Abstract
The outcome of patients with acute myeloid leukemia (AML) worsens with increasing age. Dichotomization into “younger” and “older” patients is clinically routine and often dictates treatment options. We aimed to delineate whether molecular genetic features and/or outcome measures support assorting patient populations by age, including division into “younger” and “older” groups. We analyzed 2823 adult AML patients enrolled onto frontline chemotherapy-based clinical protocols of two cooperative study groups from USA and Germany who were profiled molecularly via targeted sequencing platforms. Frequencies of gene mutations and cytogenetic findings were depicted in 5-year age increments. Clinical outcomes of 2756 AML patients were analyzed with respect to molecular features, genetic-risk groups and age. Age-associated distributions of gene mutations and cytogenetic abnormalities were similar in both cohorts. There was almost linear shortening of overall survival with increasing age among all patients (P < 0.001) and within 2022 European LeukemiaNet-defined genetic-risk groups, with survival decreasing as age increased (favorable-risk, P < 0.001; intermediate-risk, P < 0.001; adverse-risk, P < 0.001). Although mutational profiles and outcomes of the youngest patients differed from those of older patients, there was no age cut-off identifying “younger” and “older” patients. These findings support more age-associated flexibility for drug approval and trial eligibility.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) is a disease affecting predominantly older patients, but it does occur across the entire age spectrum. Patient outcomes strongly correlate with patient-associated factors such as age, race and performance status as well as disease-associated factors such as AML-associated cytogenetic [1,2,3] and molecular genetic abnormalities [4,5,6,7]. The contribution of increasing age to worsening survival has been well established [8, 9] and, as a consequence, age currently represents a major factor in the consideration of treatment options [10], including curative intensive chemotherapy regimens, eligibility for many clinical trials and has even found a place on the United States (US) Food and Drug Administration (FDA) label for one targeted therapy [11]. Notably, this aged-based distinction derives from the observation that AML in the elderly is a distinct clinical entity compared with the AML in younger patients, as well as the assumption of concurrent decreasing fitness and increasing comorbidities, an assumption that has rightly been challenged with the rise of more comprehensive and objective geriatric assessments [4, 10, 12,13,14,15].
However, our understanding of the underlying disease biology and driver mutations has tremendously improved and at last translated into both novel targeted therapies and effective combinations outside of intensive induction regimens [16,17,18,19]. Thus, in the current era of expedient genomic classification [20,21,22], the question of relevance of age alone in AML - rather than the presence or absence of targetable molecular features or genetic risk groups - is again being challenged [5, 6, 23,24,25,26,27,28,29,30,31,32]. Age as a major criterion for inclusion (or exclusion) from clinical trials could preclude patients from getting an effective therapy, and could bias and impede our understanding of the biology of disease as well as responses to therapy [33]. Distinct age cut-offs between the different study cohorts can confound interpretation of the results and could result in loss of valuable information.
In the present work, we performed a multi-dimensional analysis of mutational patterns and survival correlations through 5-year age intervals from 18 to 92 years, using two large datasets of de novo AML patients from Germany and USA. The aims of this study were: 1) to perform an unbiased characterization of the molecular landscape across the age spectrum of adult AML, 2) to analyze the survival of adult AML patients receiving similar, frontline chemotherapy on clinical trials stratified by age groups, and 3) to evaluate the rationale for age cuts used in the characterization and treatment guidance of AML via integration of molecular, clinical and survival parameters when traditional chemotherapy is utilized.
Methods
Patients and treatment
Our combined patient cohort comprised 2823 patients diagnosed with AML (other than acute promyelocytic leukemia) who were treated in the setting of frontline treatment protocols of two large cooperative groups, including 1743 patients from the Cancer and Leukemia Group B (CALGB) enrolled between 1986 and 2016 [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48], and 1080 patients enrolled on protocols of the AML Cooperative Group (AMLCG) between 1999 and 2017 [8, 49, 50] (for details see Supplementary Information). CALGB is now part of Alliance for Clinical Trials in Oncology (Alliance). Treatment of all patients included in outcome analyses included intensive cytarabine-based induction therapy. Performance of allogeneic hematopoietic stem-cell transplantation (HSCT), on or off protocol, was considered as independent variable. The treatment regimens are described in the Supplementary Information. The CONSORT diagram with the patient inclusion/exclusion criteria in this study is shown in Fig. 1. For subsequent outcome analyses, patients who received incomplete or inadequate treatment were excluded, resulting in a total of 2756 patients included in the outcome analyses (CALGB/Alliance, n = 1698; AMLCG, n = 1058; Fig. 1).
