Abstract
Background
Obesity and overweight are major risk factors for cardiovascular diseases. Although various weight control interventions have been evaluated individually, their comparative effectiveness across outcomes and populations remains uncertain.
Objectives
To evaluate the effects of weight control interventions on all-cause mortality and cardiovascular outcomes.
Methods
A comprehensive search was conducted in PubMed, Embase, Web of Science, and the Cochrane Library from inception to June 2024. Meta-analyses reporting pooled effect sizes for all-cause mortality or cardiovascular outcomes were included. Reviews without quantitative synthesis were excluded. Risk of bias and methodological quality were assessed using A Measurement Tool to Assess Systematic Reviews and the Grading of Recommendations Assessment, Development and Evaluation approach. No new meta-analysis was conducted, relevant data were re-analyzed when required to ensure consistency. This review was registered in PROSPERO (CRD42024573542).
Results
Forty-seven effect sizes from 31 articles were extracted. Among pharmacologic interventions, high- to moderate-quality evidence showed that glucagon-like peptide-1 receptor agonists (GLP1-RAs) were associated with reduced all-cause mortality, major adverse cardiovascular events, stroke, cardiovascular mortality, myocardial infarction, and heart failure among individuals with type 2 diabetes or overweight/obesity. Bariatric surgery was consistently associated with reduced risks for all cardiovascular outcomes except atrial fibrillation. For dietary strategies, low-fat diets were linked to lower all-cause mortality, while Mediterranean and Nordic diets showed benefits for stroke and cardiovascular mortality. Physical activity was associated with reduced all-cause and cardiovascular mortality. Comprehensive lifestyle interventions showed no significant cardiovascular benefit. Most evidence was of moderate or low certainty due to methodological limitations, including bias, imprecision, and inconsistency.
Conclusion
Weight control interventions are associated with improved all-cause mortality and cardiovascular outcomes. High- to moderate-quality evidence supported benefits of GLP1-RAs in individuals with type 2 diabetes or overweight. Dietary, surgical, and exercise interventions showed modest effects. No consistent cardiovascular benefit was observed for comprehensive lifestyle interventions.

Weight Control Interventions and Cardiovascular Outcomes. Associations between five categories of weight control interventions—pharmacological interventions, bariatric surgery, dietary interventions, exercise interventions, and comprehensive lifestyle interventions—and seven cardiovascular outcomes are illustrated. Beneficial associations are indicated in pink, and interventions with no observed associations are shown in green. Created in BioRender. Chen, X. (2025) https://BioRender.com/km8jw8h.
Similar content being viewed by others
Introduction
Obesity and overweight are major global public health concerns. In 2022, the World Health Organization reported that over 43% of adults were classified as having overweight (body mass index (BMI) 25–29.9 kg/m²) and nearly 16% as having obesity (BMI ≥ 30 kg/m²) [1]. These conditions are linked to adverse health outcomes such as type 2 diabetes mellitus (T2DM), hypertension, certain cancers, and cardiovascular diseases (CVDs) [2, 3]. Obesity is a major risk factor for cardiovascular diseases [4,5,6]. Therefore, effective weight control is crucial for reducing cardiovascular risk.
However, studies have suggested the ‘obesity paradox,’ where individuals with overweight or mild obesity, particularly elderly and symptomatic individuals with cardiovascular disease (e.g., heart failure) exhibit higher survival rates [7, 8]. Observational studies on weight reduction in individuals with existing CVDs show mixed results, ranging from moderate benefits to potential harms [9,10,11]. This paradox underscores the need for cautious and comprehensive evaluation of the relationship between BMI reduction and cardiovascular outcomes, as well as the impact of various weight control interventions.
Weight control interventions include pharmacological interventions, bariatric surgery, dietary interventions, exercise interventions, and comprehensive lifestyle interventions [12]. These interventions are distinct and widely applied in clinical and community settings. Pharmacological and surgical interventions are typically reserved for severe cases of obesity, whereas dietary and exercise interventions cater to a broader demographic. Comprehensive lifestyle interventions integrate multiple approaches (diet, exercise, and behavioral therapy) to ensure sustained and holistic weight management.
Numerous meta-analyses of randomized controlled trials (RCTs) and observational studies have explored the relationship between weight control interventions and cardiovascular outcomes. However, challenges persist due to study design flaws, varying follow-up periods, inconsistent results, and high heterogeneity, complicating the ability to draw definitive conclusions. These issues also present difficulties for clinicians and policymakers in identifying the most effective interventions. Although several systematic reviews exist, to our knowledge, no umbrella review has comprehensively evaluated the comparative effectiveness of diverse weight control interventions—pharmacological, surgical, dietary, exercise, and lifestyle—across a broad range of cardiovascular outcomes. Furthermore, no previous review has combined multiple grading strategies, including the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system, the A Measurement Tool to Assess Systematic Reviews (AMSTAR), and predefined evidence classes, to stratify evidence quality and interpret inconsistency across findings. This integrative and methodologically layered approach is designed to overcome limitations of prior evidence syntheses and offer practical, comparative insights. This umbrella review consolidates existing systematic reviews and meta-analyses to evaluate the overall impact of various weight control interventions on all-cause mortality and cardiovascular outcomes. Our goal is to clarify the relative benefits and limitations of each intervention, thereby providing a scientific foundation for clinical practice and public health policy.
Methods
Umbrella review methods
We conducted a systematic search, extraction, and analysis of data from published systematic reviews and meta-analyses exploring the associations between various weight control interventions and all-cause mortality as well as cardiovascular outcomes [13, 14]. The interventions included pharmacological interventions, bariatric surgery, dietary interventions, exercise interventions, and comprehensive lifestyle interventions, all evaluated within meta-analyses. Systematic reviews without meta-analyses were excluded. The study was prospectively registered in PROSPERO (Registration number: CRD42024573542).
