Abstract
Objective
To describe feeding and respiratory outcomes at discharge and at the most recent follow-up visit prior to four years old in infants evaluated for micrognathia.
Study design
Single-center retrospective analysis of 218 patients admitted and evaluated with congenital micrognathia during infancy. Outcomes were compared based on treatment (medical, mandibular distraction osteogenesis, or tracheostomy), and also compared based on syndromic status.
Results
Tube feeding was required by 81% of infants at discharge and 41% at follow-up. Respiratory support was required by 32% at discharge and 22% at follow-up. There were no differences in feeding and respiratory support at discharge and at follow-up between medical treatment and mandibular distraction osteogenesis. Tracheostomy was associated with more tube feeding and respiratory support at both discharge and at follow-up. Genetic syndromes were more likely to require tube feeding and respiratory support.
Conclusion
Long-term feeding and respiratory support are common in infants hospitalized with micrognathia.
Similar content being viewed by others
Introduction
Micrognathia is a common congenital anomaly occurring in as many as 1:1000 to 1:1600 births [1, 2]. Congenital micrognathia may present as an isolated finding, part of the Robin sequence with or without cleft palate, or as part of a more complex genetic syndrome, which may have implications for treatment options and prognosis [3, 4]. In the past decade, mandibular distraction osteogenesis (MDO) has replaced tongue-lip adhesion to become the most common surgical option for those with significant airway obstruction and may otherwise require tracheostomy [4,5,6]. However, there is currently no standardized guideline to inform clinicians the ideal candidacy for MDO, optimal timing of surgery, perioperative management, or the overall care of infants with micrognathia. Therefore, understanding the expected short-term and long-term outcomes can be important for family counseling and decision-making by the multi-disciplinary team caring for infants with micrognathia.
Several studies have reported outcomes of infants with micrognathia. A substantial proportion of hospitalized infants with micrognathia require tube feeding, and infants with underlying genetic syndromes or those needing surgical intervention are more likely to require gastrostomy tube [6,7,8,9,10,11,12,13]. MDO has high success rate (>95%) in avoiding tracheostomy [4, 6] and significantly improves obstructive sleep apnea [12, 14,15,16]. However, the majority of studies evaluating feeding and respiratory outcomes in infants with micrognathia were limited by small cohorts, included only those who underwent surgery, and/or lacked long-term follow-up after discharge from initial hospitalization.
The objective of this study is to describe the feeding and respiratory status at admission, discharge, and at follow-up visit up to four years of age. Additional outcomes including neonatal hospital length of stay (LOS), polysomnography findings, mortality, and other procedures are also evaluated. Outcomes are compared based on treatment type (medical treatment, MDO, tracheostomy), and also compared among syndromic status.
Materials/subjects and methods
This was a single-center, retrospective cohort study of infants who were admitted to The Children’s Hospital of Philadelphia (CHOP) for the evaluation and management of micrognathia between 1/1/2010 and 2/28/2019. All infants who were admitted to the hospital and evaluated by plastic surgery for micrognathia prior to one year of age were eligible except for those who underwent prior MDO or tracheostomy at outside hospital prior to admission at our center. Patients were admitted from our Special Delivery Unit (inborn), emergency department, directly from clinic, or transferred from outside hospital. Patient outcomes were followed up to four years of age or until lost to follow-up. Authors LW and KM extracted data from the electronic health record and input into REDCap (Research Electronic Data Capture) for data collection and management [17]. Author KL then independently reviewed and verified data accuracy.
The evaluation and management of micrognathia at CHOP includes multi-disciplinary input from neonatology, plastic surgery, pulmonology, otolaryngology, and genetics, following a standardized protocol formalized circa 2017 and the management of patients prior to 2017 followed similar conceptual framework [18]. Generally, at our center, infants with micrognathia who have tongue-based obstruction and moderate/severe obstructive sleep apnea (OSA) and absence of severe genetic syndromes are considered favorable MDO candidates whereas those with severe genetic syndromes, poor neurocognitive prognosis, or multi-level airway obstructions are unfavorable MDO candidates and tracheostomy is preferred [18, 19]. Patients with less severe OSA are generally managed non-surgically with prone positioning, continuous positive airway pressure or supplemental oxygen during sleep with close multidisciplinary follow-up and re-evaluation.
