Abstract
Understanding how sleep affects the glymphatic system and human brain networks is crucial for elucidating the neurophysiological mechanism underpinning aging-related memory declines. We analyzed a multimodal dataset collected through magnetic resonance imaging (MRI) and polysomnographic recording from 72 older adults. A proxy of the glymphatic functioning was obtained from the Diffusion Tensor Image Analysis along the Perivascular Space (DTI-ALPS) index. Structural and functional brain networks were constructed based on MRI data, and coupling between the two networks (SC-FC coupling) was also calculated. Correlation analyses revealed that DTI-ALPS was negatively correlated with sleep quality measures [e.g., Pittsburgh Sleep Quality Index (PSQI) and apnea-hypopnea index]. Regarding human brain networks, DTI-ALPS was associated with the strength of both functional connectivity (FC) and structural connectivity (SC) involving regions such as the middle temporal gyrus and parahippocampal gyrus, as well as with the SC-FC coupling of rich-club connections. Furthermore, we found that DTI-ALPS positively mediated the association between sleep quality and rich-club SC-FC coupling. The rich-club SC-FC coupling further mediated the association between DTI-ALPS and memory function in good sleepers but not in poor sleepers. The results suggest a disrupted glymphatic-brain relationship in poor sleepers, which underlies memory decline. Our findings add important evidence that sleep quality affects cognitive health through the underlying neural relationships and the interplay between the glymphatic system and multimodal brain networks.
Similar content being viewed by others
Introduction
The glymphatic (glial-lymphatic) system is a fluid transport pathway playing an important role in clearing waste from the brain [1, 2]. This pathway involves the flow of cerebrospinal fluid (CSF) transferring along the perivascular space into the brain interstitium, facilitated by the aquaporin-4 water channels. The efficiency of CSF movement in the glymphatic pathway, known as glymphatic system functioning, is influenced by sleep-wake cycles [3, 4]. The functioning of the glymphatic system is associated with human cognition, particularly memory, whereby lower functioning correlates with worse cognitive performance [5, 6]. Moreover, the glymphatic system plays a vital role in determining brain health, particularly in aging populations [7, 8]. Dysfunction of the glymphatic system impairs the clearance function and leads to the accumulation of toxic proteins [1, 9]. This process has recently been implicated in the pathogenesis of neurological disorders, such as Alzheimer’s disease (AD) [10], Parkinson’s disease [11], and epilepsy [12]. Therefore, studying the glymphatic system offers a novel way to investigate age-related neurobiological changes.
To measure the functioning of the glymphatic system in the human brain, previous studies mostly used invasive imaging methods like contrast signal intensity after intrathecal injection of tracers [13]. However, the invasiveness has significant limitations for human research. Recently, Taoka et al. [14] proposed the use of the Diffusion Tensor Image Analysis along the Perivascular Space (DTI-ALPS) to measure the diffusivity of the water molecules in the direction of perivascular space based on diffusion magnetic resonance imaging (dMRI) data as a proxy of glymphatic functioning. It is assumed that the glymphatic activity is correlated with the perivascular water movement at the level of lateral ventricle bodies and histological change of the glymphatic pathway is supposed to affect water diffusivity along the right-left direction. Such a method provides an alternative and non-invasive way to assess the functioning of the glymphatic system in the human brain. Due to its non-invasive nature, the DTI-ALPS method has emerged as a valuable tool for assessing glymphatic functioning and has been widely applied in clinical conditions such as sleep disorders, AD, and Parkinson’s disease [6, 15, 16]. The DTI-ALPS method was found to be highly reproducible [17] and its validity was supported by the strong correlations between DTI-ALPS and regional indices (i.e., signal unit ratio) obtained from invasive glymphatic imaging data (rs <-0.77, ps <0.001) [18].
Previous studies have examined the brain’s gray matter integrity (e.g., gray matter volume) to reflect the neural underpinning of the glymphatic system [8, 19]. Normal brain function relies on not only the brain regions themselves but also the effective communication among brain regions (i.e., brain connectivity) [20]. However, knowledge of the glymphatic effect on functional and structural connectivity between brain areas is limited. The human brain can be conceptualized as complex networks [21, 22]. Within the brain networks, brain regions are defined as nodes, and functional connectivity (FC; i.e., Pearson’s correlation between regional time series) or structural connectivity (SC; i.e., white matter connection between brain regions) are defined as edges. Studying brain connectivity is of great importance, as SC and FC have shown consistent and reliable associations with individual differences in cognitive functions [20, 23]. Disrupted SC and FC in the human brain underlay pathology of a broad range of neuropsychological diseases (e.g., AD and major depression) [24, 25].
Despite the preliminary efforts to examine functional connectivity network (FCN) or structural connectivity network (SCN) independently, there has been a growing interest in exploring the relationship between FCN and SCN in recent years. It has been well demonstrated that the connections of FCN are shaped and constrained by the underlying white matter pathways of SCN [20, 26, 27]. Consequently, a growing body of research has focused on the correlation between SC and FC (SC-FC coupling), which reflects the degree to which functional communications align with the structural brain connections. The SC-FC coupling has been found to significantly correlate with neural development [28, 29], cognitive flexibility [30], and neuropathological changes (e.g., schizophrenia and AD) [31, 32]. In addition, previous evidence suggests that SC-FC coupling could be a more sensitive potential biomarker in capturing individual cognitive ability [33] and subtle pathological abnormalities [34,35,36] compared to SC or FC alone. Therefore, with regard to the relationship between the glymphatic system and cognition [5, 6], we suspect that a change in glymphatic functioning may affect cognitive functions through the underlying brain networks, which can provide a novel and comprehensive perspective on the relationship.
Sleep complaints are common in older adults, and it is estimated that around 40%–70% of older adults have chronic sleep issues [37]. Yet, sleep quality is a key influencing factor for glymphatic functioning and cognitive functions. Long-term sleep problems could further increase the risk of neurocognitive disorders (e.g., Alzheimer’s disease) in older adults [38, 39]. Poor sleep quality, such as difficulty initiating sleep and insufficiency of sleep duration, has been shown to negatively impact the glymphatic system [13, 40], and is also associated with the decline of SC/FC strength in brain areas like the attention network, limbic system, as well as hippocampal network [41, 42]. Poor sleep quality is associated with adverse effects on cognitive functions, especially memory [43, 44]. Thus, understanding how sleep quality influences the glymphatic system and human brain networks will offer insight into the neural underpinnings of age-related memory change. Here, we hypothesized that sleep quality is associated with multimodal human brain networks, which could be mediated by glymphatic functioning in older adults.
Based on the literature reviewed above, in this study, we investigated the relationship between sleep quality, the glymphatic system, and multimodal human brain networks in older adults. We analyzed multimodal MRI data [i.e., resting-state functional magnetic resonance imaging (R-fMRI) and dMRI] and sleep measurements [i.e., Pittsburgh Sleep Quality Index (PSQI) and polysomnographic (PSG) recordings] from a group of community-dwelling older adults. First, we employed the DTI-ALPS measure as a proxy for the functioning of the glymphatic system. Then we examined the association between DTI-ALPS and multimodal brain networks and tested whether DTI-ALPS can mediate the relationship between sleep and brain networks. Last but not least, we tested whether the sleep-related brain-glymphatic relationship could underlie individual memory function, a cognitive function vulnerable to aging and poor sleep.