Cytogenetic and molecular analyses
Cytogenetic analyses of pretreatment bone marrow and/or blood samples subjected to short-term (24- or 48-h) unstimulated cultures were performed by the CALGB/Alliance- and AMLCG-approved institutional laboratories, and the results were confirmed by central karyotype review [51] (Supplementary Information).
Patients in both cohorts were profiled for molecular features via targeted sequencing platforms. The respective US and German targeted molecular panels contained 24 shared AML-associated genes. We used a variant allele frequency (VAF) cut-off ≥2%, which represents the lower limit of detection for most clinically used next generation sequencing assays, and as previously reported by our groups [5, 27]. In the analysis of mutation frequency in the entire cohort, we focused on those mutations that occurred in at least 4% of patients [5, 27, 52]. Frequency determination of selected gene mutations and cytogenetic findings in both datasets was done in age groups, first comprising patients aged 18–24 years and then by 5-year intervals until the age of 74 years and finally for patients aged 75 years or older (range, 75–92).
Statistical analyses
Definitions of clinical endpoints are provided in the Supplementary Information. Early death (ED) is defined as death within 30 days after protocol enrollment. Estimated probabilities of OS were calculated using the Kaplan-Meier method. Hazard ratios (including 95% confidence intervals [CIs]) were estimated from Cox proportional hazard models. Analyses were performed by the Alliance Statistics and Data Management Center on a database locked on February 11, 2021, using SAS 9·4, TIBCO Spotfire S + 8·2 and GraphPad Prism version 10.
Results
Clinical characteristics of AML patients
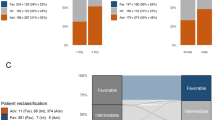
The median age was 55 years (range, 18–92 years), with 45% of patients being female. Thirty-four percent of patients belonged to the 2022 European LeukemiaNet (ELN) favorable genetic-risk group, and 27% and 39% were classified as having intermediate or adverse genetic risk, respectively. Seventy-nine percent of patients were either fully active or ambulatory (ECOG 0-1) with respect to their performance status at time of diagnosis. Twenty-six percent of patients received an allogeneic HSCT in first CR (Table 1). Pretreatment characteristics of patients in the US and German cohorts are provided in Supplementary Table 1. The median follow-up for patients who are alive is 8.2 years.
AML-related gene mutations follow different patterns over time
We analyzed the genetic alterations across the age spectrum of adult patients with AML (from 18 to 75+ years) and found age-associated frequency patterns of gene mutations and recurrent cytogenetic abnormalities. The first group comprised alterations with non-linear age-frequency distribution, which included three most common AML-associated gene mutations, that is, mutations in the NPM1 and DNMT3A genes and FLT3 internal tandem duplication (FLT3-ITD), FLT3 tyrosine kinase domain mutations (FLT3-TKD), NRAS and EZH2 mutations and mutations affecting the cohesin complex genes (RAD21, SMC1A, SMC3, SF3B1, STAG2). The second group consisted of genetic alterations whose frequency increased with age, namely mutations in the ASXL1, BCOR, IDH1, IDH2, RUNX1, SRSF2, TET2 and TP53 genes and both complex and normal karyotypes. The third group included alterations whose frequency decreased with increased age, such as mutations in the CEBPA, GATA2, KIT, KRAS, PTPN11 and WT1 genes, and core-binding factor balanced rearrangements [i.e., t(8;21)(q22;q22) and inv(16)(p13.1q22)/t(16;16)(p13.1;q22)] and rearrangements involving band 11q23 and the KMT2A (formerly MLL) gene (Fig. 2A, Supplementary Tables 2–4). These patterns were strikingly similar between the US and German cohorts (Fig. 2A). Notably, we could not identify a distinct age cut-off that could delineate a specific age at which the patterns of genetic alterations would noticeably change consistently across different mutations. This was also true for previously defined functional groups [4] (Fig. 2B, Supplementary Table 5).