Patient demographics and clinical characteristics including gestational age, birthweight, sex, race/ethnicity, syndromic status, intubation status at birth, and source of admission were directly extracted from the electronic health record. In this study, treatment group assignment was based on the initial surgical treatment of MDO (n = 119) or tracheostomy (n = 25), and those without either MDO or tracheostomy were assigned as medical treatment (n = 74). Syndromic status was characterized by confirmed syndromic (with positive genetic tests), suspected syndromic (patients with clinical features suspected to be related to a syndrome but with negative or unknown genetic tests), and non-syndromic (including isolated Robin sequence). Feeding route (exclusively by mouth, nasogastric tube, gastrostomy tube, nothing by mouth) and respiratory status (room air without respiratory support, standard nasal cannula or high-flow nasal cannula, continuous positive airway pressure or non-invasive mechanical ventilation, invasive ventilation, tracheostomy) at admission, at discharge, and at last follow-up were determined from chart review. Weight trajectory at discharge was assessed using the difference in weight Z-score at discharge from the birthweight Z-score. Obstructive apnea-hypopnea index (OAHI) and oxyhemoglobin saturation (SpO2) nadir from polysomnography were extracted for analysis.
Categorical variables were summarized by frequencies and percentages, and compared by the Fisher’s exact test. Continuous variables were summarized by medians and interquartile ranges, and compared by the non-parametric Wilcoxon rank-sum test. The comparison of polysomnography data before and after MDO was conducted by the non-parametric Wilcoxon signed-rank test for paired samples. Multivariable logistic regression models adjusting for gestational age, birthweight, sex, race/ethnicity, and intubation status at birth, were used to evaluate the association of treatment group and syndromic status with feeding and respiratory status at discharge and at follow-up. Bonferroni correction applied to a significance level of 0.05 was used in each group of comparisons (patient characteristics, MDO characteristics, polysomnography, feeding and respiratory support, and other outcomes, separated by treatment group and syndromic status) to determine statistical significance (see each table for details). All analyses were performed by using SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Results
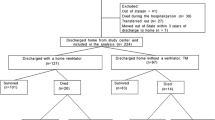
A total of 218 infants with micrognathia, including 119 (55%) who underwent MDO and 25 (12%) who underwent tracheostomy as the initial surgical treatment, were admitted to CHOP between 1/1/2010 and 2/28/2019. Table 1 describes the patient characteristics. The majority of the cohort were full-term, male, non-Hispanic white, suspected or confirmed syndromic, and not intubated at birth. There were 34 (16%) patients who were non-syndromic, 95 (44%) patients who were suspected syndromic (with negative/unknown genetic tests), and 89 (41%) who were confirmed syndromic with positive genetic tests. The majority of suspected or confirmed genetic syndromes were Stickler syndrome (41/184, 22%), followed by other chromosomal duplication/deletion syndromes (17/184, 9.2%), and then 22q11.2 deletion syndrome (11/184, 6.0%). Supplemental Table 1 shows the full list of genetic syndromes. The majority of patients were evaluated from birth hospitalization either from the Special Delivery Unit (37, 17%) or from outside hospital (135, 62%), while the remaining were readmitted from clinic (24, 11%) or emergency department (22, 10%). The median (interquartile range) age at admission was 4 (0, 24) days for the overall cohort. Patients admitted from the Special Delivery Unit were admitted earlier (as they are inborn), followed by outside hospital, emergency department, and clinic: 0 (0, 0) days vs. 4 (1, 16) days vs. 31 (13, 58) days vs. 40 (14, 105) days, p < 0.001. Patients with confirmed or suspected syndromes were admitted earlier than non-syndromic patients: 2 (0, 10) days vs. 5 (1, 25) days vs. 14 (5, 39) days, p = 0.004. Infants with micrognathia who underwent medical treatment were more likely to exclusively feed by mouth at admission (30%) compared to infants who would go on to have MDO (13%) or tracheostomy (8%), p = 0.008 (Table 1 and Fig. 1A). A larger proportion of patients who underwent medical treatment (51%) or MDO (47%) were in room air at admission compared to those received tracheostomy later (12%), p = 0.001 (Table 1 and Fig. 1D). There were no significant differences in the proportion of patients able to exclusively feed by mouth or in room air at admission based on syndromic status.