Methods
Participants
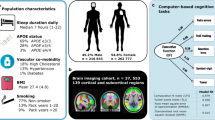
A cohort of 92 community-dwelling Chinese older adults was recruited by the Chang Gung Memorial Hospital in Taiwan (Fig. 1A). All participants were healthy with uneventful medical histories. For more details on this dataset, please refer to Siow et al. [8]. This data sample was originally collected to establish a comprehensive database of geriatric medicine for Taiwanese with no major physical or mental disabilities [8]. Among the participants of this sample, we excluded 4 participants with incomplete R-fMRI scans, 3 participants with incomplete dMRI scans, 3 participants with obvious artifacts in R-fMRI images, and 10 participants with excessive head motion (>2 mm or 2°) in R-fMRI scans. After these exclusions, a final sample of 72 healthy older adults was included in our subsequent analyses. This study was approved by the institutional review board of Chang Gung Medical Foundation, Taiwan. Informed consent was obtained from all the participants. The experimental procedure followed the Declaration of Helsinki.
A Collecting magnetic resonance imaging (MRI) and polysomnographic (PSG) data from older adults. B Constructing the functional connectivity network (FCN) and structural connectivity network (SCN), and calculating the coupling between them (i.e., SC-FC coupling; upper right panel) based on resting-state functional MRI (R-fMRI) and diffusion MRI (dMRI) data. Calculating the Diffusion Tensor Image Analysis along the Perivascular Space (DTI-ALPS) index (lower right panel) based on dMRI data. C Partial correlation analyses between DTI-ALPS and FCN, SCN, or their coupling. D Mediation analyses of glymphatic functioning on relationship between sleep quality and multimodal human brain networks. E Sleep-related neural mechanism of memory function.
Experimental design and procedure
After recruiting participants, we collected the multimodal MRI data, polysomnographic data, and behavioral assessments [e.g., Consortium to Establish a Registry for Alzheimer’s Disease Neuropsychological Battery (CERAD-NB)] from participants. Then we calculated the DTI-ALPS index to measure glymphatic functioning and constructed structural and functional human brain networks based on the MRI data. Partial correlation analyses were then performed to examine the association between the DTI-ALPS index and multimodal human brain networks. To further test whether glymphatic functioning can mediate the relationship between sleep and brain networks, mediation analyses with the DTI-ALPS index as mediator were performed. Finally, mediation analyses were conducted to examine how the brain-glymphatic relationship contribute to memory function and how this relationship may interact with sleep quality. The overall workflow of the current study is presented in Fig. 1.
Behavioral assessment
The sleep quality of participants was assessed using the global Pittsburgh Sleep Quality Index (PSQI) [45]. The PSQI is a self-report questionnaire that evaluates seven components of sleep over a 1-month period. The seven components include subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. Each component is evaluated on a 3-point scale, providing an overall score ranging from 0 to 21, with lower PSQI scores denoting better sleep quality. Memory performance was assessed using the memory domain of Consortium to Establish a Registry for Alzheimer’s Disease Neuropsychological Battery (CERAD-NB) [46] and the memory domain of Everyday Cognition Questionnaire (ECog) [47]. For the CERAD-NB, a compound score, the Memory Total Score, was calculated by summing the scores of Word List Recall, Word List Recognition, and Constructional Praxis Recall. A higher Memory Total Score indicates better overall memory performance. The Ecog memory questionnaire examines changes in memory functioning. Participants were asked to rate their current ability to perform everyday tasks compared to performing the same tasks 10 years ago. A higher Ecog memory score indicates greater subjective memory decline.
Polysomnography
In addition to self-reported measures of sleep quality, we also performed PSG on participants to objectively evaluate their sleep quality over one night. Standardized in-home PSG was utilized on participants using a portable monitoring system (Philips Alice 6 lDe; Philips Healthcare). Sleep stages were recorded in 30-s epochs following the criteria of Iber (2007) [48]. Respiratory events were scored according to the American Academy of Sleep Medicine manual (version 2.6). Apnea was defined as a cessation of inspiratory airflow for at least 10 s, and hypopnea was defined as a reduction of airflow (>30%) associated with oxygen desaturation of ≥3% or electroencephalography arousal. Several polysomnographic indices were calculated in this study, including total sleep time, sleep efficiency (ratio of total sleep time to time in bed), apnea-hypopnea index (AHI; the number of apnea and hypopnea events per hour of sleep), desaturation index (the number of oxygen desaturation per hour of sleep), and arousal index (the number of arousals per hour of sleep). Of the 72 participants in the final sample, four participants did not complete the PSG session, and subsequent analyses of PSG data were restricted to the remaining 68 participants.
MRI protocols
MR images were acquired using 8-channel head coils on 3T scanners (Discovery MR750w and Discovery MR750; GE Healthcare, Milwaukee, WI) [8]. Resting-state fMRI (R-fMRI) data were acquired using echo-planar imaging (EPI) sequence: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle (FA) = 90°, slices = 39, matrix = 64 × 64, slice thickness = 3 mm and voxel size = 3 × 3 × 3 mm3. T1-weighted images were acquired using inversion-recovery fast spoiled gradient echo sequence: TR = 8.58 ms, TE = 3.23 ms, FA = 12°, inversion time = 450 ms, matrix = 256 × 256, slices = 172, slice thickness = 1 mm and voxel size = 0.5 × 0.5 × 1 mm3. The dMRI data were collected using spin-echo echo-planar imaging sequence: TR = 6837 ms, TE = 83 ms, FA = 90°, matrix = 116 × 116, slices = 156, slice thickness = 2.2 mm and voxel size = 2.2 × 2.2 × 2.2 mm3. The diffusion-sensitizing gradients were applied along 64 noncollinear directions (b = 1000 s/mm2), together with one acquisition without diffusion weighting (b = 0 s/mm2).
Image preprocessing
The R-fMRI images were preprocessed using Statistical Parametric Mapping 12 (https://www.fil.ion.ucl.ac.uk/spm/) and Data Processing Assistant for Resting-State fMRI (DPARSF) [49] toolboxes. The first 5 volumes of R-fMRI were discarded. Images then underwent slice timing, head motion correction, and spatial normalization to the Montreal Neurological Institute (MNI) space using the normalization parameters estimated during unified segmentation of structural T1 images. After normalization, we removed the linear trends and regressed out the nuisance variables [Friston 24 head motion parameters, white matter (WM), and cerebrospinal fluid signals (CSF)] from the R-fMRI data. Finally, we performed band-pass filtering (0.01–0.08 Hz) on the images to reserve low-frequency information.
The dMRI images were preprocessed using the standard procedure of PANDA (Pipeline for Analyzing braiN Diffusion imAges) toolbox [50]. The preprocessing procedure included brain mask estimation, skull-stripping, eddy current, head motion correction, and computation of the diffusion tensor and fractional anisotropy (FA). Furthermore, the T1-weighted images were aligned to the AC-PC line and then segmented to obtain the WM binary mask (with WM probability threshold >0) in the T1 native space.
