Abstract
Myelodysplastic syndromes with somatic mutations in the splicing factor SF3B1 gene (MDS-SF3B1) result in RNA mis-splicing, erythroid dysplasia and ultimately refractory anemia. Precision medicine approaches for MDS-SF3B1 remain challenging due to both the complexity of the mis-splicing landscape and its evaluation in disease-accurate models. To uncover novel RNA mis-splicing events, isogenic SF3B1K700E and SF3B1WT iPSC lines from an MDS-SF3B1 patient were differentiated into hematopoietic cells and analyzed via unsupervised splicing event profiling using full-length RNA sequencing. This identified SF3B1K700E-specific mis-splicing of ubiquitin-like modifier activating enzyme 1 (UBA1), which encodes a key E1 protein at the apex of the ubiquitination cascade. UBA1 mis-splicing (UBA1ms) introduced protein instability and decreased total UBA1 levels, rendering mutated cells susceptible to the small-molecule UBA1 inhibitor TAK-243. Analysis of CD34+ RNA sequencing data from an MDS patient cohort confirmed unique and ubiquitous UBA1ms in MDS-SF3B1 patients, absent in other splicing factor-mutated MDS cases or healthy controls. TAK-243 selectively targeted MDS-SF3B1 primary CD34+ cells and reduced mutant cell numbers in colony-forming assays. In contrast, normal hematopoietic progenitor cells were unaffected. Altogether, we here define UBA1ms as a novel therapeutic vulnerability in SF3B1-mutant cells, introducing UBA1 inhibition as a potential avenue for future MDS-SF3B1 treatments.

Similar content being viewed by others
Introduction
Myelodysplastic neoplasms (MDS) are clonal myeloid malignancies that originate in hematopoietic stem cells, characterized by clonal expansion and ineffective hematopoiesis [1, 2]. Heterozygous, recurrent point mutations in splicing factor genes, such as SF3B1, SRSF2, and U2AF1, are the most common variants in MDS patients and result in widespread splicing defects [3, 4].
MDS with SF3B1 mutation (MDS-SF3B1) constitute a distinct subgroup of MDS, characterized by bone marrow accumulation of mutant dysplastic erythroblasts (ring sideroblasts), erythroid cytopenia, and refractory anemia [5,6,7,8,9,10]. SF3B1 encodes subunit 1 of the core RNA splicing factor 3b, involved in processing precursor mRNA to mature transcripts [11, 12]. Missense mutations in this gene alter the interaction of SF3B1 with the pre-mRNA sequence, resulting in extensive cryptic 3' splice site usage and mis-splicing of genes important for hematopoietic and erythroid differentiation, including ABCB7, ALAS2, MAP3K7, PPOX, and TMEM14C [13,14,15,16,17,18,19].
Disease-modifying therapies for MDS-SF3B1 are limited. Therapeutic target studies using experimental disease models are complicated by the dynamic nature of RNA mis-splicing in MDS: mouse models of SF3B1 mutation develop anemia but feature distinct RNA splicing from humans [20,21,22]; primary patient material is heterogeneous, has limited availability and in vitro longevity; and cell models do not fully recapitulate the complex splicing repertoire.
Patient-derived induced pluripotent stem cells (iPSCs) are scalable, tractable, and genetically faithful models, which are being increasingly utilized to understand myeloid pathobiology [23,24,25,26,27,28,29,30]. In a previous study, we established isogenic iPSC lines from MDS-SF3B1 patients and identified a signature of splicing events associated with mutant SF3B1 in hematopoietic stem and progenitor cells (HSPCs) [23]. Other work has further confirmed a splicing program paralleling the primary disease, supporting the use of SF3B1-mutant iPSCs as a physiologically relevant model [24].
In this study, we utilized RNA sequencing and splicing analysis to identify a previously unreported mis-splicing event affecting the ubiquitin-activating enzyme 1 (UBA1) gene specific to SF3B1 mutations. UBA1 is located on the X chromosome and encodes an essential multidomain protein that initiates the ubiquitination cascade, targeting proteins for degradation via the ubiquitin-proteasome system through the activation and transfer of ubiquitin to E2 enzymes. UBA1 is critical for cell survival, and is involved in protein folding and turnover, ER stress response, and DNA damage response [31,32,33]. Targeting the ubiquitin-proteasomal pathway, including UBA1, has been investigated for the treatment of hematologic malignancies and other cancers [34, 35]. We demonstrate that SF3B1 mutation-associated UBA1 mis-splicing (UBA1ms) leads to protein instability, resulting in reduced total UBA1 protein levels in SF3B1K700E cell lines. This, in turn, sensitizes SF3B1-mutant cells to the UBA1 inhibitor TAK-243. We further confirm that UBA1ms is unique to SF3B1-mutant patients in a large MDS cohort [36], and similarly enhances TAK-243 susceptibility in colony-forming assays using primary patient cells. Collectively, our findings establish UBA1ms as a targetable therapeutic vulnerability in MDS-SF3B1.
Materials and methods
Patient-derived iPSCs
The generation and characterization of human iPSC lines MDS-22.44 (SF3B1K700E) and N-22.45 (SF3B1WT), from a female MDS patient with ring sideroblasts, single-lineage dysplasia and an isolated, heterozygous SF3B1K700E variant, were previously described [23]. iPSCs were cultured on Matrigel hESC-Qualified Matrix (Corning) in mTeSR Plus (StemCell Technologies) and 1% Penicillin-Streptomycin (P/S; HyClone) and clump passaged with EZ-LiFT Stem Cell Passaging Reagent (Sigma-Aldrich). HSPCs were generated using STEMdiff Hematopoietic Kit (StemCell Technologies) and harvested on day 12. CD34+ cells were enriched using CD34 MicroBeads and positive selection with the autoMACS Pro Separator (Miltenyi Biotec). Erythroid differentiation and RNA sequencing can be found in Supplementary Methods.
Cell culture
K562 human erythroleukemia cells with a heterozygous knock-in SF3B1K700E mutation and the parental SF3B1WT line were purchased from Horizon Discovery and cultured in RPMI 1640 Medium with 10% heat-inactivated fetal bovine serum (FBS; Gibco) and 1% P/S. Media was replaced every other day. HEK293T human embryonic kidney cells were maintained in DMEM, high glucose, GlutaMAX (Gibco), containing 10% newborn calf serum (Gibco) and 1% P/S. All cells were confirmed to be Mycoplasma-negative regularly.
Primary sample collection and ethical approval
Bone marrow samples were obtained from three patients with MDS-SF3B1 and two healthy normal bone marrow (NBM) donors at Karolinska University Hospital, Huddinge, Sweden. All source material was provided with written informed consent for research use, in accordance with the Declaration of Helsinki, and the study was approved by the Ethics Research Committee at Karolinska Institutet (2017/1090-31/4, 2022-03406-02 and 2024-03119-02).
RNA splicing analysis
RNA sequencing data was assessed as previously described [8]. Briefly, differential splicing analysis was performed using rMATS v. 4.1.1 [37], with p-values calculated using the likelihood-ratio test (LRT) and adjusted with the Benjamini-Hochberg method. Sashimi plots for visualization were generated using ggsashimi v. 1.1.5 [38].
