Abstract
Risk factor exposure during childhood contributes to the early onset of cardiovascular disease and clusters with incident hypertension. We investigated cardiovascular risk factor patterns in preadolescent children, stratified by BP status, and their associations with macro- and microvasculature measures. We included children (n = 1043, ages 5–9 years) from the ExAMIN Youth SA study. Measurements included anthropometry, cardiorespiratory fitness, dietary intake, office BP, central pulse wave velocity (PWV), central retinal artery (CRAE), vein equivalent (CRVE), and their ratio (AVR), to identify factor patterns with exploratory factor analysis. We identified three factor pattern scores (FPS). FPS1 (chips, sweets, fast foods, and cookies/cake) and FPS2 (fruits, meat, milk, and socioeconomic status) were identified in both the normotensive blood pressure (BP) and incident hypertension groups, with the exceptions of fruits being absent in the incident hypertension group and fast foods absent in the normotensive group. FPS3, characterised by BMI, diastolic BP, and systolic BP, was observed only in the normotensive group. PWV associated with FPS3 (β = 0.372, p < 0.001) in the normotensive but with FPS2 (β = 0.197, p = 0.045) in the incident hypertension group. CRAE (β = −0.224, p = 0.001) and AVR (β = −0.26, p < 0.001) inversely associated with FPS3 in normotensive but with FPS1 in the incident hypertension (CRAE: β = −0.343, p < 0.001; AVR: β = −0.274, p < 0.001). CRVE was positively associated with FPS3 (β = 0.194, p = 0.002) in the normotensive group. Exposure to unhealthy dietary patterns in childhood compromises vascular health in the context of incident hypertension, beginning as early as five years of age.
Similar content being viewed by others
Introduction
Cardiovascular diseases (CVD) remain the leading causes of morbidity and premature mortality in the world, with hypertension as the primary risk factor for CVD [1]. The prevalence of primary hypertension in children has increased significantly [2]. A recent meta-analysis reported an elevated BP prevalence of 22% among children and adolescents in Africa under 18 years [2]. The early onset of hypertension is associated with CVDs in later life. A study [3] reported the association of childhood risk factors such as elevated BP, obesity, and high cholesterol with fatal and non-fatal cardiac events occurring before the age of 47 years. These findings emphasise the critical need to address modifiable risk factors early in life to prevent the progression of CVD and adverse outcomes in early adult life.
Modifiable risk factors such as obesity, and unhealthy dietary intake collectively contribute to the burden of CVD [4]. These risk factors are already present in childhood [5] and may contribute to the early deterioration of macro- and microvascular health in children [6, 7]. Such vascular changes are associated with elevated blood pressure and may contribute to the onset of premature cardiovascular disease.
Target organ injury markers such as pulse wave velocity (PWV) and retinal vessel calibres (CRAE: central retinal artery equivalent; CRVE: central retinal vein equivalent and their ratio, AVR: arterio-venous ratio) have shown to have predictive value for cardiovascular events in adults [8, 9]. The use of these markers in childhood may improve our understanding of the early mechanisms involved in CVD development at an early age. In children, obesity and lower cardiorespiratory fitness are associated with higher PWV, wider CRVE, smaller CRAE, and lower AVR [10,11,12], but no study has explored combined risk factor clusters, including BMI and physical activity, in relation to BP status and target organ injury markers. Although the individual impact of risk factors on hypertension is well-known, elevated BP in children is rarely isolated, and the combined effects of risk factor clusters on macro- and microvasculature remain underexplored [11]. This study aims to identify risk factor patterns and their associations with markers of vascular health in South African primary school-aged children stratified by blood pressure status.
Methods
Study design and participants
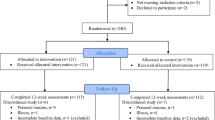
The Exercise, Arterial Modulation, and Nutrition in Youth South Africa (ExAMIN Youth SA) study is a multidisciplinary, observational cohort and analytical study that was developed to better comprehend the complicated mechanisms that are associated with the origin of early cardiovascular modification in children [12]. In this analysis, we included 1043 participants out of the original cohort of 1103 participants who were assessed at baseline [13]. A total of 37 participants were excluded for having missing data, and 23 belonged to other ethnicities. Participants were recruited from public primary schools in the North-West province of South Africa.
