Abstract
Detecting multiple parameters in salt spray environments is critical, as it significantly enhances the stability and reliability of real-time corrosion monitoring systems. However, current sensor strategies for detecting salt spray parameters face challenges such as poor timeliness, short lifespan, and low detection accuracy. This work introduces a multi-parameter micro-nano sensor based on Micro-Electro-Mechanical Systems (MEMS) technology, which integrates temperature, humidity, and conductivity detection units. Through a systematic characterization of the sensor’s performance, the sensor demonstrates excellent linearity, ideal detection ranges, and satisfactory accuracies with detection accuracies of ±0.1 °C for temperature, ±2% RH for humidity, and ±0.1 mS/cm for conductivity. This sensor offers a practical strategy for calculating the instantaneous corrosion rate of aircraft over the ocean. Additionally, based on the positive correlation between the three parameters and the liquid film thickness, a critical threshold determination method for the dynamic behavior of the sensor surface liquid film is further explored. This method macroscopically distinguishes the phase transition boundary between dry and wet states of the liquid film, offering a theoretical foundation for differentiated corrosion rate assessment and improved corrosion prediction accuracy. High-precision monitoring of environmental parameters during long-term salt spray and atmospheric exposure experiments is achieved using a self-developed online testing system. Real-time data compensation is also provided to improve the sensor’s stability and accuracy. Consequently, the proposed high-precision, miniaturized, and mass-producible multi-parameter sensor holds great promise as a competitive device for detecting salt spray environmental parameters in real-time corrosion monitoring systems for the aerospace field.

Similar content being viewed by others
Introduction
Corrosion is a critical issue affecting the health and safety of aerospace structures, especially when flight equipment is exposed to highly corrosive environments like marine atmospheres over extended periods1. Corrosion can significantly degrade the mechanical properties of essential components, including aircraft wings, surface joints, and fasteners, posing potential safety risks2,3. Real-time corrosion monitoring is particularly crucial in applications requiring high structural durability, such as aerospace, marine engineering, and transportation. Sensors for monitoring salt spray environmental parameters play a vital role in detecting corrosion conditions, as they not only monitor various parameters of the salt spray environment but also provide critical data for predicting the corrosion status of metals4. Therefore, real-time corrosion monitoring systems used in flight equipment require high-precision online measurement of salt spray environment parameters to ensure predictive maintenance, risk assessment, and reliable operation of flight equipment.
The salt spray environment is a dispersed system formed by salt-containing micro-droplets in the atmosphere, typically observed in coastal and marine areas. Seawater and marine salt spray generally have salinity levels between 3.2% and 3.7% and a pH of around 8 to 8.2. This environment contains ions such as chloride, sodium, potassium, iodide, and bromide, forming a highly corrosive electrolyte. Key parameters of salt spray environments include temperature, relative humidity, pH, chloride ion concentration, and salinity. Among these, chloride ions, which constitute over 99% of seawater anions, have strong penetration abilities that disrupt metal passivation layers, accelerating electrochemical corrosion. Temperature and humidity are primary factors influencing the alternation of wet and dry cycles on metal surfaces, determining the thickness of the surface electrolyte film5,6,7. These parameters interact to collectively affect the corrosion behavior of metal surfaces7. Consequently, accurate monitoring of temperature, humidity, and chloride ions is essential for effective corrosion monitoring.
In recent years, the emergence of miniaturized sensors has significantly advanced corrosion monitoring technologies8,9,10, particularly in the development of multi-parameter sensors for small-scale aerospace applications. These sensors facilitate continuous corrosion monitoring during equipment operation, providing precise forecasts by analyzing parameters like temperature, humidity, and salt concentration. Traditional chloride ion deposition detection requires long-term (e.g., daily, monthly, or yearly) passive collection of salt fog deposition followed by chemical analysis of the accumulated amount, which suffers from poor timeliness and the inability to provide real-time feedback. Therefore, Chang et al. proposed a multi-parameter sensor integrating temperature, humidity, chloride ion concentration, and time-of-wetness, designed specifically to monitor corrosion in compact aircraft structures11. The chloride ion sensing unit employs a dual-electrode grid structure to detect chloride concentration electrochemically, achieving a minimum detectable chloride ion mass fraction of 0.5%. However, under complex environmental conditions, external interference may reduce the sensor’s accuracy. Additionally, Zhou et al. explored a three-channel all-solid-state chloride ion sensor under marine salt spray conditions, demonstrating a sensitivity of 55.79 mV/lgCCl− and a detection limit of 4.169 × 10−4 mol/L, with excellent stability, repeatability, and a resolution exceeding 1 × 10−3 mol/L12. Nevertheless, the short lifespan of traditional internal reference electrodes limits long-term monitoring, restricting their practical application. Furthermore, Luna Innovations Inc., a company based in the United States, has developed a multi-parameter online corrosion monitoring system that provides real-time measurements of temperature (±0.3 °C), humidity (±2%RH), micro-liquid film conductivity (high- and low-frequency modes), and galvanic corrosion rate.13,14. This system evaluates corrosion severity using ISO 9223 classification standards, which range from C1 to C5. The system uses a dual-electrode system for conductivity measurements, indirectly reflecting chloride ion concentration, reducing the need for reference electrodes, and extending its operational lifespan. Building upon this study, a novel indirect conductivity detection strategy is proposed: In Cl−-dominated salt fog environments, conductivity exhibits a strong linear correlation with Cl− concentration. By combining real-time conductivity measurements with a calibration curve, Cl− concentration can be directly inferred, and dynamic deposition rates can be estimated using empirical formulas. This method offers advantages such as fast response, long-term stability, and resistance to temperature and humidity interference, making it especially suitable for monitoring Cl− deposition flux during short-term missions over the ocean for aircraft. Based on this approach, it is expected to enable the calculation of instantaneous corrosion rates and the rapid matching of corrosion categories according to ISO 9223, providing real-time decision support for anti-corrosion design. Compared to traditional methods, the conductivity-based approach demonstrates significant improvements in efficiency and sustainability. However, existing research on salt spray sensors has not yet thoroughly investigated the relationship between temperature, humidity, and liquid film thickness, which is critical for understanding the corrosion process. The thickness of the liquid film directly affects the corrosion rate, with thicker films generally promoting electrochemical corrosion.