Literature search
We conducted a comprehensive search of systematic reviews and meta-analyses of RCTs and observational studies in PubMed, Embase, Web of Science, and the Cochrane Library, covering all available literature up to June 2024. We used the following search terms for English-language meta-analyses on body weight, obesity, or overweight and CVDs: (body weight OR obesity OR overweight) AND (cardiovascular diseases) AND (systematic review OR meta-analysis). Two authors (XMC and XGZ) independently carried out electronic searches, screened titles and abstracts, and identified eligible meta-analyses through full-text review. Discrepancies were resolved by a third author (XX). Additionally, we manually examined the reference lists of all included articles to identify any potentially overlooked meta-analyses and reviews.
Eligibility criteria
We included systematic reviews and meta-analyses of RCTs and observational studies assessing weight control interventions, such as pharmacological interventions, bariatric surgery, dietary interventions, exercise interventions, and comprehensive lifestyle interventions. Eligible studies were required to report efficacy measures for all-cause mortality and cardiovascular outcomes, including CVD mortality, major adverse cardiovascular events (MACE), stroke, myocardial infarction, heart failure, and atrial fibrillation. Only studies published in English were included.
Eligible studies evaluated the effects of various weight control interventions on identical cardiovascular outcomes using metrics such as relative risk (RR), odds ratio (OR), or hazard ratio (HR). For studies reporting multiple health outcomes, data were extracted for each outcome separately. In cases where multiple meta-analyses assessed the same intervention and outcome, preference was given to the most recent, largest, and most comprehensive meta-analysis. When studies on the same outcome were published more than 24 months apart, the most recent study, typically with the largest sample size, was included. For studies published within the same 24-month period, meta-analyses with the highest number of prospective cohort studies and RCTs were prioritized. If the number of prospective studies was identical, those with the highest AMSTAR scores were selected.
Data extraction
Two reviewers, XMC and XGZ, independently extracted data from each eligible study, including the first author’s name, publication year, type of weight control intervention (pharmacological, bariatric surgery, dietary, exercise, comprehensive lifestyle), control group, study population, all-cause mortality, cardiovascular outcomes, number of included studies, study design (RCTs and observational studies), case count, total participants, and pooled overall effects (RR, OR, HR, and their 95% confidence intervals (CIs)). We also extracted information on effect models (random-effects and fixed-effects), heterogeneity (I² statistic and Cochran’s Q test P value), and publication bias assessments (Egger’s test or funnel plot P value).
For meta-analyses of observational studies with stratified analyses by study design, data extraction or re-analysis from cohort studies was prioritized. Discrepancies were resolved by a third author (XX).
Quality assessment of methods and evidence
Two reviewers (XMC and XGZ) evaluated the methodological quality of the included studies using the AMSTAR tool, a validated and reliable instrument for assessing systematic reviews and meta-analyses [15]. Additionally, the quality of evidence for each cardiovascular outcome was assessed using the GRADE approach, with ratings of “high,” “moderate,” “low,” or “very low” quality [16].
Furthermore, we categorized the outcome evidence into four classes: Class I (convincing evidence), Class II (highly suggestive evidence), Class III (suggestive evidence), Class IV (weak evidence), and NS (non-significant) [17, 18]. The specific criteria for these classifications are outlined in Table 1.
Data analysis
We re-analyzed the OR, RR, and HR using both random-effects and fixed-effects models. We calculated the I² statistic and Cochran’s Q test P value for heterogeneity, and Egger’s regression test P value for small-study effects (for meta-analyses with at least three studies). The meta-analyses reported the metrics, case numbers, and participant counts from the original studies [19]. For outcomes classified as Class I or II, we performed sensitivity analyses to evaluate the robustness of the evidence by excluding certain component studies.
If the latest meta-analysis excluded original studies included in other meta-analyses, we incorporated these data and performed a new analysis. We assessed inter-rater reliability between the two authors (XMC and XGZ) for study selection using Cohen’s kappa statistic and its 95% CIs, interpreting agreement levels according to Landis and Koch’s guidelines: slight (0.00–20), fair (0.21–40), moderate (0.41–60), substantial (0.61–80), and almost perfect (0.81-1.00) [20].
Moreover, when a meta-analysis aggregated effect estimates from observational studies and RCTs, we re-evaluated these estimates separately for each study type. When re-evaluation was not feasible, we extracted summary data and assessed heterogeneity and publication bias where possible. We defined statistical significance for heterogeneity tests as P values < 0.10 and for other tests as P values < 0.05.
For evidence synthesis, we used Review Manager version 5.4. Egger’s test and sensitivity analysis were conducted with Stata version 17.0. Cohen’s kappa statistic was computed using IBM SPSS Statistics version 26.
Results
Characteristics of included meta-analyses
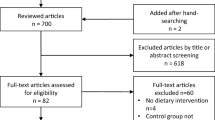
Figure 1 presents the flowchart for the literature search and screening process. A systematic search identified 15,911 unique articles, among which 31 meta-analyses met the inclusion criteria—13 derived from RCTs and 18 from cohort studies. The inter-rater agreement between the reviewers (XMC and XGZ) was high (κ = 0.8367, 95% CI: 0.7472 to 0.9262; P < 0.001). Meta-analyses of RCTs examined pharmacological, dietary, and lifestyle interventions, resulting in 28 pooled effect sizes. Meta-analyses of cohort studies assessed bariatric surgery, dietary, and exercise interventions, yielding 19 pooled effect sizes. Both meta-analysis types investigated seven cardiovascular outcomes: all-cause mortality, CVD mortality, MACE, stroke, myocardial infarction, heart failure, and atrial fibrillation. The full details of the associations between weight control interventions and cardiovascular outcomes are provided in Supplementary Table S1.
