Abstract
Low-grade appendiceal mucinous neoplasms (LAMNs) exhibit drastically different clinical course and prognosis depending on tumor stage, particularly as it relates to the extent and cellularity of peritoneal involvement. In this context, recent changes in staging guidelines have sought to clarify criteria for pT and pM categories. This study’s aim was to identify clinicopathological features associated with patient outcomes, especially as they pertain to updated stage groups. We reviewed LAMNs from 192 patients (mean age: 56.9 years, 119 (62.0%) women). The tumors consisted of 66 (34.4%) pTisM0, 16 (8.3%) pT3M0, 16 (8.3%) pT4aM0, 27 (14.1%) pTxM1a, and 67 (34.9%) pTxM1b cases. In multivariate analysis, only gross perforation was significantly associated with higher TNM group stage (p = 0.001; OR 3.3, 95% CI: 1.7–6.4). Of 165 (85.9%) patients with clinical follow-up, 51 (30.9%) had disease progression (over a mean 33.7 months, range: 4.7–121.7), whereas over significantly longer follow-up (mean 48.7 months, range: 3.1–143.9; p = 0.004), 114 (69.1%) patients did not. In multivariate analysis, higher TNM stage was significantly associated with disease progression (p = 0.029; OR 18.3, 95% CI: 1.4–246.0). In Kaplan–Meier analysis, none of 74 patients with disease limited to the appendix (pM0), 6 of 27 (22.2%) cases with peritoneal involvement by acellular mucin only (pM1a), and 45 of 64 (70.3%) tumors with intraperitoneal deposits containing neoplastic cells (pM1b) showed disease progression (p < 0.001). These differences in progression-free survival among TNM groups persisted when limiting the analysis to patients who had undergone successful cytoreductive surgery (p = 0.050). Finally, in four patients (all with pM1b disease) death was attributed to disease progression whereas there was no disease-specific mortality in the pM0 and pM1a groups (p = 0.020). These data support the designation of LAMNs with acellular peritoneal mucin as having an intermediate prognosis between cases limited to the appendix and those with intraperitoneal deposits containing neoplastic epithelium.
Similar content being viewed by others

Introduction
Low-grade appendiceal mucinous neoplasms (LAMNs) are relatively rare, albeit increasing in incidence, tumors of the appendix that are histologically characterized by mucinous epithelium with low-grade cytologic atypia, but absence of overt aggressive features, such as infiltrative growth pattern or destructive invasion with associated desmoplastic reaction of the stroma [1,2,3]. The biological behavior and clinical course of LAMNs is heterogenous and highly dependent on the extent of disease involvement (i.e., tumor stage) at presentation. Patients with disease confined to the appendix wall appear to have negligible risk of disease recurrence following appendectomy [4, 5]. In contrast, some patients present with pseudomyxoma peritonei (PMP), a clinical syndrome characterized by intraperitoneal mucinous implants and progressive accumulation of mucinous ascites, most of which are now thought to arise from peritoneal dissemination of LAMNs [6,7,8,9]. Patients with LAMN-associated PMP have slowly progressive, yet incurable disease with a high risk for recurrence, morbidity, and eventual mortality, even though reported 5- and 10-year survival rates are 50–86% and 45–68%, respectively [4, 5, 10,11,12,13,14,15,16,17].
The treatment of PMP typically involves cytoreductive surgery (CRS) followed by hyperthermic intraperitoneal chemotherapy (HIPEC) [18, 19]. In this setting, CRS involves resection of all macroscopically visible peritoneal disease and may include removal of organs with serosal involvement. However, there is little consensus on the appropriate therapy for a subset of LAMN patients with intermediate clinicopathological features such as localized periappendiceal spread without evidence of intraperitoneal involvement or peritoneal spread entirely devoid of neoplastic epithelium (so-called acellular mucin). Limited data on the risk of progression and recurrence of PMP in these settings, compounded by a historical lack of consensus on nomenclature and classification in these tumors, preclude uniformity in clinical decisions on the required aggressiveness of treatment options [20].
The incongruous histopathologic and clinical features of LAMNs have also led to a dizzying array of divergent terminologies and various classification systems both for the appendiceal neoplasm and the peritoneal disease component. For example, metastatic disease associated with appendiceal mucinous neoplasms, when limited to the peritoneum and consisting of low-grade mucinous epithelium, has been variably referred to as low-grade mucinous carcinoma peritonei, disseminated peritoneal adenomucinosis, and LAMN with peritoneal involvement [6, 21,22,23]. In a recent attempt at consensus, the Peritoneal Surface Oncology Group International (PSOGI) defined LAMNs as mucinous neoplasms with low-grade cytology and any of the following: loss of muscularis mucosae, submucosal fibrosis, “pushing” or diverticular-like growth into the wall, dissecting acellular mucin, undulating or flattened epithelial growth, and mucin or neoplastic cells outside the appendix [1]. In addition, the pathologic classification of PMP involves the evaluation of cytologic atypia in peritoneal neoplastic epithelium and comprises three categories: low-grade, high-grade, and high-grade with signet ring cells; acellular mucin as the only component of PMP is classified separately [13, 24]. The most recent edition of the World Health Organization Classification of Tumours discourages terms such as borderline, uncertain malignant potential, and mucinous cystadenoma, and considers grade 1 appendiceal mucinous neoplasms to have low-grade cytology and a pushing margin in the primary appendiceal tumor and hypocellular mucinous deposits or low-grade epithelium in peritoneal metastases [2].
In this context, recent changes in the American Joint Commission on Cancer (AJCC) staging manual have also sought to address confusion in the pathologic reporting of LAMNs [25]. Prior to the current edition, the staging of LAMNs was not specifically addressed, forcing pathologists to use the same criteria as for conventional appendiceal adenocarcinoma, whereby invasion into submucosa is staged as pT1 and into muscularis propria as pT2. In addition, the significance of acellular mucin during the staging of appendiceal neoplasms was not specifically incorporated into the manual and intraperitoneal metastatic disease spread was defined as containing neoplastic cells. Thus, the 8th edition of the manual explicitly includes LAMNs, which are staged as pTis(LAMN) when confined to the muscularis propria (and belong to prognostic stage group 0), but as pT3 when neoplastic mucinous epithelium or acellular mucin extend to the subserosal soft tissue, and as pT4a when either extends to the serosa of the appendix or the mesoappendix, including in cases of perforation when cellular or acellular mucin is continuous to the serosal surface through inflammation [25]. In addition, intraperitoneal spread of acellular mucin qualifies as metastatic disease (pM1a), whereas peritoneal deposits of neoplastic mucinous epithelium are now designated pM1b, if they comprise well-differentiated (G1) epithelium, but both belong to the same overall prognostic group (stage IVA).
