Abstract
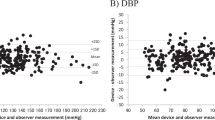
This study evaluates the accuracy of the Hipermax-BF model A7101 (Combiomed, Havana, Cuba) automatic oscillometric upper-arm sphygmomanometer for office and home use in general population as part of the HEARTS in the Americas initiative. The research was developed according to the Universal Standard AAMI/ESH/ISO ISO 81060-2:2018/Amd 1:2020. The subjects were recruited according to the requirements of age, gender, blood pressure values and upper-arm circumference. The same upper-arm sequential blood pressure measurement method was used. For measurements with the device under test, the 2-piece cuff from 22–44 cm limb circumference range was used. 92 subjects were recruited and 85 were analyzed. Mean age was 44.8 ± 14.7 years, mean upper-arm circumference was 32.3 ± 6.2, and 56.5% were female. For Validation Criterion 1, the mean value ± standard deviation of the differences in readings between the device under test and the reference device was 1.2 ± 4.9/0.8 ± 4.9 mmHg (systolic/diastolic). For both pressures, in criterion 1 the standard requires a mean value of the differences ≤ ± 5 mmHg and a standard deviation ≤ ± 8 mmHg. For Validation Criterion 2, the standard deviation of the mean blood pressure differences per subject was 4.2/4.2 mmHg (systolic/diastolic). According to Table 1 of criterion 2, for the mean values of 1.2/0.8 mmHg (systolic/diastolic), the maximum allowable standard deviation had to be < 6.84 for systolic and < 6.89 for diastolic pressure. The Combiomed Hipermax-BF A7101 automatic sphygmomanometer meets the requirements of the AAMI/ESH/ISO Universal Standard (ISO 81060-2:2018/Amd 1:2020) in the general population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data analyzed during this study are available in the Cuban Public Registry of Clinical Trials. https://rpcec.sld.cu/.
References
Campbell NRC, Schutte AE, Varghese CV, Ordunez P, Zhang X-H, Khan T, et al. São Paulo call to action for the prevention and control of high blood pressure: 2020. J Clin Hypertens. 2019;21:1744–52.
Padwal R, Campbell NRC, Schutte AE, Olsen MH, Delles C, Etyang A, et al. Optimizing observer performance of clinic blood pressure measurement: a position statement from the Lancet Commission on Hypertension Group. J Hypertens. 2019;37:1737–45. https://doi.org/10.1097/HJH.0000000000002112.
Padwal R, Berg A, Gelfer M, Tran K, Ringrose J, Ruzicka M, et al. Accuracy in measurement of blood pressure (AIM-BP) Collaborative. The Hypertension Canada blood pressure device recommendation listing: Empowering use of clinically validated devices in Canada. J Clin Hypertens (Greenwich). 2020;22:933–6. https://doi.org/10.1111/jch.13868.
How to check that a blood pressure monitor has been properly tested for accuracy. https://www.menzies.utas.edu.au/documents/research/blood-pressure-clinic/blood-pressure-devices.pdf.
Picone DS, Deshpande RA, Schultz MG, Fonseca R, Campbell NRC, Delles C, et al. Nonvalidated home blood pressure devices dominate the online marketplace in Australia: major implications for cardiovascular risk management. Hypertension. 2020;75:1593–9. https://doi.org/10.1161/HYPERTENSIONAHA.120.14719.
Sharman JE, Padwal R, Campbell NRC. Global marketing and sale of accurate cuff blood pressure measurement devices. Circulation. 2020;142:321–3. https://doi.org/10.1161/CIRCULATIONAHA.120.046205.
Patel P, Ordunez P, DiPette D, Escobar MC, Hassell T, Wyss F, et al. Improved blood pressure control to reduce cardiovascular disease morbidity and mortality: the standardized hypertension treatment and prevention project. J Clin Hypertens (Greenwich) 2016;18:1284–94.
HEARTS Technical package for cardiovascular disease management in primary health care: access to essential medicines and technology. Geneva: World Health Organization. 2019 (WHO/NMH/NVI/18.3). https://iris.paho.org/handle/10665.2/50804.
Campbell NR, Berbari AE, Cloutier L, Gelfer M, Kenerson JG, Khalsa TK, et al. Policy statement of the world hypertension league on noninvasive blood pressure measurement devices and blood pressure measurement in the clinical or community setting. J Clin Hypertens (Greenwich). 2014;16:320–2.
World Health Organization. WHO technical specifications for automated non-invasive blood pressure measuring devices with cuff. World Health Organization. 2020. https://iris.who.int/handle/10665/331749. Licence: CC BY-NC-SA 3.0 IGO.
Stergiou GS, Palatini P, Asmar R, Ioannidis JP, Kollias A, Lacy P, et al. European Society of Hypertension Working Group on Blood Pressure Monitoring. Recommendations and Practical Guidance for performing and reporting validation studies according to the Universal Standard for the validation of blood pressure measuring devices by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO). J Hypertens. 2019;37:459–66. https://doi.org/10.1097/HJH.0000000000002039. Erratum in: J Hypertens. 2020 Mar;38(3):561.
International Organization for Standardization. ISO81060-2:2018. Noninvasive sphygmomanometers: part 2: clinical investigation of intermittent automated measurement type. https://www.iso.org/standard/73339.html.
International Organization for Standardization. ISO81060-2:2018/AMD1:2020. Non-invasive sphygmomanometers —Part 2: Clinical investigation of intermittent automated measurement type - Amendment 1. https://www.iso.org/standard.