UBA1 expression plasmids
Human UBA1 transcript variant 1 (WT) cDNA open reading frame (ORF) clone (accession number: NM_003334.4, CloneID: OHu24932) in a pcDNA3.1 + /C-(K)-DYK expression vector; pcDNA3.1 + C-eGFP (eGFP); and pcDNA3.1(+) (empty vector control) were purchased from GenScript. UBA1ms cDNA ORF clone, including the 135 extra bases (MS), was subcloned into UBA1_OHu24932D_pcDNA3.1 + /C-(K)-DYK using GenScript’s gene synthesis and subcloning service. Plasmid transfection was achieved using Lipofectamine 2000 transfection reagent (Invitrogen) following manufacturer’s instructions. In brief, 2×105 cells were seeded into 24-well culture plates in growth medium without P/S the day before transfection and cultured to ≥70% confluency. Lipofectamine complexes were prepared with 5 µg of UBA1 WT, UBA1 MS variant, pcDNA3.1, or eGFP plasmid in Opti-MEM (Gibco). RNA was collected after 48 h and protein at 72 h. See Supplementary Table 1 for additional details.
Statistics
Statistical analysis was performed with Prism (v10.2, GraphPad). Data are shown as mean with standard error (SEM) unless otherwise noted. Numerical variables were compared using unpaired t-test, one-way analysis of variance (ANOVA), or two-way ANOVA, as indicated in the figure legends. For multiple test correction, p-values were adjusted by Šídák’s, Holm-Šídák’s, Tukey’s, or Dunnett’s multiple comparisons test, as specified.
Supplementary methods
Additional details of the materials and methods are provided in Supplementary Methods.
Results
SF3B1 mutation induces UBA1 ms in MDS-SF3B1
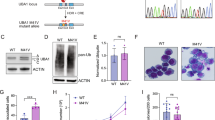
New treatment strategies for MDS-SF3B1 require a deepened understanding of mis-splicing and the molecular consequences in sculpting the pathophysiology of MDS. Here, we employed isogenic iPSC lines originally generated from an MDS-SF3B1 patient [23], harboring an isolated, heterozygous SF3B1K700E mutation (Fig. 1A), the most recurrent variant among MDS-SF3B1 cases [10]. To model MDS-SF3B1 in vitro, we performed hematopoietic differentiation of wildtype (SF3B1WT) and mutant (SF3B1K700E) iPSCs for twelve days and analyzed surface expression of HSPC markers CD34 and CD45 by flow cytometry (Fig. 1B, left). Consistent with our previous observations, SF3B1K700E HSPCs showed lower viability (supplementary Fig. 1A) but no defects in hematopoietic specification, with similar levels of CD34+ cells compared to SF3B1WT (supplementary Fig. 1B). As before, SF3B1K700E iPSCs exhibited reduced erythroid potential compared with SF3B1WT cells (Fig. 1B, right) [23]. To examine SF3B1 mutation-associated mis-splicing and uncover novel RNA mis-splicing events, we performed 5'-based full-length RNA sequencing of iPSC-derived erythroid cells to capture both immature and mature transcripts. Splicing analysis confirmed aberrant splicing of known genes, including TMEM14C and MAP3K7, in SF3B1K700E but not SF3B1WT cells (supplementary Fig. 1C), consistent with prior observations and findings in SF3B1-mutated patients [16, 24, 39]. Moreover, in this analysis we identified an RNA mis-splicing event of the UBA1 transcript (59.3 percent spliced-in [PSI]) caused by the in-frame retention of an intronic region of 135 bases between exons 5 and 6 (Fig. 1C and supplementary Fig. 1D). UBA1 encodes ubiquitin-activating enzyme E1, which catalyzes the first step of the ubiquitination pathway, and is necessary for protein homeostasis. Examination of this intron revealed an enrichment of cryptic SF3B1 binding sites, rendering it vulnerable to misrecognition (supplementary Fig. 1E and supplementary Table 4). We hypothesized that the non-conserved reads detected by RNA sequencing in SF3B1WT resulted from intronic sequences in pre-mRNA, and utilized RT-qPCR for the UBA1ms transcript to confirm that it is exclusive to SF3B1K700E cells (supplementary Fig. 1F). As SF3B1 mutations originate in the stem cell population [40], we assessed iPSC-derived CD34+ HSPCs and erythroid progenitors and observed that SF3B1K700E but not SF3B1WT cells expressed UBA1ms (Fig. 1D, E), accounting for approximately 20% of total UBA1 transcripts. To visualize the intronic sequence retention in CD34+ HSPCs, RT-PCR and gel electrophoresis of the UBA1 exon 5-6 junction was performed (supplementary Fig. 1F). A second PCR product was generated in SF3B1-mutated cells, corresponding to the 135-base pair size difference resulting from mis-splicing, which was absent in SF3B1WT (Fig. 1F and supplementary Fig. 1G). Next, we investigated whether UBA1ms affects protein expression in iPSC-derived CD34+ HSPCs. UBA1 encodes two main isoforms: nuclear UBA1a and cytosolic UBA1b. Immunoblotting against UBA1a/b revealed significantly reduced total UBA1 protein levels in SF3B1K700E compared to SF3B1WT cells (Fig. 1G, H). These results uncover previously unreported mis-splicing of UBA1 in an in vitro model of MDS-SF3B1 and suggest a decrease in UBA1 protein content in SF3B1-mutant cells.
A Origin of iPSC lines and experimental overview. B Representative flow cytometry diagrams of SF3B1WT and SF3B1K700E iPSC-derived cells after 12 days of hematopoietic differentiation (left) and another 14 days of erythroid culture (right). Colored gates indicate cells used in downstream analyses. C Sashimi plots of the mis-spliced region of UBA1 in SF3B1WT and SF3B1K700E from total RNA sequencing of iPSC-derived GlyA+ erythroblasts (n = 1). Black, canonical splice junction counts; orange, mis-spliced junction counts. y-axis, absolute read counts. D qPCR analysis of UBA1ms relative to 18S in CD34+ HSPCs (n = 4) and E erythroblasts (nWT = 6; nK700E = 7) derived from SF3B1WT and SF3B1K700E iPSCs. Mean ± SEM relative expression. Unpaired t-test. F Agarose gel electrophoresis of the PCR-amplified exon 5-6 mis-spliced region of UBA1 in iPSC-derived CD34+ cells (n = 4). The lower band corresponds to the PCR product of canonically spliced, and the upper band to mis-spliced UBA1. G Immunoblot analysis and H quantification of UBA1a/b protein levels in iPSC-derived CD34+ cells (n = 4). Actin was used as a loading control and relative signals were normalized by lane normalization factor. Mean ± SEM relative UBA1 signal intensity. Unpaired t-test with Holm-Šídák’s multiple comparisons test. *, P ≤ 0.05; ***, P ≤ 0.001. The numbers (#) below the blot indicate experimental repeats. VAF variant allele frequency, PSI percent spliced-in, RNA seq RNA sequencing, bp base pairs.