Nevertheless, studies that validate these recent changes or adequately stratify risk in terms of outcomes such as disease recurrence or progression in patients with these neoplasms are lacking. Therefore, our aim in this study was to review cases of LAMN from a large patient cohort, restage them according to these most recent criteria, and evaluate the clinicopathological features that are associated with disease progression and patient prognosis in this setting.
Materials and methods
Study cases
The study was approved by the Mount Sinai Institutional Review Board. Cases of appendiceal mucinous neoplasms were retrieved through a search of surgical pathology records over a 10-year period (January 2008–January 2018). Inclusion criteria consisted of specimens diagnosed as primary, low-grade mucinous neoplasms of the appendix. High-grade appendiceal mucinous neoplasms and appendiceal adenocarcinomas with invasive, destructive growth were excluded from the study. Specimens with mucinous neoplasms of the colon (including cecum) or small intestine were excluded. Neuroendocrine neoplasms (NENs), mixed neuroendocrine-non-neuroendocrine neoplasms (MiNENs), and goblet cell adenocarcinomas (formerly known as goblet cell carcinoids) were excluded. Specimens from endoscopic procedures only (e.g., biopsies of the appendiceal orifice during colonoscopy) were excluded. Consultation cases submitted to our Department from referring institutions were included only when microscopic slides and/or tissue blocks were available for review. Cases of LAMN diagnosed as being confined to the appendix, but which had not been completely submitted for histologic evaluation, were excluded from the study. Cases with insufficient clinical data in patient medical records were excluded.
Clinicopathological data
Data on demographic and clinicopathological features, including patient age and sex, type of index surgical procedure, method of intraperitoneal sampling, presence or absence of gross tumor perforation, histological status of surgical resection margin, stage (pT, pN, pM, and overall prognostic stage group), concurrent diagnosis of inflammatory bowel disease (IBD), and treatment administration (HIPEC, CRS, and/or systemic chemotherapy), if any, were obtained by reviewing surgical pathology microscopic slides and reports (including review of gross specimen photographs) and patient medical records (including intraoperative and admission or discharge notes). The index surgical procedure was defined based on the anatomical extent of primary resection at the time of LAMN diagnosis (or within 3 months) and characterized as simple, when consisting of an appendectomy with or without synchronous cecectomy, or extensive, when consisting of an ileocolic resection, segmental (right) colectomy, or total/subtotal colectomy. The method of diagnostic or therapeutic intraperitoneal sampling was categorized as comprising biopsies (sampling of intraperitoneal tissues and/or organs) or resection (wide excision of peritoneal organs, such as oophorectomy, omentectomy, etc.). HIPEC consisted of 40 mg mitomycin C delivered in closed fashion for 90 min and targeting an intraperitoneal temperature of 41–43 °C, as recommended by consensus guidelines [26]. CRS included surgical resection of all visible peritoneal disease, including peritoneal stripping, full thickness diaphragm resection, and resection of any involved organs. Extent of CRS was quantified using conventional completeness of cytoreduction scores (CCS), determined at the time of surgery, as follows: CCS0: no residual tumor nodules; CCS1: residual tumor nodules <2.5 mm; CCS2: residual tumor nodules ≥2.5 mm and ≤2.5 cm; CCS3: residual tumor nodules >2.5 cm [27,28,29]. In cases where CCS was not reported at the time of surgery, intraoperative notes were reviewed and CCS was assigned retrospectively. For the purposes of statistical analysis, CCS scores of 0 and 1 were considered successful CRS and were combined (CCS 0-1).
AJCC/TNM restaging
All hematoxylin and eosin (H&E)-stained sections were independently and blindly reviewed by two gastrointestinal pathologists (SJB and ADP) for diagnosis confirmation and reassessment of pathologic stage according to the current (8th) edition of the AJCC Staging Manual [25]. Disagreement was resolved by reviewing cases on a multiheaded microscope and consensus was achieved throughout. All cases, as per study inclusion criteria, were classified as low-grade/well-differentiated (G1) and were restaged and sub-grouped into five categories (pTis, pT3, pT4a, pTxM1a, and pTxM1b). Briefly, LAMNs that were confined to the appendix and/or extended into but not through the appendiceal muscularis propria were staged as pTis(LAMN). Penetration of neoplastic epithelium or acellular mucin through the muscularis propria into periappendiceal soft tissues without serosal involvement was staged as pT3, whereas serosal surface involvement of the appendix or mesoappendix was staged as pT4a. Intraperitoneal disease was confirmed by tissue sampling within 3 months of initial diagnosis and staged as pM1a or pM1b based on the absence or presence, respectively, of neoplastic cells within mucinous peritoneal deposits.
Patient outcomes
Electronic medical records (including physician notes, results of imaging studies, etc.) were extensively reviewed and patient outcomes were recorded. Primary outcome endpoints consisted of disease progression and disease-specific mortality. Disease progression was defined as evidence of intraperitoneal disease recurrence after a documented disease-free interval or evidence of increasing disease burden (disease extension) after a treatment attempt (HIPEC and/or CRS) and was established by cross-sectional imaging (including CT and MRI scanning modalities) and/or histopathological examination of peritoneal tissue. Follow-up was defined as the time from initial LAMN diagnosis to event (disease progression or disease-specific mortality) or last clinical examination and was measured in months. Only patients with at least 3 months of follow-up were included in outcome analysis.
Statistical analysis
Continuous variables (patient age and follow-up time) were compared using one-way analysis of variance. Categorical variables, including patient sex, type of index procedure, peritoneal sampling method, tumor perforation, margin status, stage group (pTNM), concurrent IBD diagnosis, treatment modalities, response, and patient outcome, were compared using Pearson chi-squared tests. Multivariate logistic regression was performed using all characteristics as independent variables, except where indicated, with calculated odds ratio (OR) and 95% confidence intervals (CI). Cohen’s kappa coefficient was calculated to evaluate for concordance in the presence of neoplastic cells within peritoneal metastases between specimens from the initial diagnosis and during disease progression. Kaplan–Meier survival analysis was used to test for differences in disease progression and disease-specific mortality during the follow-up period and log-rank test was employed for statistical significance. All statistical analysis was carried out using Statistical Package for the Social Sciences software (SPSS; Build 1.0.0.1327; copyright 2019, IBM) with p < 0.05 considered significant.