Basic procedures
The detailed study procedures have been published previously in the ExAMIN Youth SA protocol paper [12]. In brief, all eligible children received an information letter to take home to their parents or guardians. Participation was entirely voluntary; neither children nor parents were obligated to take part. Parental permission was sought through a structured consent process after families were given the opportunity to ask questions and receive full information about the study aims and procedures. Only children with completed parental consent and child assent forms were included in the study. Approval was obtained from the schools to conduct the research on their premises.
In addition to physical measurements, parents or guardians completed a questionnaire that captured information on the child’s age, sex, and parental education. The questionnaire also included items on socioeconomic and health-related variables. Socioeconomic status (SES) was quantified using a composite mean score derived from household income, parental education level, and employment status, with higher scores indicating higher SES.
Anthropometric and cardiorespiratory fitness measurements
All anthropometric measurements in this study were determined in accordance with the International Society for the Advancement of Kinanthropometry [14]. The body weight was taken with a Seca 813 numerical scale to determine weight to the closest 0.1 kg, and height with a Seca 213 stadiometer (Birmingham, United Kingdom) with a perpendicular panel to the closest 1 mm. Sex-adjusted body mass index for age (BMI z-score) was calculated using the World Health Organisation’s AnthroPlus software for children according to the child growth references for children between 5–19 years old [15]. Cardiorespiratory fitness (CRF) was measured by the 20-meter shuttle run test to objectively assess maximal aerobic capacity after a standardised 5-min warm-up [16]. The number of “stages” accomplished, where 1 stage ≈ 1 min, is calculated with a precision of 0.5 stage [14]. The test was done in the morning during school time and not during the winter season. CRF was recorded as the number of shuttles completed.
Cardiovascular measurements
Blood pressure
Office blood pressure and heart rate of all the participants were determined with validated, automated oscillometric paediatric BP monitors [Omron HBP-1100-E, (OMRON Healthcare Co., Ltd. Kyoto, Japan)] [17]. Prior to the BP measurement, the participants were requested to sit relaxed for 3–5 min with their backs supported, right arm horizontally supported, with their feet on the ground, and the cubital fossa at heart level.
Blood pressure was measured using standardised procedures in accordance with the 2017 American Academy of Paediatrics Clinical Practice Guidelines [18] for screening and management of high BP in children and adolescents. Measurements were taken on the right arm using appropriately sized cuffs. BP was measured five times at one-minute intervals, and the mean of the three readings with the least variance was used in analysis [19]. We grouped children according to normal BP range ( < 90th percentile for age, height and sex) and incident hypertension ( ≥ 90th percentile for age, sex and height or >120/80 mmHg).
Pulse wave velocity
Validated oscillometric Mobil-O-Graph monitors, along with the HMS Client-Server software package Version 4.7.1 (I.E.M. GmbH, Germany), were used to non-invasively perform pulse wave analysis [20]. These monitors were programmed to complete pulse wave analysis in duplicate using an appropriately sized cuff to the participants’ right arm, while in a sitting position. The aortic PWV was determined through this method [12].
Retinal vessels
A Static Retinal Vessel Analyser (SVA-T, Imedos Systems GmbH, Jena, Germany) connected to a fundus camera (Topcon TRC NW8) was used to capture non-invasive and nonmydriatic optic disc-centred retinal fundus images. Each child had two images taken of the right eye at an angle of 45 degrees. Vessel analysis (Visualis 2.80, Imedos Systems GmbH, Jena, Germany) was semi-automatically performed and retinal arteries and veins, running through an extent of 0.5–1 disc diameter from the optic disc margin, were selected. CRAE and CRVE were obtained by averaging the vessel diameters through the Parr-Hubbard formula, and the arterio-venous ratio was then determined (CRAE/CRVE) [21]. The mean of the two images of the right eye was used for CRAE and CRVE. The vessel diameters were expressed in measuring units, where 1 mu equalled 1 μm in the model of Gullstrand’s normal eye.