UBA1 ms decreases UBA1 protein in SF3B1 K700E cells
To investigate whether UBA1ms is a specific consequence of SF3B1 mutation, we utilized the SF3B1K700E K562 human erythroleukemia cell line. Similar to iPSCs, K562 cells harboring SF3B1 mutation recapitulate the splicing landscape of primary MDS [17, 19]. First, analysis of a recently published full-length K562 RNA sequencing dataset also displayed the UBA1ms event in SF3B1K700E but not in SF3B1WT K562 cells, which was not reported by the authors (Fig. 2A) [41]. As before, RT-qPCR of UBA1ms and visualization of the retained intron by gel electrophoresis confirmed that UBA1ms is specific to SF3B1K700E (Fig. 2B and supplementary Fig. 2A). RNA mis-splicing induced by mutant SF3B1 can trigger nonsense-mediated decay (NMD) when a premature termination codon is introduced, disrupting ribosome dynamics [13]. However, in this case, the cryptic exon resulting from UBA1ms does not contain an in-frame premature stop codon. Experiments using actinomycin D (ActD) to inhibit transcription in SF3B1K700E K562 cells confirmed that UBA1ms does not impair mRNA stability. RT-qPCR analysis revealed that UBA1ms, like canonically spliced UBA1 (UBA1WT), remains stable, whereas short-lived MYC mRNA undergoes rapid degradation (Fig. 2C). Additionally, inhibition of NMD with cycloheximide (CHX) did not result in an increase in UBA1ms (Fig. 2D, E). In contrast, the well-characterized NMD target resulting from mis-splicing of ABCB7 was only detectable upon NMD inhibition, as shown by gel electrophoresis (Fig. 2E), consistent with previous findings [39].
A Sashimi plots of the UBA1ms region from total RNA sequencing of SF3B1WT and SF3B1K700E K562 cells from previously published data [39]. B qPCR analysis of UBA1ms relative to 18S in SF3B1WT and SF3B1K700E K562 cells (n = 3). Mean ± SEM relative expression. Unpaired t-test. C qPCR analysis of UBA1WT and UBA1ms transcript levels in SF3B1K700E K562 cells after treatment with actinomycin D (ActD) for the indicated time points (n = 3). Results were normalized to 0 h, and MYC was included as a fast-degrading transcript control. Mean ± SEM relative expression, One-phase decay nonlinear curve fit (dotted line). D qPCR analysis of UBA1WT and UBA1ms in SF3B1WT and SF3B1K700E K562 cells after treatment with cycloheximide (CHX) for 4 h (n = 3). The fraction of UBA1 splice forms is shown within the bars. Mean ± SEM relative expression. Two-way ANOVA with Tukey’s multiple comparisons test. E Agarose gel electrophoresis of the PCR-amplified mis-spliced region of UBA1 (top) and ABCB7 (bottom) in SF3B1WT and SF3B1K700E K562 cells. The lower bands correspond to the PCR product of canonically spliced RNA, and the upper bands to mis-spliced RNA. Representative image from three experimental repeats. F Representative polysome profiles of SF3B1WT and SF3B1K700E K562 cells recorded at 254 nm. Pooled monosome and polysome fractions are indicated. G qPCR analysis of total UBA1, UBA1WT, and UBA1ms RNA transcript distribution in monosome and polysome fractions from SF3B1WT and SF3B1K700E K562 cells (n = 5). Housekeeping gene transcript distribution can be found in Supplementary Fig. 2C. Mean ± SEM fraction. Two-way ANOVA with Tukey’s multiple comparisons test. H Immunoblot analysis and I quantification of UBA1 isoforms in whole cell lysates from SF3B1WT and SF3B1K700E K562 cells (n = 3). Actin was used as a loading control for total UBA1 and UBA1b; Lamin B1 was used as a loading control for nuclear UBA1a, and relative signals were normalized by lane normalization. Mean ± SEM relative signal intensity. Unpaired t-test with Holm-Šídák’s multiple comparisons test. *, P ≤ 0.05; **, P ≤ 0.01; ns not significant.
To determine whether UBA1ms affects ribosome recruitment, we performed polysome profiling and RT-qPCR on monosome and polysome fractions from SF3B1K700E and SF3B1WT K562 cells (Fig. 2F). UBA1 transcripts were predominantly associated with the polysome fraction, indicating active translation irrespective of splice variant, unlike NMD targets, which are typically depleted from polysomes [42, 43]. Moreover, the distribution of UBA1 transcripts across fractions remained consistent between mutant and wild-type cells, reflecting patterns observed in housekeeping genes (Fig. 2G and supplementary Fig. 2B). However, similar to our findings in iPSC-derived CD34+ cells, total UBA1 protein levels in SF3B1K700E K562 cells were significantly reduced compared to SF3B1WT cells, owing to lower levels of both UBA1a and UBA1b isoforms (Fig. 2H, I). In summary, these data validate SF3B1 mutation-specific UBA1ms in an independent cell model and demonstrate decreased total UBA1 protein levels in mutant cells despite stable transcript levels and intact ribosome assembly.
Mis-splicing of UBA1 compromises protein stability
To determine the consequence of UBA1ms on protein stability, we first modeled its structure in silico. AlphaFold2 was used to predict protein folding of the 1058 amino acid UBA1a isoform from full-length UBA1 (UBA1WT) cDNA (ENST00000377351.8) and UBA1ms including the predicted 45 amino acid sequence (supplementary Fig. 1D). The insertion resulted in low sequence coverage, low predicted local distance difference test score (< 50%), and high predicted aligned error (supplementary Fig. 3A–C). Consequently, the model failed to confidently predict folding of amino acid residues from UBA1ms (supplementary Fig. 3D).
To experimentally test whether UBA1ms compromises protein stability, we designed expression plasmids for UBA1WT (WT) and UBA1ms (MS) fused to a C-terminal FLAG tag for transfection of HEK293T cells and subsequent assessment of recombinant protein levels (Fig. 3A and supplementary Fig. 4A). Total UBA1 transcript levels, measured by RT-qPCR after 48 h, were elevated in cells transfected with WT or MS plasmids compared to control, and expression of UBA1ms was exclusive to UBA1 MS-transfected cells (Fig. 3B). We next validated high transfection efficiency and cell viability at 72 h post-transfection (supplementary Fig. 4B, C), followed by immunoblotting to examine recombinant UBA1 translation. These results revealed one FLAG band corresponding to the predicted product from the ORF. Interestingly, FLAG-tagged UBA1 protein levels were significantly lower in MS- compared to WT-transfected cells, which was recapitulated in UBA1a/b expression levels (Fig. 3C–E). To determine whether this reduction in UBA1 levels result from impaired protein stability, we performed CHX chase assays of cells transfected with UBA1 WT or MS plasmid. Immunoblotting against FLAG at the indicated time points revealed that UBA1WT protein levels remained stable, consistent with the reported half-life exceeding 100 h [44], whereas UBA1ms protein displayed rapid decay, with a calculated half-life of 2.5 h (Fig. 3F, G). We confirmed proteasomal degradation of UBA1ms protein by co-treating transfected cells with CHX and the proteasome inhibitor MG-132. This partially restored FLAG-tagged UBA1ms protein levels, mirroring the stabilization of the short-lived endogenous cMYC protein, which served as a positive control (Fig. 3H, I). Together, these data show that UBA1 protein produced from mis-spliced mRNA is unstable and degraded, explaining the reduction in total UBA1 levels in models of mutant SF3B1.