Clinical characteristics specific to MDO are described in Table 2. Age at MDO as the initial surgical treatment was 37 (17, 70) days and time from admission to surgery for the 119 patients who underwent MDO as the initial treatment was 13 (7, 25) days. Non-syndromic patients had a tendency of earlier attempt of extubation after MDO: 5 (3, 6) days compared to 7 (5, 7) days for patients with suspected syndromes and 7 (5, 8) days for patients with confirmed syndromes, p = 0.008 (however not significant after Bonferroni’s correction). There were no failed attempts from the initial extubation for non-syndromic patients; however, 7.1% of patients with suspected syndromes and 26% of patients with confirmed syndromes failed the initial extubation attempt, p = 0.004. A total of 8 (6.7%) patients who failed extubation subsequently had tracheostomy and another 2 (1.7%) patients who were not able to extubate received tracheostomy.
Polysomnography was conducted during neonatal hospitalization in 67% of patients who did not undergo MDO and also pre-operatively in 77% of patients who underwent MDO (Supplemental Table 2). Patients who underwent MDO had a significantly higher OAHI than patients who did not undergo MDO: 45.7 (18.8, 130.1) events/hour vs. 18.3 (7.5, 36.6) events/hour, (p < 0.001) and lower SpO2 nadir: 79% (63%, 85%) vs. 84% (79%, 89%), (p = 0.005). Of the 95 patients who underwent MDO and had a pre-operative diagnostic polysomnography, 74 (78%) also were evaluated with a post-MDO polysomnography. Post-MDO polysomnography showed significant decrease in OAHI: 4.6 (1.5, 11.7) events/hour vs. 45.7 (18.8, 130.1) events/hour pre-operatively, (p < 0.001) and increase in SpO2 nadir (84% [81%, 90%] vs. 79% [63%, 85%], p = 0.013).
Other short-term outcomes at discharge from the initial hospitalization are shown in Table 3. LOS was significantly longer in patients who underwent tracheostomy or MDO vs. those with medical treatment only: 129 (67, 178) vs. 39 (29, 81) vs. 17 (9, 29) days, (p < 0.001). Infants with micrognathia had suboptimal weight gain at discharge. Z-score difference between weight at discharge and birthweight for the overall cohort was (−1.05 [−1.57, −0.67]). 43% of the overall cohort failed hearing test at discharge with those underwent tracheostomy (76%) or those with confirmed syndromes (56%) having higher likelihood of failing hearing test.
Only 19% of the study cohort were able to feed exclusively orally at discharge with those who underwent tracheostomy all need tube feeding and there were no significant differences between MDO and medical treatment. (Table 3 and Fig. 1B). Overall 32% of the study cohort still required some level of respiratory support at discharge, and there were no significant differences between MDO and medical treatment (Table 3 and Fig. 1E). Higher proportion of patients with genetic syndromes needed respiratory support at discharge but not significant after Bonferroni’s correction (p = 0.010).
The median age at last follow-up for our cohort was 3.7 years. The overall mortality during the study period was 15/218 (6.9%). The proportion of patients able to feed exclusively by mouth and in room air without respiratory support at last follow-up prior to age of four years increased to 60% and 78%, respectively, without significant differences between MDO and medical treatment (Table 3 and Fig. 1C, F). Those who underwent tracheostomy had significantly the lowest rate of feeding exclusively by mouth (14%) or in room air (18%) at last follow-up, p < 0.001 (Table 3 and Fig. 1C). A higher proportion of patients with confirmed syndromes required tube feeding (57%) at last follow-up compared to patients with suspected syndromes (35%) or non-syndromic patients (13%), p < 0.001 (Table 3 and Fig. 1C). Patients with confirmed syndromes were also more likely to still require respiratory support (32%) at last follow-up compared to patients with suspected syndromes (20%) or non-syndromic patients (3.2%), p = 0.002 (Table 3 and Fig. 1F). The overall study cohort during the study period underwent the following procedures (Table 3): cleft palate repair (30%), gastrostomy tube (43%), tongue lip adhesion (6.0%), tracheostomy (16%), tonsillectomy and adenoidectomy (8.3%), and myringotomy/tubes (39%).