A Heatmap showing the frequency of the common mutations in 5-year age intervals. Three groups with different patterns of age-related occurrence of genetic alterations are indicated by color: green, mutations with non-linear age-frequency distribution; red, genetic alterations whose frequency increased with increased age; and blue, alterations whose frequency decreased with increased age. The arrows indicate direction of changes with age for these three patterns. B Area plots showing the frequencies of mutations grouped by their biological category (as previously described by Cancer Genome Atlas Research Network [4]) in age intervals.
OS diminishes with increasing age in adult patients with AML
The 5-year OS rate of all patients in our study was 40%, and there was a positive correlation between shortening of OS and increasing age (Fig. 3A). This age-associated worsening of OS was consistent across 2022 ELN genetic-risk groups. In the 2022 ELN favorable genetic-risk patients, the estimated proportion of patients alive at 5 years ranged from 21% for those aged 75 years or older to 73% for the youngest patients (18–24 years old; Fig. 3B). Similarly, 2022 ELN intermediate genetic-risk patients followed this survival pattern, with 5-year OS rate of 4% in patients aged ≥75 years compared with 53% in those aged 18–24 years (Fig. 3C). In 2022 ELN adverse genetic-risk patients, we found, expectedly, less variability, with the lowest 5-year OS rate of 2% in patients aged 70–74 years compared with the highest 5-year OS rate of 37% in patients aged 25–29 years (Fig. 3D). Thus, although application of the 2022 ELN criteria allows risk stratification of patients with AML, age itself is also an important factor with regard to determining OS within each 2022 ELN genetic-risk group. Consistent with the aforementioned results, we found no specific age that would serve as a cut-off point to identify patients with better and those with worse OS within each of the 2022 ELN genetic-risk group, further supporting age as a continuum in AML for both biology and risk stratification.
Concerning the CR and ED rates in age intervals, we observed a trend in CR rates decreasing with age and a trend in ED rates increasing with age, although again without a clear age-dependent delineator (Table 2). Of note, all results mentioned above were comparable in the US and German cohorts when each cohort was considered separately (data not shown).
Age-dependent outcome varies along mutational subgroups
We then assessed the impact of age on outcome of select groups of patients harboring specific, recurring, AML-associated gene mutations, with focus on mutations for which approved targeted inhibitors exist, including mutations in the IDH1 and IDH2 genes, FLT3-ITD and FLT3-TKD. Of note, for these targeted inhibitors, distinct age cut-offs were introduced in the trials testing them and thus led to questions about the generalizability of results in subsequent clinical practice [7, 41, 53].
In these molecularly defined subgroups, we examined patient survival and found that age in fact has a negative prognostic impact within the IDH1 and IDH2 mutations groups and in both FLT3-ITD and FLT3-TKD (Fig. 4A–D). To further investigate the relevance of age on outcome for individual mutations, we calculated the hazard ratio of age in the mutational subgroups, considering age as a continuous variable. In this analysis, increasing age associated with inferior survival in a significant way in all prognostically relevant AML-associated gene mutations and chromosome rearrangements we analyzed, with the exception of rearrangements involving 11q23/KMT2A other than t(9;11)(p22;q23) (Fig. 5). The median age of patients with each mutation did not correlate with the magnitude of the effect that age had on risk of death, which is to say that within each individual mutation grouping, it did not matter whether a mutation was more common in younger or older patients; the spectrum of age remains an important factor in each case. In conclusion, association of age with prognosis can only in part be attributed to certain gene distributions and the reasons for age-dependent prognosis within certain gene groups remain elusive.
Discussion
To our knowledge, this is one of the first large scale cross-continent depictions of mutational patterns and outcome in AML inclusive of the entire adult age spectrum. We characterize different age-associated molecular distribution patterns that do not provide support for a molecular basis of a singular age that would justify a younger versus older age categorization. Importantly, it was not our scientific objective to define precise cut points among age groups, but instead we aimed to depict molecular patterns across ages, and test whether associated survival patterns may outweigh any age-restricted definitions.