Figure 2 provides an overview of the intervention and outcome distributions across included meta-analyses. Pharmacological strategies were most frequently assessed (n = 24, 51%), followed by bariatric surgery (n = 12, 26%) and dietary interventions (n = 7, 15%). Cardiovascular outcomes most frequently evaluated included all-cause mortality (n = 10, 21%), CVD mortality (n = 9, 19%), and stroke (n = 9, 19%). Panel A of Fig. 2 shows the proportion of studies by intervention type; Panel B illustrates the distribution of extracted effect sizes across cardiovascular outcomes. Among the 47 effect sizes, 30 were statistically significant. According to GRADE and evidence classification standards, 7 (15%) effect sizes were rated as “high” quality, 16 (34%) as “moderate” quality, and 24 (51%) as “low” or “very low” quality. Evidence classification showed that 4 (9%) were Class I, 9 (19%) were Class II, and 34 (72%) were Class III, IV, or NS evidence.
A Proportion of included studies categorized by intervention type, including pharmacological (51%), dietary (15%), bariatric surgery(26%), exercise (4%), and comprehensive lifestyle interventions (4%). B Proportion of extracted effect sizes across cardiovascular outcomes, including all-cause mortality (21%), stroke (19%), CVDmortality (19%), major adverse cardiovascular events (MACE, 15%), myocardial infarction (11%), heart failure (11%), and atrialfibrillation (4%). Colors represent distinct categories of interventions and outcomes. Percentages are based on the total number ofstudies or effect sizes.
Pharmacological interventions
Pharmacological interventions have received increasing attention for their potential cardiovascular benefits in individuals with obesity or type 2 diabetes. Figure 3 summarizes study-specific effect estimates with 95% CIs, GRADE ratings, AMSTAR scores, study designs, and evidence classifications across relevant outcomes.
High and moderate quality evidence
Mannucci et al. [21] (2020) conducted a meta-analysis encompassing seven randomized, placebo-controlled trials involving 56,004 high-risk diabetes patients without prior cardiovascular events. The findings demonstrated that glucagon-like peptide-1 receptor agonists (GLP-1 RAs) significantly reduced the risk of MACE (OR = 0.86, 95% CI: 0.81–91, I² = 0%; high, I (the quality of evidence is reported as “GRADE, evidence class”)) (Egger’s test P = 0.216). Furthermore, GLP-1 RAs were associated with a lower risk of stroke (OR = 0.83, 95% CI: 0.75–93, I² = 0%; high, III) (Egger’s test P = 0.281). While GLP-1 RAs showed a trend towards reducing all-cause mortality (OR = 0.90, 95% CI: 0.82–98, I² = 40%; moderate, IV) (Egger’s test P = 0.818), they significantly reduced the risk of CVD mortality (OR = 0.88, 95% CI: 0.70–97, I² = 18%; moderate, IV) (Egger’s test P = 0.624).
Yoshiji et al. [22] (2022) performed a meta-analysis of eight randomized, placebo-controlled trials involving 60,800 T2DM patients. Their findings demonstrated that GLP-1 RAs significantly lowered the risk of all-cause mortality (HR = 0.88, 95% CI: 0.82–94, I² = 10.5%; high, III) (Egger’s test P = 0.3546). Additionally, GLP-1 RAs were associated with a significant reduction in the risk of MACE (HR = 0.86, 95% CI: 0.80–93, I² = 44.5%; high, III) (Egger’s test P = 0.9326), CVD mortality risk (HR = 0.87, 95% CI: 0.80–94, I² = 12.8%; moderate, IV) (Egger’s test P = 0.7732), myocardial infarction risk (HR = 0.90, 95% CI: 0.83–98, I² = 27.4%; moderate, IV) (Egger’s test P = 0.9863), and heart failure risk (HR = 0.89, 95% CI: 0.82–98, I² = 2.5%; moderate, IV) (Egger’s test P = 0.8745).
Sigh et al. [23] (2024) conducted a meta-analysis comprising 10 randomized placebo-controlled trials, encompassing 29,325 individuals with overweight or obesity without diabetes. The analysis revealed that GLP-1 RAs significantly reduced the risk of MACE (OR = 0.79, 95% CI: 0.71–89, I² = 0%; high, III) (Egger’s test P = 0.6615), and decreased the risk of myocardial infarction (OR = 0.72, 95% CI: 0.61–85, I² = 0%; high, III) (Egger’s test P = 0.3531).
Adamou et al. [24] (2024) performed a meta-analysis of 11 randomized placebo-controlled trials involving 82,140 adults, demonstrating that GLP-1 RAs significantly reduced the risk of all-cause mortality (RR = 0.85, 95% CI: 0.77–93, I² = 0%; moderate, IV) (Egger’s test P = 0.7538).
Low-quality evidence
Sigh et al. [23] (2024) reported that GLP-1 RAs significantly reduced the risk of all-cause mortality (OR = 0.80, 95% CI: 0.70–92, I² = 0%; low, IV) (Egger’s test P = 0.0013).
We found no significant associations between weight loss medications (orlistat, liraglutide at 3.0 mg dose only, naltrexone and bupropion, lorcaserin, phentermine, and topiramate) and cardiovascular outcomes (including all-cause mortality, CVD mortality, myocardial infarction, heart failure, and stroke) in individuals with obesity or overweight [25]. Moreover, GLP-1 RAs did not significantly impact myocardial infarction and heart failure outcomes in high-risk diabetes patients without prior cardiovascular events [21], nor did they show a significant association with CVD mortality in individuals with overweight or obesity without diabetes [23].
Bariatric surgery
Bariatric surgery is widely regarded as the best treatment for individuals with severe obesity (BMI ≥ 40 Kg/m2). Figure 4 summarizes study-specific effect estimates with 95% CIs, stratified by study design and evidence quality, for outcomes such as all-cause mortality, MACE, heart failure, and stroke.
