Abstract
The acquired JAK2-V617F mutation plays a causal role in myeloproliferative neoplasms (MPN). Weakly activating JAK2 germline variants have been associated with MPN risk, but the underlying mechanisms remain unclear. We previously identified the JAK2-R1063H germline variant, which contributes to hereditary MPN and increased disease severity in essential thrombocythemia. Here, we studied alterations in hematopoiesis in Jak2-R1063H knock-in mice. The Jak2-R1063H mouse cohort exhibited increased mortality, stimulated thrombopoiesis and elevated D-dimers levels, indicative of thrombotic complications. Bone marrow analysis revealed myeloid bias, enhanced megakaryopoiesis and activation of inflammatory signaling. Transcriptional and functional assays of hematopoietic stem cells suggested their accelerated aging and functional decline. The Egr1 transcriptional network, including the Thbs1 gene, progressively increased in aging mice, reinforcing alterations initiated by Jak2/Stat signaling. In murine acute myelogenous leukemia models, the Jak2-R1063H cooperated with a driver oncogene in promoting leukemogenesis. Germline JAK2-R1063H was found in 10 of 200 MPN patients from local hematology centers, with a higher minor allele frequency compared to healthy controls. Patients harboring JAK2-R1063H variant exhibited an increased incidence of thrombotic complications and disease progression with shortened survival. In conclusion, our findings identify the JAK2-R1063H germline variant as a risk factor for MPN development, thrombotic complications, and leukemic transformation.

Our study, which involves a mouse model and a cohort of 200 MPN patients, characterizes the JAK2-R1063H germline mutation as a risk factor for MPN development, thrombotic complications, and leukemic transformation. These findings may have important clinical implications for managing MPN patients carrying the JAK2-R1063H germline variant.
Similar content being viewed by others
Introduction
The Janus kinase 2 (JAK2) gene encodes for a tyrosine kinase responsible for mediating a critically important signaling in immune and hematopoietic cells [1]. The most frequently occurring gain-of-function JAK2 mutation, V617F, gives rise to a constitutively active JAK2 kinase, which drives the JAK/STAT signaling that leads to excessive proliferation and survival of myeloid progenitor cells in most of the Philadelphia chromosome-negative classical myeloproliferative neoplasms (MPNs) [2]. Multiple studies have demonstrated that besides the somatic mutation(s), there is a substantial hereditable component, linked to the risk of MPN[3,4,5], and germline variations in JAK2 itself, such as the JAK2 GGCC haplotype, have been associated with JAK2-V617F somatic mutation acquisition and the development of MPNs [6, 7]. Also, non-canonical, weakly activating germline JAK2 variants may predispose progenitor cells to acquire JAK2-V617F and accelerate MPN progression to acute myeloid leukemia (AML) [8, 9]. Cases of hereditary thrombocytosis associated with some germline JAK2 variants indicate a distinct impact of JAK2 variants compared to somatic JAK2 mutations [10,11,12]. The JAK2-R1063H germline variant, functionally characterized in cooperation with JAK2-E846D, contributed to hereditary MPN with erythrocytosis and megakaryocytic atypia [13]. Mambet et al. [14] showed that patients with essential thrombocythemia (ET) carrying JAK2-R1063H and JAK2-V617F mutations had increased JAK2 signaling and disease severity, with 28% of them experiencing thrombotic events. The study also demonstrated that while most of the patients with the JAK2-R1063H mutation were heterozygous germline carriers, rare patients were (nearly) homozygous for the R1063H mutation and in rare cases the R1063H was an acquired somatic mutation [14]. Additionally, whole exome sequencing identified JAK2-R1063H in a patient with venous thromboembolism, suggesting it as a potential disease-causing variant [15]. Evaluation of the risk of MPN-associated thrombosis typically considers quantitative changes in platelets [16], but metabolic alterations mediating aberrant platelet activity and function may also play a crucial role, potentially driving hyperreactivation and increasing thrombotic risk in these patients [17]. However, the mechanism of thrombosis associated with weakly activating JAK2 germline mutations has not been described, nor it is known whether these variants modulate hematopoietic stem cell (HSC)/progenitor function as proposed for other contributors to germline MPN risk [3]. In this context, no study to date has investigated whether the JAK2-R1063H variant may promote a non-hierarchical platelet differentiation pathway, in which platelet-biased HSCs give rise directly to megakaryocyte progenitors (MkPs), bypassing canonical multipotent intermediates [18, 19], and leading to overactive platelets that increase the risk of thrombosis in the elderly [19]. To study the biology of the JAK2-R1063H variant, we generated CRISPR/Cas9-edited Jak2-R1063H mice and provide a phenotypic and molecular characterization of this model.
Materials and methods
Ethics approval and consent to participate
The study was conducted in accordance with the principles of the Declaration of Helsinki. The project was approved by the Ethics Committee of University Hospital Brno. Reference number 22-240620/EK and approval number 102/20 dated 24 June 2020. Written informed consent for participation and publication of results was obtained from all enrolled patients and/or their legal guardians. For in vivo work in mice, studies were carried out in compliance with The Ministry of Agriculture of the Czech Republic and protocols approved by the Czech Academy of Sciences Animal Welfare and Ethical Review Advisory Body (protocol 67/2020).
Mice
The mouse model bearing Jak2-R1063H mutation was created using microinjection of synthesized 5 μM ssODNs (87 bp) containing the R1063H mutation, 5 μM sgRNA mRNA and 100 ng/μl Cas9 protein into C57BL6/N-derived zygotes. In this study, 10–14- and 52–62-week-old mice were referred to as young (3 M) and old (12 M), respectively. In some experiments, 75- to 80-week-old mice were analyzed, referred to as 18 M. Since the quantification of the R1063H allele in the patient DNA samples indicated both heterozygous and homozygous state [14], we bred and analyzed the knock-in Jak2-R1063H homozygous mice (depicted as RH/RH; unless otherwise stated, the designation Jak2-R1063H mice refers to this genotype) to enhance the potential effect. In some experiments, we also analyzed heterozygous Jak2-R1063H mice (depicted as m/RH). Additional methods are described in the Supplemental File.
Results
Jak2-R1063H mutant mice exhibit stimulated thrombopoiesis and age-related decline of erythropoiesis
The mice were born in normal (expected) frequency without marked changes in spleen size and bone marrow (BM) cellularity (Supplemental Fig. 1A). However, we observed a relatively high frequency of sudden death in the cohort of Jak2-R1063H mice with median overall survival (OS) of 215 days (5/15) in comparison with the wt group (0/15) (Fig. 1A). We analyzed functionality of the Jak2-R0163H mutation in this in vivo model. Western blot (WB) analysis showed that Jak2 protein in unsorted BM cells was comparable between Jak2-R1063H and wt mice, but BM cells stimulated with EPO, TPO, G-CSF and IL-3 resulted in higher activation of Stat5 in the mutant cells (Fig. 1B). We observed phosphorylated Stat5 also in the absence of added growth factors in R1063H BM cells, suggesting weakly activated signaling in ligand-unstimulated cells (Fig. 1B). This experiment showed that the Jak2-R0163H mutation has expected regulation in mice with the ability to overstimulate downstream receptor signaling.