International Organization for Standardization. ISO 14155:2020(eng). Clinical investigations of medical devices for human subjects - Good Clinical Practices. https://www.iso.org/standard.
Ministry of Public Health of Cuba. Validation study for the clinical use of the Hipermax BF automatic blood pressure measurement device in the general population (VALIDATION OF AUTOMATIC DEVICES IN THE CLINIC: VALIDAC-1). Clin Investig Plan. 2022;Version 4:59.
Siddique S, Hameed Khan A, Shahab H, Zhang YQ, Chin Tay J, Buranakitjaroen P, et al. Office blood pressure measurement: a comprehensive review. J Clin Hypertens (Greenwich). 2021;23:440–9. https://doi.org/10.1111/jch.14169.
Asayama K, Ohkubo T, Imai Y. In-office and out-of-office blood pressure measurement. J Hum Hypertens. 2024;38:477–85. https://doi.org/10.1038/s41371-021-00486-8.
Stergiou G, Kollias A, Parati G, O’Brien E. Office blood pressure measurement: the weak cornerstone of hypertension diagnosis. Hypertension. 2018;71:813–5. https://doi.org/10.1161/HYPERTENSIONAHA.118.10850.
Parati G, Mendis S, Abegunde D, Asmar R, Mieke S, Murray A, et al. Recommendations for blood pressure measuring devices for office/clinic use in low resource settings. Blood Press Monit. 2005;10:3–10.
Velludo Veiga E, Daniel ACQG, Bortolloto LA, Machado CA, Plavinik FL, Cláudia Irigoyen A, et al. Some problems and solutions in implementing the world hypertension league recommendations for automated office assessment of blood pressure. J Clin Hypertens. 2016;18:7–9. https://doi.org/10.1111/jch.12676.
Valdés González Y, Campbell NRC, Pons Barrera E, Calderón Martínez M, Pérez Carrera A, Morales Rigau JM, et al. Implementation of a community-based hypertension control program in Matanzas, Cuba. J Clin Hypertens. 2020;22:142–9.
International Organization for Standardization. ISO 81060-2:2018/AMD 2:2024 non-invasive sphygmomanometers – part 2: clinical investigation of intermittent automated measurement type – amendment 2; 2024. Available from: https://www.iso.org/standard/83598.html.
HEARTS in the Americas regulatory pathway to the exclusive use of validated blood pressure measuring devices. Washington, D.C.: Pan American Health Organization; 2021. License: CC BY-NC-SA 3.0 IGO. https://doi.org/10.37774/9789275124864.
Sharman JE, Ordunez P, Brady T, Parati G, Stergiou G, Whelton PK, et al. The urgency to regulate validation of automated blood pressure measuring devices: a policy statement and call to action from the World Hypertension League. J Hum Hypertens. 2023;37:155–159. https://doi.org/10.1038/s41371-022-00747-0.
Acknowledgements
We would like to acknowledge the collaboration of the Group for the conduct of clinical validation studies of automatic sphygmomanometers in Cuba: Amarilys Jimenez Chiquet and Lizette Pérez Perea from the Public Health Ministry, Jorge Enrique Aguiar Pérez, Sandra Quintana Estévez, Mary Leivys Herrera Giró, Judith Castellanos Almeida and Dania Fernández Rodríguez from the Institute of Cardiology and Cardiovascular Surgery, Elaine Hernández Morales, Zuleidys Gourneo Álvarez, Emelina Despaigne Carrión and Yadira Drake Pérez from the University Hospital “General Calixto García”, Katherine Bancroft Sánchez from the Obstetric Hospital “América Arias”, Mario César Muñiz Ferrer, Ernesto Alcolea González and Dorian Alonso Martínez from the State Center for the Control of Medicines, Equipment and Devices (CECMED), Irma Millán Marrero and Edilberto González Ortiz from BioCubaFarma, as well as Peter Wood from the University of Alberta, Canada, Norm Campbell from University of Calgary, Canada, James E. Sharman from University of Tasmania, Australia and Cintia Lombardi. Thanks to the Panamerican Health Organization (PAHO) and the World Hypertension League (WHL) for joint work in the formation of technical capabilities in low and middle-income countries.
Funding
This study was funded by the Cuban Ministry of Public Health.
Author information
Authors and Affiliations
Contributions
DHV conceived the original idea, designed the procedures according to the standard, collected data, helped write the manuscript. YVG conceived the original idea, designed the procedures according to the standard, collected data, helped write the manuscript. NAR designed the procedures according to the standard, participated in data extraction and processing, provided comments on the report. RDLNG designed the procedures according to the standard, collected data, helped write the manuscript. JR provided comments on the Clinical Investigation Plan design and experimental issues according to the standard, advised data collection/processing and provided comments on the report. RP provided comments on the Clinical Investigation Plan design and experimental issues according to the standard, advised data collection/processing and provided comments on the report.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
In all cases, the ethical principles of the Declaration of Helsinki were respected and the informed consent of the participants was obtained.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hernández Véliz, D., Valdés González, Y., Armas Rojas, N.B. et al. Validation of combiomed hipermax-BF model A7101 automatic oscillometric upper-arm sphygmomanometer in general population: AAMI/ESH/ISO universal standard (ISO 81060-2:2018/Amd 1:2020). J Hum Hypertens 38, 779–785 (2024). https://doi.org/10.1038/s41371-024-00948-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41371-024-00948-9