While this study sought to challenge the relevance of chronologic age in AML, we have in fact shown that age provides further risk stratification to patients already classified by 2022 ELN in an almost continuous fashion, with substantive differences in OS between the youngest and oldest patients belonging to the same 2022 ELN genetic-risk group. The age-associated survival was less pronounced in the adverse risk group, which is not surprising given that overall outcomes of patients in this group are poor, suggesting that the weight of adverse risk disease features supersedes the importance of age-related factors in this risk group.
When we examined the prognostic impact of prognostically relevant AML-associated gene mutations and recurrent chromosome abnormalities with respect to age, we found that the established negative survival association of most of these alterations worsened with increasing age (Fig. 5). Similarly, some genetic abnormalities associated with favorable outcome such as NPM1 mutations without FLT3-ITD and inv(16) also tended to lose their favorable influence as patients aged. In contrast, the poor prognostic impact of 11q23/KMT2A rearrangements other than t(9;11) was independent of age; but will require additional validation due to the relatively small sample size. Surprisingly, this was not the case for other known adverse risk subtypes, such as TP53, complex karyotype or FLT3-ITD mutation groups, although they had relatively lower hazard ratios. This provides a rationale for individualized risk- and associated treatment-eligibility assessments that take both age and molecular features into account.
This study is limited to patients who met eligibility requirements for clinical trials, which means that subsets of real-world patients with more severe organ dysfunction, uncontrolled infections and concurrent malignancies are not included in our study. Additionally, owing to a relatively small sample size of Hispanic or Black patients, caution should be applied when considering how our findings might relate to these patient groups, and underscores the importance of diversity and inclusivity considerations for all future trial design and enrollment. Similarly, while an analysis of the contribution of allogeneic stem cell transplant to outcomes by age and ELN risk group would be ideal, lack of complete data for all patients (concurrent performance status, co-morbidity index) and differences between US and German application of transplant in consolidation preclude an unbiased look at the specific impact of transplant, and this needs to be carefully evaluated in future work.
In conclusion, choosing a precise age cut-off such as age 39 years for separating adolescents and young adult patients from older adults, or 59 years for identifying “younger” and “older” AML patients, does not seem to be supported by the results of our analyses. While our intention was to assess whether defined age cut-offs are supported by patterns of genetic alterations, our data tend to refute the existence of any uniform cut-offs across the age spectrum, whether considering the younger adults population aged 18–39 years, or the distinction between “younger and older” AML patients with age greater than 55 or 60 years defining older patients with AML.
Previous reports have already described age-associated differences in mutation frequencies [5, 6, 52, 54]. However, these observations have been limited by the comparison between only two pre-defined age groups, such as <60 versus as ≥60 years, or children/adolescents versus adults. In our study, we did analyze age using 5-year intervals, and this allowed us to observe different age patterns for different mutations. The shortcomings associated with the use of age as a dichotomized variable for therapy decisions or inclusion in clinical trials are also supported by several reports that revealed strong age disparities between clinical trials and “real” world data [33, 55, 56]. This is exemplified by 18% of AML patients <60 years harboring either IDH1 or IDH2 mutations, which would qualify them for the use of a targeted inhibitor but who might be excluded from receiving this therapy given their younger age.
The last few years have seen the exciting translation of biologic drivers in specific subsets of AML into targets of inhibitors that have demonstrated single agent activity in relapsed and refractory disease, as well as increased survival when used in combination with standard therapies [41, 57]. It is hoped that in the future, the number of patients who are treated with these targeted therapies and have a longer follow-up time will increase sufficiently to enable performing studies similar to the one we report here, including analyses of age influence on patient outcomes within the 2022 ELN genetic-risk groups and among patients harboring specific, prognostically relevant driver mutations and/or cytogenetic abnormalities. This will allow determination of whether our conclusions hold-up in patient populations treated differently from the one we studied, that is patients receiving intensive chemotherapy only. Moreover, integrated algorithms based on age, mutational profiles and performance status will likely be developed that would aid in making individual therapy decisions. Together with increased openness adopted by all clinical trials, this will give the chance to all patients to get the best available therapy tailored to their individual characteristics.