Wang et al.‘s study on environmental factors in marine atmospheric corrosion and material transfer rates in thin liquid films revealed the correlation between environmental conditions, film thickness, and concentration in corrosion dynamics15. Temperature, humidity, and conductivity exhibit a strong synergistic effect during metal corrosion. Elevated temperatures accelerate electrochemical reactions by increasing the rate of mass transfer and compromising the passive layer, significantly intensifying the corrosion process. Humidity controls the formation of the electrolyte film on metal surfaces. Higher humidity results in a thicker electrolyte film, thereby accelerating corrosion reactions, particularly under wet-dry cycling conditions where corrosion is further exacerbated. Conductivity indirectly reflects the concentration of corrosive ions, such as chloride ions, where higher conductivity corresponds to more severe corrosion. Temperature and humidity regulate the migration and accumulation of chloride ions, subsequently influencing conductivity, which ultimately determines the corrosion rate. So, monitoring these parameters is essential for accurately assessing and predicting metal corrosion behavior. Therefore, monitoring these parameters is crucial for accurately assessing and predicting metal corrosion behavior. In this context, this study proposes a method for determining critical thresholds based on the dynamic behavior of the sensor surface liquid film. By monitoring changes in the liquid film resistance, the method macroscopically differentiates the phase transition boundary between the dry state (discontinuous, chemically oxidized) and the wet state (continuous, electrochemically corrosion-driven). Combined with the differential corrosion rate assessment for dry/wet corrosion in the ISO 9223 standard, this method is expected to enable real-time matching of environmental conditions with corresponding corrosion kinetics parameters, thereby enhancing the accuracy of corrosion predictions.
Hence, in this paper, a Micro-Electro-Mechanical Systems (MEMS)-based sensor designed for high-precision detection of multiple parameters in salt spray corrosion environments for real-time monitoring systems. Polyimide (PI) film, known for its stable performance, is selected as the humidity-sensitive material to ensure high sensitivity and long-term stability in measurements. Appropriately sized interdigital electrodes are designed to enable dual-function, high-precision monitoring of time-of-wetness and conductivity. Additionally, to evaluate the effectiveness and practicality of these batch-fabricated sensors, their sensing behavior was systematically analyzed. Experimental results indicate that the salt spray sensor demonstrates excellent batch consistency, high accuracy, good stability, and a strong linear response, meeting the measurement requirements for salt spray environmental parameters in real-time corrosion monitoring systems.
Materials and methods
Sensor design
The salt spray sensor consists of three sensing units: temperature, humidity, and conductivity. These units are integrated onto a single chip using MEMS technology, as shown in Fig. 1a.
Humidity sensors are classified by their sensing mechanisms, including capacitive, resistive, resonant, and optical types. Compared with others, capacitive humidity sensors can offer high accuracy over a wide range of relative humidity and exhibit fast response times16,17. A layer of humidity-sensitive material is applied on the surface of the interdigital electrodes, where its capacitance varies with the adsorption and desorption of water molecules. In recent years, various sensitive materials are used for humidity detection, such as perovskites, porous silicon, ceramics, and MOF derivatives16. However, most of these materials lack the stability and durability required for complex salt spray environments. For long-term applications, a humidity-sensitive material with excellent physicochemical stability and environmental adaptability is necessary. PI, a classic and reliable material, exhibits outstanding temperature resistance, mechanical strength, chemical stability, electrical insulation, and low expansion coefficient, making it widely used in electronics, aerospace, medical, and optical applications17. The water absorption process of PI film involves both physical and chemical adsorption, as illustrated in Fig. 1b. Physical adsorption primarily occurs on the surface or near the surface area of the PI film, primarily relying on van der Waals forces for intermolecular adsorption. On the other hand, Chemical adsorption typically occurs at active sites on the PI film surface or within its structure, involving hydrogen bonding between water molecules and hydrophilic functional groups (such as carboxyl (-COOH) and hydroxyl (-OH) groups). The interaction between water molecules and these polar groups increases the local dipole moment, thereby significantly enhancing both the overall dipole moment and dielectric constant of the PI film. For instance, hydrogen bonding between water molecules and carboxyl groups alters the local electronic environment, increases polarizability, and thus raises the dielectric constant17.