Results
Clinicopathological characteristics
Specimens from 192 patients who had been diagnosed with LAMNs fulfilled study criteria and were included. The patients, 119 (62.0%) of whom were female and 73 (38.0%) male, had a mean age of 56.9 ± 13.8 years and a median age of 58 years (range: 19–91). Nine patients (4.7%) also carried a diagnosis of IBD. Based on inclusion criteria, all appendiceal tumors were classified as low-grade/well-differentiated (G1) and lacked infiltrative or destructive growth, complex glandular architecture, invasion with desmoplastic stroma reaction, or signet ring cells. Lymph node metastases (pN) were not present in any of the 84 (43.8%) cases that had lymph nodes available for histological examination. Invasion of adjacent organs (i.e., pT4b), lymphovascular or perineural space invasion, distant metastases other than in the peritoneum (pM1c), or high-grade epithelium were not seen in any of the cases, whether at first presentation/diagnosis or during disease progression.
Updated AJCC/TNM staging
Based on the most recent AJCC staging criteria, study cases were divided into five groups (Fig. 1). A significant proportion of patients (66, 34.4%) had presented with a LAMN confined to the appendiceal wall without extension to the subserosa or peritoneal involvement (pTisM0). In 16 cases (8.3%), neoplastic epithelium or acellular mucin extended to the appendiceal subserosal soft tissue (pT3M0) and in another 16 (8.3%) to the serosal surface (pT4aM0), neither of which had associated peritoneal disease. Regardless of primary tumor stage in the appendix, 27 cases (14.1%) showed involvement of the peritoneum by acellular mucin (pTxM1a) and another 67 cases (34.9%) had peritoneal disease consisting of low-grade mucinous epithelium (pTxM1b).
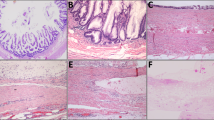
a LAMN completely confined to the appendix, which is designated pTis(LAMN). Notice the obliterated lamina propria and intact muscularis propria layer. b LAMN with acellular mucin extending into, but not through, the muscularis propria. This is still classified as pTis(LAMN). c Acellular mucin dissecting through the muscularis propria (top of image) to the subserosal soft tissue, without involving the serosa, is staged as pT3. d Acellular mucin reaching the inked serosal surface (top of image) of the mesoappendix is assigned a stage of pT4a. e Peritoneal sample from the omentum showing acellular mucin surrounding adipose tissue, which is categorized as pM1a. f Peritoneal biopsies with low-grade mucinous epithelium surrounding fibrous tissue qualify this tumor as pM1b. (H&E stains; original magnification: ×200).
With the intention to identify features that might correlate with higher overall stage, clinicopathological characteristics were evaluated in these five groups (Table 1). In univariate analysis, patient sex, while significantly different among stage subgroups (p = 0.022), did not exhibit a discernible pattern. In contrast, patients with higher stage LAMNs were more likely to have undergone more extensive surgical resection (p = 0.031) and to have had gross perforation in the appendix (p < 0.001). A concomitant diagnosis of IBD was more likely in the group of cases confined to the appendix (pTisM0), but the difference did not reach statistical significance (p = 0.080). Patient age, histological status of the resection margin, and method of peritoneal sampling were not significantly different among LAMN stage groups. Variables which were dependent on stage group (i.e., type of index surgical procedure and method of intraoperative peritoneal sampling), were excluded from multivariate analysis which showed that only gross perforation remained significantly associated with LAMN stage group (p = 0.001) and, when present, increased the likelihood of a higher overall stage with an OR of 3.3 (95% CI: 1.7–6.4).
Patient treatment, response, and outcomes
Treatment utilization, response, and disease outcomes were evaluated for 165 (85.9%) patients who had clinical follow-up of at least 3 months. These characteristics were considerably different across various LAMN stage groups (Table 2). As expected, given their respective stage, only 1 patient (2.0%) with pTisM0 and 1 (8.3%) with pT3M0 received HIPEC (both cases had gross perforation of the appendix). Conversely, more than half pT4aM0 patients (7 cases, 58.3%) and most patients with intraperitoneal acellular mucin (pTxM1a; 23 cases, 85.2%) or neoplastic epithelium (pTxM1b; 58 cases, 90.6%) were given HIPEC (p < 0.001). The majority of patients with LAMNs staged as pM1a and pM1b also received CRS (88.9% and 92.2%, respectively), significantly more compared to 1 (8.3%) pT4aM0 patient and no patients in the pTis and pT3 groups (p < 0.001). Most patients had good response to treatment and at similar rates: CRS completion scores (CCS) of 0 or 1 were achieved in the 1 patient (100%) with pT4aM0, 19 patients (90.5%) with pM1a, and 45 patients (80.4%) with pM1b stage LAMNs (difference not significant).
Over similar lengths of follow-up time among these stage groups (the differences not being significant), there was no evidence of disease progression in any of the 50 pTisM0, 12 pT3M0, and 12 pT4aM0 patients who had clinical follow-up. In contrast, significantly more patients with peritoneal spread had disease progression (p < 0.001): 6 of 27 (22.2%) cases with peritoneal acellular mucin (pM1a) and 45 of 64 (70.3%) patients with peritoneal spread composed of neoplastic epithelium (pM1b). All four deaths attributed to LAMN (disease-specific mortality) in this cohort belonged to the pM1b stage group (6.3% of patients with follow-up in this group) and all four had shown disease progression prior to death. In contrast, there was no disease-related mortality among any of the pM0 (Tis/T3/T4a) and pM1a patients, significantly less than the pM1b group (p = 0.011).