Meta-analyses of cohort studies revealed several key findings. Berger et al. [26] (2018) demonstrated that bariatric surgery significantly decreased the risk of heart failure in individuals with severe obesity (RR = 0.44, 95% CI: 0.36–55; low, I). Sutanto et al. [27] (2021) reported a substantial reduction in the risk of MACE in individuals with obesity cardiovascular diseases (OR = 0.49, 95% CI: 0.40–60; low, II). Tang et al. [28] (2022) also observed a significant reduction in MACE risk among individuals with severe obesity (RR = 0.53, 95% CI: 0.45–62; low, II). Van Veldhuisen et al. [29] (2022) found a notable decrease in all-cause mortality (HR = 0.53, 95% CI: 0.45–62)(very low, II) and stroke risk (HR = 0.64, 95% CI: 0.53–77; very low, III) in individuals with obesity. Chandrakumar et al. [30] (2023) reported significant reductions in CVD mortality (HR = 0.48, 95% CI: 0.40–57; very low, II), myocardial infarction (HR = 0.53, 95% CI: 0.44–64; very low, II), and heart failure risk (HR = 0.45, 95% CI: 0.37–55; very low, II). Cui et al. [31] observed significant decreases in all-cause mortality (OR = 0.52, 95% CI: 0.47–58; very low, II), MACE risk (OR = 0.58, 95% CI: 0.51–66; very low, II), and stroke risk (OR = 0.75, 95% CI: 0.63–89; very low, IV) in individuals with severe obesity.
However, no significant association between bariatric surgery and atrial fibrillation outcomes in individuals with obesity was observed in the included studies [30].
Dietary interventions
Dietary interventions are widely accessible strategies for weight control. Figure 5 presents study-specific effect estimates with 95% CIs and GRADE-based evidence ratings for various dietary patterns, including low-fat, Mediterranean, and Nordic diets, in relation to cardiovascular outcomes. The results are organized by study design and evidence level to enable structured comparison across interventions.
Moderate quality evidence
A meta-analysis by Ma et al. [32] (2017) included 34 RCTs involving 21,699 individuals with obesity. The analysis indicated that low-fat diets substantially decreased the risk of all-cause mortality (RR = 0.82, 95% CI: 0.71–95, I² = 0%; moderate, IV) (Egger’s test P = 0.2690). Similarly, Chen et al. [33] (2019) conducted a meta-analysis of 20 cohort studies, encompassing 682,149 individuals without cardiovascular diseases, which demonstrated that the Mediterranean diet notably reduced stroke risk (RR = 0.84, 95% CI: 0.81–8, I² = 11.5%; moderate, I) (Egger’s test P = 0.2800).
Low and very low-quality evidence
A meta-analysis by Massara et al. [34] (2022) found that the Nordic diet substantially decreased the risk of CVD mortality (OR = 0.74, 95% CI: 0.69–80; very low, II), and stroke risk (RR = 0.87, 95% CI: 0.78–97; low, I) in individuals with T2DM.
Additionally, low-fat diets did not show a significant association with CVD mortality in individuals with obesity [32], and high-protein diets were not associated with CVD mortality or stroke in individuals without CVDs [35].
Exercise interventions
Exercise interventions represent a foundational behavioral approach to weight management. Figure 6 presents cohort-based findings on their association with cardiovascular outcomes.
Liu et al. [36] (2022) conducted a meta-analysis of cohort studies and found that active physical exercise significantly decreased the risk of all-cause mortality (RR = 0.57, 95% CI: 0.49–67)(low, II) and CVD mortality (RR = 0.55, 95% CI: 0.34–68; very low, IV) in individuals with diabetes.
Comprehensive lifestyle interventions
Comprehensive lifestyle interventions, including dietary, exercise, or behavioral therapy, showed no significant association with all-cause mortality in either individuals with overweight or obesity [37, 38]. Figure 7 illustrates these null associations across cardiovascular outcomes, presenting effect estimates with 95% CIs and corresponding evidence ratings.
Heterogeneity
We re-analyzed the heterogeneity of all cardiovascular outcomes using random-effects or fixed-effects models. Approximately 32% of the re-analyzed cardiovascular outcomes exhibited significant heterogeneity (I² > 50% or P value < 0.1 for Cochran’s Q test). This heterogeneity was likely influenced by various factors, including regional differences, race, study design, study quality, sample size, gender, age, follow-up duration, and adjustments for confounding factors.
Assessment of risk of bias
In our re-analysis, we performed Egger’s test on 83% of the outcomes. The results indicated publication bias in 11 outcomes. Specifically, two all-cause mortality outcomes for bariatric surgery and one for pharmacological interventions showed significant publication bias, with P values of 0.0017, 0.0027, and 0.0013, respectively. Two CVD mortality outcomes for bariatric surgery and one for dietary interventions showed bias, with P values of 0.0000301, 0.0360, and 0.0138. Two myocardial infarction outcomes for bariatric surgery showed bias, with P values of 0.0044 and 0.0081. One heart failure outcome for bariatric surgery showed bias (P = 0.0022). One stroke outcome for bariatric surgery showed bias (P = 0.0007). One atrial fibrillation outcome for bariatric surgery showed bias (P = 0.0141). One outcome could not be tested with Egger’s due to only two meta-analyses, and the remaining outcomes showed no significant publication bias.
AMSTAR, GRADE, and evidence classification
The median AMSTAR score for cardiovascular outcomes was 9, with a range from 6 to 10 and an interquartile range of 8.5 to 10.0 (Supplementary Table S2). Detailed AMSTAR scores for each specific outcome are provided in Supplementary Table S3.
Due to limitations and downgrading factors in observational study designs—such as significant bias risk, inconsistency, indirectness, imprecision, and potential publication bias—18 meta-analyses from cohort studies were rated as “low” or “very low” quality. One stroke outcome from dietary interventions was upgraded to “moderate” quality due to confounding factors. Of the 28 meta-analyses from randomized controlled trials, 7 were rated as “high” quality, 15 were downgraded to “moderate” quality due to bias risk or imprecision, and 6 were downgraded to “low” quality due to bias risk, imprecision, or publication bias (Supplementary Table S1). Detailed GRADE classifications for each outcome are presented in Supplementary Table S4.