The electrochemical detection methods for conductivity typically include inductive or electrode-based approaches. To enable simultaneous detection of conductivity and time-of-wetness, a two-electrode system was selected for conductivity measurement. By applying a high-frequency alternating signal (voltage or current) to excite the electrodes, the impedance of the solution at a specific frequency can be measured18. The conductivity can then be calculated using the relationship between impedance and the cell constant. When the sensor is exposed to a high-humidity salt spray environment, water molecules in the air form a thin liquid film on the surface of the interdigital electrodes, as illustrated in Fig. 1c. The dissolved salts in this film create an electrolyte solution, which significantly increases ion concentration and conductivity19. Consequently, as temperature and humidity rise, the surface liquid film gradually thickens, the electrolyte concentration increases, and the conductivity reading rapidly escalates, approaching levels similar to seawater conductivity. Moreover, since seawater conductivity exhibits a strict linear correlation with Cl− concentration, and the contribution of Cl− accounts for the overwhelming majority, conductivity can be employed as an indirect indicator for evaluating Cl− concentration.
Time-of-wetness refers to the duration during which a metal surface is covered by a water film or an adsorptive electrolyte layer, and it is a key parameter influencing metal corrosion in atmospheric environments20. According to the ISO 9223 standard, time-of-wetness is defined as the period when the temperature exceeds 0 °C and the relative humidity remains above 80%. This study focuses on determining time-of-wetness through conductivity measurements using interdigital electrodes, along with real-time monitoring of the liquid film thickness. When the liquid film forms on the surface of the interdigital electrodes, its conductive properties cause a change in the impedance between the electrodes. As the liquid film thickness increases, the impedance between the electrodes decreases significantly. By utilizing high-precision impedance measurements, the variation in liquid film thickness can be quantified, which enables accurate estimation of the time-of-wetness and dry/wet states.
For temperature measurement, the temperature sensing unit employs a serpentine platinum thin-film resistor to detect ambient temperature, with an insulating layer of silicon nitride (Si3N4) deposited to provide chemical inertness and low water permeability. The highly integrated salt spray environment sensor has a compact size of just 8.5 mm × 5 mm × 0.8 mm. This sensor can be encapsulated in a specially designed and manufactured housing and attached to the metal surfaces of flight equipment, achieving high-precision measurements of temperature, humidity, and conductivity in salt spray environments.
Sensor fabrication
To begin, after a thorough cleaning with H2SO4:H2O2 = 7:1 (Step 1), NH4OH:H2O2: H2O = 1:1:7 (Step-2), HF: H2O = 1:50 (Step 2), as well as an O2 plasma treatment, a 400 μm silicon wafer with a diameter of 4 in. was ready to be processed. Then, dry oxidation for 55 min and wet oxidation for 450 min at 1100 °C produced a 2 μm SiO2 layer (Fig. 2a). Following that, the silicon wafer was thoroughly cleaned and dried and then coated with a 2.4-μm thick LC100A photoresist from Rohm and Haas by the EVG101 spin coater at the speed of 1000 r/min for 30 s. The proposed silicon wafer was exposed to a lithography machine for 15 s, and then immersed in the FHD-320 solution for 40 s for the purpose of patterning, as shown in Fig. 2b. Afterward, the Pt electrode with a thickness of 200 nm was prepared by the lift-off process, and Cr with a thickness of 50 nm was additionally applied as an adhesion layer so that the Pt electrode formed the intimated contact with the wafer, as shown in Fig. 2c. And the silicon nitride with a thickness of 500 nm was prepared by the plasma-enhanced chemical vapor deposition to protect the Pt electrode, as shown in Fig. 2d. Next, by repeating the step presented in Fig. 2b, the patterned photoresist with a thickness of 2 μm was prepared by the Photolithography Process, as shown in Fig. 2e. Then, reactive-ion etching was used to selectively remove the Si3N4 from the targeted area and all the photoresist was removed by the same step mentioned previously, as shown in Fig. 2f. Further, a chromium (Cr) film with a thickness of 50 nm and a thin gold (Au) film with a thickness of 200 nm were prepared by magnetron sputtering Cr and Au first, and then the same operation was repeated (Fig. 2b) to coat a 2.4 μm thick SU-8 3005 photoresist and complete the photoresist patterning, as shown in Fig. 2g. Finally, the patterned Au electrodes were prepared by photolithography process and ion beam etching, and all photoresists were removed by the same steps mentioned earlier, as shown in Fig. 2h.
The wafer was diced into 160 individual chips, each with dimensions of 5 mm × 8.5 mm, as shown in Fig. 2i–k. In order to evaluate the performance of the sensors, the sensor chips were packaged for facilitate testing. After the PI coating process, each chip was wire-bonded to the pins on a specially designed packaging substrate, forming the final test chip. Based on this, the overall performance of the sensor was discussed through detailed testing and analysis.