Food intake survey
A face-validity food frequency questionnaire was developed and validated [22] to gather dietary data on foods that are generally consumed by South African school children. The food intake survey included four healthy (vegetables, fruits, and fish/meat/poultry/eggs/milk), and six unhealthy food groups (cold drinks, sweets, sugar-sweetened hot drinks, cakes, fast foods, and salty snacks [23], based on the WHO Global School-based Student Health Survey [12].
Statistical analysis
Statistical analyses were performed with IBM® SPSS® Statistics version 30 software (IBM Corporation; Armonk, New York, USA), and figures were made using GraphPad Prism v5.03. All variables were tested for normality by visual inspection (QQ-plots). Normally distributed data were expressed as mean ± standard deviation, while non-normally distributed variables were expressed as mean sum ranks. Chi-square tests were used to compare the proportions of dichotomous variables. We performed independent t-tests to compare mean values between groups, while the Mann-Whitney U-test was used for food group data.
Factor analysis using the dimension reduction function of SPSS was used to identify risk factor patterns. Principal component analysis was used, and factors with an eigenvalue > 1.5 were retained. The Oblimin rotation method was used to obtain independent factors. A factor loading of ≥0.5 was used to interpret the factor patterns that were automatically calculated by the statistical software and used in further analyses. Pearson and partial correlations (adjusted for age, sex, ethnicity, and PWV was additionally adjusted for heart rate and mean arterial pressure) were performed to determine the associations of PWV, CRAE, CRVE, and AVR with each cardiovascular risk factor pattern in the normotensive and incident hypertension groups.
Multiple regression analysis was performed to test the independent associations of PWV, CRAE, CRVE, and AVR with each cardiovascular risk factor pattern in the normotensive and incident hypertension groups. Covariates considered for entry in the models included age, sex, and ethnicity, and additionally, mean arterial pressure and heart rate with PWV as the dependent variable.
Results
Characteristics of the study groups
Table 1 displays the characteristics of the study group stratified by BP ranges. Sex, age and ethnic distribution were similar between the groups (all p > 0.05). The children with incident hypertension had higher anthropometric measures, BP, and heart rate (all p < 0.001) than those in the normotensive group. Children with incident hypertension had higher mean PWV than the normotensive group (p < 0.001). CRAE was narrower and AVR lower in the incident hypertension group (both p < 0.001), whereas no differences were observed for CRVE and dietary intake between the groups.
Factor analysis
Table 2 contains the factor pattern scores (FPS) from the exploratory factor analysis performed in the normotensive and incident hypertension groups. The analysis identified two similar patterns for both the normotensive group and the incident hypertension groups. The third pattern was identified for only the normotensive group. The first-factor pattern score (FPS1) consisted of chips, sweets, cookies/cakes and fast foods (the latter only in the incident hypertension group); FPS 2 consisted of fruits (only in the normotensive BP group), meat, milk and socioeconomic score; and FPS3 consisted of systolic BP, diastolic BP, and BMI z-score (Fig. 1).
Bivariate and partially adjusted correlations
We performed bivariate correlations of PWV and the retinal markers with each FPS in the normotensive (Supplementary Table 1) and incident hypertension groups (Supplementary Table 2). We performed additional correlation analyses between dietary components, blood pressure, BMI, and vascular measures in normotensive and incident hypertension children (Supplementary Tables 3–6).
In the normotensive group, PWV correlated with FPS3 (r = 0.415; p < 0.001), AVR correlated with FPS2 (r = 0.158, p = 0.028) and FPS3 (r = −0.142; p = 0.048). After adjusting for age, sex, and ethnicity (Table 3), we confirmed the positive correlation between PWV and FPS3 (r = 0.350; p < 0.001), while an inverse correlation between AVR and FPS3 (r = −0.263; p < 0.001) emerged. A positive correlation of CRVE with FPS2 (r = 0.193; p = 0.008) and an inverse correlation of CRAE with FPS3 (r = −0.152; p = 0.0036) were additionally observed.