Disease progression
Among cases with peritoneal disease spread that progressed, there was significant concordance in terms of the presence or absence of neoplastic cells in specimens from the original diagnostic procedure compared to specimens obtained at the time of disease progression (Table 2). Of 33 cases with peritoneal spread by neoplastic cells (pM1b), 31 (93.9%) also had low-grade epithelium at disease progression. Conversely, of six cases with intraperitoneal mucin (pM1a) that progressed, two (33.3%) had acellular mucin only at disease progression (84.6% proportionate agreement; p = 0.043). However, this agreement could only be characterized as fair (κ = 0.316), given that most (4 of 6, 66.7%) of the original pM1a cases that progressed on the basis of histologic examination did so with the presence of neoplastic epithelium. There were no cases that progressed with high-grade neoplastic epithelium (i.e., G2 or G3).
In order to identify prognostic features associated with disease progression in LAMNs, we compared 51 (30.9%) cases with documented disease progression over a mean follow-up of 33.7 ± 28.4 months (mean: 26.0, range: 4.7–121.7) to 114 (69.1%) patients with no evidence of disease during a significantly longer mean follow-up of 48.7 ± 31.2 months (mean: 41.3, range: 3.1–143.9; p = 0.004) in terms of various clinicopathological characteristics (Table 3). In univariate analysis, absence of a concomitant diagnosis of IBD (p = 0.039), a more extensive index surgical procedure (p < 0.001), and higher AJCC/TNM stage group (p < 0.001) were all significantly associated with disease recurrence. In contrast, patient age and sex, gross perforation of the appendix, appendiceal margin status, and extent of peritoneal sampling at the time of diagnosis were not associated with disease progression. Unsurprisingly, since type of index surgery is a variable not independent of group stage (see Table 1), it lost significance in multivariate analysis, as did a concurrent IBD diagnosis. AJCC/TNM stage remained significantly associated with disease progression in multivariate analysis (p = 0.029) and a higher overall stage group increased the likelihood of disease progression with an OR of 18.3 (95% CI: 1.4–246.0). As expected, incomplete cytoreduction was associated with disease progression: of 65 patients with successful CRS (i.e., CCS 0–1), less than half (31 cases, 47.7%) had disease progression, whereas out of 13 patients with incomplete CRS scores (CCS 2–3), most (10 cases, 76.9%) exhibited progression. The difference was significant in multivariate analysis (p = 0.036) with less complete cytoreduction (i.e., higher CCS scores) increasing the likelihood of disease progression by an OR of 7.3 (95% CI: 1.1–46.4).
Progression-free and disease-specific survival
To demonstrate the significance of stage group in disease progression and survival, Kaplan–Meier survival analysis was employed (Fig. 2). None of the 74 (0%) patients with disease limited to the appendix wall and periappendiceal serosa (pTis/T3/T4a M0) had disease progression (Fig. 2a). In contrast, 6 of 27 (22.2%) patients with peritoneal acellular mucin (pTxM1a) and 45 of 64 (70.3%) cases with peritoneal neoplastic epithelium (pTxM1b) progressed over the follow-up period, a significant difference (p < 0.001; log-rank test). Since incompleteness of CRS was significantly associated with disease progression in multivariate analysis, we wanted to determine whether AJCC/TNM stage group was still associated with disease progression once treatment effect had been taken into account. We therefore limited progression-free survival analysis to cases where complete or near complete CRS had been achieved (CCS 0–1) and there were still significant differences among these stage groups in terms of disease progression (p = 0.050; Fig. 2b). Finally, disease-specific survival (DSS) analysis (Fig. 2c) showed that cases with peritoneal metastases composed of low-grade mucinous epithelium (pTxM1b) had significantly higher mortality due to disease compared with cases without peritoneal spread of neoplastic cells (pTx M0/M1a; p = 0.020).
a Kaplan–Meier curves of cumulative progression-free survival (PFS), defined as the absence of peritoneal disease recurrence or extension during clinical follow-up, for LAMNs of different AJCC/TNM stage groups. b Kaplan–Meier curves of PFS by LAMN stage group, limited to cases with successful cytoreduction (i.e., with CCS of 0–1). c Kaplan–Meier curves of cumulative disease-specific survival (DSS) in cases with peritoneal disease comprised of neoplastic epithelium at the time of LAMN diagnosis (pM1b) compared with cases without (pM0/M1a). Cross hatches (+) indicate censoring of patients (death or last follow-up) and vertical step lines indicate events (i.e., disease progression or mortality).
Discussion
In this study, LAMNs confined to the appendiceal wall and serosal surface without peritoneal spread at the time of diagnosis had no risk of disease progression, while those with peritoneal disease consisting of neoplastic low-grade epithelium had significant risk. Crucially, LAMNs presenting with peritoneal spread of acellular mucin had an intermediate risk of disease progression between the two, supporting recent updates in the staging of these neoplasms.
Our findings provide additional evidence that patients with LAMNs confined to the appendix have no risk of disease progression or recurrence. This includes LAMNs with extension into the muscularis propria (pTis) as well as those with neoplastic epithelium or acellular mucin in the subserosal soft tissue (pT3), without involvement of the serosal surface. In fact, including 82 such cases in the current study, there are now over 300 LAMN cases with no serosal involvement reported in the literature and none had disease recurrence [4, 5, 30,31,32,33,34,35]. Collectively, these results support current recommendations that patients with such neoplasms should be considered adequately treated by appendectomy alone and would further suggest that the pT3 category in the current AJCC staging can probably be down-staged, from the current prognostic group IIA (if pM0) closer to the pTis(LAMN) designation and overall prognostic stage group 0.
A clinical dilemma that might arise in this setting concerns prognosis and subsequent follow-up requirements for tumors with positive surgical resection margins, whether due to acellular mucin or neoplastic epithelium. There was no disease recurrence or progression among 4 pTisM0 and 2 pT4aM0 cases with positive resection margins in the current study, nor did we find that the status of the appendectomy margin overall is statistically associated with disease progression in our series. These data confirm results from prior studies on surgical margins in LAMNs, and would corroborate prior recommendations for conservative management in these cases [30, 36]. However, determination of margin status in LAMN resections is inherently fraught with difficulties due to the presence of mucin and its tendency to contaminate histological sections. Thus, if improperly designated cases with “positive” margins due to mucin extravasation have been incorporated, the negligible recurrence rates that have been reported in this setting would have been overstated. Indeed, cases wherein the tumor is contiguous with the margin should probably enjoy the benefit of continued clinical scrutiny [6, 37].