Regarding evidence classification, four outcomes were categorized as Class I evidence: bariatric surgery (heart failure), dietary interventions (stroke, CVD mortality), and pharmacological interventions (MACE). Eight outcomes from bariatric surgery, covering different populations and including all-cause mortality, MACE, CVD mortality, myocardial infarction, and heart failure, as well as one all-cause mortality outcome from exercise interventions, were rated as Class II evidence. Among the remaining 34 outcomes, 6 (13%) were rated as Class III evidence, 12 (26%) as Class IV evidence, and 16 (34%) as non-significant (Supplementary Table S2). Sensitivity analyses for all Class I and II outcomes showed no change in the direction or significance of associations.
Discussion
Main conclusions and possible explanations
This umbrella review examined the relationships between different weight control interventions and both all-cause mortality and cardiovascular outcomes. Interventions assessed included pharmacological interventions, bariatric surgery, dietary interventions, exercise interventions, and comprehensive lifestyle interventions. Out of 15,911 articles, we identified 31 meta-analyses with 47 pooled effect sizes—19 from observational studies and 28 from RCTs. The analysis indicated that pharmacological interventions, bariatric surgery, dietary interventions, and exercise interventions positively impact cardiovascular outcomes. However, comprehensive lifestyle interventions did not show a significant association with cardiovascular outcomes.
Among pharmacological interventions, GLP-1 RAs are significantly associated with improved cardiovascular outcomes in individuals with diabetes, obesity, or overweight. These outcomes include reductions in all-cause mortality [21, 22], CVD mortality [21, 22], MACE [21,22,23], stroke [21, 24], myocardial infarction [22, 23], and heart failure [22]. Clinical trials have demonstrated that liraglutide and semaglutide, both GLP-1 RAs, reduce cardiovascular events in individuals with T2DM. Furthermore, a meta-analysis of RCTs supports these findings, showing reduced risks of cardiovascular outcomes (all-cause mortality, CVD mortality, MACE, stroke, heart failure) in individuals with T2DM with or without established cardiovascular disease [39]. GLP-1 RAs function by targeting the incretin pathway, enhancing postprandial insulin release, reducing glucagon secretion, improving glucose uptake, and maintaining optimal glucose levels [40, 41]. Additionally, GLP-1 RAs increase satiety through direct action on the hypothalamus and by slowing gastric emptying, contributing to overall weight loss [42]. Beyond glucose control, GLP-1 RAs exhibit multifaceted effects on various organ systems. In the cardiovascular system, they promote vasodilation, neurohormonal regulation, natriuresis, anti-inflammatory actions, weight reduction, lipid profile improvement, and plaque inhibition [40, 41]. At the molecular level, GLP-1 RAs provide cardiovascular protection through multiple mechanisms. Firstly, GLP-1 RAs increase nitric oxide (NO) production via the GLP-1 receptor-dependent AMPK/Akt/eNOS signaling pathway, improving endothelial function and inducing vasodilation [43, 44]. Secondly, GLP-1 RAs inhibit the proliferation and migration of vascular smooth muscle cells (VSMCs), regulate their phenotypic transformation, and prevent vascular remodeling and the progression of atherosclerosis [45, 46]. Additionally, GLP-1 RAs modulate macrophage polarization, promoting a shift towards the anti-inflammatory M2 phenotype, thereby reducing inflammatory cell infiltration and foam cell formation in arterial walls [47,48,49]. Lastly, GLP-1 RAs help maintain myocardial energy metabolism balance and alleviate cardiomyocyte dysfunction by activating the PI3K/Akt and p38 MAPK pathways, as well as the Nrf-2/heme oxygenase-1 (HO-1) axis [50,51,52]. In various animal models, GLP-1 RAs have demonstrated significant cardiovascular protective effects. For instance, in a high-fat diet-induced atherosclerosis mouse model, liraglutide significantly slowed the progression of atherosclerosis by inhibiting inflammation and foam cell formation [53]. Similarly, in myocardial infarction rat models, exenatide reduced infarct size and improved ventricular function, effectively mitigating myocardial damage and the risk of heart failure [54]. Furthermore, GLP-1 RAs modulate multiple signaling pathways, such as the AMPK-TXNIP pathway, to inhibit hyperglycemia-induced cardiomyocyte pyroptosis, further validating their potential application in preventing and treating cardiovascular diseases. These mechanisms collectively provide cardiovascular protection, making GLP-1 RAs crucial in managing cardiovascular health.
Low-fat diets are strongly associated with reduced all-cause mortality in individuals with obesity [32], while the Mediterranean diet shows a strong beneficial association with reduced stroke incidence [33]. A low-fat diet typically provides ≤30% of energy from fat. The Mediterranean diet emphasizes olive oil, fish, vegetables, fruits, and whole grains. These dietary interventions may offer cardiovascular protection by improving lipid profiles, lowering blood pressure, enhancing insulin sensitivity, preventing inflammation, reducing oxidative stress, improving endothelial function, and optimizing glucose metabolism [55,56,57,58]. One cohort study found low-fat diets associated with reduced all-cause mortality and CVD mortality risk [59]. Another cohort study found that a 2-point increase in the Mediterranean diet score correlates with approximately a 10% reduction in cardiovascular disease incidence [60]. Adherence to the Mediterranean diet has been linked to lower all-cause mortality [61] and reduced incidence of cerebrovascular disease [62], hypertension [63], and atrial fibrillation [64]. Our findings align with the Dietary Guidelines for Americans [65], supporting the Mediterranean diet as a crucial measure for CVD prevention. Due to the limited number of studies included, additional large-scale prospective research is required to further elucidate the relationships between dietary interventions and cardiovascular outcomes.