Evaluating the sensing behavior of the sensor
The sensing behavior of the proposed salt spray environment sensor was evaluated based on detection range, linearity, hysteresis, accuracy, repeatability, stability, and overall performance of the monitoring system.
The operational thresholds of the salt fog sensor were determined through comprehensive analysis of environmental parameters specified in ISO 9223, MIL-STD-810, and GJB150A-2009. The detection ranges were ultimately defined as: temperature (−30 to 80 °C), relative humidity (30–100% RH), and conductivity (0–60 mS/cm), ensuring compatibility with multi-standard testing requirements for both natural atmospheric corrosion and accelerated aging protocols.
First, the sensor chip was placed securely inside a temperature- and humidity-controlled chamber, and a commercial temperature and humidity meter (TH20R-EX, Shenzhen Huahanwei Technology Co., Ltd., China) was used to calibrate the real-time temperature and humidity within the chamber. A digital multimeter (SDM3055, Shenzhen Dingyang Technology Co., Ltd., China) was employed to measure the impedance changes of the temperature and conductivity sensing units, as well as the capacitive response of the humidity sensing unit, with data acquisition and analysis conducted via computer. Next, to evaluate the performance of the conductivity sensing unit, KCl standard solutions with a conductivity range of 5–60 mS/cm (5.03, 10.1, 14.96, 20.01, 25.1, 29.98, 35.17, 40.12, 45.2, 50.06, 54.9, and 60.18 mS/cm) were prepared using deionized water (18 MΩ) in appropriate proportions. When the sensor chip was immersed in the solution, the electrochemical workstation (Gamry Reference 600+, Gamry Electrochemical Instruments, Inc., USA) was used to measure solution impedance, with the scanning frequency set from 10 Hz to 1000 kHz to determine the optimal operating frequency for the sensing unit. Subsequently, at this optimal frequency, a 90 mV alternating current source was applied to the sensor for linear testing. The interdigital electrodes’ response to chloride concentration is influenced solely by conductivity and not by electrolyte type under conditions of AC < 100 mV and frequencies between 1 KHz and 500 KHz. Finally, the sensor and commercial temperature-humidity meter were placed in an outdoor atmospheric environment, with a humidifier containing a 5% NaCl solution positioned nearby and windbreak panels added. When the environmental humidity exceeds 50% RH, the humidifier automatically activates, dispersing chloride ions above the sensor to simulate a salt mist environment. The humidifier stops working automatically when the humidity exceeds 80% RH to prevent excessive moisture. Additionally, a circuit monitoring system filters, calibrates, and compensates the sensor output signals to enhance the accuracy and stability of the sensor’s measurements.
Results and discussion
Performance calibration of as-prepared sensors
The resistance variation of the temperature sensing unit was measured in a temperature- and humidity-controlled chamber using a digital multimeter, with actual temperature values referenced to the standard temperature readings from a commercial temperature and humidity meter. Under constant humidity conditions (60% RH), the chamber temperature was varied within the range of −30 to 80 °C. As the temperature increased, the sensor exhibited a significant linear relationship (R² > 0.9999) between measured resistance and actual temperature, as shown in Fig. 3a. Additionally, the maximum hysteresis of the temperature sensing unit was only 0.45%, indicating good accuracy and consistency under varying temperature change rates, as illustrated in Fig. 3b. Subsequently, the temperature sensing unit was placed in a temperature- and humidity-controlled chamber at a constant 30 °C to perform a 6-h stability and accuracy test. The results showed that, influenced by airflow within the chamber, the resistance of the temperature sensing unit displayed a cosine-like fluctuation with an average value of 781.4877 Ω and a relative standard deviation (RSD) of 0.0149%, indicating extremely low fluctuation, as depicted in Fig. 3c. This stable output characteristic demonstrates that the sensing unit is capable of reliable long-term environmental monitoring, particularly in applications requiring high-precision and stability.
a Linear fit of the dynamic continuous temperature response test results. b Temperature hysteresis characteristics of the sensor. c Stability test of the temperature sensing sensor. d Exponential and linear fits of the dynamic continuous humidity response test results. e Humidity hysteresis characteristics of the sensor. f Stability test of the humidity sensing unit. g Frequency response of the sensor in a standard conductivity solution. h Linear fit of the continuous conductivity response test results. i Stability test of the conductivity sensing unit
The capacitive humidity sensor absorbs water molecules from the air by the PI film, which leads to the change of dielectric constant and capacitance. As the relative humidity of the environment increases, more water molecules are adsorbed onto the PI film, increasing its dielectric constant and resulting in a higher capacitance value. Conversely, when humidity decreases, water molecules desorb from the film, reducing both the dielectric constant and capacitance.