Data availability
Patient data used in survival analyses were obtained from the Alliance Statistics and Data Management Center and the AMLCG database. Individual participant data will not be shared. Legal restrictions prohibit us from publicly sharing raw sequencing data, which, however, could be made available upon reasonable request and permission of the local ethics committee.
References
Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100:4325–36.
Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–65.
Mrózek K. Molecular cytogenetics in acute myeloid leukemia in adult patients: practical implications. Pol Arch Intern Med. 2022;132:16300.
Cancer Genome Atlas Research Network, Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–74.
Metzeler KH, Herold T, Rothenberg-Thurley M, Amler S, Sauerland MC, Görlich D, et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood. 2016;128:686–98.
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–21.
Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77.
Büchner T, Berdel WE, Haferlach C, Haferlach T, Schnittger S, Müller-Tidow C, et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: a study by the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol. 2009;27:61–9.
Bloomfield CD, Lawrence D, Byrd JC, Carroll A, Pettenati MJ, Tantravahi R, et al. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 1998;58:4173–9.
Abdallah M, Xie Z, Ready A, Manogna D, Mendler JH, Loh KP. Management of acute myeloid leukemia (AML) in older patients. Curr Oncol Rep. 2020;22:103.
Montesinos P, Recher C, Vives S, Zarzycka E, Wang J, Bertani G, et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med. 2022;386:1519–31.
Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–5.
Scheepers ERM, Vondeling AM, Thielen N, van der Griend R, Stauder R, Hamaker ME. Geriatric assessment in older patients with a hematologic malignancy: a systematic review. Haematologica. 2020;105:1484–93.
Min G-J, Cho B-S, Park S-S, Park S, Jeon Y-W, Shin S-H, et al. Geriatric assessment predicts nonfatal toxicities and survival for intensively treated older adults with AML. Blood. 2022;139:1646–58.
Klepin HD, Geiger AM, Tooze JA, Kritchevsky SB, Williamson JD, Ellis LR, et al. The feasibility of inpatient geriatric assessment for older adults receiving induction chemotherapy for acute myelogenous leukemia. J Am Geriatr Soc. 2011;59:1837–46.
Chan SM, Thomas D, Corces-Zimmerman MR, Xavy S, Rastogi S, Hong WJ, et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat Med. 2015;21:178–84.
Levine RL. Molecular pathogenesis of AML: translating insights to the clinic. Best Pr Res Clin Haematol. 2013;26:245–8.
Sasine JP, Schiller GJ. Emerging strategies for high-risk and relapsed/refractory acute myeloid leukemia: novel agents and approaches currently in clinical trials. Blood Rev. 2015;29:1–9.
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17.
Duncavage EJ, Schroeder MC, O’Laughlin M, Wilson R, MacMillan S, Bohannon A, et al. Genome sequencing as an alternative to cytogenetic analysis in myeloid cancers. N Engl J Med. 2021;384:924–35.
Sekeres MA, Guyatt G, Abel G, Alibhai S, Altman JK, Buckstein R, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020;4:3528–49.
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–19.
Liersch R, Müller-Tidow C, Berdel WE, Krug U. Prognostic factors for acute myeloid leukaemia in adults-biological significance and clinical use. Br J Haematol. 2014;165:17–38.
Saad M, Loh KP, Tooze JA, Pardee TS, Ellis LR, Powell BL, et al. Geriatric assessment and survival among older adults receiving postremission therapy for acute myeloid leukemia. Blood. 2020;136:2715–9.
Eisfeld A-K, Kohlschmidt J, Mrózek K, Blachly JS, Walker CJ, Nicolet D, et al. Mutation patterns identify adult patients with de novo acute myeloid leukemia aged 60 years or older who respond favorably to standard chemotherapy: an analysis of Alliance studies. Leukemia. 2018;32:1338–48.