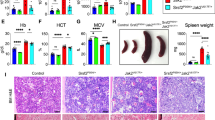
A Increased mortality rate of the mutant mice. Kaplan-Meier survival analysis of m/m (wt) (n = 15) and RH/RH (n = 15) mice. Mantle-Cox test was used to assess statistical significance of obtained results (p = 0.0350). B Increased baseline (blank line) and cytokine-stimulated Jak2/Stat5 activation in lysates of total BM cells obtained from RH/RH 3-month-old animals (n = 2, pooled cells), when compared to age-matched controls (n = 2), identified by WB analysis. C PLT counts for m/m (wt) and RH/RH are shown at the indicated times (up); comparison of PLT counts for m/m (wt), heterozygous and homozygous Jak2-R1063H mice at 3 and 12 months of age (down), n ≥ 10 mice per group, per each time point. D Phenotypic MkPs stratification based on CD48 expression. Representative flow cytometry plot (up) and quantification of CD48_low non-hierarchical MkPs at indicated time-points (n ≥ 3 mice per group) (down). E Tpo concentration in plasma (n ≥ 4 mice per group). F Workflow of transplantation of unsorted Jak2-R1063H-positive or wt BM cells to lethally irradiated C57BL/6NCrl recipients and PLT counts after transplantation (n ≥ 10 mice per group). G RBC, hemoglobin (HGB) and hematocrit (HCT) levels at the indicated times (n ≥ 10 mice per group, per each time point) (up). Comparison of RBC, HGB and HCT counts for m/m (wt), heterozygous and homozygous Jak2-R1063H mice at 3 and 12 months of age, n ≥ 10 mice per group, per each time point (down). H Epo concentration in plasma (n ≥ 4 mice per group). I Altered coupling of mouse (m) Jak2-R1063H or human (h) JAK2-R1063H to hematopoietic receptors. Mouse Jak2-Flag and human JAK2-Flag mutants were transiently expressed in HEK293 cells in which mouse V5-tagged Epor and human HA-tagged EPOR/TPOR were stably expressed. Interaction was examined by co-immunoprecipitation with Flag/V5 antibody. Immunoblot band intensity was quantified by ImageJ software and normalized to loading control and human JAK2 wt. All data are presented as mean ± SD and unpaired t-test with Welsch’s correction was used for group comparison. *p < 0.05.
The Jak2-R1063H mice displayed significantly increased platelet (PLT) counts when compared to wt mice during life-span (Fig. 1C top), but only with age the counts exceeded the physiological levels for C57BL/6N background [20]. Analysis of 3- and 12-month-old heterozygous Jak2-R1063H animals (m/RH) showed intermediate phenotype, supporting a dose-dependent effect of the Jak2-R1063H mutation and excluding an unexpected phenotype associated with the homozygous state (Fig. 1C bottom). To investigate whether the observed hematopoietic alterations might be driven (at least partially) by an expansion of platelet-biased HSCs (i.e. recently described non-hierarchical megakaryopoiesis pathway) [18, 19] we measured the expansion of Lin−/cKit+/CD41+/CD48− cells. This expansion was pronounced from 12 months of age, preceding the age-associated increase observed in m/m (wt) animals (Fig. 1D). These findings suggest that Jak2-R1063H progressively activates a platelet-biased differentiation trajectory through involvement of direct pathway from HSCs to MkPs [18, 19].
The plasma thrombopoietin (Tpo) level was normal (Fig. 1E). To further confirm that the observed trend towards increased PLTs is a cell autonomous defect, unsorted BM cells from wt or Jak2-R1063H mice were transplanted into the lethally irradiated recipients. The onset of increased PLTs was observed 9 weeks after transplantation and persisted till 17 weeks, when the experiment was terminated (Fig. 1F, Supplemental Fig. 1B).
The red blood cell (RBC) characteristics were normal but with more pronounced signs of anemia in aged Jak2-R1063H group than in wt mice (Fig. 1G top) and analysis of 3- and 12-month-old heterozygous Jak2-R1063H animals (m/RH) showed an intermediate phenotype, further supporting a dosage-dependent impact of the mutation also on erythroid parameters (Fig. 1G bottom). We also observed normal maturation of RBCs (data not shown), non-altered iron metabolism (the exception was increased ferritin in old Jak2-R1063H mice, Supplemental Fig. 1C), but increased erythropoietin (Epo) levels (Fig. 1H). White blood cell (WBC) parameters remained unaltered at all examined time points (Supplemental Fig. 1D).
Although the WB analysis (Fig. 1B) revealed activation caused by the Jak2-R0163H mutation downstream of EpoR and TpoR, it was documented in the presence of excess of ligand, with limited functional relevance for in vivo context. We hypothesized, that the observed phenotype in thrombopoiesis/erythropoiesis could be (at least partially) modulated by different coupling affinity of the Jak2-R0163H kinase to EpoR and TpoR, as seen with human G-CSFR, when JAK2-V617F/R1063H mutant couples to G-CSFR with higher affinity than JAK2-V617F [14]. We performed co-immunoprecipitation experiments in unstimulated HEK293 cells stably expressing mouse EpoR and TpoR. As shown in Fig. 1I left, less EpoR co-immunoprecipitated with Jak2-R1063H. Due to technical issues with very weak TpoR signal, we were unable to reliably evaluate the TpoR/Jak2-R1063H co-IP results (data not shown). However, when we used HEK293 cells stably expressing human EPOR and TPOR, more TPOR and less EPOR co-immunoprecipitated with human JAK2-R1063H (Fig. 1I right). This result suggested that due to the decreased binding affinity with EpoR, the Jak2-R1063H signaling is slightly compromised but not physiologically manifested as anemia (in young animals) due to the compensation by slightly increased production of Epo. Vice versa, increased coupling of Jak2-R1063H with TpoR promotes mild thrombocytosis without elevated circulating Tpo.
Jak2-R1063H alters normal hematopoietic development and causes premature HSC aging and functional decline of HSC compartment
While the total number of LSK cells in the BM remained unchanged in the young animals, the Jak2-R1063H model displayed increased number of HSCs, specifically long-term hematopoietic stem cells (LT-HSCs) at a young age (3 M); the proportion of populations of short-term hematopoietic stem cells (MPP1) and multipotent progenitors (MPP2) remained unchanged at this stage (Fig. 2A). In aged mice (12 M), Jak2-R1063H mice exhibited increased frequency in the LSK population, mainly due to the increase in MPP1 and MPP2 (Fig. 2A). The levels of myeloid-biased multipotent progenitors (MPP3) and lymphoid-biased multipotent progenitors (MPP4) were not significantly altered (Supplemental Fig. 2).
A Quantification of absolute number of distinct stem and progenitor populations in BM. Lin− c-Kit+ Sca-1+ (LSK), Lin− c-Kit+ Sca-1+ CD48− CD150+ (HSC), Lin− c-Kit+ Sca-1+ CD48− CD150+ CD34− CD135−(LT-HSC), Lin− c-Kit+ Sca-1+ CD48− CD150+ CD34+ CD135- (MPP1), Lin− c-Kit+ Sca-1+ CD48+ CD150+ CD34+ CD135− (MPP2) (n ≥ 4 mice per group). B Relevant unique downregulated and upregulated GO terms in RH/RH HSCs, as calculated using Cytoscape platform (STRING App). C GSEA for transcriptional activation and HSC dysregulation of young (3 M) RH/RH vs. m/m, NOM p values < 0.05. D Hierarchical clustering of expression levels of Stat5 targets clearly distinguishes old Jak2-R1063H HSCs from wt (m/m) HSC (left) and from their young Jak2-R1063H counterparts (right). Heatmap representations of differential expression of Stat5 target genes. Red indicates upregulation, blue indicates downregulation of gene expression, and color intensity indicates the level of differential expression. Rows in the heatmaps represent individual samples. E Principal component analysis (PCA) (n = 4 mice per group, only RH/RH old mice (12 M) n = 3). F Volcano plot depicting expression levels of genes upregulated (red points) and downregulated (blue points) during aging (3 M versus 12 M) in m/m and RH/RH HSCs; FDR < 0.05, │log2FC│>1 (up left). Venn diagrams of intersecting upregulated or downregulated genes |log2FC | ≥ 2, p < 0.05 comparing m/m and RH/RH HSCs during aging (up right). Relevant unique upregulated and downregulated GO terms and KEGG pathways in RH/RH HSCs, as calculated using Cytoscape platform (STRING App) (down). G Heatmap of top 25 genes representing mouse LT-HSC compartment. H Workflow of transplantation of unsorted BM cells to lethally irradiated recipients (n = 5 mice per group) and frequencies of BM HSC and MPP 17 weeks after transplantation. Data are presented as mean ± SD and unpaired t-test with Welsch’s correction was used for group comparison. *p < 0.05.