In the incident hypertension group, there was a positive correlation between PWV and FPS2 (r = −0.261; p = 0.009). CRAE (r = −0.313 p = 0.001) and AVR (r = −0.356; p < 0.001) both correlated inversely with FPS1, while CRVE correlated negatively with FPS2 (r = −0.384; p < 0.001). After adjustments for age, sex, and ethnicity (PWV was additionally adjusted for MAP and HR) (Table 4), we confirmed the previous correlations of AVR (r = −0.343; p < 0.001) and CRAE (r = −0.331; p = 0.001) with FPS1. PWV was positively correlated with FPS2 (r = 0.225, p = 0.029).
Multiple linear regression analysis
We performed multiple regression analysis using the backward elimination method to determine the associations between measures of the macro- and microvasculature and the risk factor pattern scores (Fig. 2a–h, Supplementary Tables 7–10). PWV associated with FPS3 (Fig. 2a) in the normotensive group (adj. R2 = 0.174, β = 0.372, p < 0.001) and with FPS2 (Fig. 2b) in the incident hypertension group (adj. R2 = 0.178, β = 0.197, p = 0.045). CRAE associated with FPS3 (Fig. 2c) in the normotensive group (adj. R2 = 0.293, β = −0.224, p = 0.001) and with FPS1 (Fig. 2d) in the incident hypertension group (adj. R2 = 0.269, β = −0.329, p < 0.001). CRVE associated with FPS3 (Fig. 2e) in the normotensive group only (adj. R2 = 0.359 β = 0.194, p = 0.002) and no association with either FPS1 or FPS2 in the incident hypertension group (Fig. 2f). AVR associated with FPS3 (Fig. 2g) in the normotensive group (adj. R2 = 0.158, β = −0.274, p < 0.001) and with FPS1 (Fig. 2h) in the incident hypertension group (adj. R2 = 0.22, β = −0.321, p = 0.008).
Panel a–h indicate each independent model specific to each blood pressure group and main dependent variable. PWV pulse wave velocity, CRAE central retinal artery equivalent, CRVE central retinal artery equivalent, AVR arterial veinratio, BP blood pressure, SES socio-economic status, BMI z-score Body Mass Index Z-scores.
Discussion
This study identified cardiovascular risk factor patterns based on BP status and examined associations of macro- and microvasculature measures with the identified risk factor pattern scores in children between 5–9 years old. We observed adverse associations of both micro- and macrovascular health measures with factor patterns consisting of highly processed and fast foods (chips, sweets, and cookies/cakes) as well as meat, milk and SES in children with incident hypertension. Cardiorespiratory fitness was considered for entry into the factor analysis but did not load significantly onto any of the derived patterns, suggesting it did not contribute meaningfully to the risk factor clustering observed in our cohort.
In the incident hypertension group, the two identified food pattern scores reflect distinct but complementary dietary intake patterns. FPS1, comprising chips, sweets, fast foods (incident hypertension group), and cakes/cookies, represents a pattern of highly processed, energy-dense foods that are rich in added sugars, saturated fats, and salt. This pattern was significantly associated with narrower CRAE and lower AVR, markers of adverse microvascular health [24]. FPS2 captures the influence of meat and dairy products that are common among higher SES groups with greater financial ability to access these foods [25]. Notably, FPS2 was significantly associated with increased PWV, suggesting a link to increased arterial stiffness [26]. This reflects the contribution of added salt, sugar and saturated fats in meats and dairy products, alongside the role of SES in shaping access to food quality. It is important to note that fruits were not part of either FPS in the incident hypertension group, limiting any protective dietary influence on vascular outcomes in this subgroup. Together, these findings illustrate that while both FPSs are characterised by processed food consumption, the impact of SES appears more pronounced on large artery stiffness, potentially through enabling access to unfavourable but affordable foods.