Eisfeld AK, Kohlschmidt J, Mims A, Nicolet D, Walker CJ, Blachly JS, et al. Additional gene mutations may refine the 2017 European LeukemiaNet classification in adult patients with de novo acute myeloid leukemia aged <60 years. Leukemia. 2020;34:3215–27.
Herold T, Rothenberg-Thurley M, Grunwald VV, Janke H, Goerlich D, Sauerland MC, et al. Validation and refinement of the revised 2017 European LeukemiaNet genetic risk stratification of acute myeloid leukemia. Leukemia. 2020;34:3161–72.
Wetzler M, Mrózek K, Kohlschmidt J, Dombret H, Döhner H, Pilorge S, et al. Intensive induction is effective in selected octogenarian acute myeloid leukemia patients: prognostic significance of karyotype and selected molecular markers used in the European LeukemiaNet classification. Haematologica. 2014;99:308–13.
Prassek VV, Rothenberg-Thurley M, Sauerland MC, Herold T, Janke H, Ksienzyk B, et al. Genetics of acute myeloid leukemia in the elderly: mutation spectrum and clinical impact in intensively treated patients aged 75 years or older. Haematologica. 2018;103:1853–61.
Greif PA, Konstandin NP, Metzeler KH, Herold T, Pasalic Z, Ksienzyk B, et al. RUNX1 mutations in cytogenetically normal acute myeloid leukemia are associated with a poor prognosis and up-regulation of lymphoid genes. Haematologica. 2012;97:1909–15.
Mendler JH, Maharry K, Radmacher MD, Mrózek K, Becker H, Metzeler KH, et al. RUNX1 mutations are associated with poor outcome in younger and older patients with cytogenetically normal acute myeloid leukemia and with distinct gene and microRNA expression signatures. J Clin Oncol. 2012;30:3109–18.
Tsui SP, Ip HW, Saw NY, Zhang C, Cheung AK, Ng NK, et al. Redefining prognostication of de novo cytogenetically normal acute myeloid leukemia in young adults. Blood Cancer J. 2020;10:104.
Habr D, McRoy L, Papadimitrakopoulou VA. Age is just a number: considerations for older adults in cancer clinical trials. J Natl Cancer Inst. 2021;113:1460–4.
Kolitz JE, George SL, Marcucci G, Vij R, Powell BL, Allen SL, et al. P-glycoprotein inhibition using valspodar (PSC-833) does not improve outcomes for patients under age 60 years with newly diagnosed acute myeloid leukemia: Cancer and Leukemia Group B study 19808. Blood. 2010;116:1413–21.
Blum W, Sanford BL, Klisovic R, DeAngelo DJ, Uy G, Powell BL, et al. Maintenance therapy with decitabine in younger adults with acute myeloid leukemia in first remission: a phase 2 Cancer and Leukemia Group B study (CALGB 10503). Leukemia. 2017;31:34–9.
Baer MR, George SL, Caligiuri MA, Sanford BL, Bothun SM, Mrózek K, et al. Low-dose interleukin-2 immunotherapy does not improve outcome of patients age 60 years and older with acute myeloid leukemia in first complete remission: Cancer and Leukemia Group B study 9720. J Clin Oncol. 2008;26:4934–9.
Kolitz JE, George SL, Dodge RK, Hurd DD, Powell BL, Allen SL, et al. Dose escalation studies of cytarabine, daunorubicin, and etoposide with and without multidrug resistance modulation with PSC-833 in untreated adults with acute myeloid leukemia younger than 60 years: final induction results of Cancer and Leukemia Group B study 9621. J Clin Oncol. 2004;22:4290–301.
Walker AR, Marcucci G, Yin J, Blum W, Stock W, Kohlschmidt J, et al. Phase 3 randomized trial of chemotherapy with or without oblimersen in older AML patients: CALGB 10201 (Alliance). Blood Adv. 2021;5:2775–87.
Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med. 1994;331:896–903.
Moore JO, George SL, Dodge RK, Amrein PC, Powell BL, Kolitz JE, et al. Sequential multiagent chemotherapy is not superior to high-dose cytarabine alone as postremission intensification therapy for acute myeloid leukemia in adults under 60 years of age: Cancer and Leukemia Group B study 9222. Blood. 2005;105:3420–7.