Transcriptional analysis of HSCs sorted at 3 and 12 months of age revealed 54 genes and 124 genes, respectively, to be expressed differentially between the wt and Jak2-R1063H (i.e. differentially expressed genes (DEGs), FDR ≤ 0.05; Supplemental Table 1). Processes related to cell cycle were downregulated in the young mutant HSCs (Fig. 2B left). These data suggested accumulation of less cycling, relatively more quiescent cells in the young R1063H BM when compared to wt. Also examples of other significantly downregulated GSEA in the mutant 3-month-old HSCs (G2M checkpoint gene set, double strand repair by homologous recombination gene set, Fig. 2C) are consistent with more quiescent HSC phenotype [21,22,23]. These data indicated that downregulation of pathways related to cycling/cell cycle progression, known to be downregulated in old HSCs [21, 24], are consequences of premature aging of R1063H HSCs in 3-month-old mice. Also, significantly upregulated processes in the R1063H HSCs, translation and ribosomal protein genes expression (Fig. 2C left) and activated mTorc1 signaling (Supplemental Fig. 3A) are compatible with HSC aging [24, 25]. In the 12-month-old mice, lack of differential expression of the cell cycle-related gene sets likely reflects their comparative expression achieved by physiological aging of the wt HSC controls, but the processes associated with ribosomal genes expression remained enriched in the mutant HSCs of the 12-month-old animals (Fig. 2B right).
Then we questioned whether a significant increase of LSK, that is predominantly caused by significant increase of MPP1/MPP2 populations in aged R1063H murine BM (Fig. 2A), could result from a pro-proliferative signaling, known to be activated in MPP1/MPP2 cell subsets [26]. Earlier studies showed that loss of STAT5 expression in hematopoiesis specifically reduces numbers of MPP1/MPP2 [27], so opposite effect would be expected for augmented STAT5 activity. We assessed the expression of previously defined key STAT5 target genes in hematopoiesis [28] and found that their increased expression clearly distinguishes R1063H and wt HSCs in 12 M (Fig. 2D left). This differential expression was age-dependent, not present in 3-month-old HSCs, and distinguished also HSCs derived from young and old mutant mice (Fig. 2D right). These data suggested that the transcriptional differences in HSC compartment between the young and aged mutant animals, reinforced during aging, could be primarily linked to life-lasting chronic stress-induced conditions caused by the weakly activating Jak2 mutation.
To better characterize the extent of intrinsic R1063H HSCs alterations during aging, we constructed the principal component analysis and identified age as the main source of transcriptional variation in HSC and also LK compartment (Fig. 2E). The skewing of transcriptional activation of aged HSCs was more pronounced in Jak2-R1063H population (Fig. 2F). Analysis of DEGs during aging confirmed, besides the mentioned JAK/STAT activation, also upregulation of PI3K-Akt signaling and the down-regulation or loss of immune system process and hematopoietic differentiation (Fig. 2F). These data likely reflect a known phenomenon of diminished differentiation and impaired immune response associated with a decrease of lymphoid-biased HSCs in aged hematopoiesis [24, 29, 30]. HSC compartment at 12 M also showed downregulation of majority of LT-HSC specific genes [31] in Jak2-R1063H cells, confirming faster, premature functional decline and relative exhaustion of this cell population in mutant animals (Fig. 2G). Using publicly available RNA-seq datasets (GSE123401) [32], we show that the transcriptional features of exhaustion/premature aging of Jak2-R1063H HSCs in BM of young mice are comparable to those that characterize HSC of young mice carrying Vav-Cre Jak2-V617F (see Supplemental Methods and Supplemental Fig. 3B–D).
Next, we aimed to determine whether the observed less cycling, more quiescent HSC status in young Jak2-R1063H mice is intrinsically driven and maintained after transplantation into wt recipients [33, 34]. We non-competitively transplanted total BM derived from control and R1063H littermates into CD45.1 recipients and assessed HSC numbers 17 weeks post-transplantation. The frequency for Jak2-R1063H HSCs was decreased compared to wt control mice, while the MPP frequencies were comparable (Fig. 2H). These results support our findings that Jak2-R1063H mutation results, at least in part, in HSC-autonomous premature aging/functional decline, but do not exclude microenvironmental contribution (driven by cell-extrinsic factors present in the Jak2-R1063H BM niche) to the pathological premature aging and functional decline of hematopoiesis in Jak2-R1063H mice.
Myeloid bias, enhanced megakaryopoiesis and inflammatory signatures in Jak2-R1063H mutant hematopoiesis
Based on the above data (Fig. 1D), we reasoned that the direct pathway of HSC differentiation to MkPs is promoted by Jak2-R1063H in aged mice. We questioned if the canonical megakaryopoiesis pathway, which functions in parallel with the non-hierarchical pathway [19], is also enhanced and whether it may function more prominently at younger age. To explore the functional consequences of Jak2-R1063H expression in the myeloid progenitor compartment further, and in view of described myeloid bias and augmented megakaryocyte expansion associated with hematopoietic aging [34, 35], we analyzed immunophenotypically-defined LK cells (Lin−/Sca1−/cKithigh) in the Jak2-R1063H murine BM. We observed non-significant increase in megakaryocytic/erythroid progenitors (MEP) at 3 months of age (Fig. 3A, left), and substantial myeloid bias in aged mice, characterized by a disproportionate increase in CMP cells and a marked decrease in MEP cells (Fig. 3A, right). At the level of committed cell subsets downstream of MEP, we observed significantly increased megakaryocytic and erythroid progenitors in young R1063H mice (Fig. 3B left); the trend towards expansion of pre-CFU-Es and megakaryocyte progenitor skewing persisted also in old mice (Fig. 3B, left).
A Quantification of absolute number of myeloid precursors in BM. Lin− c-Kit+ Sca-1− (LK), Lin− c-Kit+ Sca-1− CD34+ FcgRII/IIIlow (CMP), Lin− c-Kit+ Sca-1− CD34+ FcgRII/III+(GMP) and Lin−c-Kit+ Sca-1- CD34- FcgRII/IIIlow (MEP) (n ≥ 4 mice per group). B Quantification of absolute number of erythroid/megakaryocytic committed subsets - Lin− c-Kit+Sca-1−/CD41−/CD150low/CD105+ (CFU-E), Lin−c-Kit+Sca-1−/CD41−/ CD 150+/CD105+ (Pre–erythroid CFU), Lin−c-Kit+Sca-1−/CD41+/CD150+ (MkP), Lin−c-Kit+Sca-1−/ CD41low /CD150+/CD105− (Pre-MegE) and Lin−c-Kit+Sca-1−/CD41low/CD150−/CD105- (Pre-GM) (n ≥ 4 mice per group). C Phosphorylation of Stat1 (Tyr 694), Stat3 (Tyr 705) and Erk1/2 (Thr 202/Tyr 204) proteins in m/m (wt) and RH/RH c-Kit+ enriched fraction of total BM. Total Erk protein and Vinculin served as loading control. WB shows the baseline (blank line) and cytokine-stimulated protein activities in protein lysates from enriched c-kit+ cells, starved for 40 hours and unstimulated or stimulated with indicated cytokines for 15 min. D GSEA for selected pathways from Hallmark gene sets enriched in RH/RH vs. m/m in LK cells, NOM p values < 0.05. Data are presented as mean ± SD and unpaired t-test with Welsch’s correction was used for group comparison. *p < 0.05.