Additionally, we found an inverse association of CRAE and AVR with FPS1, suggesting that children consuming high amounts of highly processed foods exhibited narrower retinal arteries. This is consistent with previous studies that link high levels of carbohydrates and sugary soft drinks to narrower retinal arteries in children [27]. Studies suggest that a possible mechanism for these highly processed refined sugar foods leading to narrower retinal arteries is related to metabolic responses to elevated blood sugar levels. A key antioxidant, Glutamate Cysteine Ligase, is mobilised and diverted to metabolise the surplus glucose in the blood [28]. This results in a reduced availability of antioxidants to counteract oxidative stress, while the level of reactive oxygen species increases. Consequently, increased reactive oxygen species lead to vasoconstriction and subsequent arteriolar narrowing and elevated BP [29, 30]. The unhealthy dietary patterns rich in trans fats, sugars and salt may also contribute to systemic inflammation and obesity [31, 32]. This aligns with findings from a previous study that also found that narrower arterioles were associated with obesity and elevated BP, indicative of early CVD development [33]. The narrowing of retinal arterioles observed in our sample of children is particularly concerning, as it serves as an early indicator of vascular dysfunction strongly related to modifiable risk factors [34].
In the normotensive group, we observed positive associations of both PWV and AVR with FPS3, which comprised systolic BP, diastolic BP and BMI. In addition, both CRAE and AVR associated inversely with FPS3. These associations seem physiologically feasible in the normotensive context whereby the main component for PWV is BP [13, 24]. Similarly for the microvascular measures it is known that higher BP contribute to arteriolar narrowing of the retina. Both the average BMI z-scores and the BIA-derived fat mass index data (Supplementary Table 11) indicate that widespread excess adiposity was not present in our sample. Nevertheless, the positive association with PWV suggests that variations in BMI, even within the slightly elevated levels, can still influence arterial structure and function. This indicates that children with BP levels below clinical concern display the physiological dynamics of BP and body composition on vascular health preservation.
Our findings have shown that the combined effect of unhealthy diet and SES was observed only in children with incident hypertension, not in the normotensive group. This suggests that these factors are particularly relevant in the development of hypertension, demonstrating the need for healthier dietary choices in this group. In normotensive children, traditional risk factors such as genetic predisposition, birth weight, BP, and adiposity primarily drive macro- and microvascular alterations [35]. However, among children in our study with incident hypertension, modifiable factors, particularly unhealthy dietary intake, appear to play a more dominant role in early vascular compromise. The observed associations of PWV, CRAE, and AVR with unhealthy dietary intake, and at times SES, suggest that poor nutrition, compounded by socioeconomic disparities, may accelerate vascular dysfunction in children with incident hypertension as early as five years of age. Our observations demonstrate the need for further investigation into the potential protective effects of wholesome dietary patterns, such as fruits, on vascular health in this population and context.
This study has strengths and limitations to report. This paper reports on a cross-sectional analysis using only baseline data from the ExAMIN Youth SA study, a prospective cohort study [36]. While this allowed us to explore associations between dietary intake patterns, vascular measures, and BP status, the cross-sectional nature of the analysis limits our ability to establish causality. Additionally, we recruited children in the North-West province of South Africa from public schools that were in urban areas, which may not reflect the sociodemographic profile of the entire country. Despite these limitations, this study made use of a Static Retinal Vessel Analyser to non-invasively observe the health status of the retinal microvasculature in children with minimal discomfort. To the best of our knowledge, this is the first study to assess both macro- and microvascular health measures with composite risk factor patterns to determine the modifiable contributors to incident hypertension risk in children under 9 years of age.
Our findings emphasise the importance of screening children from an early age for blood pressure, as those with incident hypertension appeared more susceptible to the combined influence of poor diet. This enables targeted lifestyle interventions, particularly dietary modifications that can alleviate risk. We recommend the promotion of healthier food intake patterns, alongside nutrition education, and improving access to nutritious foods. Early intervention strategies that target both dietary habits are vital to breaking the cycle of cardiovascular disease onset in children that may track into adulthood.