DTI-ALPS index
The DTI-ALPS index was initially developed by Taoka et al. [14] to quantify the functioning of the glymphatic system with diffusion MRI data. This method evaluates the movement of water molecules along the perivascular space at the level of lateral ventricle bodies. At this level, the perivascular water flow around the medullary vessels primarily follows a direction perpendicular to the ventricular wall, mainly parallel to the right-left direction (i.e., the x-axis of the image coordinate). It is assumed that the glymphatic activity is correlated with the perivascular water movement, and histological change of the glymphatic pathway is supposed to affect water diffusivity along the right-left direction. Unlike invasive methods that measure the behavior of the tracer after injection, the DTI-ALPS can noninvasively evaluate the glymphatic functioning at a given time point. Although the DTI-ALPS index is highly reproducible in measuring glymphatic functioning, it is sensitive to head motion during scan [51]. Taoka et al. [52] reported a reduced DTI-ALPS with head rotation compared to that with low head motion. Therefore, head motion could reduce the reliability of DTI-ALPS, and participants with severe head motion were excluded in our preprocessing step.
To calculate the DTI-ALPS index, we extracted diffusivity coefficients from two major fiber bundles passing through the perivascular space: the projection fibers that pass along the lateral ventricular wall in the craniocaudal direction (i.e., the z-axis of the image coordinate), and the association fibers that go through at the more lateral area in the anterior–posterior direction (i.e., the y-axis of the image coordinate). Therefore, the diffusivity along the x-axis was defined as the mean tensor values of overlapping areas between perivascular space and projection fibers (Dxproj) or association fibers (Dxassoc), which can be extracted from the preprocessed dMRI data. For standardization, the tensor value of projection fiber in the anterior–posterior direction (Dyproj) and the tensor value of association fiber in the craniocaudal direction (Dzassoc) was also extracted. To avoid bias due to manually drawn region of interest (ROI) on projection and association fibers, we used an atlas-based approach to define the ROIs [8, 53, 54]. Briefly, the FA and other diffusion metrics maps were transformed from the individual native space to the standard MNI space, and ROIs were extracted from the ICBM-DTI-81 template in the MNI space. The ROI of projection fibers was defined as superior and posterior corona radiata in ICBM-DTI-81, and the ROI of association fibers was defined as superior longitudinal fasciculus in ICBM-DTI-81 (Fig. 2A). The range of the ROIs was then limited to the area that perpendicular to the lateral ventricle bodies. To avoid the inclusion of CSF voxels, a mask of FA > 0.2 was further applied to the ROIs. The mean diffusion values of these ROIs were extracted, and the DTI-ALPS index was then computed as follows [8, 14]:
To further avoid the possible difference of choosing ROI from different sides (i.e., left and right), we calculated DTI-ALPS from bilateral ROIs and then averaged to obtain a mean DTI-ALPS index for subsequent analyses [18].
Functional brain network construction
The functional connectivity network (FCN) of the human brain was constructed using the preprocessed R-fMRI data for each participant (Fig. 1B). Nodes of both networks were defined by parcellating the brain gray matter into 246 regions based on the Brainnetome atlas [55]. To allow modular-level examination, we further assigned the brain regions into seven functional modules [56] by evaluating the overlap area between brain regions and specific modules and assigning each region to the module with the most overlapping voxels. The assigned functional modules included the visual network (VN), the somatomotor network (SMN), the dorsal attention network (DAN), the ventral attention network (VAN), the limbic system (Limbic), the control network (CN), and the default mode network (DMN). Subcortical regions were not initially considered in Yeo’s parcellation. Hence, we further grouped the subcortical regions into one module and labeled it as subcortical module (Sub).
For constructing the functional connectivity (i.e., edge of FCN), we first extracted the regional blood oxygen level-dependent signals by averaging signals of all voxels within each brain region. Pearson’s correlations between all pairs of regional signals were computed and transformed by Fisher’s Z transformation to improve normality. The Fisher’s Z-transformed correlation coefficients were defined as the weights of function connectivity. Given the controversies regarding the biological explanation of negative connectivity [57, 58], the negative connectivity was set to zero, and our analyses were restricted to positive connectivity. Finally, a 246 × 246 whole-brain functional connectivity matrix was constructed for each participant.
Structural brain network construction
The structural connectivity network (SCN) of the human brain was constructed using the preprocessed dMRI data for each participant (Fig. 1B). Consistent with FN, nodes of SN were also defined based on the Brainnetome atlas. Since the Brainnetome atlas was originally defined in the standard MNI space, to construct SCN on native space dMRI data, we further transformed the atlas into the native diffusion space for each participant using the typical procedure of Gong et al. (2009). Specifically, we coregistered the individual FA image to the T1-weighted image and then nonlinearly registered it to the ICBM152 template to obtain the inverse warping transformation parameters. Then we applied the parameters to inversely wrap the Brainnetome atlas from MNI space to individual native diffusion space.
For constructing the structural connectivity (i.e., edge of SCN), we used the deterministic tractography based on fiber assignment by continuous tracking (FACT) algorithm of the Diffusion Toolkit [59]. Briefly, seeds were first placed at voxels with FA values higher than 0.2 within the WM mask obtained in preprocessing. Then, a streamline was started from each seed and was terminated when it reached a voxel with a turning angle greater than 45° or FA value less than 0.2. Through this procedure, all streamlines within the WM mask were constructed. The weight of each structural connectivity was defined as fiber density [60, 61] between brain regions. The fiber density was computed as follows:
where Wuv is the weight of the edge between region u and v, Su and Sv are the cortical surface of region u and v, Ef is the set of all existed streamlines between region u and v, and l(f) is the length of streamline f. Therefore, for each participant, a 246 × 246 undirected structural connectivity matrix was constructed.
Coupling between functional and structural brain networks
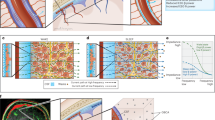
The coupling between FCN and SCN was examined at two different levels. (1) Whole-brain connectivity. Here, we first examined the SC-FC coupling across all connected structural connectivity. Briefly, we first extracted the non-zero elements (i.e., connected SC) from the SC matrix and then extracted the corresponding elements from the FC matrix. The SC-FC coupling strength was computed as the Spearman correlation between the weights of extracted SC and FC. (2) Rich-club connectivity. The rich-club organization is a phenomenon in which a group of highly connected nodes (i.e., hub regions) densely connect to each other forming a “rich-club”. The rich-club is critically important for facilitating information communication in the whole-brain network, acting as an anatomical foundation for linking the functional network in the human brain [31, 62]. The rich-club nodes were defined as regions with the top 20% weighted degree (i.e., the sum of edge weights owned by each region) [63,64,65]. After defining the rich-club nodes, we extracted three groups of edges based on the rich-club organization [62], which are rich-club edges (connections between rich-club nodes), feeder edges (connections between rich-club and non-rich-club nodes), and local edges (connections between non-rich-club nodes) (Fig. 3C). Then SC-FC coupling was computed within these three groups respectively.
A Functional connectivity that was positively correlated with DTI-ALPS. B Structural connectivity that was positively correlated with DTI-ALPS. C Network representation of rich-club, feeder, and local connections. D Negative correlation between DTI-ALPS and SC-FC coupling in rich-club connections [SC-FC Coupling (RC)].
Statistical analyses
Data analyses were performed using Matrix Laboratory (MATLAB) 2022a and SPSS v.28. To test whether the functioning of the glymphatic system is associated with sleep quality, we conducted two-sided partial correlation between the DTI-ALPS index and sleep indices (e.g., PSQI and AHI), controlling for age, sex, and types of MRI scanners. False discovery rate (FDR) procedure was further conducted to correct for multiple comparisons.