We questioned whether megakaryocytic differentiation program (Figs. 1D and 3B) and thrombopoiesis in aged Jak2-R1063H mice (Fig. 1C) could be explained on the level of differential STAT activation downstream Jak2-R1063H mutation. It was previously reported that in JAK2-V617F CD34+ progenitors, intrinsically increased STAT1 phosphorylation promoted enhanced megakaryocytic and reduced erythroid differentiation and ET-like phenotype [36]. To evaluate differences in signaling caused by Jak2-R1063H kinase, we examined the phosphorylation levels of Stat1, Stat3, and Erk1/2 within the c-kit+-enriched BM population. WB analysis revealed increased baseline Stat1 phosphorylation level for Jak2-R1063H cells, with a marked increase after the short pulse stimulation with IL3, EPO and also TPO in old mice (Fig. 3C right); in the young Jak2-R1063H mice, the activation could be better demonstrated for Stat3 (Fig. 3C left). Cell-intrinsic inflammatory properties were demonstrated for JAK2-V617F myeloid progenitors [37, 38]. To test this aspect, we could only directly compare (at the gene expression level) LK cells from the 3-month-old mice, where individual myeloid populations did not differ significantly between mutant and wt animals (Fig. 3A). We observed interferon gamma response, TNFα signaling via NF-κB and inflammatory response gene sets significantly enriched in the Jak2-R1063H mutant LKs (Fig. 3D). These data are consistent with chronic inflammatory signaling mediated by Jak2-R1063H in the mouse BM compartment.
Gene expression profiles suggest a key role for Egr1 in transcriptional regulatory network of aged Jak2-R1063H progenitors
Among the genes differentially overexpressed across the HSC/progenitor Jak2-R1063H populations, we identified an immediate early response gene Egr1 (Supplemental Table 1), a known transcriptional master regulator of HSC aging, quiescence and overall HSC/progenitor functionality [39, 40]. Egr1 was significantly upregulated in the Jak2-R1063H 12 M HSCs vs. wt 12 M HSCs and also in the Jak2-R1063H 12 M HSCs vs. 3 M mutant HSCs (Fig. 4A). In addition, although increased Egr1 transcript levels in Jak2-R1063H 12 M LK cells vs. wt 12 M LK cells did not reach significance (Fig. 4A), some of the key Egr1 targets, as mentioned bellow, were significantly upregulated in both HSC/LK mutant populations. We searched for overlap between the putative EGR1 target genes in the ChIP-seq datasets (ENCODE transcription factor dataset) [41], and genes differentially upregulated in Jak2-R1063H 12 M HSCs vs. 3 M HSCs (Supplemental Table 1) and found that many of upregulated genes in 12-month-old mutant HSCs are targets of EGR1; many of these target genes were also upregulated in 12-month-old R1063H HSCs vs. 12-month-old wt HSCs. These data suggested a progressive augmentation of a subset of Egr1 transcriptional signature in the Jak2-R1063H HSCs/progenitors.
A, B Differential expression of Egr1 (A) and Thbs1 (B) genes in HSC and LK compartments of Jak2-R1063H (RH/RH) and wt (m/m) animals. Boxplots generated from RNA-seq data. Student’s t test with Bonferroni correction, *p < 0.05; CPM counts per million. C, D Correlation curves depicting a significant strong positive correlation of Egr1 and Thbs1 expression levels (C) and a significant very strong positive coregulation of Egr1 and Pim1 (D) genes in HSC and LK compartments. Pearson correlation analysis combined values of both wt (m/m, black dots) and Jak2-R1063H (RH/RH, red dots). CPM counts per million, p value, R correlation coefficient.
Among the genes functionally validated as responsible for HSC aging [42], JUNB, ZFP36, IER2 or Cited2, are proposed EGR1 targets in biomedical database [41] and showed different expression pattern (Supplemental Table 1). The most differentially upregulated Egr1 target in 12 M mutant HSCs compared to wt (Supplemental Table 1) was Thbs1, the gene encoding thrombospondin 1. Thbs1 was also significantly upregulated in 12 M mutant LKs and HSCs vs. 3 M mutant HSCs (Fig. 4B). Thbs1 is a ligand for CD36 and CD47 receptors and it is implicated in angiogenesis, tumor growth and thromboembolism [43, 44]. Correlation analysis [45] revealed a strong positive correlation of Egr1 expression with the Thbs1 expression in HSC/LK populations, suggesting their functional relationship in these cells (Fig. 4C). One of the differentially upregulated Stat5-target genes was Pim1 (Fig. 2D), that is commonly found upregulated in JAK2-V617F polycythemia vera (PV) and other myeloid malignancies [46]. Pim kinases are considered to be “weak oncogenes” and it seems that upregulation of this gene alone is not sufficient to promote overt disease such as PV or AML [46, 47], but may cooperate in its development. Egr1 expression and Pim1 expression revealed a very strong positive coregulation in HSC/LK populations in 12 M mice (Fig. 4D), but such a possible relationship in Jak2-R1063H progenitors requires further functional analyses.
Jak2-R1063H mutation represents risk factor for thrombosis
Our previous analysis of MPN patients bearing concomitantly JAK2-R1063H and V617F mutations revealed increased incidence of thrombotic events [14], so we asked whether the increased mortality in the cohort of Jak2-R1063H mice (which occurred suddenly precluding pathological analysis) (Fig. 1A) could be related to platelet pathology, similar to that observed for JAK2-V617F [17, 48]. We measured the levels of D-dimers as a marker of thrombosis and found them significantly increased in the young Jak2-R1063H group (Fig. 5A). Using Seahorse flux analyzer, we measured extracellular acidification rate (ECAR) that reflects cellular glycolysis, and oxygen consumption rate (OCR) as a measure of mitochondrial metabolism. We showed significant increase in basal/ATP-linked respiration, while maximal respiration remained unchanged (Fig. 5B), suggesting higher utilization of mitochondrial ATP by Jak2-R1063H PLTs (Supplemental Fig. 4). In subsequent RNA-seq analysis we demonstrated strong enrichment of fatty acid metabolism genes, including type II scavenger receptor CD36 (Fig. 5C), which was shown to promote PLT activation and thrombosis by activating redox sensing/signaling events [49]. As mentioned above, the most differentially upregulated gene in 12 M mutant HSCs compared to wt was Thbs1, the gene encoding thrombospondin 1, a CD36 ligand (Fig. 4B). Also, as noted above, the hyperactive PLTs produced by the platelet-biased HSC trajectory are prone to thrombosis [19]. Overall, the presence of the germline Jak2-R1063H mutation leads to a significant increase in PLTs in mice (Fig. 1C), independent of Tpo levels (Fig. 1D), and to increased thrombotic markers (Fig. 5A).