Conclusion
Our study established a significant association between frequent consumption of diets high in fast foods, meat, and milk, alongside household socioeconomic factors, and compromise in micro- and macrovascular health in children with incident hypertension.
Summary
What is known about the topic
-
1.
Early exposure to cardiovascular risk factors such as obesity, elevated blood pressure and unhealthy dietary patterns is associated with adverse vascular changes in children.
-
2.
Retinal vessel diameters and pulse wave velocity are validated early markers of vascular dysfunction and predictors of future cardiovascular disease.
-
3.
Socioeconomic status is a key determinant of dietary behaviour, which in turn affects cardiovascular risk trajectories from a young age.
What this study adds
-
1.
Distinct patterns of dietary intake and socioeconomic factors are differentially associated with macro- and microvascular alterations in children, depending on their blood pressure status.
-
2.
Among children with incident hypertension, unhealthy dietary patterns showed stronger associations with vascular dysfunction, as reflected by narrower arteriolar diameters and increased arterial stiffness.
-
3.
The findings highlight the importance of early screening and targeted dietary interventions to mitigate vascular compromise and reduce long-term cardiovascular risk.
Data availability
The data used in this study are not publicly however, anonymised summary data relevant to the analyses presented in this manuscript can be made available upon reasonable request to the corresponding author, subject to institutional and ethical approvals.
References
Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–37.
Song P, Zhang Y, Yu J, Zha M, Zhu Y, Rahimi K, et al. Global prevalence of hypertension in children: a systematic review and meta-analysis. JAMA Pediatr. 2019;173:1154–63.
Jacobs DR, Woo JG, Sinaiko AR, Daniels SR, Ikonen J, Juonala M, et al. Childhood cardiovascular risk factors and adult cardiovascular events. N Engl J Med. 2022;386:1877–88.
Pool LR, Aguayo L, Brzezinski M, Perak AM, Davis MM, Greenland P, et al. Childhood risk factors and adulthood cardiovascular disease: a systematic review. J Pediatr. 2021;232:118–26.e23.
Rodrigues AN, Abreu GR, Resende RS, Goncalves WL, Gouvea SA. Cardiovascular risk factor investigation: a pediatric issue. Int J Gen Med. 2013;6:57–66.
Köchli S, Endes K, Steiner R, Engler L, Infanger D, Schmidt-Trucksäss A, et al. Obesity, high blood pressure, and physical activity determine vascular phenotype in young children. Hypertension. 2019;73:153–61.
Newman AR, Andrew NH, Casson RJ. Review of paediatric retinal microvascular changes as a predictor of cardiovascular disease. Clin Exp Ophthalmol. 2017;45:33–44.
Meyer ML, Klein BE, Klein R, Palta P, Sharrett AR, Heiss G, et al. Central arterial stiffness and retinal vessel calibers: the atherosclerosis risk in communities study - neurocognitive study (ARIC-NCS). J Hypertens. 2020;38:266–73.
Elmoselhi AB, Shankhwar V, Qaisar R, Hamoudi R, Brix B, Salon A, et al. Retinal vascular changes and arterial stiffness during 8-month isolation and confinement: the SIRIUS-21 space analog mission. Front Physiol [Internet]. 2024 May 27 [cited 2025 Jan 27];15. Available from: https://www.frontiersin.org/journals/physiology/articles/10.3389/fphys.2024.1374309/full.
Lona G, Hauser C, Köchli S, Infanger D, Endes K, Schmidt-Trucksäss A, et al. Association of blood pressure, obesity and physical activity with arterial stiffness in children: a systematic review and meta-analysis. Pediatr Res. 2022;91:502–12.
Feber J, Ahmed M. Hypertension in children: new trends and challenges. Clin Sci (Lond). 2010;119:151–61.