To examine the relationship between the glymphatic system and brain networks, we conducted two-side partial correlation between the DTI-ALPS index and weights of FC (i.e., Fisher’s Z-transformed Pearson correlation coefficient) or weights of SC (i.e., fiber density) or SC-FC coupling, controlling for age, sex, and types of MRI scanners (Fig. 1C). For the partial correlation analyses of SC and FC, FDR procedure was further conducted to correct for multiple comparisons (i.e., 30135 connections).
To test whether glymphatic functioning could mediate the association between sleep quality and brain networks, we conducted mediation analyses using the PROCESS macro implemented in SPSS (pre-set Model No.4) (Fig. 1D). A bootstrapping approach (5000 times) was used to assess the significance of the models [66]. In the mediation analyses, sleep index (i.e., PSQI or polysomnographic indices) was included as the independent variable, brain network measure (i.e., weights of FC, weights of SC, or SC-FC coupling) was included as the dependent variable, with DTI-ALPS acting as the mediator. Variables of age, sex, and types of MRI scanners were included as covariates.
Finally, we would like to focus on the relationship between the glymphatic system and memory function. We tested whether brain networks could mediate the association between glymphatic functioning and memory functions, and how sleep quality moderates this relationship. We conducted moderated mediation analyses using the PROCESS macro implemented in SPSS (pre-set Model No. 14). In the analyses, DTI-ALPS was included as the independent variable, memory functions (i.e., Memory Total Score and Ecog Memory) were included as the dependent variable, brain network measures were included as the mediator, and sleep indices were included as the moderator (Fig. 1E). For PSQI scores, participants were divided into two groups according to a typical cut-off, where participants scored lower than or equal to 5 in PSQI were labeled as good sleepers, otherwise were labeled as poor sleepers [45, 67]. For polysomnographic indices, participants were divided into three groups using 16th, 50th, and 84th percentiles. Variables of age, sex, and types of MRI scanners were included as covariates.
Results
Demographic and behavioral data
After data filtering, data from 72 healthy older adults (age 73.31 ± 7.20 years, 42 females) were included in the main analyses in our study (Table 1). Participants had a considerable variety in PSQI scores, ranging from 2 to 17 (mean ± SD: 6.486 ± 3.692). No significant effect of age (r = −0.095, p = 0.427) and sex (t = −0.424, p = 0.673) on PSQI was found in the current sample. We observed that sleep efficiency (r = −0.307, p = 0.011) and total sleep time (r = −0.250, p = 0.040) showed significant correlations with age, but other polysomnographic indices did not show significant correlations with age (ps > 0.05). No significant sex differences were found in polysomnographic indices (ps > 0.05).
Association between DTI-ALPS index and sleep metrics
In order to estimate the glymphatic system, we computed a DTI-ALPS index based on the dMRI data to evaluate the glymphatic functioning for each participant. A negative correlation was found between the DTI-ALPS index and PSQI scores (r = −0.381, p = 0.001, FDR corrected; Fig. 2B), which indicates that poorer sleep quality is associated with worse functioning of the glymphatic system. Besides the total score, we further explored the associations between component scores of PSQI and DTI-ALPS. Only the components of sleep quality (r = −0.349, p = 0.003, FDR corrected) and sleep duration (r = −0.298, p = 0.011, FDR corrected) showed significant negative correlations. As for the polysomnographic indices, a significant negative correlation was observed between the DTI-ALPS index and AHI (r = −0.314, p = 0.011, FDR corrected; Fig. 2C). The AHI measure is related to respiratory problems during sleep, suggesting that apnea or hypopnea during sleep might hamper the functioning of the glymphatic system.
To test whether the above relationships vary with their demographic characteristics (i.e., age and sex), we divided the participants into young-old (<median age) and old-old groups (≥median age) (here median age = 71 years) or male and female groups. Group comparisons between these subgroups are presented in Table 2. Partial correlation analysis revealed that PSQI only showed a negative correlation in the old-old group (r = −0.418, p = 0.011), while AHI only showed a negative correlation in the young-old group (r = −0.479, p = 0.007). As for sex groups, both the PSQI and AHI negatively correlated with the DTI-ALPS index in the male group (PSQI: r = −0.561, p = 0.002; AHI: r = −0.416, p = 0.034), but not in the female group (PSQI: r = −0.221, p = 0.171; AHI: r = −0.231, p = 0.163).
Neural correlates of DTI-ALPS index
In this section, we explored how the glymphatic system was associated with FCN, SCN, and their relationship in the human brain. For the FCN, DTI-ALPS showed significant positive or negative correlation with the strength (i.e., z-transformed Pearson’s correlation coefficient) of several functional connectivity (ps < 0.05, FDR corrected; Fig. 3A). These functional connectivities involved a broad range of regions (e.g., left inferior temporal gyrus, middle temporal gyrus, insula, and parahippocampal gyrus). From the aspect of the functional module, the DTI-ALPS-related functional connectivity involved all the modules, especially the ventral attention network and limbic network. For the SCN, DTI-ALPS showed a positive or negative correlation with fiber density of several structural connectivity (ps < 0.05, FDR corrected; Fig. 3B). These connectivities also involved several medial or subcortical brain regions (e.g., thalamus, precuneus, anterior cingulate cortex, and orbital frontal gyrus). From the functional module aspect, the DTI-ALPS-related structural connectivity involved most of the modules, except for the visual network and somatomotor network. Regarding the relationship between DTI-ALPS and SC-FC coupling strength, no significant correlation was observed at whole-brain SC-FC coupling (r = −0.107, p = 0.381). However, after dividing the connections according to rich-club structure, we found a significant negative correlation in SC-FC coupling of rich-club connections (r = −0.305, p = 0.011; Fig. 3D), but not feeder (rs = −0.177, p = 0.146) or local (rs = 0.071, p = 0.564) connections. In subgroups of age or sex, we observed group differences in SC-FC coupling of rich-club connections. Specifically, a negative correlation between DTI-ALPS and SC-FC coupling of rich-club connections was only observed in the female group (r = −0.345, p = 0.029) and the young-old group (r = −0.443, p = 0.011).
Mediation of DTI-ALPS index on association between sleep quality and human brain networks
Given the association between glymphatic functioning and sleep quality or brain networks, we examined whether sleep quality is also associated with the above human brain networks and whether glymphatic functioning can mediate this association. First, partial correlation analysis revealed that PSQI score was positively correlated with SC-FC coupling of rich-club connections (r = 0.251, p = 0.037; Fig. 4A), but no significant correlations with SC-FC coupling at other conditions or mean strength of the DTI-ALPS related FC or SC (ps > 0.05). No significant partial correlations were observed between AHI and SC-FC coupling of rich-club connections (r = 0.130, p = 0.302; Fig. 4B) or other brain network measures (ps > 0.05). Following the correlation analyses, we built a mediation model with sleep index (i.e., PSQI or AHI) as the independent variable, brain network measure as the dependent variable, and DTI-ALPS index as the mediator. As shown in Fig. 4C, D, the DTI-ALPS index significantly mediated the association between PSQI score and SC-FC coupling of rich-club connections [β = 0.093, SE = 0.058, bootstrapped 95% confidence interval (CI) = 0.004–0.233]. While taking AHI as an independent variable, the mediation effect of the DTI-ALPS index was also significant (β = 0.094, SE = 0.052, bootstrapped 95% CI = 0.013–0.213). Both models indicated that, worse sleep quality was associated with lower glymphatic functioning, which led to higher coupling between rich-club SC and FC. No significant mediation results were found in other brain network measures.