A Maps for ORF cDNA in pcDNA3.1 + /C-(K)-DYK plasmids for UBA1 transcript variant 1 (WT) and UBA1ms (MS) and experimental design for evaluation of UBA1ms in HEK293T cells. B qPCR analysis of UBA1 transcript levels in HEK293T cells 48 h following transfection with WT or MS relative to pcDNA3.1 control transfected cells. The fraction of UBA1 splice forms is shown within bars (n = 3). Mean ± SEM relative fold change. Two-way ANOVA with Tukey’s multiple comparisons test. C Immunoblot analysis and quantification of D FLAG-tagged and E UBA1a/b protein levels in HEK293T cells 72 h after transfection with WT, MS or control. Actin was used as a loading control and the highest relative FLAG and lowest relative UBA1a/b signals were used to normalize results (n = 4). Mean ± SEM relative signal intensity. Welch ANOVA with Dunnett’s multiple comparisons test. F Representative immunoblot analysis and G quantification of FLAG-tagged protein levels in HEK293T cells 72 h post-transfection with WT, MS, or control plasmids and treatment with 50 µg/ml cycloheximide (CHX) for the indicated time points (n = 3). Actin was used as a loading control and signals were normalized to relative signals at 0 h for each group. Mean ± SEM relative signal intensity, interpolation of a one-phase decay non-linear regression curve (dotted line). H Representative immunoblot analysis and I quantification of FLAG-tagged UBA1ms and cMYC protein levels in HEK293T cells 72 h post-transfection with the MS plasmid and treatment with 50 µg/ml cycloheximide (CHX) and 10 µM MG-132 for 6 h (n = 4). Actin was used as a loading control and signals were normalized to untreated controls. Mean ± SEM relative signal intensity. Two-way ANOVA with Tukey’s multiple comparisons test. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ns not significant.
SF3B1 mutation sensitizes K562 cells to UBA1 inhibition
UBA1 plays a critical role in cellular proteostasis and loss of UBA1 induces cell death [32, 33, 45], indicating a minimum level of UBA1 activity is required for cell survival. We hypothesized that a reduction in UBA1 protein content in SF3B1-mutant cells would confer higher sensitivity to targeted UBA1 inhibition compared to SF3B1WT cells. To test this, the selective UBA1 inhibitor TAK-243 [34] (Fig. 4A) was employed to investigate the effect of UBA1 inhibition on cell viability, clonal competition, and colony-forming unit (CFU) potential in SF3B1-mutated cells. First, we treated SF3B1WT and SF3B1K700E K562 cells with increasing concentrations of TAK-243 and conducted flow cytometry and luminescence-based assays after 72 h to determine IC50. TAK-243 induced apoptosis in a concentration-dependent manner and, importantly, with significantly higher sensitivity in SF3B1K700E K562 cells (Fig. 4A, B and supplementary Fig. 5A–D). Additionally, TAK-243 reduced K48-linked ubiquitin levels in a dose-dependent manner and led to a marked increase in apoptosis-associated cleaved PARP1 and cleaved Caspase-3 in SF3B1K700E compared to wild-type (supplementary Fig. 5E–J). We confirmed UBA1 specificity, as mutated cells did not exhibit differential sensitivity to other commonly used drugs to treat MDS and AML, cytarabine, daunorubicin, pladienolide B, and venetoclax, compared to SF3B1WT cells (supplementary Fig. 6A–D). Furthermore, small interfering RNA (siRNA)-mediated knockdown followed by TAK-243 exposure significantly reduced the IC50 compared to control-transfected SF3B1WT and SF3B1K700E cells (Fig. 4C–F supplementary Fig. 6E), further supporting the selective vulnerability of SF3B1-mutant cells to UBA1 inhibition.
A Dose-response curves of SF3B1WT and SF3B1K700E K562 cells treated with TAK-243 or DMSO for 72 h (n = 3). Chemical structure of the small-molecule UBA1 inhibitor TAK-243 (MLN7243) displayed under the curve. Data points represent mean ± SEM live cell fractions, normalized to control-treated samples. Interpolated sigmoidal, four parameter logistic (4PL), standard curves of mean (solid line) and 95% confidence interval (dotted line). Live cells were defined as Aqua/Apotracker Green double negative singlets, assessed by flow cytometry (see supplementary Fig. 5A for gating strategy). IC50 of TAK-243 are quantified in B. Mean ± SEM nM TAK-243. Unpaired t-test. C Immunoblot analysis and D quantification of UBA1a/b protein levels in SF3B1WT and SF3B1K700E K562 cells 72 h after transfection with negative control siRNA (control) or UBA1 siRNA (siUBA1). Actin was used as a loading control and results were normalized to the highest relative signal. Mean ± SEM relative UBA1a/b signals. Two-way ANOVA with Tukey’s multiple comparisons test. E Dose-response curves of SF3B1WT and SF3B1K700E K562 cells 72 h after transfection with negative control siRNA (control) or UBA1 siRNA (siUBA1), treated with TAK-243 or DMSO for 72 h (n = 3). Data points represent mean ± SEM live cell fractions, normalized to control-treated samples. Interpolated sigmoidal, 4PL, standard curves of mean (solid line) and 95% confidence interval (dotted line). Live cells were defined as Aqua/Apotracker Green double negative singlets, assessed by flow cytometry. IC50 of TAK-243 are quantified in F. Mean ± SEM nM TAK-243. Two-way ANOVA with Tukey’s multiple comparisons test. G Experimental strategy to assess the effect of targeted UBA1 inhibition by TAK-243 treatment on SF3B1WT and SF3B1K700E cells. H SF3B1K700E VAF in co-cultures of 1:1 SF3B1WT and SF3B1K700E K562 cells after 72 h of treatment with 50 nM TAK-243 or DMSO, as determined by ddPCR (n = 3). Mean ± SEM. Unpaired t-test. I CFU counts per 1000 seeded SF3B1WT and SF3B1K700E K562 cells treated with indicated concentrations of TAK-243 for 7 days (nDMSO = 7, n15 nM = 5, n32 nM = 3; n50 nM = 4). Mean ± SEM. Two-way ANOVA with Dunnett’s multiple comparisons test. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ns not significant.
We next assessed the sensitivity of SF3B1-mutated K562 cells to TAK-243 through competitive culture between wild-type and mutant cells with short-term, 72-h TAK-243 treatment (quantification of variant allele frequency [VAF] by droplet digital PCR [ddPCR]), as well as CFU assays with continuous TAK-243 exposure for seven days (Fig. 4G). Indeed, TAK-243 significantly reduced SF3B1K700E mutational burden in co-cultures, albeit with modest effect (Fig. 4H), likely attributed to the proliferative advantage of SF3B1WT cells in vitro [23] (supplementary Fig. 6F), as supported by a reduction in SF3B1K700E VAF after 72 h in control conditions. In addition, SF3B1K700E cells exhibited lower overall CFU potential compared to SF3B1WT (Fig. 4I), consistent with previous findings [23]. UBA1 inhibition affected CFU potential in both SF3B1K700E and SF3B1WT cells in a concentration-dependent manner. TAK-243 treatment with 32 nM or 50 nM induced a significantly differential effect on CFU potential, but 50 nM severely impaired wild-type cells (supplementary Fig. 6G). Notably, the calculated IC50 values reflected the enhanced sensitivity of SF3B1-mutated cells to UBA1 inhibition compared to SF3B1WT (supplementary Fig. 6G). Together, these results define a therapeutic window in which SF3B1K700E cells, but not SF3B1WT cells, are selectively sensitized to UBA1 inhibition, providing an opportunity to diminish the SF3B1-mutant population while preserving wild-type clones.