Multivariable logistic regression analyses for feeding and respiratory outcomes at follow-up are shown in Table 4. MDO was not associated with feeding or respiratory status at follow-up compared to medical treatment. Tracheostomy was significantly associated with lower odds of feeding exclusively by mouth (aOR: 0.09 [0.02–0.39]) as well as lower odds of room air without any support (aOR: 0.03 [0.01–0.12]) at follow-up. Patients with confirmed syndromes were less likely to feed exclusively by mouth at follow-up (aOR: 0.19 [0.06–0.63]) compared to non-syndromic patients. As a secondary analysis, adding cleft repair or admission source as a covariate in these multivariable models yielded similar results.
Discussion
This retrospective cohort study of 218 infants with micrognathia admitted between 1/1/2010 and 2/28/2019 for evaluation of micrognathia with median age at last follow-up of 3.7 years demonstrates a significant number of patients continued to require tube feeding and respiratory support at discharge and also throughout the first four years of life. Patients who underwent MDO during their neonatal hospitalization had significant improvement in obstructive sleep apnea following surgery. Although patients who needed MDO surgery generally had more severe obstructive sleep apnea compared to those underwent medical treatment only, after MDO they were equally likely to be in room air without respiratory support and feeding exclusively by mouth both at hospital discharge and at follow-up. Not surprisingly, the majority of those who required tracheostomy still needed tube feeding and respiratory support even at last follow-up close to four years of age. A large proportion of infants with micrognathia required gastrostomy (43%) and myringotomy/tubes (39%) during the study period. These findings suggest that infants with micrognathia require multidisciplinary support after neonatal hospitalization and patients selected to undergo MDO have favorable outcomes similar to those without surgical treatment despite suffering from more airway obstruction initially.
In our cohort, 81% of infants with micrognathia needed tube feeding at discharge and 41% at follow-up up to four years of age continued to require tube feeding. Overall, 43% of patients had gastrostomy placement. Similar to prior studies, syndromic patients were less likely to exclusively feed by mouth at discharge and at follow-up, and more likely to have gastrostomy [7, 10]. The exact proportion of patients requiring feeding tubes differs slightly likely due to patient selection and variation of practice from different institutions [13]. We found that infants with micrognathia had poor weight gain during their neonatal hospitalization, which was more pronounced in those undergoing MDO, similar to Dorise et al.’s study [20]. Other studies have shown that infants with micrognathia have initial poor weight gain and then improved weight gain following MDO [21,22,23,24], but may take 1-3 years for catch-up growth [11, 25]. Unfortunately, the longitudinal weight trajectory after discharge was not recorded in our study. These findings suggest that infants with micrognathia are at risk for poor growth. A large proportion of patients in our cohort required tube feedings following discharge, therefore attention to nutrition early as well as close outpatient follow-up for continued feeding therapy and nutrition monitoring is important for optimal growth.
Our patients who underwent MDO had significant improvement in polysomnography parameters including OAHI and SpO2 nadir, confirming the findings from prior studies in this larger cohort [12, 14]. Similar to prior studies [9, 26], we also showed infants with micrognathia are at risk of hearing loss (43% failed hearing at discharge), emphasizing the importance of audiology evaluation in infants with micrognathia and subsequent follow-up.
Management of patients with micrognathia at our institution involves multidisciplinary evaluation and treatment. We had formalized a protocol for general guidance and standardization of care that was launched circa 2017 (n = 78) [18], and the overall management of patients prior to 2017 also followed similar conceptual framework (n = 140). Our management decisions are similar to published treatment algorithms from other centers [27,28,29,30], with the exception that tongue-lip adhesions are not routinely considered at our center. Additional quality improvement studies evaluating protocol compliance and outcomes are warranted to provide optimal care.
There are several strengths and important implications from our study. Our cohort is one of the largest (218 patients) whereas the majority of prior studies on micrognathia have small number of patients mostly fewer than 50. Our cohort was from the recent decade with the majority of the operative group undergoing MDO, which is consistent with the current trend [6] thereby providing more updated information regarding current practice. Furthermore, we also followed our patients up to four years of age in this study allowing the assessment of long-term feeding and respiratory outcomes and the need for additional procedures. Our study suggests infants with micrognathia with or without surgical intervention require long-term multidisciplinary support as many of these patients still require tube feeding and respiratory support years after discharge.