A Positive correlation between PSQI and SC-FC coupling in rich-club connections [SC-FC Coupling (RC)]. B No significant correlation between AHI and SC-FC coupling in rich-club connections [SC-FC Coupling (RC)]. C DTI-ALPS positively mediated the association between PSQI and SC-FC coupling of rich-club (RC) connectivity. D DTI-ALPS positively mediated the association between AHI and SC-FC coupling of RC connectivity. CI is 95% bootstrapping confidence interval of indirect effect. **p < 0.01, *p < 0.05, †p < 0.1.
Moderated mediation model of memory function
Finally, we wanted to examine the neural mechanism underpinning memory in older adults. First, we examined the association between DTI-ALPS or SC-FC coupling, and the memory scores. Only SC-FC coupling of whole-brain connections showed a negative correlation with the Ecog memory score (r = −0.254, p = 0.035). However, after dividing the sample into two groups [i.e., good sleeper (N = 36) and poor sleeper (N = 36)] according to PSQI, we observed significant moderating effects of PSQI on the association between SC-FC coupling of whole-brain connections and Memory Total Score (b = 4.188, SE = 1.614, bootstrapped 95% CI = 0.942–7.300), and the association between SC-FC coupling of rich-club connections and Ecog memory score (b = −0.745, SE = 0.356, bootstrapped 95% CI = −1.457 to −0.056). For Memory Total Score, follow-up analysis revealed that SC-FC coupling of whole-brain connections negatively correlated with Memory Total Score (b = −2.796, SE = 1.039, bootstrapped 95% CI = −4.872 to −0.721) in good sleepers, while no significant correlation was observed in poor sleepers (b = 1.392, SE = 1.183, bootstrapped 95% CI = −0.971 to 3.755). For the Ecog memory score, follow-up analysis revealed that SC-FC coupling of rich-club connections positively correlated with Ecog memory score (b = 0.575, SE = 0.237, bootstrapped 95% CI = 0.101–1.049) in good sleepers, while no significant correlation was observed in poor sleepers (b = −0.170, SE = 0.236, bootstrapped 95% CI = −0.641 to 0.302).
Based on the above findings, we performed a moderated mediation analysis to test whether SC-FC coupling can mediate the association between DTI-ALPS and memory scores, and how sleep quality moderates this relationship. As shown in Fig. 5, we found that rich-club SC-FC coupling mediated the association between DTI-ALPS and the Ecog memory score, but the association between rich-club SC-FC coupling and Ecog memory score was moderated by PSQI (index of moderated mediation = 0.572, bootstrapped 95% CI = 0.007–1.376). Specifically, the mediating effect of rich-club SC-FC coupling was significant only in good sleepers (b = −0.427, SE = 0.259, bootstrapped 95% CI = −1.020 to −0.017) but not in poor sleepers (b = 0.145, SE = 0.189, bootstrapped 95% CI = −0.212 to 0.561).
A PSQI moderated the mediating effect of rich-club SC-FC coupling on the association between DTI-ALPS and Ecog Memory. B Rich-club SC-FC coupling negatively mediated association between DTI-ALPS and Ecog Memory in good sleepers. C Rich-club SC-FC coupling failed to mediate the association between DTI-ALPS and Ecog Memory in poor sleepers. CI is 95% bootstrapping confidence interval of conditional indirect effect.
Discussion
In this study, we investigated the interplay between sleep, glymphatic functioning, and human brain networks in older adults and its impact on memory performance. First, we observed that the DTI-ALPS index correlated with both self-reported and polysomnographic measures of sleep quality. Moreover, the DTI-ALPS index was associated with multimodal brain networks, involving a broad range of brain areas. Among the brain network measures, we found that the DTI-ALPS index mediated the association between sleep quality and SC-FC coupling of rich-club connections. Furthermore, the SC-FC coupling was associated with memory functions in older adults, and its mediation on the association between DTI-ALPS index and memory functions was moderated by sleep quality. These findings clearly reveal the effect of sleep on the multimodal human brain network through the glymphatic system, which in turn affects memory performance in older adults. Therefore, maintaining efficient glymphatic functioning seems crucial for promoting healthy aging [68].
Glymphatic functioning and sleep quality
Consistent with our observation, previous findings also showed that poor sleep quality could disrupt the functioning of the glymphatic system [13, 40]. Our study, incorporating the non-invasive DTI-ALPS approach, extended the findings in community-dwelling older adults. In regard to component scores of PSQI, only components of sleep quality and sleep duration were associated with DTI-ALPS. Similar to the finding, several previous studies reported glymphatic outcomes related to total sleep time. Deposition of amyloid-β and tau were found to be negatively correlated with sleep time [69,70,71]. Velocity of CSF flow was also found to increase after unrestricted sleep [72]. However, we failed to find an association with total sleep time obtained by PSG recording. The in-lab sleep duration may not accurately reflect the night-to-night sleep quality [40], which may limit its association with glymphatic functioning. In addition to subjective sleep quality (i.e., PSQI), we also found negative correlations between the DTI-ALPS index and AHI, which is in line with previous findings demonstrating that people with Obstructive Sleep Apnea (OSA) had impaired glymphatic functioning [15, 73]. Following this line of thought, hypoxia might be an important component of poor sleep quality that underlies the dysfunction of the glymphatic system. Previous research has identified that hypoxia during sleep was associated with declined cognitive functions (e.g., memory) and increased risk of Alzheimer’s disease (AD) [74, 75]. Since abnormal accumulation of amyloid β‐protein (Aβ) in cerebrospinal fluid is the main cause of AD [76], it is reasonable to speculate that hypoxia during sleep might increase the Aβ deposition through disrupting the clearance function of the glymphatic system and further increase the risk of Alzheimer’s disease in older adults [5].
The association between glymphatic functioning and sleep quality varied with age and sex. The PSQI and AHI showed significant correlations in different age groups. That may be due to their different nature in evaluating sleep quality [40]. Older participants may experience more frequent sleep problems [77], whose long-term sleep quality (i.e., PSQI score) is more likely to reflect in glymphatic functioning. However, younger participants in our sample have more variable sleep quality, whose glymphatic functioning may be more likely affected by their present sleep status (e.g., hypoxia in sleep). As for sex difference, one recent study found that females experienced more sudden glymphatic changes with the aging process compared to males [78]. With respect to sleep quality, the sleep-related effect may show a similar pattern on glymphatic functioning, and thus, only males with gradual sleep-related changes showed a correlation between sleep measures and glymphatic functioning.
Glymphatic functioning correlates with structural and functional brain networks
Functional connectivity was associated with glymphatic functioning, as revealed by our findings, involving the middle and inferior temporal gyrus, insula, medial frontal cortex, and parahippocampal gyrus. These regions participate in a broad range of cognitive functions, including memory [79,80,81]. Notably, these regions are commonly observed areas of tau/amyloid-β deposition in Alzheimer’s disease [82, 83], and other neurodegenerative diseases (e.g., frontotemporal dementia) [84, 85]. They are thought of as the early-stage deposition network of the toxic proteins (e.g., amyloid-β), and such deposition pattern is responsible for subsequent atrophy progress starting from these areas [86, 87]. Regarding the role of the glymphatic system in clearing damaging waste, an active glymphatic system might be protective against the deposition of toxic protein (e.g., amyloid-β) in these brain regions. In other words, dysfunction of the glymphatic system could adversely impact the functional activity of the early-stage deposition network due to the accumulation of toxic proteins and hence disrupted the functional interaction among widespread brain regions. Similarly, the DTI-ALPS-related SC connected the bilateral superior parietal lobule, precuneus, and anterior cingulate cortex, which are also part of areas involving the deposition of toxic proteins [82, 83].