UBA1 ms in MDS-SF3B1 patients confers sensitivity to targeted UBA1 inhibition
We further probed the increased sensitivity of SF3B1-mutated cells to UBA1 inhibition by treating patient iPSC-HSPCs with increasing concentrations of TAK-243 over 24 h and measuring cell viability by flow cytometry (Fig. 5A and supplementary Fig. 7A). As before, TAK-243 was more potent at inducing cell death in SF3B1K700E iPSC-HSPCs compared to SF3B1WT cells (supplementary Fig. 7B), resulting in a significantly lower IC50 in SF3B1K700E HSPCs (Fig. 5B). Importantly, splicing analysis of RNA sequencing data from bone marrow mononuclear cells (BM MNC) of the original MDS-SF3B1 patient used for the iPSC reprogramming [23] confirmed that UBA1ms is a common event in both the mutant cell line and the primary cells (Fig. 5C). To evaluate the clinical relevance of our findings and to verify how UBA1ms is recapitulated in patients, we performed splicing analysis in our previously reported MDS cohort [36]. Specifically, we examined the mis-splicing event in 124 patients with MDS with ring sideroblasts and a VAF ≥ 20% (SF3B1mt, n = 83; SRSF2mt, n = 15; U2AF1mt, n = 4; and splicing factorWT, n = 22) compared to healthy donors (n = 16) using full-length total RNA sequencing data from CD34+ BM MNCs. UBA1ms was abundant (57.31 ± 1.78 PSI) and exclusive to SF3B1-mutated patients but not present in other splicing factor-mutated or wild-type cases nor in healthy donors (Fig. 5D, E). To validate these results, we assessed an independent long-read single-cell RNA sequencing dataset [46]. Although the UBA1ms event was not originally reported, splicing analysis confirmed it to be exclusive to SF3B1-mutated cases (supplementary Fig. 8A). This transcriptomic data also enabled verification that UBA1ms results in a full-length transcript. Total UBA1 transcript levels were not significantly different between CD34+ cells from sex-matched MDS-SF3B1 cases and healthy donors in our cohort. However, UBA1 transcript levels were overall higher in female compared to male individuals (supplementary Fig. 8B), likely due to an escape from X inactivation [47].
A Experimental strategy to assess the effect of UBA1 inhibition by TAK-243 on the viability of HSPCs derived from SF3B1WT and SF3B1K700E iPSCs. B IC50 of TAK-243 in SF3B1WT and SF3B1K700E iPSC-derived HSPCs treated with TAK-243 or DMSO for 24 h (n = 3), quantified from dose-response curves in supplementary Fig. 7B. Mean ± SEM. Unpaired t-test. C Sashimi plots of the mis-spliced region in UBA1 from total RNA sequencing of SF3B1K700E iPSC-derived erythroblasts and primary CD34+ BM MNCs from the original MDS-SF3B1 patient. D Sashimi plots of read counts of the mis-spliced region in UBA1 in MDS-RS patients and healthy donors, grouped by splicing factor mutation status, from our previously published data [36] (nSF3B1 = 83, nSRSF2 = 15, nU2AF1 = 4, nSFWT = 22, nNBM = 16). E Violin plots of UBA1 intron 5 mis-splicing PSI from total RNA sequencing of CD34+ BM MNCs from the same patient cohort, organized by splicing factor mutation. F qPCR analysis of UBA1ms relative to 18S in CD34+ (filled circles) or CD34- (empty circles) cells from primary BM MNCs of healthy donors (NBM; n = 6) and SF3B1-mutated MDS patients (SF3B1mt; n = 7). Mean ± SEM relative expression. Unpaired t-test G Experimental strategy to assess the effect of UBA1 inhibition on colony growth and composition in CD34+-enriched BM MNCs from MDS-SF3B1 patients and healthy controls. H Effect of UBA1 inhibition on CFU counts relative to DMSO and I frequency of SF3B1WT and SF3B1mt colonies relative to total CFU counts from MDS patient (n = 3) or healthy control (n = 2) cells treated with 32 nM TAK-243 or DMSO for 14 days. Numbers within brackets indicate colonies assessed by ddPCR. Mean ± SEM. Unpaired t-test. *, P ≤ 0.05; ***, P ≤ 0.001; ns not significant. SF3B1mt SF3B1-mutated, MDS-RS MDS with ring sideroblasts, NBM normal bone marrow from healthy donors.
Among the SF3B1 mutation cohort, SF3B1K700E patients (50 of 83) exhibited similar levels of UBA1ms compared to patients with other SF3B1 variants (28 of 33) (Supplementary Fig. 8C–E). Additionally, mis-splicing levels were consistent across SF3B1K700E VAFs (Supplementary Fig. 8F). We confirmed the presence of UBA1ms in HSPCs from MDS-SF3B1 patients and its absence in NBM, by RT-qPCR and RT-PCR, using primary CD34+ cells (and CD34- cells when cell count was limited, as indicated) (Fig. 5F and supplementary Fig. 8G).
Given the abundance of UBA1ms in MDS-SF3B1 patients and the instability of UBA1ms protein, we hypothesized a partial loss of functional UBA1 protein that could be exploited therapeutically. To assess whether targeted UBA1 inhibition through TAK-243 in primary HSPCs from MDS patients would selectively reduce SF3B1-mutant cells, as observed with K562 cells, we performed CFU assays with CD34+ BM MNCs from sex-matched SF3B1-mutated MDS patients and healthy donors (Fig. 5G). Indeed, TAK-243 exposure significantly reduced the CFU potential relative to DMSO of cells from MDS patients compared to healthy donors (Fig. 5H). Single-colony genotyping by ddPCR confirmed that the treatment effect in SF3B1-mutated patient cells largely resulted from a reduction of mutated colonies, whereas CFU formation from residual wild-type cells was less affected (Fig. 5I). Taken together, we confirm extensive UBA1ms exclusive to SF3B1-mutated patients, conferring a sensitivity of mutant cells to targeted inhibition of UBA1 with TAK-243 in both iPSC-derived and primary HSPCs.
Discussion
A detailed understanding of mis-splicing and ensuing molecular consequences in splicing factor-mutated MDS is key for the development of improved therapies. Advances in single-cell and integrative genomics have empowered the discovery of novel MDS-SF3B1 disease mechanisms, presenting potential therapeutic modalities [8, 19, 46]. Here, we leveraged in vitro models of MDS-SF3B1 and full-length RNA sequencing data with unsupervised analysis to identify UBA1 as a target of mis-splicing with adverse effects on UBA1 protein levels. This finding was corroborated by MDS patient cohort analyses, in which UBA1ms was exclusively present in SF3B1-mutant individuals. Our study underscores the relevance of iPSCs as a valuable tool in hematological research and highlights an SF3B1-driven RNA mis-splicing event with therapeutic implications.