A D-dimers concentration in plasma (n ≥ 4 mice per group). B Combined PLTs OCR profiles from m/m (wt) and RH/RH mice (young (3 M)). Quantification of basal, ATP linked and maximal respiration are shown in right (n ≥ 6 mice per group). C GSEA enrichment plots for Hallmark “Fatty acid metabolism” gene set enriched in RH/RH vs. m/m (wt) PLTs. Heatmap of top ten most upregulated and downregulated genes from Hallmark “Fatty acid metabolism” gene set (n = 3 mice per group, middle). Transcripts per million of CD36 gene in PLT from m/m and RH/RH mice (young (3 M), right). D Workflow of pulmonary ECs isolation and representative flow cytometry plot (left). GSEA for selected pathways enriched in Jak2-V617F ECs [50] that are not over-represented in Jak2-R1063H mice (right). E Workflow. Kaplan-Meier survival analysis of m/m (n = 6) and RH/RH (n = 5) mice intravenously injected with 200 MLL-AF9 leukemic cells. MLL-AF9 splenocytes isolated from a leukemic mouse were transplanted into non-irradiated 10- to 14- weeks old m/m (wt) and RH/RH recipients. Mantle-Cox test was used to assess statistical significance of obtained results (p = 0.0133). F Workflow. GFP sorted 100 LSK cells transduced with MLL-AF9 oncogene were plated in semisolid media and total number of colonies were evaluated after 7 days. For serial re-plating, colonies were harvested from the methylcellulose media, washed, counted and seeded again. G Analysis of colonies grown in semisolid media with the increasing concentration of ruxolitinib, when Y-axis indicates the proportion of colonies to absolute numbers (n = 3 mice per group (left), 3 independent experiments in right). Data are presented as mean ± SD and unpaired t-test with Welsch’s correction was used for group comparison. *p < 0.05.
Endothelial cells (ECs) play a critical role in maintaining vascular homeostasis and regulating thrombotic responses. In the context of JAK2-V617F-driven MPNs, ECs harboring the mutation have been shown to adopt a pro-inflammatory, pro-adhesive, and pro-thrombotic phenotype, contributing to the higher incidence of vascular events in these patients [50, 51]. Nevertheless, analysis of pulmonary ECs in the Jak2-R1063H model did not reveal similar features (Fig. 5D), thus does not provide evidence of the link between ECs and Jak2-R1063H-associated pathobiology.
Cooperative role of Jak2-R1063H mutation in leukemic development
The presence of JAK2-R1063H has been proposed as a potential risk factor for progression from MPN to AML [9]. In order to mimic leukemic transformation, we used model based on overexpression of the oncogenic fusion protein MLL-AF9 [52]. MLL-AF9 splenocytes were transplanted into wt or mutant mice, allowing us to determine the effects of the mutant niche on leukemic progression. We observed quicker leukemic onset in Jak2-R1063H than in wt mice (Fig. 5E, Supplemental Fig. 5) suggesting cell extrinsic effect of Jak2-R1063H mutation which might favor leukemogenesis. In addition, Jak2-R1063H HSCs transduced with MLL-AF9 oncogene exhibited both enhanced colony-forming potential upon MLL-AF9 introduction and increased re-plating capacity, confirming also the intrinsic effect of Jak2-R1063H mutation in promoting leukemic development (Fig. 5F). A previous in vitro sensitivity assay of Ba-F3/EPOR cells showed that human JAK2-R1063H and JAK2-V617F/R1063H double mutant cells are significantly more sensitive to JAK2 inhibitor, ruxolitinib, compared to wt cells [14]. The colony assay of wt and Jak2-R1063H BM cells under increasing concentration of ruxolitinib showed similar results. The consistent result was observed also by cultivating wt and Jak2-R1063H LSKs transduced with MLL-AF9, suggesting potential therapeutic implication (Fig. 5G). Structural modeling of interactions in the JAK2 wt and the R1063H mutant active site with ruxolitinib suggested stronger binding into the mutant (Supplemental Fig. 6). These findings provide a plausible structural explanation for the increased sensitivity of the R1063H mutant to JAK2 inhibition by ruxolitinib.
Clinical outcomes of MPN patients carrying the JAK2-R1063H germline variant support that it is a risk-conferring variant
Samples from 200 consecutive MPN patients (71 ET, 46 primary myelofibrosis (PMF), 83 PV) were analyzed for the presence of JAK2-R1063H variant. This variant was found in 10 patients (5%) – 6 ET (8.4% of all ET patients), 3 PV (3.6% of all PV patients) and 1 PMF (2.2% of all PMF patients) (Fig. 6A left). All JAK2-R1063H-positive MPN patients were also JAK2-V617F-positive, except for 3 CALR-positive ET patients (Fig. 6A middle). No significant difference was found between JAK2-R1063H positive and negative patients in terms of age at diagnosis (60 vs. 52 years), sex (female percentage 60% vs. 62%), and occurrence of bleeding (0 vs. 6%) (Supplemental Table 2A). However, there was a trend towards a higher incidence of thrombotic events (33% vs. 22%) and especially disease progression – i.e. transformation to AML or post-PV fibrotic progression (33% vs. 13%) in JAK2-R1063H-positive group (Fig. 6A right, Supplemental Table 2A). All progressions to AML were observed in patients with an initial diagnosis of ET. Moreover, patients with JAK2-R1063H had shorter median OS (8.4 years vs. not reached) (Fig. 6B). Comparison of the healthy Czech population from the 1000 Czech Genomes Project [53] with a group of our 200 MPN patients showed that the minor allele frequency (MAF) of the JAK2-R1063H in the healthy population is lower than in the group of MPN patients (1.67% vs. 2.50%; not statistically significant). The aggregate frequency of the JAK2-R1063H allele in general European population is much lower (0.58%) [54].
A Descriptive characteristics of MPN patients negative and positive for the JAK2-R1063H variant. Left graph shows higher proportion of ET in the JAK2-R1063H-positive group. Middle graph shows mutational landscape within the JAK2-R1063H-negative and R1063H-positive patient groups. Right graph shows the rate of thrombotic events and disease progression (transformation and post-PV fibrotic progression) in the JAK2-R1063H-negative and R1063H-positive group of patients. None of the patients who progressed in the JAK2-R1063H-positive group had a mutation in the TP53 gene; 4 patients with TP53 mutation were among the 19 patients who progressed in the JAK2-R1063H-negative group. B Kaplan-Meier curve of OS of MPN patients negative and positive for the JAK2-R1063H variant (left). Patients with the JAK2-R1063H mutation who progressed to sAML (n = 3) were treated with hydroxyurea (HU) (n = 2) and one patient received HU plus interferon. For patients with the JAK2-R1063H mutation who did not progress (n = 7), treatment included HU (n = 2), anagrelide (n = 3), or a combination of HU and anagrelide (n = 2). Kaplan-Meier estimate of OS in patients with and without JAK2-R1063H mutation (right upper panel). CI confidence interval, n/n censored = number of dead patients/number of censored patients, NR not reached. p value was obtained through statistical testing of OS with Log-rank test. 40 patients with missing data were not included in the survival analysis. For Cox regression model of hazard ratio estimate of death (right lower panel): CI confidence interval, HR hazard ratio. p value was obtained through Chi-squared test. 40 patients were excluded due to missing data. For additional clinical data see Supplemental Tables 2A, B.
Discussion
Because JAK2 activities and downstream signaling have pleiotropic effects in hematopoiesis [55, 56], we asked whether weakly activated Jak2, a lifelong consequence of the JAK2-R1063H variant, leads to the evolution of an age-associated phenotype.
In our mouse model, HSCs and progenitors expressing Jak2-R1063H variant revealed some characteristics of accelerated (premature) aging, including selected transcriptional characteristics [24, 25], myeloid-skewed differentiation [34] or impairment of HSCs [30] with observed loss of LT-HSC gene expression signature and decreased repopulation potential following transplantation [33, 34]. Increased anemia in aged R1063H mice would then also reflect more progressive aging-related changes in hematopoiesis in the mutant BM [35]. Physiologically, HSCs of 3-month-old mice exhibit a quiescent transcriptional signature and MPP1/MPP2 exhibit a cycling/proliferative signature [26]. The fact that the Jak2-R1063H-positive HSCs are at 3 months even more quiescent and accumulated in greater numbers at this age suggests that they have an increased proliferative history from early development, which has contributed to their accelerated aging and myeloid bias [57]. The exhaustion/premature aging of Jak2-R1063H HSCs belong to phenotypic features that share similarities with the oncogenic V617F mutation, as we documented by comparative analysis with the Vav-Cre Jak2-V617F mouse model [32]. The most striking difference in the hematopoietic phenotype of the two mutations is the absence of granulocytosis in our model. Even though we have previously described statistically higher WBC and neutrophils in the double mutant JAK2-V617F/R1063H ET patients [14], the effect on granulocytosis in these patients could be caused by cumulative effect on JAK2 signaling of the two JAK2 (germline R1063H and somatic V617F) mutations. Specific differences in granulopoiesis in humans and mice may also play a role [58].