We also identified 16 cases of LAMN with acellular mucin extending to the serosal surface of the appendix or mesoappendix, but without evidence of intraperitoneal spread (pT4aM0). In one case, mucin was present locally in the periappendiceal right lower quadrant (RLQ) area, but was included in this group (as opposed to being staged as pM1a), given that AJCC criteria for the pM1a designation describe “disseminated peritoneal mucinous deposits.” This is perhaps the least well-defined and studied subcategory within LAMN lesions, with few cases reported overall and specific details on the cellular composition of the extra-appendiceal disease component generally lacking. A literature search uncovered ~118 cases of LAMNs (including this study) with acellular mucin present in, but localized to, the appendiceal serosa and/or surrounding periappendiceal area (i.e., without disseminated peritoneal disease) [5, 32, 33, 35, 36, 38]. Overall, among these reported cases, only 3 (2.5%) had disease recurrence during follow-up. Among 12 patients with pT4aM0 LAMNs and clinical follow-up in our study, none had disease recurrence over an average of ~3.5 years (median 3 years). However, more than half of these patients (58.3%) had received intraoperative HIPEC and one patient with mucin in the RLQ had also undergone CRS, interventions that could have affected their favorable outcome.
Only a few of these prior studies additionally evaluated cases where such localized extra-appendiceal deposits contained neoplastic cells and together they found that a total 8 of 22 (36.4%) such patients developed mucinous ascites during follow-up, including two who eventually died of disease [5, 38]. The difference by an order of magnitude in the rate of disease progression between LAMNs with localized acellular vs. cellular periappendiceal mucin deposits would suggest that they do not belong together under the same AJCC stage group IIB. Thus, while not distinguishing between acellular mucin and neoplastic epithelium when staging LAMNs may be applicable for the pTis and pT3 categories, pT4a may require a modification in order to separate cases that are associated with a documented worse prognosis. Certainly, they would seem to belong to a higher overall prognostic stage level compared to pT3M0 tumors, which are currently designated IIA.
Similar to resection margin status, a number of considerations might influence the proper designation of pT4a stage in these cases. Acellular mucin, or even neoplastic epithelium, can appear on the serosal surface of the appendix due to intraoperative contamination or improper handling during gross sectioning, leading to inappropriate upstaging of the tumor. Given that the rate of disease progression in pT4aM0 LAMNs is not negligible, particularly in cases due to the presence of neoplastic epithelium, care must be taken to exclude residual disease in the surrounding area of the peritoneum (RLQ). In many institutions, including ours, surgical evaluation of the peritoneum (all 4 quadrants) is routine during diagnostic laparoscopy in these cases, as is radiologic surveillance every 6 months for 5 years [28]. In a related matter, a number of studies have found significant coexistence of appendiceal diverticular disease with the presence of LAMNs, suggesting that diverticulae may form due to increased intraluminal pressure in the setting of LAMN or may preexist and be secondarily involved by the mucinous neoplasm itself [4, 39, 40]. In fact, whether ruptured or not, appendiceal diverticulae may simulate features of LAMN and cause erroneous overdiagnosis [41,42,43].
In our study, gross perforation of the appendix was the only variable significantly associated with a higher pathologic LAMN stage in multivariate analysis. As with diverticulae, gross perforation might provide a means of egress for neoplastic epithelium or acellular mucin through the appendiceal wall, upstaging the tumor. In addition, perforation may be secondary to acute appendicitis, mucosal herniation or true tumor extension [6]. Even though LAMNs are by definition not invasive tumors with destructive growth, their pushing growth or dissecting mucin may cause appendiceal perforation or, conversely, perforation due to luminal pressure and/or inflammation (appendicitis) may allow tumor to escape. The latter may be considered a situation where the tumor is incorrectly upstaged, particularly since AJCC staging criteria consider tumor cells or acellular mucin continuous with the serosal surface through inflammation as pT4a [25]. Regardless of which came first or issues of semantics, gross perforation of the appendix would almost certainly lead to tumor found outside the appendix and a higher assigned stage. However, in and of itself, this may not necessarily be a sign of tumor aggressiveness. Supporting this contention, gross perforation was not associated with disease progression in our study and there was no progression among pT4aM0 cases. Perforation may allow mucin and/or tumor cells to escape to the serosal surface and surrounding periappendiceal area, but it probably does not lead to peritoneal disease spread unless the tumor has the biological capacity to do so.
One of the major changes in the most recent AJCC staging of appendiceal neoplasms concerns the definition of peritoneal involvement by acellular mucin as metastatic disease, designated as pM1a, even though it is understood to have the most favorable prognosis among the histological phenotypes associated with PMP [1, 6]. Prior studies have seen mixed results among patients with such tumors, with approximately half the series reporting no disease progression and the rest describing only one case each with recurrence out of different case number totals and after varying lengths of follow-up [5, 13, 33, 35, 44,45,46,47,48,49,50,51]. Taking the results of these studies together, the overall rate of recurrence in this group appears to be ~4–5%, although some of these series with smaller numbers of cases reported rates as high as 10–25% [37, 46, 49, 51]. We report herein that 22.2% of cases with acellular peritoneal mucin at presentation had subsequent disease progression, despite the fact that all cases that progressed had received HIPEC and had undergone CRS, as well as receiving adjuvant systemic chemotherapy in one case. There could be many reasons why our cases with acellular mucin in the peritoneum (pM1a) had a higher rate of progression compared with the average of what is reported in the literature. Studies may contain important differences in study population (our institution is a referral center for the treatment of LAMNs), length of follow-up time (our study had a median of 3 years), method of surveillance (we included progression as determined by imaging studies), and number of cases within this stage subgroup (our study had a fairly large number of such cases: 27).
Statistical analysis (such as Kaplan–Meier survival) within the same study population is best suited at comparing recurrence rates among stage groups and we found that patients with pM1a LAMNs had significantly higher rate of progression compared to pM0 cases and significantly lower compared to pM1b patients, differences that persisted when the analysis was limited to patients that had undergone successful cytoreduction. However, the presence of acellular peritoneal mucin was not associated with an increase in disease-specific mortality, which was significantly less than that observed with pM1b tumors. A recent, large prospective series has confirmed that peritoneal disease classified as acellular mucin according to the recent PSOGI consensus (equivalent to pM1a in the AJCC/TNM staging system) has a favorable prognosis and significantly higher 10-year survival compared to low-grade PMP [47]. Thus, while our data supports the recent designation of acellular peritoneal mucin as pM1a, we believe that it does not necessarily belong to the same overall prognostic group as pM1b (i.e., stage IVA), given the significantly lower risk for disease progression and disease-specific mortality.