Despite beneficial associations between bariatric surgery and cardiovascular outcomes in individuals with obesity, both with and without cardiovascular diseases (all-cause mortality [29, 31], CVD mortality [30], MACE [27, 28, 31], stroke [29, 31], myocardial infarction [30], heart failure [26, 30], atrial fibrillation [30]), the evidence quality remains low. Bariatric surgery is a last-line treatment for severe obesity that is unmanageable through lifestyle changes or medication alone. The low quality of evidence regarding cardiovascular outcomes associated with this intervention is due to the inclusion of non-randomized studies, the predominance of individuals with severe obesity, the complexity of the surgery, insufficient long-term follow-up data, and risk of complications. An umbrella review of bariatric surgery outcomes found similar results, indicating beneficial associations with cardiovascular outcomes but also highlighting the low quality of evidence [66]. Therefore, more high-quality, long-term studies are needed to confirm the true effects of these interventions.
Exercise interventions are associated with improved cardiovascular outcomes in individuals with diabetes, although evidence quality is low. Variability in intervention types and adherence levels contribute to high heterogeneity. Despite limited studies, evidence suggests exercise interventions may benefit cardiovascular health. Future research should focus on high-quality RCTs, optimizing protocols, ensuring long-term adherence, and exploring the impacts of different exercise types.
Comprehensive lifestyle interventions do not show significant associations with all-cause mortality and cardiovascular outcomes, possibly due to smaller BMI, intervention complexity, and adherence variability. Future studies should optimize protocols, enhance long-term adherence, and conduct large-scale RCTs. Research should focus on specific components and implementation methods to improve effectiveness.
Strengths and limitations of this umbrella review
This umbrella review synthesizes the most comprehensive and up-to-date evidence on various weight control interventions and their associations with cardiovascular outcomes. By integrating findings across pharmacological, surgical, dietary, exercise-based, and lifestyle interventions, we offer a broad comparative perspective that is not readily available in individual meta-analyses. This integrative approach enhances the practical utility of existing evidence for clinical and public health decision-making. In addition, the stratification of evidence quality using both the GRADE framework and an objective evidence classification scheme allows for a more nuanced interpretation of the strength of findings.
To provide a balanced interpretation of our findings, we highlight several key strengths and limitations of this umbrella review. The review was conducted using a rigorous and systematic methodology, including independent literature screening, data extraction, and quality appraisal. The combined application of AMSTAR, GRADE, and evidence classification enhances the transparency and credibility of the evaluation. Given the global burden of overweight and obesity, this study has significant clinical and societal relevance in informing obesity management strategies. However, several limitations should be noted. For certain interventions, only a limited number of meta-analyses were available, potentially affecting the robustness of conclusions. Furthermore, discrepancies were observed between GRADE assessments and evidence classification results in several outcomes, likely due to the subjective nature of GRADE criteria. Thus, we recommend that both methods be considered together when interpreting the strength of evidence and guiding recommendations.
Conclusions
This umbrella review highlights that pharmacological interventions, particularly GLP-1 RAs, significantly improve all-cause mortality and cardiovascular outcomes. Dietary interventions, including low-fat and Mediterranean diets, also demonstrate beneficial associations, although the number of studies is limited. Bariatric surgery and exercise interventions offer cardiovascular protection, though evidence quality is lower. Comprehensive lifestyle interventions show no significant benefits, likely due to their complexity and variable adherence. Future research should prioritize high-quality trials to optimize and evaluate long-term effects, providing a robust foundation for effective weight management strategies in cardiovascular disease prevention and management.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
WHO. Obesity and overweight. WHO.https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#.
Chen Y, Ma L, Han Z, Xiong P. The global burden of disease attributable to high body mass index in 204 countries and territories: findings from 1990 to 2019 and predictions to 2035. Diabetes Obes Metab. 2024;26:3998–4010.
Gregg EW, Shaw JE. Global health effects of overweight and obesity. N Engl J Med. 2017;377:80–81.
Global BMIMC, Di Angelantonio E, Bhupathiraju ShN, Wormser D, Gao P, Kaptoge S, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786.
Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: a report From the American Heart Association. Circulation. 2022;145:e153–e639.
Gribsholt SB, Schmidt M, Kristiansen EB, Richelsen B, Sørensen HT. Risk of cardiovascular disease after hospital-diagnosed overweight or obesity. Endocr Connect. 2024;13:e230452.
Alebna PL, Mehta A, Yehya A, daSilva-deAbreu A, Lavie CJ, Carbone S. Update on obesity, the obesity paradox, and obesity management in heart failure. Prog Cardiovasc Dis. 2024;82:34–42.
Tutor AW, Lavie CJ, Kachur S, Milani RV, Ventura HO. Updates on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2023;78:2–10.
Lavie CJ, De Schutter A, Patel DA, Romero-Corral A, Artham SM, Milani RV. Body composition and survival in stable coronary heart disease: impact of lean mass index and body fat in the “obesity paradox. J Am Coll Cardiol. 2012;60:1374–1380.
Chen Y, Koirala B, Ji M, Commodore-Mensah Y, Dennison Himmelfarb CR, Perrin N, et al. Obesity paradox of cardiovascular mortality in older adults in the United States: a cohort study using 1997-2018 National Health Interview Survey data linked with the National Death Index. Int J Nurs Stud. 2024;155:104766.
Lv Y, Zhang Y, Li X, Gao X, Ren Y, Deng L, et al. Body mass index, waist circumference, and mortality in subjects older than 80 years: a Mendelian randomization study. Eur Heart J. 2024;45:2145–2154.
Laddu D, Neeland IJ, Carnethon M, Stanford FC, Mongraw-Chaffin M, Barone Gibbs B, et al. Implementation of obesity science into clinical practice: a scientific statement From the American Heart Association. Circulation. 2024;150:e7–e19.
Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Health. 2015;13:132–140.
Papatheodorou S. Umbrella reviews: what they are and why we need them. Eur J Epidemiol. 2019;34:543–546.
Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394.
Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. Cmaj. 2009;181:488–493.