Our data suggest that in Jak2-R1063H-positive hematopoiesis, cell-autonomous signaling with a likely contribution of a pro-inflammatory milieu promotes pro-thrombotic complications and risk for malignant development. Our data revealed intrinsic activation of Jak2/Stat5 signaling in the BM of young Jak2-R1063H-mutant mice and augmented activation of a set of Stat5 target genes in old animals’ BM cells. We also observed differential Stat3 and Stat1 activation in Jak2-R1063H-positive c-kit progenitors, with more exhibited Stat1 activation in aged mutant progenitors. In agreement with the Jak2-R1063H mouse phenotype, STAT1 is known to support megakaryocytic differentiation and to create a bias towards an ET phenotype [36]. Importantly, we observed age-dependent alterations in the activation of two distinct pathways for megakaryopoiesis, which give rise to PLTs [18, 19]. The traditional “myeloid bias” (which includes increased MkPs) was detectable in both, 3 M and 12 M old mutant mice, whereas accumulated stress (marked by Egr1 expression signature) appears to lead to an augmented direct MkP differentiation trajectory from platelet-biased HSCs only during aging. Further studies will be needed to clarify the role of JAK2-R1063H (as well as the role of other germline mutations associated with thrombocytosis) [10,11,12], in the regulation of both pathways.
In previous studies, chronic inflammatory conditions and Egr1 overexpression have been connected with acquisition of molecular/phenotypical markers of aged-state of hematopoiesis [59, 60]. A key factor modulating the phenotype of Jak2-R1063H mice appears to be the overexpression of Egr1 in aged HSCs/progenitors. Given that the expression of Egr1 (and Egr1 targets) increases with animal age and that STAT and EGR1 signaling are directly or indirectly interconnected [61,62,63], we conclude that stress/inflammatory response-associated Egr1 signaling in aging mice likely reinforces some alterations initiated by Jak2/Stat target genes’ expression.
Patients with MPNs are known to be at an elevated risk of thrombosis, but the mechanism for MPN-related coagulation activation is not yet fully characterized [16]; there is evidence that JAK2-mutated ECs may contribute to the MPN pro-thrombotic state [45, 51]. We observed increased mortality in Jak2-R1063H mouse cohort and elevated levels of D-dimers, indicative of thrombotic complications, but the ECs from these mice did not exhibit pro-adherent and pro-thrombotic features. Whereas MPN patients typically exhibit activated OXPHOS and mTORC1 signaling pathways and enhanced mitochondrial activities in PLTs, both leading to PLT hyperactivity [17], Jak2-R1063H-positive PLTs metabolic abnormalities were associated with fatty acid metabolism. This metabolic shift is critical, since it was shown that mitochondrial ATP, needed for PLT granule secretion and thrombus formation, is primarily derived from fatty acid oxidation [64]. Thrombosis in MPN patients is a multifactorial event involving chronic inflammation [65], functionally hyper-reactive PLTs produced by direct megakaryocyte differentiation pathway from platelet-biased HSCs [66] and other complex interactions among various blood cells [16], so role of these processes and cell types in the thrombosis risk caused by the Jak2-R1063H mutation cannot be excluded. Differential overexpression of Thbs1, coregulated with Egr1 in the mutant HSCs and LKs, may be involved in chronic inflammatory responses within the progenitors and promote the observed aging-related HSC defects [67]. Whether and how is thrombospondin 1 secreted and promotes the observed PLT activation and contributes to thrombosis remains to be determined.
In this study, we focused primarily on the cell intrinsic alterations caused by Jak2-R1063H that underlie the observed murine phenotype. The exception was the assessment of the effect of Jak2-R1063H on leukemia progression, in which we demonstrated both, cell-intrinsic as well as cell-extrinsic effect of the mutation on leukemia progression. The cell-autonomous factor contributing to the accelerated leukemic development could be overexpression of Pim1 oncogene [46]. Pim1 expression was shown to be significantly increased in hematopoietic progenitors of Jak2-V617F knock-in mice [47] and was overexpressed in JAK2-V617F-positive ET patients [36, 68]. Whether a possible inflammatory microenvironment (mediated by R1063H-positive hematopoietic cells and nonhematopoietic niche cells) produces inflammatory factors that can act as the cell-extrinsic factors supporting the expansion of MLL-AF9 leukemic cells [69] remains undefined and should be further addressed by future research endeavors.
Earlier clinical studies have suggested a higher risk of thrombotic events in patients with JAK2-R1063H variant [14, 15] as well as a higher risk of transformation to AML [9], and our clinical evidence is consistent with these studies. Interestingly, all transformations to AML in our JAK2-R1063H-positive group occurred in patients initially diagnosed with ET; two of these ET patients carried the CALR driver mutation, none of them carried a mutation in the TP53 gene. The frequency of the rare germline low-penetrance risk alleles predisposing to sporadic MPN may be population-specific [70]. The studied JAK2 variant has a MAF of 1.67% in the Czech population, which is relatively high; the available database for JAK2-R1063H across multiple ethnicities reveals that the MAF is much lower for most populations [54]. Missing analyses of more diverse cohorts is a limitation of our study; further population studies will be needed to better delineate the overall disease risk conferred by this variant.
Data availability
RNA-seq data are available in the BioStudies database (http://www.ebi.ac.uk/biostudies) under accession number S-BSST12345, E-MTAB-14686, E-MTAB-14685 and E-MTAB-15355.
References
Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunological Rev. 2009;228:273–87.
Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017;129:667–79.
Bao EL, Nandakumar SK, Liao X, Bick AG, Karjalainen J, Tabaka M, et al. Inherited myeloproliferative neoplasm risk affects haematopoietic stem cells. Nature. 2020;586:769–75.
Tapper W, Kralovics R, Harutyunyan A, Zoi K, Leung W, Godfrey A, et al. Genetic variation at MECOM, TERT, JAK2 and MYB predispose to myeloproliferative neoplasm. Nat Commun. 2015;2015:6691.
Zoi K, Cross N. Genomics of Myeloproliferative Neoplasms. J Clin Oncol. 2017;35:947–54.
Olcaydu D, Harutyunyan A, Jäger R, Berg T, Gisslinger B, Pabinger I, et al. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet. 2009;41:450–4.
Kilpivaara O, Mukherjee S, Schram AM, Wadleigh M, Mullally A, Ebert BL, et al. A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat Genet. 2009;41:455–9.
Lanikova L, Babosova O, Swierczek S, Wang L, Wheeler DA, Divoky V, et al. Coexistence of gain-of-function JAK2 germ line mutations with JAK2V617F in polycythemia vera. Blood. 2016;128:2266–70.
Benton CB, Boddu PC, DiNardo CD, Bose P, Wang F, Assi R, et al. Janus kinase 2 variants associated with the transformation of myeloproliferative neoplasms into acute myeloid leukemia. Cancer-Am Cancer Soc. 2019;125:1855–66.
Mead AJ, Chowdhury O, Pecquet C, Dusa A, Woll P, Atkinson D, et al. Impact of isolated germline mutation on human hematopoiesis. Blood. 2013;121:4156–65.
Brooks SA, Luty SB, Lai HY, Morse SJ, Nguyen TK, Royer LR, et al. JAK2 results in cytokine hypersensitivity without causing an overt myeloproliferative disorder in a mouse transduction-transplantation model. Exp Hematol. 2016;44:24–9.