To verify the sensitivity performance of the fabricated humidity sensing unit, humidity tests were conducted at a constant temperature. As shown in the curve in Fig. 3d, the capacitance of the sensor increases exponentially with the gradual increase in humidity. This trend is consistent with the sensing mechanism of PI-based humidity sensors discussed in Chapter 2. At lower humidity levels, water molecules primarily undergo physical adsorption on the surface of the PI film, typically involving only a single or a few layers of water molecules, which has minimal effect on the dielectric constant of the PI film, resulting in minor capacitance changes. However, as humidity increases, more water molecules interact chemically with active sites within the PI film, leading to stable adsorption within the film. At higher humidity levels, additional water molecules are physically adsorbed onto the existing water layers, forming a multilayer network that significantly enhances the dielectric constant of the PI film. This mechanism rationally explains the exponential growth in capacitance observed in the PI-based humidity sensing unit. Meanwhile, to more intuitively verify the performance of the sensing unit, the capacitance values were converted into corresponding humidity values using an exponential fitting formula, and a linear fit (R² > 0.99) was performed with the actual humidity, as shown by the straight line in Fig. 3d.
Additionally, Fig. 3e presents the adsorption-desorption hysteresis characteristics of the sensor, with a low hysteresis value of only 1.79%, indicating minimal lag. A six-hour stability test was also conducted at 60% RH. The results show that the capacitance of the sensing unit exhibited cosine-like fluctuations influenced by airflow cycles, with an average value of 864.741 pF and a RSD of 0.0151%, as illustrated in Fig. 3f. Moreover, the instantaneous fluctuation in capacitance was approximately 0.1401 pF, as shown in the inset of Fig. 3f. This demonstrates that the humidity sensor not only performs excellently in short-term response but also maintains high stability in long-term monitoring, making it suitable for humidity detection in harsh environments.
To establish standard experimental conditions simulating the conductivity values of seawater, KCl solutions with conductivities ranging from 5 to 60 mS/cm were prepared at a standard temperature of 25 °C using high-purity potassium chloride (≥99.99%) and ultrapure water (>18.2 MΩ·cm). First, to determine the optimal excitation frequency, the conductivity sensing unit was sequentially placed in standard solutions of varying conductivities, with impedance scanning performed across a frequency range from 0 to 1 MHz under a constant 90 mV voltage. As shown in Fig. 3g, as the frequency and solution conductivity increased, the impedance between the electrodes gradually decreased, with each concentration curve displaying parallel distribution, indicating that the sensor can differentiate solutions of various conductivities. Considering subsequent circuit system design, 2.5 kHz was selected as the optimal operating frequency. Subsequently, under appropriate constant current excitation (90 mV, 2.5 kHz), twelve standard conductivity solutions ranging from 5 to 60 mS/cm were continuously tested. As shown in Fig. 3h, a strong linear relationship (R² > 0.99) was observed between the measured and standard conductivities. Additionally, the six-hour test results in a 36.7 mS/cm NaCl solution indicated a slight drift in the conductivity sensing unit during prolonged measurements, as shown in Fig. 3i. This drift is primarily attributed to ion accumulation caused by polarization effects in the high ion concentration environment, which impedes normal current flow. However, in the short-term test of 90 s, the conductivity sensing unit demonstrated outstanding stability, with an RSD of just 0.112%, suggesting minimal polarization impact over short durations and high reliability.
Overall, compared with conventional single-function sensors, the developed sensor enables in-situ synchronous detection of temperature, humidity, and conductivity, exhibiting notable superiority in detection range, sensitivity, environmental adaptability, and device miniaturization Table 1. It is particularly well-suited for real-time, multi-parameter online corrosion monitoring in complex salt fog environments, especially under conditions requiring long-term operational stability and strong resistance to environmental interference. Furthermore, benefiting from its innovative design featuring multi-parameter in-situ sensing, broad environmental tolerance, and enhanced anti-interference capabilities, the proposed sensor offers a highly reliable, embedded solution for corrosion monitoring in marine structures and aerospace platforms.
Correlation analysis between multiple parameters
In complex and dynamically changing salt spray environments, multi-parameter sensors designed for real-time corrosion monitoring face dual challenges: achieving high-precision measurements and mitigating multiple environmental interferences. To enhance the sensor’s anti-interference capability as well as its measurement accuracy and stability, this study systematically analyzes the correlations among key monitoring parameters, including temperature, humidity, and conductivity. Additionally, by examining the wetting and evaporation characteristics of the electrode surface, the intrinsic relationship between liquid film thickness and its resistance variation is thoroughly investigated. This analysis clarifies the impact of key parameters on the liquid film state, providing critical insights for optimizing sensor performance.
First, standard conductivity solutions were placed in a thermostatic water bath, and the sensor was tested at various temperatures using an electrochemical workstation to analyze the effect of temperature on conductivity. Experimental results indicated a positive correlation, with conductivity values increasing as temperature rose at a given concentration, as shown in Fig. 4a. Next, the sensor and a commercial temperature and humidity meter were placed in a temperature- and humidity-controlled chamber. By setting a constant temperature and adjusting the relative humidity in the chamber, the sensor’s response was evaluated, with data recorded using a digital multimeter. As shown in Fig. 4b, under humidity conditions of 40%, 60%, and 80% RH, the sensor’s temperature response remained consistent, indicating that humidity changes had a negligible effect on temperature measurement and demonstrating the excellent stability of the temperature sensing unit. Subsequently, a constant relative humidity was set, and the temperature in the chamber was adjusted to examine the temperature drift characteristics of the sensor’s humidity detection unit. As shown in Fig. 4c, the sensor’s response to humidity was consistent across various temperatures, displaying high sensitivity and a positive linear temperature coefficient, indicating the sensor’s robust temperature compensation capability.