Kruger R, Monyeki MA, Schutte AE, Smith W, Mels CMC, Kruger HS, et al. The Exercise, Arterial Modulation and Nutrition in Youth South Africa Study (ExAMIN Youth SA). Front Pediatr [Internet]. 2020 Apr 29 [cited 2024 Dec 6];8. Available from: https://www.frontiersin.org/journals/pediatrics/articles/10.3389/fped.2020.00212/full
Kruger R, Kruger HS, Monyeki MA, Pienaar AE, Roux SBL, Gafane-Matemane LF, et al. A demographic approach to assess elevated blood pressure and obesity in prepubescent children: the ExAMIN Youth South Africa study. J Hypertens. 2021;39:2190–9.
Stewart A, Marfell-Jones M, Olds T, De Ridder J. International Standards for Anthropometric Assessment. 137. Potchefstroom, South Africa: ISAK; 2011.
de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–7.
Liu NY, Plowman SA, Looney MA. The reliability and validity of the 20-meter shuttle test in American students 12 to 15 years old. Res Q Exerc Sport. 1992;63:360–5.
Joukar F, Yeganeh S, Naghipour M, Hassanipour S, Nikbakht HA, Mansour-Ghanaei F. Validation of Omron HBP-1100-E professional blood pressure measuring device according to the american association for the advancement of medical instrumentation protocol: The PERSIAN Guilan Cohort Study (PGCS). Med Devices (Auckl). 2020;13:231–6.
Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904.
Kruger R, Monyeki MA, Schutte AE, Smith W, Mels CMC, Kruger HS, et al. The exercise, arterial modulation and nutrition in Youth South Africa Study (ExAMIN Youth SA). Front Pediatr [Internet]. 2020 Apr 29 [cited 2024 Sept 18];8. Available from: https://www.frontiersin.org/journals/pediatrics/articles/10.3389/fped.2020.00212/full
Weiss W, Gohlisch C, Harsch-Gladisch C, Tölle M, Zidek W, van der Giet M. Oscillometric estimation of central blood pressure: validation of the Mobil-O-Graph in comparison with the SphygmoCor device. Blood Press Monit. 2012;17:128–31.
Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–80.
Kruger HS, Makore P, van Zyl T, Faber M, Ware LJ, Monyeki MA, et al. Validation of a short food group questionnaire to determine intakes from healthy and unhealthy food groups in 5–9-year-old South African children. J Hum Nutr Diet. 2024;37:234–45.
Pedro TM, MacKeown JM, Norris SA. Variety and total number of food items recorded by a true longitudinal group of urban black South African children at five interceptions between 1995 and 2003. Public Health Nutr. 2008;11:616–23.
Köchli S, Smith W, Lona G, Goikoetxea-Sotelo G, Breet Y, Botha-Le Roux S, et al. Obesity, blood pressure and retinal microvascular phenotype in a bi-ethnic cohort of young children. Atherosclerosis. 2022;350:51–7.
Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87:1107–17.
Leed A, Sheridan E, Baker B, Bamford S, Emmanouilidis E, Stewart F, et al. Dietary intake and arterial stiffness in children and adolescents: a systematic review. Nutrients. 2023;15:2092.
Gopinath B, Flood VM, Wang JJ, Smith W, Rochtchina E, Louie JCY, et al. Carbohydrate nutrition is associated with changes in the retinal vascular structure and branching pattern in children. Am J Clin Nutr. 2012;95:1215–22.
Pinho N, Bombaça AC, Wiśniewski JR, Dias-Lopes G, Saboia-Vahia L, Cupolillo E, et al. Nitric oxide resistance in Leishmania (Viannia) braziliensis involves regulation of glucose consumption, glutathione metabolism and abundance of pentose phosphate pathway enzymes. Antioxidants (Basel). 2022;11:277.
Kearney FM, Fagan XJ, Al-Qureshi S. Review of the role of refined dietary sugars (fructose and glucose) in the genesis of retinal disease. Clin Exp Ophthalmol. 2014;42:564–73.