Wang D, Benito PJ, Rubio-Arias J, Ramos-Campo DJ, Rojo-Tirado MA. Exploring factors of adherence to weight loss interventions in population with overweight/obesity: an umbrella review. Obes Rev. 2024:e13783.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174.
Mannucci E, Dicembrini I, Nreu B, Monami M. Glucagon-like peptide-1 receptor agonists and cardiovascular outcomes in patients with and without prior cardiovascular events: an updated meta-analysis and subgroup analysis of randomized controlled trials. Diab Obes Metab. 2020;22:203–211.
Yoshiji S, Minamino H, Tanaka D, Yamane S, Harada N, Inagaki N. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular and renal outcomes: a meta-analysis and meta-regression analysis. Diab Obes Metab. 2022;24:1029–1037.
Singh S, Garg A, Tantry US, Bliden K, Gurbel PA, Gulati M. Safety and efficacy of glucagon-like peptide-1 receptor agonists on cardiovascular events in overweight or obese non-diabetic patients. Curr Probl Cardiol. 2024;49:102403.
Adamou A, Barkas F, Milionis H, Ntaios G. Glucagon-like peptide-1 receptor agonists and stroke: a systematic review and meta-analysis of cardiovascular outcome trials. Int J Stroke. 2024;19:876–87.
Capristo E, Maione A, Lucisano G, Russo MF, Mingrone G, Nicolucci A. Effects of weight loss medications on mortality and cardiovascular events: a systematic review of randomized controlled trials in adults with overweight and obesity. Nutr Metab Cardiovasc Dis. 2021;31:2587–2595.
Berger S, Meyre P, Blum S, Aeschbacher S, Ruegg M, Briel M, et al. Bariatric surgery among patients with heart failure: a systematic review and meta-analysis. Open Heart. 2018;5:e000910.
Sutanto A, Wungu CDK, Susilo H, Sutanto H. Reduction of major adverse cardiovascular events (MACE) after bariatric surgery in patients with obesity and cardiovascular diseases: a systematic review and meta-analysis. nutrients. 2021;13:3568.
Tang BR, Zhang Y, Wang YF, Wang XR, An ZL, Yu XJ. Effect of bariatric surgery on long-term cardiovascular outcomes: a systematic review and meta-analysis of population-based cohort studies. Surg Obes Relat Dis. 2022;18:1074–1086.
van Veldhuisen SL, Gorter TM, van Woerden G, de Boer RA, Rienstra M, Hazebroek EJ, et al. Bariatric surgery and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J. 2022;43:1955–1969.
Chandrakumar H, Khatun N, Gupta T, Graham-Hill S, Zhyvotovska A, McFarlane SI. The effects of bariatric surgery on cardiovascular outcomes and cardiovascular mortality: a systematic review and meta-analysis. Cureus. 2023;15:e34723.
Cui B, Wang G, Li P, Li W, Song Z, Sun X, et al. Disease-specific mortality and major adverse cardiovascular events after bariatric surgery: a meta-analysis of age, sex, and BMI-matched cohort studies. Int J Surg. 2023;109:389–400.
Ma C, Avenell A, Bolland M, Hudson J, Stewart F, Robertson C, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. Bmj. 2017;359:j4849.
Chen GC, Neelakantan N, Martín-Calvo N, Koh WP, Yuan JM, Bonaccio M, et al. Adherence to the Mediterranean diet and risk of stroke and stroke subtypes. Eur J Epidemiol. 2019;34:337–349.
Massara P, Zurbau A, Glenn AJ, Chiavaroli L, Khan TA, Viguiliouk E, et al. Nordic dietary patterns and cardiometabolic outcomes: a systematic review and meta-analysis of prospective cohort studies and randomised controlled trials. Diabetologia. 2022;65:2011–2031.
Mantzouranis E, Kakargia E, Kakargias F, Lazaros G, Tsioufis K. The Impact of high protein diets on cardiovascular outcomes: a systematic review and meta-analysis of prospective cohort studies. Nutrients. 2023;15:1372.
Liu X, Wu Z, Li N. Association between physical exercise and all-cause and CVD mortality in patients with diabetes: an updated systematic review and meta-analysis. Afr Health Sci. 2022;22:250–266.
Kritchevsky SB, Beavers KM, Miller ME, Shea MK, Houston DK, Kitzman DW, et al. Intentional weight loss and all-cause mortality: a meta-analysis of randomized clinical trials. PLoS ONE. 2015;10:e0121993.
Singh N, Stewart RAH, Benatar JR. Intensity and duration of lifestyle interventions for long-term weight loss and association with mortality: a meta-analysis of randomised trials. BMJ Open. 2019;9:e029966.
Marsico F, Paolillo S, Gargiulo P, Bruzzese D, Dell’Aversana S, Esposito I, et al. Effects of glucagon-like peptide-1 receptor agonists on major cardiovascular events in patients with Type 2 diabetes mellitus with or without established cardiovascular disease: a meta-analysis of randomized controlled trials. Eur Heart J. 2020;41:3346–3358.
Ferhatbegović L, Mršić D, Macić-Džanković A. The benefits of GLP1 receptors in cardiovascular diseases. Front Clin Diab Health. 2023;4:1293926.
Heuvelman VD, Van Raalte DH, Smits MM. Cardiovascular effects of glucagon-like peptide 1 receptor agonists: from mechanistic studies in humans to clinical outcomes. Cardiovasc Res. 2020;116:916–930.
Del Prato S, Gallwitz B, Holst JJ, Meier JJ. The incretin/glucagon system as a target for pharmacotherapy of obesity. Obes Rev. 2022;23:e13372.
Koska J, Sands M, Burciu C, D’Souza KM, Raravikar K, Liu J, et al. Exenatide protects against glucose- and lipid-induced endothelial dysfunction: evidence for direct vasodilation effect of GLP-1 receptor agonists in humans. Diabetes. 2015;64:2624–2635.