Our study has some limitations. Our retrospective study is subject to selection bias where patients were evaluated and selected to undergo MDO vs. tracheostomy based on multidisciplinary expertise. We attempted to adjust the bias in multivariable logistic regressions to include baseline characteristics and syndromic status, however, our comparative findings should be viewed as associations rather than causal relationships. Nevertheless, the favorable outcomes after MDO in our study are consistent with prior studies. Future studies should focus on identifying ideal candidates for MDO and optimizing postoperative care such as pain management, timing of extubation, feeding and respiratory support, and multidisciplinary follow-up to further improve outcomes. Although our study is one of the largest single-center cohort of micrognathia, the sample size may not be powered enough for the multiple outcomes studied especially after adjustment for multiple comparisons. However, our results will provide insights for the design of future studies. There are many genetic syndromes associated with micrognathia as was observed in our study [2, 3], and sub-analysis of specific syndrome was not possible with the size of this single-center cohort, but should be the focus of future multi-center studies. The long-term impact of micrognathia and surgical intervention on dental [31, 32], speech [33,34,35,36,37], or neurodevelopmental outcomes [38, 39], was not captured in this retrospective cohort, but are important outcomes to be included in future clinical trials.
In conclusion, patients who underwent tracheostomy were associated with tube feeding and respiratory support at follow-up and patients who underwent MDO had similar favorable long-term feeding and respiratory outcomes compared to those needing only medical treatment. A large proportion of infants with micrognathia still required feeding assistance and/or respiratory support at follow-up up to four years. Improved understanding of potential trajectories of feeding and respiratory outcomes provided by this study can facilitate planning for multidisciplinary care and family counseling, with referral to appropriate support services.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Vettraino IM, Lee W, Bronsteen RA, Harper CE, Aughton D, Comstock CH. Clinical outcome of fetuses with sonographic diagnosis of isolated micrognathia. Obstet Gynecol. 2003;102:801–5.
Sanz-Cortés M, Gómez O, Puerto B 68 - Micrognathia and Retrognathia. In: Copel JA, D’Alton ME, Feltovich H, Gratacós E, Krakow D, Odibo AO et al. (eds). Obstetric Imaging: Fetal Diagnosis and Care (Second Edition). Elsevier, 2018, pp 321-327.e1.
Evans KN, Sie KC, Hopper RA, Glass RP, Hing AV, Cunningham ML. Robin sequence: from diagnosis to development of an effective management plan. Pediatrics. 2011;127:936–48.
Breik O, Tivey D, Umapathysivam K, Anderson P. Mandibular distraction osteogenesis for the management of upper airway obstruction in children with micrognathia: a systematic review. Int J Oral Maxillofac Surg. 2016;45:769–82.
Chocron Y, Barone N, Zammit D, Gilardino MS. Efficacy and complications of mandibular distraction osteogenesis for airway obstruction in the robin sequence population: a comprehensive literature review. J Craniofac Surg. 2022;33:1739.
Resnick CM, Rottgers SA, Wright JM, Vyas RM, Goldstein JA, Swanson JW, et al. Surgical outcome and treatment trends in 1289 infants with micrognathia: a multicenter cohort. Plast Reconstr Surg. 2024;154:155e–166e.
El Ghoul K, Calabrese CE, Koudstaal MJ, Resnick CM. A comparison of airway interventions and gastrostomy tube placement in infants with Robin sequence. Int J Oral Maxillofac Surg. 2020;49:734–8.
Khansa I, Hall C, Madhoun LL, Splaingard M, Baylis A, Kirschner RE, et al. Airway and feeding outcomes of mandibular distraction, tongue-lip adhesion, and conservative management in Pierre robin sequence: a prospective study. Plast Reconstr Surg. 2017;139:975e–983e.
Glynn F, Fitzgerald D, Earley MJ, Rowley H. Pierre Robin sequence: An institutional experience in the multidisciplinary management of airway, feeding and serous otitis media challenges. Int J Pediatr Otorhinolaryngol. 2011;75:1152–5.