Chiu CJ, Taylor A. Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog Retin Eye Res. 2011;30:18–53.
Babalola OO, Akinnusi E, Ottu PO, Bridget K, Oyubu G, Ajiboye SA, et al. The impact of ultra-processed foods on cardiovascular diseases and cancer: Epidemiological and mechanistic insights. Asp Mol Med. 2025;5:100072.
Monda A, de Stefano MI, Villano I, Allocca S, Casillo M, Messina A, et al. Ultra-processed food intake and increased risk of obesity: a narrative review. Foods. 2024;13:2627.
Lona G, Hauser C, Köchli S, Infanger D, Endes K, Faude O, et al. Blood pressure increase and microvascular dysfunction accelerate arterial stiffening in children: modulation by physical activity. Front Physiol. 2020;11:613003.
Mitchell P, Cheung N, de Haseth K, Taylor B, Rochtchina E, Islam FMA, et al. Blood pressure and retinal arteriolar narrowing in children. Hypertension. 2007;49:1156–62.
Matjuda EN, Engwa GA, Letswalo PB, Mungamba MM, Sewani-Rusike CR, Nkeh-Chungag BN. Association of hypertension and obesity with risk factors of cardiovascular diseases in children aged 6–9 years old in the Eastern Cape Province of South Africa. Children (Basel). 2020;7:25.
Kruger R, Monyeki MA, Schutte AE, Smith W, Mels CMC, Kruger HS, et al. The exercise, arterial modulation and nutrition in youth South Africa study (ExAMIN Youth SA). Front Pediatr [Internet]. 2020 Apr 29 [cited 2025 June 3];8. Available from: https://www.frontiersin.org/journals/pediatrics/articles/10.3389/fped.2020.00212/full
Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–7.
Acknowledgements
The authors extend their gratitude to all parents and children who voluntarily participated in the study, as well as the school principals for their support. Appreciation is also given to the research staff and students for their dedication to data collection. This study is part of an ongoing research project funded by the South African Medical Research Council (SAMRC) Extra Mural Unit and the National Research Foundation (NRF) of South Africa through competitive support for Y-Rated Researchers (Unique Identification Number: 112141) and the NRF Equipment Related Training and Travel Grant (Unique Identification Number: 109905). Additionally, the research presented in this paper was supported by the South African Medical Research Council under a Self-Initiated Research Grant and the South African Research Chairs Initiative (SARChI) of the Department of Science and Innovation and National Research Foundation (NRF) of South Africa (Unique Identification Number: 86895). The views expressed are solely those of the authors and do not necessarily represent the official views of the SAMRC. Any opinion, findings, and conclusions or recommendations expressed in this study are those of the authors, and therefore, the NRF does not accept any liability in this regard. The funding bodies had no role in the study’s conceptualisation, data collection, manuscript preparation or decision to publish.
Funding
Open access funding provided by North-West University.
Author information
Authors and Affiliations
Contributions
JN contributed to the data analysis, interpretation of results, and drafting of the manuscript. SD contributed to manuscript writing and critical revisions. LL contributed to data acquisition, methodology, and interpretation. SW contributed to data acquisition, quality control, and manuscript review. KR conceived and supervised the study, funding acquisition, provided critical revisions to the manuscript, and approved the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was ethically approved by the provincial Department of Education and the Health Research Ethics Committee of the North-West University (NWU-0041-22-A1) and registered in a clinical trials registry (NCT04056377). All methods were performed following the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines [37] and the International Society for the Advancement of Kinanthropometry [14]. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41371_2025_1074_MOESM1_ESM.docx
Associations of Cardiovascular and Retinal Markers with Risk Factor Patterns in Children with and without Incident Hypertension
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nsamba, J., Swanepoel, D., Lammertyn, L. et al. Risk factor patterns and vascular health in children with incident hypertension: The ExAMIN Youth SA study. J Hum Hypertens 39, 859–866 (2025). https://doi.org/10.1038/s41371-025-01074-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41371-025-01074-w