Besides functional or structural network alone, glymphatic functioning is also associated with the coupling between the two networks (i.e., SC-FC coupling of rich-club connections). The rich-club is considered the backbone of the human brain network that facilitates global brain communication and supports higher cognitive functions [62, 88]. These highly connected rich-club nodes are crucial in integrating information from different brain regions. Disruption of connections within the rich-club is thought to induce abnormal information transfer and resource allocation in the whole-brain [31, 89]. Notably, we found a negative correlation between DTI-ALPS and rich-club SC-FC coupling. Lower SC-FC coupling may indicate more dynamic and less stringent brain communication in the rich-club pathways [31, 32]. Therefore, our findings suggest that active flow in the glymphatic system may be able to sustain flexible global information transfer on the relatively fixed structural pathways. Although emerging evidence has reported the relationship between glymphatic functioning and brain health [8, 68], to our knowledge, there still lacks evidence on communication within the human brain. From the perspective of network neuroscience, our findings provide novel insights that the glymphatic system is protective of neural interaction in the human brain, which can also be the missing link between glymphatic functioning and the pathologies of neurodegenerative diseases (e.g., AD).
Glymphatic functioning mediated the association between sleep quality and rich-club SC-FC coupling
Another main finding is that glymphatic functioning mediated the association between sleep quality (i.e., PSQI and AHI) and rich-club SC-FC coupling, where poorer sleep quality was associated with lower glymphatic functioning, which then led to higher rich-club SC-FC coupling. Aging is accompanied by adverse changes in several biological processes. Decreased sleep quality is one of the most prominent changes [37, 38]. Meanwhile, the declined sleep quality leads to disrupted brain functions and cognitive decline [90, 91]. The poor sleep quality is associated with decreased FC or SC [41, 92], less efficient functional integration [93], and less dynamic changes in FC [94], suggesting a weaker and inflexible functional communication in the whole-brain network. Similar to these findings, we found a stronger rich-club SC-FC coupling with poor sleep quality in older adults. Previous studies have firmly established that FCN is constrained by the underlying anatomical white matter pathways of the SCN. However, the SCN does not absolutely determine the FCN [95, 96], allowing functional dynamics to deviate from the white matter pathways so as to cater for the fluid nature of human cognition [30, 97]. With regard to the integrating role of rich-club structure, the current finding consistently revealed more stringent functional integration in older adults with poorer sleep quality.
Furthermore, we found that glymphatic functioning mediated the above relationship. The proper regulation of the central nervous system (CNS) microenvironment is critical for normal brain functionality. The glymphatic system acts as a gateway clearing toxic substances (e.g., metabolic waste) from the brain through the turnover of the interstitial fluid (ISF) [1, 2]. This process of moving waste is facilitated by aquaporin-4 (AQP4) water channels on astroglia, combining CSF-ISF and promoting waste removal [98], and loss of AQP4 polarization reduces glymphatic functioning [99, 100]. However, lower sleep quality can lead to the loss of AQP4 polarization [77] and alter the expression of AQP4 [101], which in turn induce abnormal accumulation of toxic waste and exacerbates neuroinflammation. Moreover, aging also has an impact on the AQP4. Older adults showed decreased AQP4 polarization on the astroglia processes encircling the cortical-penetrating arterioles compared to younger ones [68]. The dysregulation of AQP4 is also a proposed mechanism underlying neurodegenerative diseases [102, 103]. Therefore, our finding suggests that poor sleep quality in older adults may gradually impair normal brain function (i.e., SC-FC coupling) by deactivating the restorative glymphatic system. A similar mediating effect was also revealed in AHI, which further highlights the important role of hypoxia. Our findings support that simultaneously considering both SCN and FCN is able to provide a more comprehensive understanding of the neural mechanism underlying individual differences [27, 95], and the SC-FC coupling could be a more sensitive biomarker [33, 36].
Moderated SC-FC coupling underlay the memory function of older adults
Impaired memory is a common complaint among older adults with poor sleep quality [37, 38]. Our results provide a novel perspective on the interplay between sleep, the glymphatic system, and multimodal brain networks. Specifically, the rich-club SC-FC coupling mediated the association between glymphatic functioning and Ecog memory, but the mediating effect was only significant in good sleepers. As mentioned above, rich-club connections are central pathways responsible for efficient global integration and human cognitive functions [62, 88]. Therefore, a low rich-club SC-FC coupling, which indicates flexible communication in the rich-club, is supportive of better memory performance in older adults. Combined with the mediation results of DTI-ALPS, we can further infer that sleep quality is crucial in affecting memory functions through the underlying glymphatic functioning and human brain networks. As for the different mediating effects between poor and good sleepers, one possible explanation is that a normal glymphatic-brain relationship in good sleepers might be able to support normal memory function, while a disrupted glymphatic-brain relationship in poor sleepers fails to support it. This different pattern may be related to the resilience of the glymphatic system. Previous studies on recovery sleep revealed that recovery sleep after sleep deprivation can partially restore cognitive functions (e.g., response inhibition and memory) [104, 105] and brain connectivity (e.g., hippocampal connectivity) [106]. These findings may suggest that the glymphatic system has resilience against a certain degree of poor sleep. Once the sleep quality is lower than a certain threshold (e.g., a cut-off of PSQI), the supporting effect of the glymphatic system on the brain network may collapse and result in the different mediating patterns in different sleep quality levels, as we observed. Recently, it has been argued that binary classification of sleep quality may lose some detailed information and induce bias from the arbitrary cut-off. However, binary classification also has its advantages in simplicity and clinical relevance when used in screening or intervention. Our findings here may also give rise to the biological significance of dividing into good and poor sleepers. Similar to our findings, Dai et al. [31] also observed relatively higher SC-FC coupling in older adults with Alzheimer’s disease, who presented with significant memory impairment relative to their healthy counterparts. Both findings convergently indicate that less stringent FC in the human brain might favor memory performance. Regarding the imperative role of the glymphatic system in protecting against cognitive decline, some recent animal studies have shed light on treatment for cognitive decline by promoting glymphatic functioning [107, 108]. For example, rodent studies found a voluntary exercise that can accelerate glymphatic transport [109] and polyunsaturated fatty acid supplements that can improve glymphatic clearance and protect cognitive function [110]. Future research may verify if these treatments are effective in humans, and the strength of rich-club SC-FC coupling can be a sensitive biomarker for evaluating the effectiveness of interventions.
Limitation
There are several limitations of our study that are worth discussing. First, because our study is a cohort one, we could not offer insight into the longitudinal changes in sleep quality, glymphatic functioning, and human brain networks, and cannot address the cause-effect relationships between sleep, glymphatic functioning and human brain networks. Second, the DTI-ALPS method is a deductive measure of glymphatic functioning. It has been pointed out that the measured diffusivity may reflect the mixing of water movement at various speeds [17, 111]. Future studies using diffusion MRI data with multiple b values are needed to verify this. Moreover, this method can only evaluate water diffusivity outside the lateral ventricles. It has been confirmed that the glymphatic system has different functions in different areas [112, 113], which cannot fully be captured by DTI-ALPS. Future studies combining multiple methods would provide more comprehensive evidence of these functions. Third, our data were collected on a healthy sample. Generalization of our findings to clinical populations should be done with caution. Fourth, due to resource constraints, we did not collect PET data that offers direct measurement of the levels of toxic proteins. Further studies adopting PET data of the relevant proteins (e.g., amyloid-β) could add empirical evidence to the relationship between dysfunction of the glymphatic system and brain health. Also, due to resource and participants’ time constraints, we did not conduct a comprehensive neuropsychological assessment for each participant. To overcome these constraints, future studies may consider employing digital neuropsychological assessment systems to improve the efficiency of data collection.