The relationship between conductivity (\({C}_{t}\)) and temperature (\(T\)) is as follows21:
where \({C}_{t}\) is conductivity at temperature t, \({C}_{{cal}}\) is conductivity at the standard temperature (25 °C), \({T}_{{cal}}\) is standard temperature, and \(\alpha\) is temperature coefficient of the solution at the standard temperature By using this formula, the linear relationship between the temperature-compensated standard conductivity and the measured conductivity, as well as the RSD (only 0.936), can be obtained, as shown in Fig. 5a.
Based on the research by Islam, Tarikul, and others on temperature compensation for humidity22, the relationship between relative humidity (\({{RH}}_{{comp}}\)) and temperature (\(T\)) is defined as follows22:
The relative humidity at temperature \(T\) is denoted as \({{RH}}_{{comp}}\), and the parameters \({b}_{0},\,{b}_{1},\,{b}_{2}{,{b}}_{3}\) and \({b}_{4}\) represent the least squares fitting coefficients. By using this formula, the linear relationship between the temperature-compensated true humidity and the measured humidity, as well as the RSD (only 0.971), can be obtained, as shown in Fig. 5b.
Besides, in complex salt spray environments, sensors frequently undergo cycles of drying, humidity, and moisture alternation. As illustrated in Fig. 6a, with the gradual increase in humidity, water molecules in the air adhere to the sensor surface until a continuous liquid film is formed, transitioning the sensor into a moist state. When the temperature rises, leading to the gradual evaporation of water (resulting in decreased humidity), the sensor surface returns to a dry state. To comprehensively assess this dynamic process, this study integrates a comb-shaped electrode conductivity sensing unit, accurately identifies dry and wet states by real-time monitoring of liquid film resistance changes, quantifies moistening time and evaporation characteristics, and elucidates the intrinsic relationships between liquid film thickness, resistance variation, and environmental parameters. This provides a reliable foundation for optimizing sensor performance and real-time monitoring of corrosion behavior in salt spray environments.
When a conductive liquid film forms on the electrode surface, the resistance decreases significantly, enabling precise identification of the critical point and duration of the sensor’s wetted state. As shown in Fig. 6b, under constant temperature and humidity conditions (30 °C), the sensor remains in a dry state at low humidity levels, where the output resistance is extremely high, approaching infinity. As the humidity gradually increases to approximately 75% RH (Phase a), water vapor begins to condense on the sensor surface, causing a significant decrease in resistance. This is attributed to the formation of a conductive liquid film by water molecules on the electrode surface, which markedly enhances the electrolyte’s conductivity. When the humidity further rises above 90% RH (Phase b), the liquid film thickness increases gradually, and the resistance decrease becomes less pronounced, indicating the formation of a stable multilayer structure in the liquid film. This result aligns with the liquid film thickness and atmospheric corrosion rate model proposed by Tomashov et al.23. Specifically, when the liquid film thickness is small (<10 nm), the corrosion rate is low, corresponding to the dry atmospheric corrosion stage. As the liquid film thickness increases to the range of 10 nm to 1 µm, corrosion is predominantly electrochemical, with the rate stabilizing and exhibiting two distinct phases: rapid decline followed by slow stabilization. This experiment demonstrates that the sensor exhibits high sensitivity to changes in humidity, and its resistance signal effectively reflects the formation and evolution of the liquid film. These findings provide critical data support for further studies on corrosion behavior under humid environmental conditions.
Subsequently, 10 μL of deionized water was dropped onto the sensor electrode surface using a micropipette, while maintaining constant temperature and humidity conditions (30 °C, 60% RH) in the environmental chamber. The evaporation characteristics of the liquid film were then monitored. As shown in Fig. 6c, the resistance evolution could be categorized into five distinct stages:
-
(1)
Initial Stage: The resistance increases slightly as the liquid film transitions from uneven contact to complete wetting of the electrode surface, forming stable conductive pathways. The minimal resistance change during this phase reflects the initial adjustment characteristics of the liquid film-electrode interface.
-
(2)
Brief Decline Stage: The resistance decreases slightly, possibly due to localized thickening of the liquid film caused by uneven distribution, which optimizes the conductive pathways5. Additionally, ions from the air (e.g., Na⁺, Cl−) may dissolve or adsorb onto the liquid film, enhancing its conductivity and further reducing resistance.
-
(3)
Gradual Rise Stage: As the liquid film continues to evaporate, its thickness decreases gradually, leading to a progressive weakening of conductivity and a slow increase in resistance6. This phase demonstrates the sensitive response of impedance to changes in liquid film thickness, highlighting the dynamic equilibrium of the evaporation process.
-
(4)
Abrupt Transition Stage: The resistance undergoes a significant abrupt increase, indicating that the liquid film is nearing its critical evaporation point. Most conductive pathways are disrupted, leaving only residual liquid film. This transition corresponds to critical changes in liquid film thickness, marking the acceleration phase of evaporation.