SF3B1 is the most frequently mutated component of the spliceosome machinery, associated with widespread disruption of RNA splicing [3, 8, 9, 13, 16, 18, 21]. Previously characterized SF3B1-mutant mis-spliced transcripts typically introduce a premature stop codon and are therefore targeted for NMD. This is not the case for UBA1ms, which encodes a 45-amino-acid insertion within the inactive adenylation domain of UBA1. Consistent with this, we confirmed mRNA stability, absence of NMD, and successful engagement of UBA1ms mRNA with the translation machinery. Although a recent study reported decreased UBA1 RNA and protein expression in splicing gene–mutant MDS, albeit without a link to RNA mis-splicing of UBA1 [48], our data do not show a difference in UBA1 transcript expression levels between SF3B1-mutant individuals and healthy donors. Instead, we attribute the reduction of total UBA1 to the instability of the UBA1ms protein product, as evidenced by its short half-life in our in vitro model compared to UBA1WT [44]. Consequently, the rapid degradation of UBA1ms prevents its detection by immunoblotting, leaving only the canonically spliced UBA1 isoforms visible in SF3B1-mutant cells. Previous work by us and others has described stage-specific, pervasive mis-splicing due to SF3B1 mutation and induction of pro-survival mechanisms in mutated cells [8, 46]. In contrast, lower UBA1 protein levels due to UBA1ms would likely challenge mutant cell viability as UBA1 is essential for protein homeostasis and cell survival [32, 33]. Curiously, a recent study reported an adaptive stress response in cells with a partial reduction in UBA1 activity that confers cellular resilience [49], which would be in line with the clonal advantage of SF3B1-mutated hematopoietic stem cells.
Somatic, heterozygous mutations in the UBA1 gene have recently gained attention in VEXAS (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) syndrome [50]. In this case, UBA1b expression is specifically lost and the accumulation of a catalytically impaired isoform leads to disruption of the ubiquitin-proteasome system. In contrast, mis-splicing of UBA1, driven by cryptic SF3B1 splice site recognition, impacts both isoforms, leading to reduced levels of UBA1a and UBA1b without causing a complete loss of function. The shared convergence of UBA1, as a target of mis-splicing in MDS-SF3B1 and somatic mutation in VEXAS, leads to two distinct clonal disorders. VEXAS manifests with inflammatory and hematological symptoms, with approximately half of affected patients also presenting with MDS [51]; however, MDS-SF3B1 individuals typically exhibit a non-inflammatory phenotype and patient cells have been associated with a low inflammatory profile compared to other low-risk MDS subgroups [52]. Despite the different clinical phenotypes, it is tempting to speculate that exploiting UBA1 dysfunction might be a plausible treatment strategy in both myeloid disorders. Indeed, a recent study of a VEXAS cell model showed selective susceptibility to UBA1 modulation with the specific inhibitor TAK-243 [45], also used in this study. In line with this report, our data indicate a differential response of SF3B1-mutated cells to TAK-243 compared to SF3B1WT, potentially supporting the specificity and applicability of UBA1 inhibition in the development of pharmacological intervention for MDS-SF3B1. Utilizing a combination of cell models, HSPCs derived from iPSCs, and primary cells from MDS patients, we observed cell type-specific and concentration-dependent responses to UBA1 inhibition. While relative differences in sensitivity were modest, mutant cells consistently presented an increased susceptibility to UBA1 inhibition compared with wild-type cells.
SF3B1 mutations are a favorable prognostic factor in MDS. Nevertheless, patients frequently develop transfusion-dependency, and allogenic stem cell transplantation remains the only curative option. While current treatment regimens primarily aim to manage the anemia, they do not specifically target the mutant HSPC compartment. A therapeutic strategy which depletes mutated cells in the bone marrow necessitates the presence of wild-type HSPCs to replenish the exhausted cells. Our results suggest that TAK-243 selectively reduces mutated cells while preserving the residual wild-type cells. Although TAK-243 did not completely eradicate mutant cells in CFU assays, it may present an opportunity for a mild, minimally toxic pharmacological intervention to decrease mutational burden and delay allogenic stem cell transplantation. Future work is warranted exploring combination treatments with other targets of SF3B1 mutations. Given a role for UBA1 in the DNA damage response, and an observed increase in DNA damage in SF3B1-mutant cells, exploiting synthetic lethality, such as combining PARP or ATR inhibitors with TAK-243, could present a strategy to selectively eliminate mutant clones [41, 53]. Interestingly, TAK-243 has been shown to induce apoptosis in several hematological cancer models, including acute myeloid leukemia [35, 54, 55] and is currently being evaluated in a clinical trial for intermediate-2 or high-risk refractory MDS and leukemias (NCT03816319). Results from this trial will provide valuable insights into the dosing and toxicity profiles, informing decision-making for future clinical utility for MDS-SF3B1. In conclusion, we identify UBA1 as a previously unrecognized target of SF3B1-mutant mis-splicing, which introduces a specific vulnerability of SF3B1-mutated cells to directed UBA1 inhibition.
Data availability
The raw data for the RNA sequencing and corresponding metadata and intermediate analysis has been deposited as SND-ID: 2024-128 (Bulk RNA sequencing of erythroblasts from a pair of SF3B1-mutated and SF3B1-wildtype induced pluripotent stem cell (iPSC) lines) on the Swedish National Data Service’s research data repository. These data have been uploaded with restricted access due to their personally identifiable information status but are accessible upon reasonable request through SND at: https://doi.org/10.48723/3hs1-0v44. Other raw data are available from the corresponding author upon request.
References
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DAG, Gralnick HR, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–99.
Woll PS, Kjällquist U, Chowdhury O, Doolittle H, Wedge DC, Thongjuea S, et al. Myelodysplastic syndromes are propagated by rare and distinct human cancer stem cells in vivo. Cancer Cell. 2014;25:794–808.
Yoshida K, Sanada M, Shiraishi Y, Nowak D, Nagata Y, Yamamoto R, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478:64–69.
Kennedy JA, Ebert BL. Clinical implications of Genetic mutations in Myelodysplastic syndrome. J Clin Oncol. 2017;35:968–74.
Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–28.
Tehranchi R, Invernizzi R, Grandien A, Zhivotovsky B, Fadeel B, Forsblom AM, et al. Aberrant mitochondrial iron distribution and maturation arrest characterize early erythroid precursors in low-risk myelodysplastic syndromes. Blood. 2005;106:247–53.
Malcovati L, Karimi M, Papaemmanuil E, Ambaglio I, Jädersten M, Jansson M, et al. SF3B1 mutation identifies a distinct subset of myelodysplastic syndrome with ring sideroblasts. Blood. 2015;126:233–41.
Moura PL, Mortera-Blanco T, Hofman IJ, Todisco G, Kretzschmar WW, Björklund AC, et al. Erythroid Differentiation Enhances RNA Mis-Splicing in SF3B1-Mutant Myelodysplastic Syndromes with Ring Sideroblasts. Cancer Res. 2024;84:211–25.
