver Spring). 2016;24:1054–60.
Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, et al. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes Surg. 2010;20:369–74.
Blum K, Thanos PK, Gold MS. Dopamine and glucose, obesity, and reward deficiency syndrome. Front Psychol. 2014;5:919.
Delbès AS, Castel J, Denis RGP, Morel C, Quiñones M, Everard A, et al. Prebiotics supplementation impact on the reinforcing and motivational aspect of feeding. Front Endocrinol (Lausanne). 2018;9:273.
van Galen KA, Booij J, Schrantee A, Adriaanse SM, Unmehopa UA, Fliers E, et al. The response to prolonged fasting in hypothalamic serotonin transporter availability is blunted in obesity. Metabolism. 2021;123:154839.
Darcey VL, Guo J, Chi M, Chung ST, Courville AB, Gallagher I, et al. Striatal dopamine tone is positively associated with body mass index in humans as determined by PET using dual dopamine type-2 receptor antagonist tracers. Mol Psychiatry. 2025;30:3708–17
Ochner CN, Kwok Y, Conceição E, Pantazatos SP, Puma LM, Carnell S, et al. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg. 2011;253:502–7.
Halmi KA, Mason E, Falk JR, Stunkard A. Appetitive behavior after gastric bypass for obesity. Int J Obes. 1981;5:457–64.
Alabduljabbar K, Al-Najim W, le Roux CW. Food preferences after bariatric surgery: a review update. Intern Emerg Med. 2023;18:351–8.
Brougher J, Aziz U, Adari N, Chaturvedi M, Jules A, Shah I, et al. Self-administration of right vagus nerve stimulation activates midbrain dopaminergic nuclei. Front Neurosci. 2021;15:782786.
Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, et al. A neural circuit for gut-induced reward. Cell. 2018;175:665.e23–678.e23.
Guerrero-Hreins E, Foldi CJ, Oldfield BJ, Stefanidis A, Sumithran P, Brown RM. Gut-brain mechanisms underlying changes in disordered eating behaviour after bariatric surgery: a review. Rev Endocr Metab Disord. 2022;23:733–51.
Alabdulkader S, Al-Alsheikh AS, Miras AD, Goldstone AP. Obesity surgery and neural correlates of human eating behaviour: a systematic review of functional MRI studies. Neuroimage Clin. 2024;41:103563.
Alhadeff AL. Monitoring in vivo neural activity to understand gut-brain signaling. Endocrinology. 2021;162:bqab029.
le Roux CW, Bueter M, Theis N, Werling M, Ashrafian H, Löwenstein C, et al. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1057–66.
Gero D, File B, Alceste D, Frick LD, Serra M, Ismaeil AE, et al. Microstructural changes in human ingestive behavior after Roux-en-Y gastric bypass during liquid meals. JCI Insight. 2021;6:e136842.
Gautron L, Elmquist JK, Williams KW. Neural control of energy balance: translating circuits to therapies. Cell. 2015;161:133–45.
Smith KR, Papantoni A, Veldhuizen MG, Kamath V, Harris C, Moran TH, et al. Taste- related reward is associated with weight loss following bariatric surgery. J Clin Invest. 2020;130:4370–81.
Nance K, Eagon JC, Klein S, Pepino MY. Effects of sleeve gastrectomy vs. Roux-en-Y gastric bypass on eating behavior and sweet taste perception in subjects with obesity. Nutrients. 2017;10:E18–23.
Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept. 2008;149:15–25.
Hao Z, Townsend RL, Mumphrey MB, Patterson LM, Ye J, Berthoud HR. Vagal innervation of intestine contributes to weight loss after Roux-en-Y gastric bypass surgery in rats. Obes Surg. 2014;24:2145–51.
Bueter M, Löwenstein C, Ashrafian H, Hillebrand J, Bloom SR, Olbers T, et al. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes Surg. 2010;20:616–22.
Johannessen H, Revesz D, Kodama Y, Cassie N, Skibicka KP, Barrett P, et al. Vagal blocking for obesity control: a possible mechanism-of-action. Obes Surg. 2017;27:177–85.
Shikora SA, Wolfe BM, Apovian CM, Anvari M, Sarwer DB, Gibbons RD, et al. Sustained weight loss with vagal nerve blockade but not with sham: 18-month results of the ReCharge Trial. J Obes. 2015;2015:365604.