-
(5)
Complete Evaporation Stage: The resistance rises sharply, rapidly reaching 100 MΩ, signifying the complete evaporation of the liquid film. The medium between the electrodes transitions from liquid to air, and the resistance approaches the system’s saturation limit. This phase marks the termination of the liquid film evaporation process6.
This process vividly illustrates the dynamic transition of the liquid film from complete coverage to total evaporation. It demonstrates the sensor’s ability to accurately capture changes in liquid film thickness, providing critical data for comprehensive evaluations of time-of-wetness and evaporation characteristics.
Performance of the monitoring system in exposed atmospheres
In this paper, the circuit monitoring system proposed in this study integrates multiple sensors and control components, utilizing AC (Alternating Current) impedance, DC (Direct Current) voltage, and pulse signals as the signal types for measuring conductivity, temperature, and humidity, respectively, as illustrated in Fig. 6. The core control unit is an STM32F103 microcontroller, which supports data acquisition, processing, and communication, ensuring high efficiency and low power consumption. Additionally, the system incorporates a multi-channel 24-bit SPI Δ-Σ Analog-to-Digital Converter for digitizing analog signals and enables remote monitoring through LTE and Wi-Fi interfaces. During startup, the system performs initialization and calibration to ensure data accuracy and reliability, making it suitable for applications such as water quality monitoring and salt spray environment surveillance.
To validate the performance of the designed system in complex salt fog environments and its dynamic response to various environmental variables, a series of experimental methods were further developed to simulate real-world application scenarios. The sensor was fixed alongside a commercial thermometer and hygrometer in the same area (exposed to real atmospheric conditions) to ensure synchronization of temperature and humidity data. Real-time monitoring of key parameters such as humidity, temperature, and conductivity was achieved through multi-sensor collaborative measurement, supported by a high-precision signal acquisition and data processing module to ensure the stability and anti-interference ability of the monitoring system under harsh conditions Fig. 7. This experiment aimed to comprehensively evaluate the reliability, sensitivity, and applicability of the sensor system in multi-parameter correlation analysis, providing important reference for the further optimization of salt fog environmental monitoring.
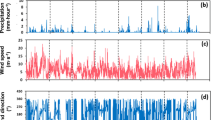
The results, as shown in Fig. 8b, c, reveal periodic fluctuations in temperature and relative humidity over a 15-day long-term monitoring period, exhibiting a typical day-night variation pattern that correlates with climate changes and the periodic fluctuations in temperature and humidity. As shown in Fig. 8d, the variations in conductivity show a significant positive correlation with changes in humidity, a phenomenon consistent with electrochemical behavior in thin liquid film environments. This indicates that humidity is the primary driving factor behind the changes in salt fog conductivity, while environmental temperature plays a secondary role. Overall, the periodic variations in temperature, humidity, and conductivity align with natural environmental cycles, suggesting that the sensor system used can effectively capture and reflect changes in these environmental parameters, providing reliable data support for subsequent environmental monitoring. The sensor holds significant application potential in marine atmospheric corrosion monitoring or salt fog environmental dynamics research.
a Schematic diagram of sensor testing in an exposed atmospheric environment. b Dynamic temperature response of the sensor under atmospheric environmental conditions. c Dynamic humidity response of the sensor under atmospheric environmental conditions. d Dynamic conductivity response of the sensor under atmospheric environmental conditions
Conclusions
In this paper, a salt spray environmental parameter monitoring sensor for real-time corrosion monitoring system is proposed and fabricated using MEMS technology. The conductivity sensing utilizes embedded digital microelectrodes, temperature measurement is conducted using a thin-film platinum resistor, and humidity detection employs PI as the sensing medium, coupled with micro-interdigitated electrodes. A high-precision signal acquisition and processing circuit was developed and integrated with the sensor chip, creating a comprehensive monitoring system capable of accurately tracking multiple environmental parameters. The temperature detection accuracy of the sensor is ±0.1 °C, the humidity measurement accuracy is ±2% RH within a range of 30% to 100% RH, and the conductivity detection accuracy is 0.1 mS/cm. More importantly, the proposed salt spray environment sensor exhibits outstanding sensing performance, including high sensitivity, a broad linear response range, and reliable stability. These features make it highly suitable for real-time corrosion monitoring systems, offering significant potential for application in harsh salt spray environments. Although the study could not explore the quantitative relationships between environmental parameters, liquid films, and conductivity in detail due to experimental limitations, its qualitative experiments clearly demonstrate its significance in corrosion rate analysis. Furthermore, the sensor’s excellent performance in both laboratory and simulated tests further highlights its need for precision and durability in aerospace applications.
References
Li, L., Chakik, M. & Prakash, R. A review of corrosion in aircraft structures and graphene-based sensors for advanced corrosion monitoring. Sensors 21, 2908 (2021).
Abbas, M. & Shafiee, M. An overview of maintenance management strategies for corroded steel structures in extreme marine environments. Mar. Struct. 71, 102718 (2020).
Benavides, S. Corrosion Control in the Aerospace Industry, (Woodhead Publishing, UK, 2009).