McGhee H, Gehle D, Shope C, Wen CC, Marston AP, Discolo C, et al. Feeding performance and outcomes in infants with robin sequence undergoing mandibular distraction osteogenesis. Cleft Palate Craniofac J. 2024;61:295–301.
Harris JA, Caprio RM, Resnick CM. Do infants with robin sequence have improved feeding and weight gain after mandibular distraction? J Oral Maxillofac Surg. 2021;79:1331–8.
Kosyk MS, Carlson AR, Zapatero ZD, Kalmar CL, Liaquat S, Bartlett SP, et al. Multimodal treatment of robin sequence utilizing mandibular distraction osteogenesis and continuous positive airway pressure. Cleft Palate Craniofac J. 2023;60:993–1001.
Padula MA, Naing K, Wenger TL, Ahmad I, Coghill CH, Wild KT, et al. Spectrum of disease in hospitalized newborns with congenital micrognathia: a cohort of 3,236 infants at North American tertiary-care intensive care units. J Pediatr. 2024;265:113799.
Cielo CM, Taylor JA, Vossough A, Radcliffe J, Thomas A, Bradford R, et al. Evolution of obstructive sleep apnea in infants with cleft palate and micrognathia. J Clin Sleep Med. 2016;12:979–87.
Ehsan Z, Weaver KN, Pan BS, Huang G, Hossain MM, Simakajornboon N. Sleep outcomes in neonates with Pierre robin sequence undergoing external mandibular distraction: a longitudinal analysis. Plast Reconstruct Surg. 2020;146:1103.
da Costa AL, Manica D, Schweiger C, Kuhl G, Sekine L, Fagondes SC, et al. The effect of mandibular distraction osteogenesis on airway obstruction and polysomnographic parameters in children with Robin sequence. J Cranio-Maxillofac Surg. 2018;46:1343–7.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inf. 2019;95:103208.
The Children’s Hospital of Philadelphia Clinical Pathway for Evaluation/Treatment of the Neonate/Infant with Micrognathia/Retrognathia and Concern for Airway Obstruction. https://www.chop.edu/clinical-pathway/neonate-infant-micrognathia-retrognathia-and-concern-airway-obstruction-clinical-pathway (accessed 15 Aug2023).
Zhang RS, Lin LO, Hoppe IC, Bartlett SP, Taylor JA, Swanson JW. Risk factors for perioperative respiratory failure following mandibular distraction osteogenesis for micrognathia: a retrospective cohort study. Plast Reconstruct Surg. 2019;143:1725.
Dorise B, Trivedi A, Galea C, Walker K, Mehta B. Feeding practices and growth of infants with Pierre Robin Sequence. Int J Pediatr Otorhinolaryngol. 2019;118:11–14.
Gary CS, Marczewski S, Vitagliano PM, Sawh-Martinez R, Wu R, Steinbacher DM. A quantitative analysis of weight gain following mandibular distraction osteogenesis in robin sequence. J Craniofac Surg. 2018;29:676.
van Nunen DPF, Griot JPWD, Kruisinga F, Broers CJM, Breugem CC. A comparison of weight gain following mandibular distraction osteogenesis and tongue-lip adhesion in the treatment of robin sequence. Journal Craniofac Surgery. 2021;32:e578.
Harris JA, Hashim E, Larson K, Caprio RM, Gordon AM, Resnick CM. Early weight gain in infants with Robin sequence after mandibular distraction. Int J Oral Maxillofac Surg. 2022;51:1305–10.
Liu J, Sun J, Chen Y, Li F. Study on the effect of bilateral mandible distraction osteogenesis about the nutrition status of infants with pierre-robin sequence. Front. Pediatr. 2021;9:771333.
Khansa I, Aldabbeh S, Pearson GD, Baylis A, Madhoun LL, Schoenbrunner A, et al. Airway and feeding outcomes in Pierre robin sequence: a comparison of three management strategies. Cleft Palate Craniofac J. 2023;60:689–94.
Hamilton S, Dzioba A, Husein M. A retrospective study of patients with Robin sequence: Patient characteristics and their impact on clinical outcomes. Int J Pediatr Otorhinolaryngol. 2020;129:109769.