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377:454–64.
Roboz GJ, Mandrekar SJ, Desai P, Laumann K, Walker AR, Wang ES, et al. A randomized trial of 10 days of decitabine alone or with bortezomib in previously untreated older patients with acute myeloid leukemia: CALGB 11002 (Alliance). Blood Adv. 2018;2:3608–17.
Attar EC, Johnson JL, Amrein PC, Lozanski G, Wadleigh M, DeAngelo DJ, et al. Bortezomib added to daunorubicin and cytarabine during induction therapy and to intermediate-dose cytarabine for consolidation in patients with previously untreated acute myeloid leukemia age 60 to 75 years: CALGB (Alliance) study 10502. J Clin Oncol. 2013;31:923–9.
Stone RM, Berg DT, George SL, Dodge RK, Paciucci PA, Schulman P, et al. Granulocyte-macrophage colony-stimulating factor after initial chemotherapy for elderly patients with primary acute myelogenous leukemia. N Engl J Med. 1995;332:1671–7.
Lee EJ, George SL, Caligiuri M, Szatrowski TP, Powell BL, Lemke S, et al. Parallel phase I studies of daunorubicin given with cytarabine and etoposide with or without the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age or older with acute myeloid leukemia: Results of Cancer and Leukemia Group B study 9420. J Clin Oncol. 1999;17:2831–9.
Uy GL, Mandrekar SJ, Laumann K, Marcucci G, Zhao W, Levis MJ, et al. A phase 2 study incorporating sorafenib into the chemotherapy for older adults with FLT3-mutated acute myeloid leukemia: CALGB 11001. Blood Adv. 2017;1:331–40.
Moore JO, Dodge RK, Amrein PC, Kolitz J, Lee EJ, Powell B, et al. Granulocyte-colony stimulating factor (filgrastim) accelerates granulocyte recovery after intensive postremission chemotherapy for acute myeloid leukemia with aziridinyl benzoquinone and mitoxantrone: Cancer and Leukemia Group B study 9022. Blood. 1997;89:780–8.
Schiffer CA, Davis RB, Schulman P, Cooper B, Coyle T, Lee E, et al. Intensive post remission therapy of acute myeloid leukemia (AML) with cytoxan/etoposide (CY/VP16) and diazaquone/mitoxantrone (AZQ/MITO). Blood. 1991;78:460.
Büchner T, Schlenk RF, Schaich M, Döhner K, Krahl R, Krauter J, et al. Acute Myeloid Leukemia (AML): different treatment strategies versus a common standard arm- combined prospective analysis by the German AML Intergroup. J Clin Oncol. 2012;30:3604–10.
Braess J, Amler S, Kreuzer K-A, Spiekermann K, Lindemann HW, Lengfelder E, et al. Sequential high-dose cytarabine and mitoxantrone (S-HAM) versus standard double induction in acute myeloid leukemia-a phase 3 study. Leukemia. 2018;32:2558–71.
Mrózek K, Carroll AJ, Maharry K, Rao KW, Patil SR, Pettenati MJ, et al. Central review of cytogenetics is necessary for cooperative group correlative and clinical studies of adult acute leukemia: the Cancer and Leukemia Group B experience. Int J Oncol. 2008;33:239–44.
Eisfeld A-K, Mrózek K, Kohlschmidt J, Nicolet D, Orwick S, Walker CJ, et al. The mutational oncoprint of recurrent cytogenetic abnormalities in adult patients with de novo acute myeloid leukemia. Leukemia. 2017;31:2211–8.
Silva P, Neumann M, Schroeder MP, Vosberg S, Schlee C, Isaakidis K, et al. Acute myeloid leukemia in the elderly is characterized by a distinct genetic and epigenetic landscape. Leukemia. 2017;31:1640–4.
Marcucci G, Maharry K, Wu Y-Z, Radmacher MD, Mrózek K, Margeson D, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:2348–55.