Luque Paz D, Kralovics R, Skoda RC. Genetic basis and molecular profiling in myeloproliferative neoplasms. Blood. 2023;141:1909–21.
Kapralova K, Horvathova M, Pecquet C, Fialova Kucerova J, Pospisilova D, Leroy E, et al. Cooperation of germ line JAK2 mutations E846D and R1063H in hereditary erythrocytosis with megakaryocytic atypia. Blood. 2016;128:1418–23.
Mambet C, Babosova O, Defour J-P, Leroy E, Necula L, Stanca O, et al. Cooccurring JAK2 V617F and R1063H mutations increase JAK2 signaling and neutrophilia in myeloproliferative neoplasms. Blood. 2018;132:2695–9.
Lee E-J, Dykas DJ, Leavitt AD, Camire RM, Ebberink E, García de Frutos P, et al. Whole-exome sequencing in evaluation of patients with venous thromboembolism. Blood Adv. 2017;1:1224–37.
Reeves BN, Beckman JD. Novel Pathophysiological Mechanisms of Thrombosis in Myeloproliferative Neoplasms. Curr Hematologic Malignancy Rep. 2021;16:304–13.
He F, Laranjeira ABA, Kong T, Lin S, Ashworth KJ, Liu A, et al. Multiomic profiling reveals metabolic alterations mediating aberrant platelet activity and inflammation in myeloproliferative neoplasms. J Clin Investig. 2024;134:e17225631.
Carrelha J, Mazzi S, Winroth A, Hagemann-Jensen M, Ziegenhain C, Högstrand K, et al. Alternative platelet differentiation pathways initiated by nonhierarchically related hematopoietic stem cells. Nat Immunol. 2024;25:1007–19.
Poscablo DM, Worthington AK, Smith-Berdan S, Rommel MGE, Manso BA, Adili R, et al. An age-progressive platelet differentiation path from hematopoietic stem cells causes exacerbated thrombosis. Cell. 2024;187:3090–107. e21.
Barrios M, Rodríguez-Acosta A, Gil A, Salazar AM, Taylor P, Sánchez EE, et al. Comparative hemostatic parameters in BALB/c, C57BL/6 and C3H/He mice. Thromb Res. 2009;124:338–43.
Beerman I, Seita J, Inlay MA, Weissman IL, Rossi DJ. Quiescent Hematopoietic Stem Cells Accumulate DNA Damage during Aging that Is Repaired upon Entry into Cell Cycle. Cell Stem Cell. 2014;15:37–50.
Kaisrlikova M, Kundrat D, Koralkova P, Trsova I, Lenertova Z, Votavova H, et al. Attenuated cell cycle and DNA damage response transcriptome signatures and overrepresented cell adhesion processes imply accelerated progression in patients with lower-risk myelodysplastic neoplasms. Int J Cancer. 2024;154:1652–68.
Shin JJ, Schröder MS, Caiado F, Wyman SK, Bray NL, Bordi M, et al. Controlled Cycling and Quiescence Enables Efficient HDR in Engraftment-Enriched Adult Hematopoietic Stem and Progenitor Cells. Cell Rep. 2020;32:108093.
Sun D, Luo M, Jeong M, Rodriguez B, Xia Z, Hannah R, et al. Epigenomic Profiling of Young and Aged HSCs Reveals Concerted Changes during Aging that Reinforce Self-Renewal. Cell Stem Cell. 2014;14:673–88.
Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75.
Cabezas-Wallscheid N, Klimmeck D, Hansson J, Lipka DB, Reyes A, Wang Q, et al. Identification of Regulatory Networks in HSCs and Their Immediate Progeny via Integrated Proteome, Transcriptome, and DNA Methylome Analysis. Cell Stem Cell. 2014;15:507–22.
Wang Z, Emmel G, Lim HS, Zhu W, Kosters A, Ghosn EEB, et al. Stromal STAT5-Mediated Trophic Activity Regulates Hematopoietic Niche Factors. Stem Cells. 2023;41:944–57.
Baker SJ, Rane SG, Reddy EP. Hematopoietic cytokine receptor signaling. Oncogene. 2007;26:6724–37.
Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–61.
Kasbekar M, Mitchell CA, Proven MA, Passegué E. Hematopoietic stem cells through the ages: A lifetime of adaptation to organismal demands. Cell Stem Cell. 2023;30:1403–20.
Gao S, Wu Z, Kannan J, Mathews L, Feng X, Kajigaya S, et al. Comparative Transcriptomic Analysis of the Hematopoietic System between Human and Mouse by Single Cell RNA Sequencing. Cells. 2021;10:973.
Uras IZ, Maurer B, Nivarthi H, Jodl P, Kollmann K, Prchal-Murphy M, et al. CDK6 coordinates JAK2 (V617F) mutant MPN via NF-κB and apoptotic networks. Blood. 2019;133:1677–90.
Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–87.
Ho Y-H, del Toro R, Rivera-Torres J, Rak J, Korn C, García-García A, et al. Remodeling of Bone Marrow Hematopoietic Stem Cell Niches Promotes Myeloid Cell Expansion during Premature or Physiological Aging. Cell Stem Cell. 2019;25:407–18. e6.
Rundberg Nilsson A, Soneji S, Adolfsson S, Bryder D, Pronk CJ. Human and Murine Hematopoietic Stem Cell Aging Is Associated with Functional Impairments and Intrinsic Megakaryocytic/Erythroid Bias. Plos One. 2016;11:e0158369.
Chen E, Beer PA, Godfrey AL, Ortmann CA, Li J, Costa-Pereira AP, et al. Distinct Clinical Phenotypes Associated with JAK2V617F Reflect Differential STAT1 Signaling. Cancer Cell. 2010;18:524–35.
Kleppe M, Koche R, Zou L, van Galen P, Hill CE, Dong L, et al. Dual Targeting of Oncogenic Activation and Inflammatory Signaling Increases Therapeutic Efficacy in Myeloproliferative Neoplasms. Cancer Cell. 2018;33:29–43.e7.
Van Egeren D, Kamaz B, Liu S, Nguyen M, Reilly CR, Kalyva M, et al. Transcriptional differences between JAK2-V617F and wild-type bone marrow cells in patients with myeloproliferative neoplasms. Exp Hematol. 2022;107:14–9.
Kulkarni R. Early Growth Response Factor 1 in Aging Hematopoietic Stem Cells and Leukemia. Front Cell Develop Biol. 2022;10:92576141.
Desterke C, Bennaceur-Griscelli A, Turhan AG. EGR1 dysregulation defines an inflammatory and leukemic program in cell trajectory of human-aged hematopoietic stem cells (HSC). Stem Cell Res Ther. 2021;12:419.
Rouillard AD, Gundersen GW, Fernandez NF, Wang Z, Monteiro CD, McDermott MG, et al. The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database. 2016;2016:baw10064.
Colom Díaz PA, Mistry JJ, Trowbridge JJ. Hematopoietic stem cell aging and leukemia transformation. Blood. 2023;142:533–42.
Hatakeyama K, Kikushige Y, Ishihara D, Yamamoto S, Kawano G, Tochigi T, et al. Thrombospondin-1 is an endogenous substrate of cereblon responsible for immunomodulatory drug–induced thromboembolism. Blood Adv. 2024;8:785–96.
Petrik J, Lauks S, Garlisi B, Lawler J. Thrombospondins in the tumor microenvironment. Semin Cell Developmental Biol. 2024;155:3–11.
McLaughlin JN, Mazzoni MR, Cleator JH, Earls L, Perdigoto AL, Brooks JD, et al. Thrombin modulates the expression of a set of genes including thrombospondin-1 in human microvascular endothelial cells. J Biol Chem. 2005;280:22172–80.