Visconte V, Makishima H, Jankowska A, Szpurka H, Traina F, Jerez A, et al. SF3B1, a splicing factor is frequently mutated in refractory anemia with ring sideroblasts. Leukemia. 2012;26:542–5.
Papaemmanuil E, Cazzola M, Boultwood J, Malcovati L, Vyas P, Bowen D, et al. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N Engl J Med. 2011;365:1384–95.
Wang C, Chua K, Seghezzi W, Lees E, Gozani O, Reed R. Phosphorylation of spliceosomal protein SAP 155 coupled with splicing catalysis. Genes Dev. 1998;12:1409–14.
Cretu C, Schmitzová J, Ponce-Salvatierra A, Dybkov O, De Laurentiis EI, Sharma K, et al. Molecular Architecture of SF3b and Structural Consequences of Its Cancer-Related Mutations. Mol Cell. 2016;64:307–19.
Darman RB, Seiler M, Agrawal AA, Lim KH, Peng S, Aird D, et al. Cancer-Associated SF3B1 Hotspot Mutations Induce Cryptic 3’ Splice Site Selection through Use of a Different Branch Point. Cell Rep. 2015;13:1033–45.
Nikpour M, Scharenberg C, Liu A, Conte S, Karimi M, Mortera-Blanco T, et al. The transporter ABCB7 is a mediator of the phenotype of acquired refractory anemia with ring sideroblasts. Leukemia. 2013;27:889–96.
Pellagatti A, Cazzola M, Giagounidis AAN, Malcovati L, Della Porta MG, Killick S, et al. Gene expression profiles of CD34+ cells in myelodysplastic syndromes: Involvement of interferon-stimulated genes and correlation to FAB subtype and karyotype. Blood. 2006;108:337–45.
Conte S, Katayama S, Vesterlund L, Karimi M, Dimitriou M, Jansson M, et al. Aberrant splicing of genes involved in haemoglobin synthesis and impaired terminal erythroid maturation in SF3B1 mutated refractory anaemia with ring sideroblasts. Br J Haematol. 2015;171:478–90.
Lieu YK, Liu Z, Ali AM, Wei X, Penson A, Zhang J, et al. SF3B1 mutant-induced missplicing of MAP3K7 causes anemia in myelodysplastic syndromes. Proc Natl Acad Sci USA. 2022;119:e2111703119.
Shiozawa Y, Malcovati L, Gallì A, Sato-Otsubo A, Kataoka K, Sato Y, et al. Aberrant splicing and defective mRNA production induced by somatic spliceosome mutations in myelodysplasia. Nat Commun. 2018;9:3649.
Sarchi M, Clough CA, Crosse EI, Kim J, Baquero Galvis LD, Aydinyan N, et al. Mis-splicing of Mitotic Regulators Sensitizes SF3B1-Mutated Human HSCs to CHK1 Inhibition. Blood Cancer Discov. 2024;5:353–70.
Mupo A, Seiler M, Sathiaseelan V, Pance A, Yang Y, Agrawal AA, et al. Hemopoietic-specific Sf3b1-K700E knock-in mice display the splicing defect seen in human MDS but develop anemia without ring sideroblasts. Leukemia. 2017;31:720–7.
Obeng EA, Chappell RJ, Seiler M, Chen MC, Campagna DR, Schmidt PJ, et al. Physiologic Expression of Sf3b1K700E Causes Impaired Erythropoiesis, Aberrant Splicing, and Sensitivity to Therapeutic Spliceosome Modulation. Cancer Cell. 2016;30:404–17.
Yeo GW, Van Nostrand E, Holste D, Poggio T, Burge CB. Identification and analysis of alternative splicing events conserved in human and mouse. Proc Natl Acad Sci USA. 2005;102:2850–5.
Asimomitis G, Deslauriers AG, Kotini AG, Bernard E, Esposito D, Olszewska M, et al. Patient-specific MDS-RS iPSCs define the mis-spliced transcript repertoire and chromatin landscape of SF3B1-mutant HSPCs. Blood Adv. 2022;6:2992–3005.
Clough CA, Pangallo J, Sarchi M, Ilagan JO, North K, Bergantinos R, et al. Coordinated missplicing of TMEM14C and ABCB7 causes ring sideroblast formation in SF3B1-mutant myelodysplastic syndrome. Blood. 2022;139:2038–49.
Kotini AG, Carcamo S, Cruz-Rodriguez N, Olszewska M, Wang T, Demircioglu D, et al. Patient-Derived iPSCs Faithfully Represent the Genetic Diversity and Cellular Architecture of Human Acute Myeloid Leukemia. Blood Cancer Discov. 2023;4:318–35.
Chao MP, Gentles AJ, Chatterjee S, Lan F, Reinisch A, Corces MR, et al. Human AML-iPSCs Reacquire Leukemic Properties after Differentiation and Model Clonal Variation of Disease. Cell Stem Cell. 2017;20:329–344.e7.
Wheeler EC, Vora S, Mayer D, Kotini AG, Olszewska M, Park SS, et al. Integrative RNA-omics Discovers GNAS alternative splicing as a phenotypic driver of splicing factor–mutant neoplasms. Cancer Discov. 2022;12:836–55.
Kotini AG, Chang CJ, Boussaad I, Delrow JJ, Dolezal EK, Nagulapally AB, et al. Functional analysis of a chromosomal deletion associated with myelodysplastic syndromes using isogenic human induced pluripotent stem cells. Nat Biotechnol. 2015;33:646–55.
Chang CJ, Kotini AG, Olszewska M, Georgomanoli M, Teruya-Feldstein J, Sperber H, et al. Dissecting the contributions of cooperating gene mutations to cancer phenotypes and drug responses with patient-derived iPSCs. Stem Cell Rep. 2018;10:1610–24.
Kotini AG, Chang C-J, Chow A, Yuan H, Ho T-C, Wang T, et al. Stage-Specific Human Induced Pluripotent Stem Cells Map the Progression of Myeloid Transformation to Transplantable Leukemia. Cell Stem Cell. 2017;20:315–328.e7.
Moudry P, Lukas C, Macurek L, Hanzlikova H, Hodny Z, Lukas J, et al. Ubiquitin-activating enzyme UBA1 is required for cellular response to DNA damage. Cell Cycle. 2012;11:1573–82.
McGrath JP, Jentsch S, Varshavsky A. UBA 1: an essential yeast gene encoding ubiquitin-activating enzyme. EMBO J. 1991;10:227–36.
Kulkarni M, Smith HE. E1 ubiquitin-activating enzyme UBA-1 plays multiple roles throughout C. elegans development. PLoS Genet. 2008;4:e1000131.
Hyer ML, Milhollen MA, Ciavarri J, Fleming P, Traore T, Sappal D, et al. A small-molecule inhibitor of the ubiquitin activating enzyme for cancer treatment. Nat Med. 2018;24:186–93.
Barghout SH, Patel PS, Wang X, Xu GW, Kavanagh S, Halgas O, et al. Preclinical evaluation of the selective small-molecule UBA1 inhibitor, TAK-243, in acute myeloid leukemia. Leukemia. 2019;33:37–51.