Mizuno, D., Suzuki, S., Fujita, S. & Hara, N. Corrosion monitoring and materials selection for automotive environments by using Atmospheric Corrosion Monitor (ACM) sensor. Corros. Sci. 83, 217–225 (2014).
Zhang, K. et al. Monitoring atmospheric corrosion under multi-droplet conditions by electrical resistance sensor measurement. Corros. Sci. 236, 112271 (2024).
Thee, C. et al. Atmospheric corrosion monitoring of a weathering steel under an electrolyte film in cyclic wet–dry condition. Corros. Sci. 78, 130–137 (2014).
Chen, F. F. et al. A microclimate model to simulate neutral salt spray testing for corrosion inhibitor evaluation and functional coating development. Prog. Org. Coat. 111, 327–335 (2017).
Wang, B. et al. Identification of corrosion factors in blast furnace gas pipe network with corrosion big data online monitoring technology. Corros. Sci. 230, 111906 (2024).
Komary, M. et al. Low-cost technologies used in corrosion monitoring. Sensors 23, https://doi.org/10.3390/s23031309 (2023).
Han, L. & Song, S. A measurement system based on electrochemical frequency modulation technique for monitoring the early corrosion of mild steel in seawater. Corros. Sci. 50, 1551–1557 (2008).
Chang, M., Yuan, S. F. & Wang, C. C. A miniaturized multi-parameter monitoring technology for aircraft structural corrosion environments. Equip. Environ. Eng. 6, 15–21 (2014).
Zhou, Z., Su, X. G. & Li, M. A funnel-type dual electrode chloride ion concentration detection method under liquid/gas conditions. Transducer Microsyst. Technol. 40, 149–152 (2021).
Demo, J., Friedersdorf, F., Andrews, C. & Putic, M. Wireless corrosion monitoring for evaluation of aircraft structural health. In IEEE Aerospace Conference, Big Sky, MT, USA, https://doi.org/10.1109/AERO.2012.6187362 (2012).
Demo, J. & Friedersdorf, F. Aircraft corrosion monitoring and data visualization techniques for condition based maintenance. In IEEE Aerospace Conference, Big Sky, MT, USA, https://doi.org/10.1109/AERO.2015.7119048 (2015).
Wang, Y., Liu, Y. H. & Mu, X. L. Influence of environmental factors in marine atmospheric corrosion processes on material transfer within thin liquid films. J. Chin. Soc. Corros. Prot. 43, 1015–1021 (2023).
Guo, P. et al. An all‐printed, fast‐response flexible humidity sensor based on hexagonal‐WO3 nanowires for multifunctional applications. Adv. Mater. 35, 202304420 (2023).
Wang, W. et al. Structural optimization of polyimide-film humidity sensors for new energy vehicles. ACS Appl. Mater. Interfaces 16, 49733–49744 (2024).
Chen, X. et al. Integrated interdigital electrode and thermal resistance micro-sensors for electric vehicle battery coolant conductivity high-precision measurement. J. Energy Storage 58, 108–118 (2023).
Situm, A. et al. Monitoring alterations in a salt layer’s deliquescence properties during the atmospheric corrosion of a metal surface using a quartz crystal microbalance. Corros. Sci. 229, 111845 (2024).
Schindelholz, E. & Kelly, R. G. Wetting phenomena and time of wetness in atmospheric corrosion: a review. Corros. Rev. 30, 135–170 (2012).
Jiang, C. et al. Batch fabrication and testing of multiparameter integrated sensor for marine environmental measurements. IEEE Sens. J. 24, 6743–6753 (2024).
Islam, T. U., Zaheer & Gangopadhyay, A. Temperature effect on capacitive humidity sensors and its compensation using artificial neural networks. Sens. Transducers 191, 126–134 (2015).
Tomashov, N. D. Theory of Corrosion and Protection of Metals (New York, McMillan, 1966).
Wu, C., Gao, W., Zou, J., Jin, Q. & Jian, J. Design and batch microfabrication of a high precision conductivity and temperature sensor for marine measurement. IEEE Sens. J. 20, 10179–10186 (2020).
Možek, M., Pečar, B. & Vrtačnik, D. Cost-efficient oceanographic instrument with microfabricated sensors for measuring conductivity, temperature and depth of seawater. Sensors 24, 3940 (2024).
Acknowledgements
This work was financially supported by the grant from the National Science Foundation of China (62271272) and the Open Fund Project of Key Laboratory of Ocean Observation Technology, MNR (2023klootA09). This study was also sponsored by Ningbo Science and Technology Project (2022Z092).
Author information
Authors and Affiliations
Contributions
Rentao Cao: conceptualization, methodology, data curation, writing–original draft. Jiawen Yin: conceptualization, methodology, formal analysis, writing–review and editing. Hanyang Tong: validation. Shengkang Lu: software, validation. Shouhong Li: project administration, resources. Wanlei Gao: formal analysis. Jie Zou: formal analysis. Qinghui Jin: resources, formal analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cao, R., Yin, J., Tong, H. et al. High precision integrated sensors for multi-parameter online monitoring in salt spray environments. Microsyst Nanoeng 11, 159 (2025). https://doi.org/10.1038/s41378-025-00988-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41378-025-00988-2