Bellon M, Nicot C. Targeting Pim kinases in hematological cancers: molecular and clinical review. Mol Cancer. 2023;22:18.
Dutta A, Nath D, Yang Y, Le BT, Rahman MF-U, Faughnan P, et al. Genetic ablation of Pim1 or pharmacologic inhibition with TP-3654 ameliorates myelofibrosis in murine models. Leukemia. 2022;36:746–59.
Liu W, Pircher J, Schuermans A, Ul Ain Q, Zhang Z, Honigberg MC, et al. Jak2V617F clonal hematopoiesis promotes arterial thrombosis via platelet activation and cross talk. Blood. 2024;143:1539–50.
Yang M, Cooley BC, Li W, Chen Y, Vasquez-Vivar J, Scoggins NiO, et al. Platelet CD36 promotes thrombosis by activating redox sensor ERK5 in hyperlipidemic conditions. Blood. 2017;129:2917–27.
Guadall A, Lesteven E, Letort G, Awan Toor S, Delord M, Pognant D, et al. Endothelial Cells Harbouring the JAK2V617F Mutation Display Pro-Adherent and Pro-Thrombotic Features. Thromb Haemost. 2018;118:1586–99.
Farina M, Russo D, Hoffman R. The possible role of mutated endothelial cells in myeloproliferative neoplasms. Haematologica. 2021;106:2813–23.
Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL–AF9. Nature. 2006;442:818–22.
the Analysis of Czech Genome for Theranostics (A-C-G-T) project [Internet]. [cited 29.11.2024]. Available from: https://database.acgt.cz/.
NCBI SNP database - National Institutes of Health (NIH) [Internet]. [cited 29.11.2024]. Available from: https://www.ncbi.nlm.nih.gov/snp/rs41316003#frequency_tab
Perner F, Perner C, Ernst T, Heidel FH. Roles of JAK2 in Aging, Inflammation, Hematopoiesis and Malignant Transformation. Cells. 2019;8:854.
Akada H, Akada S, Hutchison RE, Sakamoto K, Wagner K-U, Mohi G. Critical Role of Jak2 in the Maintenance and Function of Adult Hematopoietic Stem Cells. Stem Cells. 2014;32:1878–89.
Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9:115–28.
Nauseef WM. Human neutrophils ≠ murine neutrophils: Does it matter?. Immunol Rev. 2023;314:442–56.
Bogeska R, Mikecin A-M, Kaschutnig P, Fawaz M, Büchler-Schäff M, Le D, et al. Inflammatory exposure drives long-lived impairment of hematopoietic stem cell self-renewal activity and accelerated aging. Cell Stem Cell. 2022;29:1273–84. e8.
Caiado F, Pietras EM, Manz MG. Inflammation as a regulator of hematopoietic stem cell function in disease, aging, and clonal selection. J Exp Med. 2021;218.
Alvarez JV, Frank DA. Genome-wide analysis of STAT target genes: Elucidating the mechanism of STAT-mediated oncogenesis. Cancer Biol Ther. 2004;3:1045–50.
Schiavone D, Avalle L, Dewilde S, Poli V. The immediate early genes Fos and Egr1 become STAT1 transcriptional targets in the absence of STAT3. FEBS Lett. 2011;585:2455–60.
Tian S, Tapley P, Sincich C, Stein R, Rosen J, Lamb P. Multiple signaling pathways induced by granulocyte colony-stimulating factor involving activation of JAKs, STAT5, and/or STAT3 are required for regulation of three distinct classes of immediate early genes. Blood. 1996;88:4435–44.
Kulkarni PP, Ekhlak M, Singh V, Kailashiya V, Singh N, Dash D Fatty acid oxidation fuels agonist-induced platelet activation and thrombus formation: Targeting β-oxidation of fatty acids as an effective anti-platelet strategy. FASEB J.2023;37:e22768.
Gangaraju R, Song J, Kim SJ, Tashi T, Reeves BN, Sundar KM, et al. Thrombotic, inflammatory, and HIF-regulated genes and thrombosis risk in polycythemia vera and essential thrombocythemia. Blood Adv. 2020;4:1115–30.
Snoeck HW. Direct megakaryopoiesis. Curr Opin Hematol. 2025;32:213–20.
Ramalingam P, Butler J, Poulos M. Endothelial mTOR Preserves Hematopoietic Stem Cell Fitness By Suppressing Thrombospondin1. Blood. 2022;140:1678.
Schwemmers S, Will B, Waller CF, Abdulkarim K, Johansson P, Andreasson B, et al. JAK2V617F-negative ET patients do not display constitutively active JAK/STAT signaling. Exp Hematol. 2007;35:1695–703.
Burocziova M, Grusanovic S, Vanickova K, Kosanovic S, Alberich-Jorda M. Chronic inflammation promotes cancer progression as a second hit. Exp Hematol. 2023;128:30–7.
Bellanné-Chantelot C, Rabadan Moraes G, Schmaltz-Panneau B, Marty C, Vainchenker W, Plo I. Germline genetic factors in the pathogenesis of myeloproliferative neoplasms. Blood Rev. 2020;42:100710.
Acknowledgements
This work was supported by the Czech Health Research Council (NU21/03/00338), the project National Institute for Cancer Research (Programme EXCELES, ID Project No. LX22NPO5102) - Funded by the European Union - Next Generation EU, and by the Czech Science Foundation, 24-11730S. Further support to animal facility BIOCEV was provided from these projects: LM 2018126 Czech Center for Phenogenomics by the Ministry of Education, Youth and Sports (MEYS CR) and OP RDE CZ.02.1.01/0.0/0.0/18_046/0015861 CCP Infrastructure Upgrade II by MEYS and ESIF; further support to team members from Masaryk University was provided from these projects: Ministry of Health of Czech Republic project FNBr 65269705 and MEYS CR European Regional Development Fund Project ACGT No. CZ.02.1.01/0.0/0.0/16_026/0008448. We thank J. Labaj and J. Balounova (both Czech Center for Phenogenomics) for their assistance with the phenotyping analyzes and O. Babosova for assistance with immunoprecipitation assays. We also thank M. Perina (Department of Experimental Biology, Faculty of Science, Palacky University, Olomouc) for structural modeling of JAK2 interactions with ruxolitinib, using AlphaFold 3.0. We also acknowledge the CF Genomics CEITEC MU supported by the NCMG research infrastructure (LM2023067 funded by MEYS CR).
Author information
Authors and Affiliations
Contributions
V.Z. performed the research, analyzed data, and contributed to the manuscript writing; M.B. and S.G. performed transplantation mice experiments and helped with mice analyzes, J.G. performed mice related in vitro assays, L.J. contributed to murine progenitor in vitro assays and to some data analyses, P.K. designed the CRISPR/Cas9 targeting and help with mouse model generation, A.P. conducted the Seahorse experiments, L.B. and D.K. analyzed RNA-seq data, D.H. and J.O. performed pulmonary endothelial cells analysis, M.A.-J. and V.K. provided financial support, I.J. performed mutational analyses of patient samples, L.N. performed statistical analysis of clinical data, B.W. was responsible for patients’ samples collection, S.P. was responsible for ACGT project management, M.D. was responsible for coordination of the clinical part of the study, V.D. provided financial support, analyzed the data and wrote the manuscript, L.L. provided financial support, performed the research, designed the experiments, analyzed data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zimolova, V., Burocziova, M., Berkova, L. et al. Germline Jak2-R1063H mutation interferes with normal hematopoietic development and increases risk of thrombosis and leukemic transformation. Leukemia 39, 2745–2757 (2025). https://doi.org/10.1038/s41375-025-02737-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41375-025-02737-w