Ludmir EB, Subbiah IM, Mainwaring W, Miller AB, Lin TA, Jethanandani A, et al. Decreasing incidence of upper age restriction enrollment criteria among cancer clinical trials. J Geriatr Oncol. 2020;11:451–4.
Ludmir EB, Mainwaring W, Lin TA, Miller AB, Jethanandani A, Espinoza AF, et al. Factors associated with age disparities among cancer clinical trial participants. JAMA Oncol. 2019;5:1769–73.
DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378:2386–98.
Acknowledgements
We thank the patients who participated in clinical trials and their families supporting them, and the recruiting centers. This work is dedicated to the memory and work of our mentors Thomas Büchner and Clara D. Bloomfield. We thank Christopher Manring and the CALGB/Alliance Leukemia Tissue Bank at The Ohio State University Comprehensive Cancer Center, Columbus, OH, for sample processing and storage services and Lisa J. Sterling for data management. This work was supported by funding from the Deutsche José Carreras Leukämie-Stiftung to TH (DJCLS 10R/2021), R01 CA262496, R01CA284595-01, R01CA283574-01, R01 LM013879, Leukemia & Lymphoma Society and the American Cancer Society. ClinicalTrials.gov Identifiers: NCT00266136, NCT01382147. Support to Alliance for Clinical Trials in Oncology and Alliance Foundation Trials programs is listed at https://acknowledgments.alliancefound.org. Trial Registration Numbers: NCT00048958 (CALGB 8461), NCT00899223 (CALGB 9665), and NCT00900224 (CALGB 20202). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
Research reported in this publication was supported in part by Deutsche José Carreras Leukämie-Stiftung to TH (DJCLS 10 R/2021); Bavarian Cancer Research Center (BZKF) Translational Group, start-up funding 2023 to PAG (TLG/20/LMU/Greif). Research reported in this publication was supported by the resources of the Pelotonia Institute for Immuno-Oncology, an allocation of computing resources from The Ohio Supercomputer Center and Shared Resources (Leukemia Tissue Bank). Research reported in this publication was supported in part by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821, U10CA180882, and U24CA196171 (to the Alliance for Clinical Trials in Oncology), UG1CA189824, UG1CA233338, UG1CA233331, and 5P30CA016058; R01CA262496 (A-KE, ASM), R01CA284595-01, R01CA283574-01 (A-KE), R01LM013879, the Coleman Leukemia Research Foundation; Leukemia & Lymphoma Society (A-KE), the American Cancer Society (A-KE). Support to Alliance for Clinical Trials in Oncology and Alliance Foundation Trials programs is listed at http://acknowledgments.alliancefound.org. The funders had no role in this manuscript’s design, data collection and analysis, decision to publish or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
TH, A-KE, MC, KL, DN, VJ, KM, and KHM designed the study; TH, A-KE, MC, KL, DN, KM, VJ, AMNB, CS, DG, UK, WEB, BJW, JSB, JB, CJW, MCW, CCO, SO, and KHM contributed to the data analysis and interpretation; DN, VJ and MCW performed the statistical analysis; TH, A-KE, MC, KL, DN, VJ, and KM wrote the manuscript; TH, A-KE, MR-T, SS, DG, KM, UK, WEB, WH, KS, PAG, ASM, AJC, WGB, BLP, JEK, JOM, RJM, RAL, RMS, and JCB were involved directly or indirectly in the care of patients and/or sample procurement. All authors read and agreed on the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were conducted in accordance with relevant guidelines and regulations. All study protocols were approved by the Institutional Review Boards of participating centers in accordance with the Declaration of Helsinki. All patients provided written informed consent for inclusion on the clinical trial and genetic analyses. Cancer Therapy Evaluation Program approval was obtained on September 13, 2024. Additional details are provided in the Supplementary Information.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cusan, M., Larkin, K., Nicolet, D. et al. Multi-dimensional analysis of adult acute myeloid leukemia cross-continents reveals age-associated trends in mutational landscape and treatment outcomes (Acute Myeloid Leukemia Cooperative Group & Alliance for Clinical Trials in Oncology). Leukemia 39, 2926–2934 (2025). https://doi.org/10.1038/s41375-025-02644-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41375-025-02644-0