The distinction between cellular and acellular peritoneal disease may be partly artificial, since presumably even in cases with acellular deposits, neoplastic cells would have had to be present at one point, in order to produce the mucin. Supporting this hypothesis, studies that have looked into adequate sampling considerations have found that additional tissue blocks can help identify neoplastic cells in ~17% of instances, thus upstaging such cases to pM1b [52]. Importantly, out of 6 pM1a cases that recurred in our study, 4 (66.7%) did so with low-grade neoplastic epithelium, raising the question of whether that had been the case, and appropriate stage, all along. Nevertheless, the significant differences in rates of disease progression and DSS among these stage groups overall suggests that there are real, biologically important distinctions between them. Perhaps it is a matter of the number and/or density of neoplastic cells present within peritoneal pools of mucin that determine both long-term disease behavior as well as the rate at which they are being detected [48, 53, 54].
As expected, we observed increasing treatment utilization with higher LAMN stage. However, since this was not an independent relationship, we attempted to incorporate a measure of treatment response in the analysis of parameters influencing disease progression by evaluating the success of cytoreduction with CCS. Successful CRS was significantly associated with lower rates of disease progression in multivariate analysis: 52.3% of patients with CCS 0–1 avoided disease progression during clinical follow-up. In addition, disease progression was significantly associated with AJCC/TNM stage group even in patients with highly complete CRS procedures, suggesting that LAMN stage remains the most important predictor of patient outcomes. Two patients with disease confined to the appendix (1 pTis and 1 pT3) received HIPEC, but both had evidence of gross perforation in the appendix. In addition, 7 (58.3%) patients with periappendiceal acellular mucin (pT4aM0) received HIPEC, including three cases with gross perforation and one with positive resection margin. Lack of established guidelines regarding treatment recommendations in this setting and confusion concerning terminology, classification, and prognosis, probably contribute to treatment overuse, which is not without adverse side effects. Concerted efforts by pathologists to achieve and enforce consensus in nomenclature, classification, and staging would go a long way toward achieving more consistent management approaches [55].
Interestingly, 4.7% of the patients in our study had a concurrent diagnosis of IBD. While this is almost fourfold higher that the prevalence of IBD in the U.S. general population, our medical center is a major referral center for the diagnosis, treatment, and surveillance of patients with IBD, which would explain the increased rate in this patient cohort. Most of these patients (7 of 9, 77.8%) had pTisM0 stage LAMNs, further suggesting that increased surveillance in these patients, including with such modalities as colonoscopy and CT scanning, may have led to increased detection of incidental, early-stage LAMNs. In addition, these patients were evenly distributed among IBD subtypes (five ulcerative colitis and four Crohn disease), arguing against inflammation of the colonic mucosa being a predisposing or contributing factor during the pathogenesis of LAMNs. Furthermore, even though a concurrent IBD diagnosis was significantly correlated with the absence of disease recurrence in univariate analysis, the association disappeared once stage group was incorporated during multivariate analysis. Finally, a large case-control study did not find differences in the overall prevalence of appendiceal mucinous cystadenomas between patients with IBD and non-IBD controls [56].
There are some limitations in this retrospective study, such as the possibility of selection and referral bias, given that our medical center treats a large patient population with these neoplasms. We tried to limit this effect by establishing strictly defined and comprehensive inclusion and exclusion criteria and by reviewing all consecutive cases identified through our search. Sampling bias from differences in handling gross pathology specimens or assaying for peritoneal disease during surgery could have influenced the rates of detection of neoplastic epithelium in our cases. Nevertheless, given that this is a series from a single institution, the limited number of surgeons operating on these patients and a uniform grossing procedure established for the handling of such cases, should minimize this effect. Our study had significant follow-up, particularly for patients without disease progression (mean 48.7 months), thus minimizing transfer bias and allowing us to interpret the results with some confidence.
In conclusion, we report that disease progression in LAMNs is significantly associated with AJCC/TNM prognostic stage groups, particularly as they pertain to the extent and type of peritoneal disease at the time of diagnosis. Patients with LAMNs having no peritoneal involvement (pM0) incurred no disease progression during follow-up, while those with peritoneal deposits containing neoplastic cells (pM1b) demonstrated significantly worse outcomes and those with intraperitoneal acellular mucin only (pM1a) had intermediate risk of disease progression. Thus, while the recent changes in AJCC/TNM staging classification are supported by our data, additional modifications specific to LAMNs, such as downstaging pT3, distinguishing cellular from acellular mucin in the pT4a category, and separating pM1a from pM1b within prognostic stage group IVA, might be warranted, especially if confirmed in future studies.
Change history
03 May 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41379-021-00811-z
References
Carr NJ, Cecil TD, Mohamed F, Sobin LH, Sugarbaker PH, Gonzalez-Moreno S, et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: the results of the Peritoneal Surface Oncology Group International (PSOGI) modified Delphi process. Am J Surg Pathol. 2016;40:14–26.
Misdraji J, Carr NJ, Pai RK (Reetesh). Appendiceal mucinous neoplasm. In: Lokuhetty D, White VA, Watanabe R, Cree IA, editors. WHO classification of tumours—digestive system tumors. 5th ed. Lyon, France: International Agency for Research on Cancer (IARC); 2019. p. 144–6.
Shaib WL, Goodman M, Chen Z, Kim S, Brutcher E, Bekaii-Saab T, et al. Incidence and survival of appendiceal mucinous neoplasms: a SEER analysis. Am J Clin Oncol. 2017;40:569–73.
Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27:1089–103.
Pai RK, Beck AH, Norton JA, Longacre TA. Appendiceal mucinous neoplasms: clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am J Surg Pathol. 2009;33:1425–39.
Carr NJ, Bibeau F, Bradley RF, Dartigues P, Feakins RM, Geisinger KR, et al. The histopathological classification, diagnosis and differential diagnosis of mucinous appendiceal neoplasms, appendiceal adenocarcinomas and pseudomyxoma peritonei. Histopathology. 2017;71:847–58.