Burneo JG, Faught E, Knowlton R, Morawetz R, Kuzniecky R. Weight loss associated with vagus nerve stimulation. Neurology. 2002;59:463–8.
Obeidat F, Shanti H, Mismar A, Albsoul N, Al-Qudah M. The magnitude of antral resection in laparoscopic sleeve gastrectomy and its relationship to excess weight loss. Obes Surg. 2015;25:1928–32.
Dixon AF, Dixon JB, O’Brien PE. Laparoscopic adjustable gastric banding induces prolonged satiety: a randomized blind crossover study. J Clin Endocrinol Metab. 2005;90:813–9.
Shoar S, Khorgami Z, Brethauer SA, Aminian A. Banded versus nonbanded Roux-en-Y gastric bypass: a systematic review and meta-analysis of randomized controlled trials. Surg Obes Relat Dis. 2019;15:688–95.
Lemmens L. Banded gastric bypass: better long-term results? A cohort study with minimum 5-year follow-up. Obes Surg. 2017;27:864–72.
Cifuentes L, Camilleri M, Acosta A. Gastric sensory and motor functions and energy intake in health and obesity-therapeutic implications. Nutrients. 2021;13:1158–63.
Stefanidis A, Forrest N, Brown WA, Dixon JB, O’Brien PB, Kampe J, et al. An investigation of the neural mechanisms underlying the efficacy of the adjustable gastric band. Surg Obes Relat Dis. 2016;12:828–38.
Duca FA, Waise TMZ, Peppler WT. The metabolic impact of small intestinal nutrient sensing. Nat Commun. 2021;12:903–9.
Ruban A, Ashrafian H, Teare JP. The EndoBarrier: duodenal-jejunal bypass liner for diabetes and weight loss. Gastroenterol Res Pr. 2018;2018:7823182.
Lo T, Lee Y, Tseng CY, Hu Y, Connelly MA, Mantzoros CS, et al. Daily transient coating of the intestine leads to weight loss and improved glucose tolerance. Metabolism. 2022;126:154917.
Busch CBE, Meiring S, van Baar ACG, Gastaldelli A, DeFronzo R, et al. Insulin sensitivity and beta cell function after Duodenal Mucosal Resurfacing (DMR): an open-label, mechanistic, pilot study. Gastrointest Endosc. 2024;25:S0016-5107(24)00049-X.
Travagli RA. The vagus nerve. In Encyclopedia of Gastroenterology. 2nd ed. Oxford, UK: Academic Press; 2020. pp. 676–82.
Dent J, Sun WM, Anvari M. Modulation of the pumping function of the gastric body and anthropic contractions. Dig Dis Sci. 1994;39:28S–31S.
Wickremasinghe AC, Johari Y, Laurie C, Shaw K, Playfair J, Beech P, et al. Delayed gastric emptying after sleeve gastrectomy is associated with poor weight loss. Obes Surg. 2022;32:3922–31.
Clarke GS, Page AJ, Eldeghaidy S. The gut-brain axis in appetite, satiety, food intake, and eating behavior: Insights from animal models and human studies. Pharm Res Perspect. 2024;12:e70027.
Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015;8:624–36.
Zhang X, Val-Laillet D. Obesity animal models for acupuncture and related therapy research studies. Evid Based Complement Altern Med. 2021;2021:66633979.
Yao J, Chen L, Zhang L, Zhou S, Zheng Q, Feng X, et al. Effect of auriculotherapy and intervention types on weight control: A systematic review and meta-analysis protocol. Med ((Baltim)). 2019;98:e169–e173.
Schukro RP, Heiserer C, Michalek-Sauberer A, Gleiss A, Sator-Katzenschlager S. The effects of auricular electroacupuncture on obesity in female patients-a prospective randomized placebo-controlled pilot study. Complement Ther Med. 2014;22:21–25.
Zhang K, Zhou S, Wang C, Xu H, Zhang L. Acupuncture on obesity: clinical evidence and possible neuroendocrine mechanisms. Evid Based Complement Altern Med. 2018;2018:6409389.
Yi CX, la Fleur SE, Fliers E, Kalsbeek A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim Biophys Acta. 2010;1802:416–31.
Matsuhisa M, Yamasaki Y, Shiba Y, Nakahara I, Kuroda A, Tomita T. Important role of the hepatic vagus nerve in glucose uptake and production by the liver. Metabolism. 2000;49:11–16.
Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 2009;10:99–109.
Bojsen-Møller KN. Mechanisms of improved glycemic control after gastric bypass Roux- en-Y. Dan Med J. 2015;62:B5057–B5065.
Cardin S, Walmsley K, Neal DW, Williams PE, Cherrington AD. Involvement of the vagus nerves in the regulation of basal hepatic glucose production in conscious dogs. Am J Physiol Endocrinol Metab. 2002;283:E958–E964.
Powley TL. Vagal circuitry mediating cephalic-phase responses to food. Appetite. 2000;34:184–8.
Spector D, Shikora S. Neuro-modulation and bariatric surgery for type 2 diabetes mellitus. Int J Clin Pract. Suppl. 2010;166:53–58.
le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–5.
Salehi M, D’Alessio DA. Effects of glucagon like peptide-1 to mediate glycemic effects of weight loss surgery. Rev Endocr Metab Disord. 2014;15:171–9.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39.
Brierley DI, de Lartigue G. Reappraising the role of the vagus nerve in GLP-1-mediated regulation of eating. Br J Pharm. 2022;179:584–99.
Plamboeck A, Veedfald S, Deacon CF, Hartmann B, Wettergren A, Svendsen LB, et al. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1117–1127.
Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–31.
Barton JR, Londregan AK, Alexander TD, Entezari AA, Covarrubias M, Waldman SA. Enteroendocrine cell regulation of the gut-brain axis. Front Neurosci. 2023;17:1272955.
Lampropoulos C, Alexandrides T, Tsochatzis S, Kehagias D, Kehagias I. Are the changes in gastrointestinal hormone secretion necessary for the success of bariatric surgery? A critical review of the literature. Obes Surg. 2021;31:4575–84.
Wilson-Pérez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D, et al. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide 1 receptor deficiency. Diabetes. 2013;62:2380–5.
Ma J, Vella A. What has bariatric surgery taught us about the role of the upper gastrointestinal tract in the regulation of postprandial glucose metabolism?. Front Endocrinol ((Lausanne)). 2018;9:324–30.
Wallenius V, Dirinck E, Fändriks L, Maleckas A, le Roux CW, Thorell A. Glycemic control after sleeve gastrectomy and roux-en-y gastric bypass in obese subjects with type 2 diabetes mellitus. Obes Surg. 2018;28:1461–72.
Romero F, Nicolau J, Flores L, Casamitjana R, Ibarzabal A, Lacy A, et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux- En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012;26:22–29.
An Z, Wang H, Mokadem M. Role of the autonomic nervous system in mechanism of energy and glucose regulation post bariatric surgery. Front Neurosci. 2021;15:770690.
Yousseif A, Emmanuel J, Karra E, Millet Q, Elkalaawy M, Jenkinson AD, et al. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obes Surg. 2014;24:241–52.
Kral JG, Paez W, Wolfe BM. Vagal nerve function in obesity: therapeutic implications. World J Surg. 2009;33:1995–2002.
Blasi C. Obesity and related type 2 diabetes: a failure of the autonomic nervous system controlling gastrointestinal function? Gastrointest Disord. 2020;2:423–47.
Emiliano AB. Mining the mechanistic underpinnings of bariatric surgery: A gateway to novel and non-invasive obesity therapies? Mol Metab. 2023;68:101663.
Berthoud HR, Earle T, Zheng H, Patterson LM, Phifer C. Food signals related gastrointestinal cells activate caudal brainstem neurons that express both NMDA and AMPA receptors. Brain Res. 2001;915:143–54.
Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206.
Papouin T, Ladépêche L, Ruel J, Sacchi S, Labasque M, Hanini M, et al. Synaptic and extrasynaptic NMDA receptors are controlled by different endogenous coagonists. Cell. 2012;150:633–46.
Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol. 2016;13:389–401.
Minaya DM, Larson RW, Podlasz P, Czaja K. Glutamate-dependent regulation of food intake is altered with age through changes in NMDA receptor phenotypes in vagal afferent neurons. Physiol Behav. 2018;189:26–31.
Ritter RC. A tale of two endings: modulation of satiety by NMDA receptors on or near central and peripheral vagal afferent terminals. Physiol Behav. 2011;105:94–99.