Conclusion
By examining human brain networks, our findings provide novel connectomic insights into the restorative role of the glymphatic system. The current study revealed the association between glymphatic functioning and multimodal human brain networks in older adults, and the mediating effect of glymphatic functioning on the relationships between sleep and rich-club SC-FC coupling that consequently affects memory. This adds to existing evidence that sleep quality is crucial for promoting cognitive health through the underpinned neural relationships and the interplay between the glymphatic system and multimodal brain networks.
Data availability
The processed data used in this study are available upon reasonable request from the corresponding authors. The raw data are not publicly available due to a lack of informed consent from the participants and ethical approval for public data sharing.
References
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111.
Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–309.
Boespflug EL, Iliff JJ. The emerging relationship between interstitial fluid-cerebrospinal fluid exchange, amyloid-beta, and sleep. Biol Psychiatry. 2018;83:328–36.
Hablitz LM, Pla V, Giannetto M, Vinitsky HS, Staeger FF, Metcalfe T, et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun. 2020;11:4411.
Kamagata K, Andica C, Takabayashi K, Saito Y, Taoka T, Nozaki H, et al. Association of MRI indices of glymphatic system with amyloid deposition and cognition in mild cognitive impairment and Alzheimer disease. Neurology. 2022;99:e2648–2660.
Steward CE, Venkatraman VK, Lui E, Malpas CB, Ellis KA, Cyarto EV, et al. Assessment of the DTI-ALPS parameter along the perivascular space in older adults at risk of dementia. J Neuroimaging. 2021;31:569–78.
Hauglund NL, Pavan C, Nedergaard M. Cleaning the sleeping brain - the potential restorative function of the glymphatic system. Curr Opin Physiol. 2020;15:1–6.
Siow TY, Toh CH, Hsu JL, Liu GH, Lee SH, Chen NH, et al. Association of sleep, neuropsychological performance, and gray matter volume with glymphatic function in community-dwelling older adults. Neurology. 2022;98:e829–e838.
Xu Z, Xiao N, Chen Y, Huang H, Marshall C, Gao J, et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Abeta accumulation and memory deficits. Mol Neurodegener. 2015;10:58.
Reeves BC, Karimy JK, Kundishora AJ, Mestre H, Cerci HM, Matouk C, et al. Glymphatic system impairment in Alzheimer’s disease and idiopathic normal pressure hydrocephalus. Trends Mol Med. 2020;26:285–95.
Massey A, Boag MK, Magnier A, Bispo D, Khoo TK, Pountney DL. Glymphatic system dysfunction and sleep disturbance may contribute to the pathogenesis and progression of Parkinson’s disease. Int J Mol Sci. 2022;23:12928.
Zhao X, Zhou Y, Li Y, Huang S, Zhu H, Zhou Z, et al. The asymmetry of glymphatic system dysfunction in patients with temporal lobe epilepsy: a DTI-ALPS study. J Neuroradiol. 2023;50:562–7.
Chong PLH, Garic D, Shen MD, Lundgaard I, Schwichtenberg AJ. Sleep, cerebrospinal fluid, and the glymphatic system: a systematic review. Sleep Med Rev. 2022;61:101572.
Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radio. 2017;35:172–8.
Lee HJ, Lee DA, Shin KJ, Park KM. Glymphatic system dysfunction in obstructive sleep apnea evidenced by DTI-ALPS. Sleep Med. 2022;89:176–81.
Chen H-L, Chen P-C, Lu C-H, Tsai N-W, Yu C-C, Chou K-H, et al. Associations among cognitive functions, plasma DNA, and Diffusion Tensor Image along the Perivascular Space (DTI‐ALPS) in patients with Parkinson’s disease. Oxid Med Cell Longev. 2021;2021:4034509.
Taoka T, Ito R, Nakamichi R, Nakane T, Kawai H, Naganawa S. Diffusion Tensor Image Analysis ALong the Perivascular Space (DTI-ALPS): revisiting the meaning and significance of the method. Magn Reson Med Sci. 2024;23:268–90.
Zhang W, Zhou Y, Wang J, Gong X, Chen Z, Zhang X, et al. Glymphatic clearance function in patients with cerebral small vessel disease. Neuroimage. 2021;238:118257.
Carotenuto A, Cacciaguerra L, Pagani E, Preziosa P, Filippi M, Rocca MA. Glymphatic system impairment in multiple sclerosis: relation with brain damage and disability. Brain. 2022;145:2785–95.
Park HJ, Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342:1238411.
Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98.
Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13:336–49.
Smith S. Linking cognition to brain connectivity. Nat Neurosci. 2016;19:7–9.
Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: a review. Neurosci Biobehav Rev. 2015;56:330–44.
Pievani M, Filippini N, van den Heuvel MP, Cappa SF, Frisoni GB. Brain connectivity in neurodegenerative diseases-from phenotype to proteinopathy. Nat Rev Neurol. 2014;10:620–33.
Honey CJ, Thivierge JP, Sporns O. Can structure predict function in the human brain? Neuroimage. 2010;52:766–76.
Wang Z, Dai Z, Gong G, Zhou C, He Y. Understanding structural-functional relationships in the human brain: a large-scale network perspective. Neuroscientist. 2015;21:290–305.
Baum GL, Cui Z, Roalf DR, Ciric R, Betzel RF, Larsen B, et al. Development of structure-function coupling in human brain networks during youth. Proc Natl Acad Sci USA. 2020;117:771–8.
Hagmann P, Sporns O, Madan N, Cammoun L, Pienaar R, Wedeen VJ, et al. White matter maturation reshapes structural connectivity in the late developing human brain. Proc Natl Acad Sci USA. 2010;107:19067–72.
Medaglia JD, Huang W, Karuza EA, Kelkar A, Thompson-Schill SL, Ribeiro A, et al. Functional alignment with anatomical networks is associated with cognitive flexibility. Nat Hum Behav. 2018;2:156–64.
van den Heuvel MP, Sporns O, Collin G, Scheewe T, Mandl RC, Cahn W, et al. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry. 2013;70:783–92.
Dai Z, Lin Q, Li T, Wang X, Yuan H, Yu X, et al. Disrupted structural and functional brain networks in Alzheimer’s disease. Neurobiol Aging. 2019;75:71–82.
Zhao S, Wang G, Yan T, Xiang J, Yu X, Li H, et al. Sex differences in anatomical rich-club and structural-functional coupling in the human brain network. Cereb Cortex. 2021;31:1987–97.
Zhang J, Zhang Y, Wang L, Sang L, Yang J, Yan R, et al. Disrupted structural and functional connectivity networks in ischemic stroke patients. Neuroscience. 2017;364:212–25.
Ma J, Liu F, Yang B, Xue K, Wang P, Zhou J, et al. Selective aberrant functional–structural coupling of multiscale brain networks in subcortical vascular mild cognitive impairment. Neurosci Bull. 2021;37:287–97.