Carr NJ, Emory TS, Sobin LH. Epithelial neoplasms of the appendix and colorectum: an analysis of cell proliferation, apoptosis, and expression of p53, CD44, bcl-2. Arch Pathol Lab Med. 2002;126:837–41.
Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol. 1995;19:1390–1408.
Szych C, Staebler A, Connolly DC, Wu R, Cho KR, Ronnett BM. Molecular genetic evidence supporting the clonality and appendiceal origin of Pseudomyxoma peritonei in women. Am J Pathol. 1999;154:1849–55.
Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis—a clinicopathological analysis of 109 cases with emphasis on distinguishing pathological features, site of origin, prognosis, and relationship to Pseudomyxoma-peritonei. Am J Surg Pathol. 1995;19:1390–1408.
Bradley RF, Stewart JHT, Russell GB, Levine EA, Geisinger KR. Pseudomyxoma peritonei of appendiceal origin: a clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol. 2006;30:551–9.
Carr NJ, Finch J, Ilesley IC, Chandrakumaran K, Mohamed F, Mirnezami A, et al. Pathology and prognosis in pseudomyxoma peritonei: a review of 274 cases. J Clin Pathol. 2012;65:919–23.
Davison JM, Choudry HA, Pingpank JF, Ahrendt SA, Holtzman MP, Zureikat AH, et al. Clinicopathologic and molecular analysis of disseminated appendiceal mucinous neoplasms: identification of factors predicting survival and proposed criteria for a three-tiered assessment of tumor grade. Mod Pathol. 2014;27:1521–39.
Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–56.
Chua TC, Yan TD, Smigielski ME, Zhu KJ, Ng KM, Zhao J, et al. Long-term survival in patients with pseudomyxoma peritonei treated with cytoreductive surgery and perioperative intraperitoneal chemotherapy: 10 years of experience from a single institution. Ann Surg Oncol. 2009;16:1903–11.
Gough DB, Donohue JH, Schutt AJ, Gonchoroff N, Goellner JR, Wilson TO, et al. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg. 1994;219:112–9.
Sugarbaker PH, Alderman R, Edwards G, Marquardt CE, Gushchin V, Esquivel J, et al. Prospective morbidity and mortality assessment of cytoreductive surgery plus perioperative intraperitoneal chemotherapy to treat peritoneal dissemination of appendiceal mucinous malignancy. Ann Surg Oncol. 2006;13:635–44.
Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42.
Jacquet P, Stephens AD, Averbach AM, Chang D, Ettinghausen SE, Dalton RR, et al. Analysis of morbidity and mortality in 60 patients with peritoneal carcinomatosis treated by cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy. Cancer. 1996;77:2622–269.
Moran B, Baratti D, Yan TD, Kusamura S, Deraco M. Consensus statement on the loco-regional treatment of appendiceal mucinous neoplasms with peritoneal dissemination (pseudomyxoma peritonei). J Surg Oncol. 2008;98:277–82.
Misdraji J. Appendiceal mucinous neoplasms: controversial issues. Arch Pathol Lab Med. 2010;134:864–70.
Misdraji J. Mucinous epithelial neoplasms of the appendix and pseudomyxoma peritonei. Mod Pathol. 2015;28 Suppl 1:S67–79.
Panarelli NC, Yantiss RK. Mucinous neoplasms of the appendix and peritoneum. Arch Pathol Lab Med. 2011;135:1261–8.
Choudry HA, Pai RK. Management of mucinous appendiceal tumors. Ann Surg Oncol. 2018;25:2135–44.
Overman MJ, Asare EA, Compton CC, Hanna NH, Kakar S, et al. Appendix—carcinoma. In: Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al., editors. AJCC cancer staging manual. 8th ed. New York: Springer; 2017. p. 237–50.
Turaga K, Levine E, Barone R, Sticca R, Petrelli N, Lambert L, et al. Consensus guidelines from The American Society of Peritoneal Surface Malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann Surg Oncol. 2014;21:1501–5.
Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–74.
Solomon D, Bekhor E, Leigh N, Maniar YM, Totin L, Hofstedt M, et al. Surveillance of low-grade appendiceal mucinous neoplasms with peritoneal metastases after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: are 5 years enough? A multisite experience. Ann Surg Oncol. 2020;27:147–53.
Tabrizian P, Shrager B, Jibara G, Yang MJ, Romanoff A, Hiotis S, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis: outcomes from a single tertiary institution. J Gastrointest Surg. 2014;18:1024–31.
Arnason T, Kamionek M, Yang M, Yantiss RK, Misdraji J. Significance of proximal margin involvement in low-grade appendiceal mucinous neoplasms. Arch Pathol Lab Med. 2015;139:518–21.
Foster JM, Sleightholm RL, Wahlmeier S, Loggie B, Sharma P, Patel A. Early identification of DPAM in at-risk low-grade appendiceal mucinous neoplasm patients: a new approach to surveillance for peritoneal metastasis. World J Surg Oncol. 2016;14:243.
McDonald JR, O’Dwyer ST, Rout S, Chakrabarty B, Sikand K, Fulford PE, et al. Classification of and cytoreductive surgery for low-grade appendiceal mucinous neoplasms. Br J Surg. 2012;99:987–92.
Wong M, Barrows B, Gangi A, Kim S, Mertens RB, Dhall D. Low-grade appendiceal mucinous neoplasms: a single institution experience of 64 cases with clinical follow-up and correlation with the current (eighth edition) AJCC staging. Int J Surg Pathol. 2020;28:252–8.
Esquivel J, Garcia SS, Hicken W, Seibel J, Shekitka K, Trout R. Evaluation of a new staging classification and a Peritoneal Surface Disease Severity Score (PSDSS) in 229 patients with mucinous appendiceal neoplasms with or without peritoneal dissemination. J Surg Oncol. 2014;110:656–60.
Umetsu SE, Shafizadeh N, Kakar S. Grading and staging mucinous neoplasms of the appendix: a case series and review of the literature. Hum Pathol. 2017;69:81–9.
Tiselius C, Kindler C, Shetye J, Letocha H, Smedh K. Computed tomography follow-up assessment of patients with low-grade appendiceal mucinous neoplasms: evaluation of risk for Pseudomyxoma peritonei. Ann Surg Oncol. 2017;24:1778–82.