Todisco G, Creignou M, Bernard E, Björklund A-C, Moura PL, Tesi B, et al. Integrated genomic and transcriptomic analysis improves disease classification and risk stratification of MDS with ring sideroblasts. Clin Cancer Res. 2023;29:4256–67.
Shen S, Park JW, Lu ZX, Lin L, Henry MD, Wu YN, et al. rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc Natl Acad Sci USA 2014;111:E5593–E5601.
Garrido-Martín D, Palumbo E, Guigó R, Breschi A. ggsashimi: Sashimi plot revised for browser- and annotation-independent splicing visualization. PLoS Comput Biol 2018;14:e1006360.
Dolatshad H, Pellagatti A, Liberante FG, Llorian M, Repapi E, Steeples V, et al. Cryptic splicing events in the iron transporter ABCB7 and other key target genes in SF3B1-mutant myelodysplastic syndromes. Leukemia. 2016;30:2322–31.
Mortera-Blanco T, Dimitriou M, Woll PS, Karimi M, Elvarsdottir E, Conte S, et al. SF3B1-initiating mutations in MDS-RSs target lymphomyeloid hematopoietic stem cells. Blood. 2017;130:881–90.
Bland P, Saville H, Wai PT, Curnow L, Muirhead G, Nieminuszczy J. et al. SF3B1 hotspot mutations confer sensitivity to PARP inhibition by eliciting a defective replication stress response. Nat Genet 2023;55:1311–23.
Sterne-Weiler T, Martinez-Nunez RT, Howard JM, Cvitovik I, Katzman S, Tariq MA, et al. Frac-seq reveals isoform-specific recruitment to polyribosomes. Genome Res. 2013;23:1615–23.
Yoshikawa H, Larance M, Harney DJ, Sundaramoorthy R, Ly T, Owen-Hughes T, et al. Efficient analysis of mammalian polysomes in cells and tissues using ribo mega-SEC. Elife 2018;7:e36530.
Mathieson T, Franken H, Kosinski J, Kurzawa N, Zinn N, Sweetman G, et al. Systematic analysis of protein turnover in primary cells. Nat Commun. 2018;9:689.
Chiaramida A, Obwar SG, Nordstrom AEH, Ericsson M, Saldanha A, Ivanova EV, et al. Sensitivity to targeted UBA1 inhibition in a myeloid cell line model of VEXAS syndrome. Blood Adv. 2023;7:7445–56.
Cortés-López M, Chamely P, Hawkins AG, Stanley RF, Swett AD, Ganesan S, et al. Single-cell multi-omics defines the cell-type-specific impact of splicing aberrations in human hematopoietic clonal outgrowths. Cell Stem Cell. 2023;30:1262–1281.e8.
San Roman AK, Godfrey AK, Skaletsky H, Bellott DW, Groff AF, Harris HL, et al. The human inactive X chromosome modulates expression of the active X chromosome. Cell Genomics. 2023;3:100259.
Wei Y, Zheng H, Li Z, Lockyer PP, Darbaniyan F, Kanagal-Shamanna R, et al. Downregulation of UBA1 expression in myelodysplastic neoplasm. Leukemia. 2024;10:2284–8.
Hunt LC, Pagala V, Stephan A, Xie B, Kodali K, Kavdia K, et al. An adaptive stress response that confers cellular resilience to decreased ubiquitination. Nat Commun. 2023;14:7348.
Beck DB, Ferrada MA, Sikora KA, Ombrello AK, Collins JC, Pei W, et al. Somatic Mutations in UBA1 and severe adult-onset autoinflammatory disease. N Engl J Med. 2020;383:2628–38.
Georgin-Lavialle S, Terrier B, Guedon AF, Heiblig M, Comont T, Lazaro E, et al. Further characterization of clinical and laboratory features in VEXAS syndrome: large-scale analysis of a multicentre case series of 116 French patients*. Br J Dermatol. 2022;186:564–74.
Schneider M, Rolfs C, Trumpp M, Winter S, Fischer L, Richter M, et al. Activation of distinct inflammatory pathways in subgroups of LR-MDS. Leukemia 2023;37:1709–18.
Singh S, Ahmed D, Dolatshad H, Tatwavedi D, Schulze U, Sanchi A, et al. SF3B1 mutations induce R-loop accumulation and DNA damage in MDS and leukemia cells with therapeutic implications. Leukemia. 2020;34:2525–30.
Best S, Hashiguchi T, Kittai A, Bruss N, Paiva C, Okada C, et al. Targeting ubiquitin-activating enzyme induces ER stress-mediated apoptosis in B-cell lymphoma cells. Blood Adv. 2019;3:51–62.
Zhuang J, Shirazi F, Singh RK, Kuiatse I, Wang H, Lee HC, et al. Ubiquitin-activating enzyme inhibition induces an unfolded protein response and overcomes drug resistance in myeloma. Blood. 2019;133:1572–84.
Acknowledgements
The authors thank the patients and healthy donors for their participation in this research; the MedH Flow Cytometry Core Facility financed by the Infrastructure Board at Karolinska Institutet, the Live Cell Imaging Core Facility, and Science for Life Laboratory at Karolinska Institutet for experimental help and support; and Professors Nico Dantuma at Karolinska Institutet and Cristian Bellodi at Lund University for fruitful discussions. VL was supported by Vetenskapsrådet (grant number 2020–01902), Cancer Research KI (Karolinska Institutet), Dr Åke Olsson Foundation (Dnr 2020-00310), KID doctoral student Funding (Karolinska Institutet; Dnr 20211-00512); KI Research Foundation grant (Dnr 2022-02170), and Felix Mindus contribution to Leukemia Research (2020-02585); IL was supported by Dr Åke Olsson Foundation; PLM was supported by Cancerfonden (grant number 21 0340), the Dr Åke Olsson Foundation (Dnr 2024-00303), the Axel and Eva Wallström Foundation (Dnr 2024-00311), KI Research Foundation grant (Dnr 2024-02330) and the Myelodysplastic Syndromes foundation Inc. (grant number 1142079); EHL was supported by Cancerfonden (grant number 19 0200), Vetenskapsrådet (grant number 211133), and Knut and Alice Wallenberg Foundation (grant number 2017.0359).
Funding
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
JT, PLM and VL designed the study; JT, SH, KMK, TMB, PLM, IL and DCK conducted experiments; IB and ACB assisted with biobank material; AGD, EP, EPP, NH, JB, EHL provided cells, supervision and resources; JT, SH, KMK, GT, TMB, EHL, PLM, VL analyzed and interpreted data; JT and VL wrote the manuscript, and all authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thier, J., Hofmann, S., Kirchhof, K.M. et al. SF3B1-mutant models of RNA mis-splicing uncover UBA1 as a therapeutic target in myelodysplastic neoplasms. Leukemia 39, 2801–2811 (2025). https://doi.org/10.1038/s41375-025-02740-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41375-025-02740-1
This article is cited by
-
Splicing-driven post-translational dysregulation: a new frontier for precision cancer medicine and immunotherapy
Clinical and Translational Oncology (2026)