Wright J, Campos C, Herzog T, Covasa M, Czaja K, Ritter RC. The reduction in food intake by cholecystokinin requires the activation of hindbrain NMDA-type glutamate receptors. Am J Physiol Regul Comp Physiol. 2011;301:R448–455.
Petersen J, Ludwig MQ, Juozaityte V, Ranea-Robles P, Svendsen C, Hwang E, et al. GLP-1-directed NMDA receptor antagonism for the treatment of obesity. Nature. 2024;629:1133–41.
Popik P, Kos T, Zhang Y, Bisaga A. Memantine reduces consumption of highly palatable food in a binge-eating model in rats. Amino Acids. 2011;40:4 77–485.
Lipton SA. Failure and success of NMDA receptor antagonists: The molecular basis for the use of open-channel blockers, such as memantine, in the treatment of acute and chronic neurological insults. NeuroRx. 2004;1:101–10.
Christensen NR, Čalyševa J, Fernandes EFA, Lüchow S, Clemmensen LS, Haugaard-Kedström LM, et al. PDZ domains as drug targets. Adv Ther ((Weinh)). 2019;2:1800143.
Fadahunsi N, Petersen J, Metz S, Jakobsen A, Vad Mathiesen C, Silke Buch- Rasmussen A, et al. Targeting postsynaptic glutamate receptor scaffolding proteins PSD-95 and PICK1 for obesity treatment. Sci Adv. 2024;10:eadg2636.
Cull-Candy S, Brickley S, Farrant M. Subunit of the NMDA receptor: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–35.
Vance KM, Rogers RC, Hermann GE. NMDA receptors control vagal afferent excitability in the nucleus of the solitary tract. Brain Res. 2015;1595:84–91.
Treece BR, Ritter RC, Burns GA. Lesions of the dorsal vagal complex abolish increase in meal size ducted by NMDA receptor blockade. Brain Res. 2000;872:37–43.
Stroebel D, Paoletti P. Architecture and function of NMDA receptor: an evolutionary perspective. J Physiol. 2021;599:2615–38.
Burnell ES, Irvine M, Fang G, Sapkota K, Jane DE, Monaghan DT. Positive and negative allosteric modulators of n-methyl-d-aspartate (NMDA) receptors: structure-activity relationships and mechanisms of action. J Med Chem. 2019;62:3–23.
Tadross JA, le Roux CW. The mechanisms of weight loss after bariatric surgery. Int J Obes ((Lond)). 2009;33:S28–32.
Hansen KB, Yi F, Perszyk RE, Furukawa H, Wollmuth LP, Gibb AJ, et al. Structure, function, and allosteric modulation of NMDA receptors. J Gen Physiol. 2018;150:1081–105.
Achat-Mendes C, Platt DM, Spealman RD. Antagonism of metabotropic glutamate 1 attenuates the behavioral effects of cocaine and methamphetamine in squirrel monkeys. J Pharm Exp Ther. 2012;343:214–24.
Bird MK, Lawrence AJ. Group I metabotropic glutamate receptors: involvement in drug-seeking and drug-induced plasticity. Curr Mol Pharm. 2009;2:83–94.
Gibbs TT, Russek SJ, Farb DH. Sulfated steroids as endogenous neuromodulators. Pharm Biochem Behav. 2006;84:555–67.
Benarroch EE. Neurosteroids: endogenous modulators of neuronal excitability and plasticity. Neurology. 2007;68:945–7.
Jiang H. Hypothalamic GABAergic neurocircuitry in the regulation of energy homeostasis and sleep/wake control. Med Rev. 2022;2:531–40.
Blasi C. Influence of benzodiazepines on body weight and food intake in obese and lean Zucker rats. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:561–77.
Tysoe O. NMDA receptor antagonist coupled to GLP1 analog in a highly effective experimental weight loss drug. Nat Rev Endocrinol. 2024;20:446.
Frick LD, Hankir MK, Borner T, Malagola E, File B, Gero D. New insights into the physiology of nutrient sensing and gut-brain communication in the surgical and experimental therapy of obesity. Obes Surg. 2023;33:2906–16.
Author information
Authors and Affiliations
Contributions
All contributions were from the single author.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Blasi, C. Mechanisms of metabolic surgery effectiveness in obesity and type 2 diabetes: a puzzle with some known pieces. Int J Obes 49, 1995–2004 (2025). https://doi.org/10.1038/s41366-025-01853-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-025-01853-y