Fournier K, Rafeeq S, Taggart M, Kanaby P, Ning J, Chen HC, et al. Low-grade appendiceal mucinous neoplasm of uncertain malignant potential (LAMN-UMP): prognostic factors and implications for treatment and follow-up. Ann Surg Oncol. 2017;24:187–93.
Yantiss RK, Shia J, Klimstra DS, Hahn HP, Odze RD, Misdraji J. Prognostic significance of localized extra-appendiceal mucin deposition in appendiceal mucinous neoplasms. Am J Surg Pathol. 2009;33:248–55.
Dupre MP, Jadavji I, Matshes E, Urbanski SJ. Diverticular disease of the vermiform appendix: a diagnostic clue to underlying appendiceal neoplasm. Hum Pathol. 2008;39:1823–6.
Lamps LW, Gray GF Jr., Dilday BR, Washington MK. The coexistence of low-grade mucinous neoplasms of the appendix and appendiceal diverticula: a possible role in the pathogenesis of pseudomyxoma peritonei. Mod Pathol. 2000;13:495–501.
Hissong E, Goncharuk T, Song W, Yantiss RK. Post-inflammatory mucosal hyperplasia and appendiceal diverticula simulate features of low-grade appendiceal mucinous neoplasms. Mod Pathol. 2020;33:953–61.
Hsu M, Young RH, Misdraji J. Ruptured appendiceal diverticula mimicking low-grade appendiceal mucinous neoplasms. Am J Surg Pathol. 2009;33:1515–21.
Lowes H, Rowaiye B, Carr NJ, Shepherd NA. Complicated appendiceal diverticulosis versus low-grade appendiceal mucinous neoplasms: a major diagnostic dilemma. Histopathology. 2019;75:478–85.
Carr NJ, McCarthy WF, Sobin LH. Epithelial noncarcinoid tumors and tumor-like lesions of the appendix. A clinicopathologic study of 184 patients with a multivariate analysis of prognostic factors. Cancer. 1995;75:757–68.
Roxburgh CS, Fenig YM, Cercek A, Shia J, Rassam RM, Paty PB, et al. Outcomes of low-grade appendiceal mucinous neoplasms with remote acellular mucinous peritoneal deposits. Ann Surg Oncol. 2019;26:118–24.
Jackson SL, Fleming RA, Loggie BW, Geisinger KR. Gelatinous ascites: a cytohistologic study of pseudomyxoma peritonei in 67 patients. Mod Pathol. 2001;14:664–71.
Baratti D, Kusamura S, Milione M, Bruno F, Guaglio M, Deraco M. Validation of the recent PSOGI pathological classification of Pseudomyxoma peritonei in a single-center series of 265 patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2018;25:404–13.
Choudry HA, Pai RK, Shuai Y, Ramalingam L, Jones HL, Pingpank JF, et al. Impact of cellularity on oncologic outcomes following cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for Pseudomyxoma peritonei. Ann Surg Oncol. 2018;25:76–82.
Guaglio M, Sinukumar S, Kusamura S, Milione M, Pietrantonio F, Battaglia L, et al. Clinical surveillance after macroscopically complete surgery for low-grade appendiceal mucinous neoplasms (LAMN) with or without limited peritoneal spread: long-term results in a prospective series. Ann Surg Oncol. 2018;25:878–84.
Reghunathan M, Kelly KJ, Valasek MA, Lowy AM, Baumgartner JM. Histologic predictors of recurrence in mucinous appendiceal tumors with peritoneal dissemination after HIPEC. Ann Surg Oncol. 2018;25:702–8.
Young RH, Gilks CB, Scully RE. Mucinous tumors of the appendix associated with mucinous tumors of the ovary and pseudomyxoma peritonei. A clinicopathological analysis of 22 cases supporting an origin in the appendix. Am J Surg Pathol. 1991;15:415–29.
Al-Azzawi M, Misdraji J, van Velthuysen MF, Shia J, Taggart MW, Yantiss RK, et al. Acellular mucin in pseudomyxoma peritonei of appendiceal origin: what is adequate sampling for histopathology? J Clin Pathol. 2020;73:220–2.
Horvath P, Yurttas C, Birk P, Struller F, Konigsrainer A. Cellularity in low-grade Pseudomyxoma peritonei impacts recurrence-free survival following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Langenbecks Arch Surg. 2018;403:985–90.
Bhatt A, Mishra S, Prabhu R, Ramaswamy V, George A, Bhandare S, et al. Can low grade PMP be divided into prognostically distinct subgroups based on histological features? A retrospective study and the importance of using the appropriate classification. Eur J Surg Oncol. 2018;44:1105–11.
Choudry HA, Pai RK, Parimi A, Jones HL, Pingpank JF, Ahrendt SS, et al. Discordant diagnostic terminology and pathologic grading of primary appendiceal mucinous neoplasms reviewed at a high-volume center. Ann Surg Oncol. 2019;26:2607–14.
Orta L, Trindade AJ, Luo J, Harpaz N. Appendiceal mucinous cystadenoma is a neoplastic complication of IBD: case-control study of primary appendiceal neoplasms. Inflamm Bowel Dis. 2009;15:415–21.
Acknowledgements
The authors would like to thank Dr. John D. Paulsen for critical comments on this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: There was a typographical error in table 3. The acronym for cytoreduction completeness score should be CCS.
Rights and permissions
About this article
Cite this article
Ballentine, S.J., Carr, J., Bekhor, E.Y. et al. Updated staging and patient outcomes in low-grade appendiceal mucinous neoplasms. Mod Pathol 34, 104–115 (2021). https://doi.org/10.1038/s41379-020-0628-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41379-020-0628-7
This article is cited by
-
Simple appendectomy is a safe and effective surgical approach for low-grade appendiceal mucinous neoplasm at the appendiceal root with negative margins: a retrospective comparative cohort study
World Journal of Surgical Oncology (2026)
-
Mortality Risk in Mucinous Appendiceal Neoplasms: Insights from a National Database
Indian Journal of Surgical Oncology (2026)
-
Zufallsbefund muzinöse Neoplasie der Appendix
Die Chirurgie (2023)
-
Clinicopathologic parameters and outcomes of mucinous neoplasms confined to the appendix: a benign entity with excellent prognosis
Modern Pathology (2022)