Paes EC, van Nunen DPF, Speleman L, Muradin MSM, Smarius B, Kon M, et al. A pragmatic approach to infants with Robin sequence: a retrospective cohort study and presence of a treatment algorithm. Clin Oral Investig. 2015;19:2101–14.
Dauria D, Marsh JL. Mandibular distraction osteogenesis for Pierre robin sequence: what percentage of neonates need it? J Craniofac Surg. 2008;19:1237.
Schaefer RB, Stadler JAI, Gosain AK. To distract or not to distract: an algorithm for airway management in isolated Pierre robin sequence. Plast Reconstr Surgery. 2004;113:1113–25.
Thimmappa B, Hopkins E, Schendel SA. Management of micrognathia. NeoReviews. 2009;10:e488–e493.
Adhikari AN, Heggie AAC, Shand JM, Bordbar P, Pellicano A, Kilpatrick N. Infant mandibular distraction for upper airway obstruction: a clinical audit. Plast Reconstr Surg Glob Open. 2016;4:e812.
Barrero CE, Ryan IA, Salinero L, McGraw JR, Pontell ME, Bartlett SP, et al. Radiographic evidence of dental complications following mandibular distraction osteogenesis: inverted-l versus oblique osteotomy. Plast Reconstr Surg. 2024;154:725e–36e.
Morzycki A, Budden C, Skulsky S, Cuglietta L, Guilfoyle R. Long term speech and feeding outcomes in patients with Pierre Robin sequence. J Craniofac Surg. 2022;33:475.
Logjes RJH, Mermans JF, Coerts MJ, Lissenberg-Witte BI, Breugem CC, Don Griot JPW. Long-term speech outcome in patients with Robin sequence after cleft palate repair and tongue-lip adhesion: A 21-year retrospective analysis. J Cranio-Maxillofac Surg. 2023;51:209–16.
Morice A, Renault F, Soupre V, Chapuis C, Trichet Zbinden C, Kadlub N, et al. Predictors of speech outcomes in children with Pierre Robin sequence. J Cranio-Maxillofac Surg. 2018;46:479–84.
Kosyk MS, Zapatero ZD, Kalmar CL, Carlson AR, Cohen M, Swanson JW, et al. Speech outcomes following mandibular distraction osteogenesis for robin sequence: midchildhood results. Plast Reconst Surg. 2023;151:149.
Schwaiger M, Cook H, Jordan Z, Edmondson S-J, Mischak I, Wallner J, et al. Robin sequence: 5-year speech outcomes—a case-control study. Plast Reconst Surg. 2021;147:676.
Thouvenin B, Djadi-Prat J, Chalouhi C, Pierrot S, Lyonnet S, Couly G, et al. Developmental outcome in Pierre Robin sequence: A longitudinal and prospective study of a consecutive series of severe phenotypes. Am J Med Genet Part A. 2013;161:312–9.
Naros A, Steiner-Wilke I, Kaiser N, Bacher M, Koos B, Blumenstock G, et al. Neurocognitive development in isolated Robin sequence treated with the Tuebingen palatal plate. Clin Oral Investig. 2022;26:4817–23.
Acknowledgements
We thank Matthew Devine for querying the electronic health record system for eligible patients and Emma Escobar for extracting polysomnography results.
Funding
This study was partially supported by Dr. Cielo’s K23 HL135346 grant and Divisional Grant from the Children’s Hospital of Philadelphia Divisions of Neonatology and Plastic, Reconstructive and Oral Surgery. Open access funding provided by SCELC, Statewide California Electronic Library Consortium.
Author information
Authors and Affiliations
Contributions
KL conceptualized the study design, acquired the data, analyzed the data, interpreted the data, and composed the initial draft of the manuscript. LW and KM acquired the data and revised the manuscript. JS, JT, JL, and CC conceptualized the study design, interpreted the data, and revised the manuscript. All authors approved the final draft of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interests.
Ethics
This study was approved by the Institutional Review Board of CHOP (IRB 17-014107) with a waiver of consent/parental permission per 45 CFR 46.116(d). This study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lai, KC., Walker, L.M., Moran, K. et al. Short-term and four-year feeding and respiratory outcomes of infants with micrognathia. J Perinatol 45, 1119–1128 (2025). https://doi.org/10.1038/s41372-025-02224-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-025-02224-1