Wei R, Ma S, Wang C, Ke J, Yang J, Li W, et al. Exenatide exerts direct protective effects on endothelial cells through the AMPK/Akt/eNOS pathway in a GLP-1 receptor-dependent manner. Am J Physiol Endocrinol Metab. 2016;310:E947–E957.
Shi L, Ji Y, Jiang X, Zhou L, Xu Y, Li Y, et al. Liraglutide attenuates high glucose-induced abnormal cell migration, proliferation, and apoptosis of vascular smooth muscle cells by activating the GLP-1 receptor, and inhibiting ERK1/2 and PI3K/Akt signaling pathways. Cardiovasc Diabetol. 2015;14:18.
Zhao L, Li AQ, Zhou TF, Zhang MQ, Qin XM. Exendin-4 alleviates angiotensin II-induced senescence in vascular smooth muscle cells by inhibiting Rac1 activation via a cAMP/PKA-dependent pathway. Am J Physiol Cell Physiol. 2014;307:C1130–C1141.
Wang N, Gao J, Jia M, Ma X, Lei Z, Da F, et al. Exendin-4 induces bone marrow stromal cells migration through bone marrow-derived macrophages polarization via PKA-STAT3 signaling pathway. Cell Physiol Biochem. 2017;44:1696–1714.
Yang L, Chen L, Li D, Xu H, Chen J, Min X, et al. Effect of GLP-1/GLP-1R on the polarization of macrophages in the occurrence and development of atherosclerosis. Mediat Inflamm. 2021;2021:5568159.
Vinué Á, Navarro J, Herrero-Cervera A, García-Cubas M, Andrés-Blasco I, Martínez-Hervás S, et al. The GLP-1 analogue lixisenatide decreases atherosclerosis in insulin-resistant mice by modulating macrophage phenotype. Diabetologia. 2017;60:1801–1812.
Lu K, Chang G, Ye L, Zhang P, Li Y, Zhang D. Protective effects of extendin‑4 on hypoxia/reoxygenation‑induced injury in H9c2 cells. Mol Med Rep. 2015;12:3007–3016.
Chang G, Zhang D, Yu H, Zhang P, Wang Y, Zheng A, et al. Cardioprotective effects of exenatide against oxidative stress-induced injury. Int J Mol Med. 2013;32:1011–1020.
Zhao SM, Gao HL, Wang YL, Xu Q, Guo CY. Attenuation of high glucose-induced rat cardiomyocyte apoptosis by exendin-4 via intervention of HO-1/Nrf-2 and the PI3K/AKT signaling pathway. Chin J Physiol. 2017;60:89–96.
Li J, Liu X, Fang Q, Ding M, Li C. Liraglutide attenuates atherosclerosis via inhibiting ER-induced macrophage derived microvesicles production in T2DM rats. Diabetol Metab Syndr. 2017;9:94.
Chen J, Wang D, Wang F, Shi S, Chen Y, Yang B, et al. Exendin-4 inhibits structural remodeling and improves Ca(2+) homeostasis in rats with heart failure via the GLP-1 receptor through the eNOS/cGMP/PKG pathway. Peptides. 2017;90:69–77.
Xiao Y, Xiao X, Zhang X, Yi D, Li T, Hao Q, et al. Mediterranean diet in the targeted prevention and personalized treatment of chronic diseases: evidence, potential mechanisms, and prospects. Epma j. 2024;15:207–220.
Stewart RAH. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;379:1388.
Fitó M, Guxens M, Corella D, Sáez G, Estruch R, de la Torre R, et al. Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch Intern Med. 2007;167:1195–1203.
Gay HC, Rao SG, Vaccarino V, Ali MK. Effects of different dietary interventions on blood pressure: systematic review and meta-analysis of randomized controlled trials. Hypertension. 2016;67:733–739.
Zhao Y, Li Y, Wang W, Song Z, Zhuang Z, Li D, et al. Low-carbohydrate diets, low-fat diets, and mortality in middle-aged and older people: a prospective cohort study. J Intern Med. 2023;294:203–215.
Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92:1189–1196.
Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608.
Misirli G, Benetou V, Lagiou P, Bamia C, Trichopoulos D, Trichopoulou A. Relation of the traditional Mediterranean diet to cerebrovascular disease in a Mediterranean population. Am J Epidemiol. 2012;176:1185–1192.
Jennings A, Berendsen AM, de Groot L, Feskens EJM, Brzozowska A, Sicinska E, et al. Mediterranean-style diet improves systolic blood pressure and arterial stiffness in older adults. Hypertension. 2019;73:578–586.
Neumann FA, Jagemann B, Makarova N, Börschel CS, Aarabi G, Gutmann F, et al. Mediterranean diet and atrial fibrillation: lessons learned from the AFHRI case-control study. Nutrients. 2022;14:3615.
Phillips JA. Dietary Guidelines for Americans, 2020–2025. Workplace health Saf. 2021;69:395–395.
Liao J, Yin Y, Zhong J, Chen Y, Chen Y, Wen Y, et al. Bariatric surgery and health outcomes: an umbrella analysis. Front Endocrinol. 2022;13:1016613.
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
XMC: Conceptualization, Methodology, Investigation, Data curation, Visualization, Writing-original draft, Writing-review & editing, Formal analysis, Supervision. XGZ: Methodology, Investigation, Data curation, Formal analysis, Project administration, Writing-review & editing. XX: Investigation, Data curation, Formal analysis, Visualization, Writing-review & editing. XF: Resources, Validation, Supervision, Writing-review & editing. FW: Resources, Validation, Supervision, Writing-review & editing. SHF: Resources, Validation, Supervision, Writing-review & editing. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors have completed the ICMJE uniform disclosure form and declare no conflicts of interest.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, X., Zhang, X., Xiang, X. et al. Effects of weight control interventions on cardiovascular outcomes: an umbrella review of systematic reviews and meta-analyses. Int J Obes 49, 1911–1920 (2025). https://doi.org/10.1038/s41366-025-01860-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-025-01860-z