Reijmer YD, Schultz AP, Leemans A, O’Sullivan MJ, Gurol ME, Sperling R, et al. Decoupling of structural and functional brain connectivity in older adults with white matter hyperintensities. Neuroimage. 2015;117:222–9.
Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2020;15:311–8.
Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94:19–36.
Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev. 2015;19:29–38.
Sangalli L, Boggero IA. The impact of sleep components, quality and patterns on glymphatic system functioning in healthy adults: a systematic review. Sleep Med. 2023;101:322–49.
Amorim L, Magalhaes R, Coelho A, Moreira PS, Portugal-Nunes C, Castanho TC, et al. Poor sleep quality associates with decreased functional and structural brain connectivity in normative aging: a MRI multimodal approach. Front Aging Neurosci. 2018;10:375.
Kaufmann T, Elvsashagen T, Alnaes D, Zak N,Pedersen PO, Norbom LB, et al. The brain functional connectome is robustly altered by lack of sleep. Neuroimage. 2016;127:324–32.
Sabia S, Fayosse A, Dumurgier J, van Hees VT, Paquet C, Sommerlad A, et al. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun. 2021;12:2289.
Spira AP, Chen-Edinboro LP, Wu MN, Yaffe K. Impact of sleep on the risk of cognitive decline and dementia. Curr Opin Psychiatry. 2014;27:478–83.
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–65.
Farias ST, Mungas D, Reed BR, Cahn-Weiner D, Jagust W, Baynes K, et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22:531.
Iber C. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. Westchester, IL: American Academy of Sleep Medicine; 2007.
Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics. 2016;14:339–51.
Cui Z, Zhong S, Xu P, He Y, Gong G. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci. 2013;7:42.
Tatekawa H, Matsushita S, Ueda D, Takita H, Horiuchi D, Atsukawa N, et al. Improved reproducibility of diffusion tensor image analysis along the perivascular space (DTI-ALPS) index: an analysis of reorientation technique of the OASIS-3 dataset. Jpn J Radio. 2023;41:393–400.
Taoka T, Ito R, Nakamichi R, Kamagata K, Sakai M, Kawai H, et al. Reproducibility of diffusion tensor image analysis along the perivascular space (DTI-ALPS) for evaluating interstitial fluid diffusivity and glymphatic function: CHanges in Alps index on Multiple conditiON acquIsition eXperiment (CHAMONIX) study. Jpn J Radio. 2022;40:147–58.
Yokota H, Vijayasarathi A, Cekic M, Hirata Y, Linetsky M, Ho M, et al. Diagnostic performance of glymphatic system evaluation using diffusion tensor imaging in idiopathic normal pressure hydrocephalus and mimickers. Curr Gerontol Geriatr Res. 2019;2019:5675014.
Hsu JL, Wei YC, Toh CH, Hsiao IT, Lin KJ, Yen TC, et al. Magnetic resonance images implicate that glymphatic alterations mediate cognitive dysfunction in Alzheimer disease. Ann Neurol. 2023;93:164–74.
Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, et al. The Human Brainnetome Atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016;26:3508–26.
Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65.
Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–83.
Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905.
Wang R, Benner T, Sorensen AG, Wedeen VJ. Diffusion toolkit: a software package for diffusion imaging data processing and tractography. Proc Intl Soc Mag Reson Med. 2007;15:3720.
Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159.
Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA. 2009;106:2035–40.
van den Heuvel MP, Sporns O. Rich-club organization of the human connectome. J Neurosci. 2011;31:15775–86.
Collin G, van den Heuvel MP, Abramovic L, Vreeker A, de Reus MA, van Haren NE, et al. Brain network analysis reveals affected connectome structure in bipolar I disorder. Hum Brain Mapp. 2016;37:122–34.
van den Heuvel MP, Mandl RC, Stam CJ, Kahn RS, Hulshoff Pol HE. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci. 2010;30:15915–26.
Ma J, Chen X, Gu Y, Li L, Cam-CAN, Lin Y, et al. Trade-offs among cost, integration, and segregation in the human connectome. Netw Neurosci. 2023;7:604–31.
Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York: Guilford Publications; 2017.
Neumann N, Lotze M, Domin M. Sex-specific association of poor sleep quality with gray matter volume. Sleep. 2020;43:zsaa035.
Benveniste H, Liu X, Koundal S, Sanggaard S, Lee H, Wardlaw J. The glymphatic system and waste clearance with brain aging: a review. Gerontology. 2019;65:106–19.
Shokri-Kojori E, Wang GJ, Wiers CE, Demiral SB, Guo M, Kim SW, et al. beta-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci USA. 2018;115:4483–8.
Wei M, Zhao B, Huo K, Deng Y, Shang S, Liu J, et al. Sleep deprivation induced plasma amyloid-beta transport disturbance in healthy young adults. J Alzheimers Dis. 2017;57:899–906.
Barthelemy NR, Liu H, Lu W, Kotzbauer PT, Bateman RJ, Lucey BP. Sleep deprivation affects tau phosphorylation in human cerebrospinal fluid. Ann Neurol. 2020;87:700–9.
Lee WJ, Jung KH, Park HM, Sohn CH, Lee ST, Park KI, et al. Periodicity of cerebral flow velocity during sleep and its association with white-matter hyperintensity volume. Sci Rep. 2019;9:15510.
Roy B, Nunez A, Aysola RS, Kang DW, Vacas S, Kumar R. Impaired glymphatic system actions in obstructive sleep apnea adults. Front Neurosci. 2022;16:884234.
Jackson ML, Howard ME, Barnes M. Cognition and daytime functioning in sleep-related breathing disorders. Prog Brain Res. 2011;190:53–68.
Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–9.
Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608.
Voumvourakis KI, Sideri E, Papadimitropoulos GN, Tsantzali I, Hewlett P, Kitsos D, et al. The dynamic relationship between the glymphatic system, aging, memory, and sleep. Biomedicines. 2023;11:2092.
Han F, Liu X, Yang Y, Liu X. Sex-specific age-related changes in glymphatic function assessed by resting-state functional magnetic resonance imaging. bioRxiv [Preprint]. 2023. Available from https://doi.org/10.1101/2023.04.02.535258.
Ward AM, Schultz AP, Huijbers W, Van Dijk KR, Hedden T, Sperling RA. The parahippocampal gyrus links the default‐mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp. 2014;35:1061–73.
Gogolla N. The insular cortex. Curr Biol. 2017;27:R580–6.
Ranganath C. Working memory for visual objects: complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neuroscience. 2006;139:277–89.
Cho H, Choi JY, Hwang MS, Kim YJ, Lee HM, Lee HS, et al. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol. 2016;80:247–58.
Sepulcre J, Sabuncu MR, Becker A, Sperling R, Johnson KA. In vivo characterization of the early states of the amyloid-beta network. Brain. 2013;136:2239–52.
Kovacs GG. Molecular pathological classification of neurodegenerative diseases: turning towards precision medicine. Int J Mol Sci. 2016;17:189.
Liao YZ, Ma J, Dou JZ. The role of TDP-43 in neurodegenerative disease. Mol Neurobiol. 2022;59:4223–41.
Chételat G, Villemagne VL, Bourgeat P, Pike KE, Jones G, Ames D, et al. Relationship between atrophy and β‐amyloid deposition in Alzheimer disease. Ann Neurol. 2010;67:317–24.
Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73:1216–27.
Van Den Heuvel MP, Kahn RS, Goñi J, Sporns O. High-cost, high-capacity backbone for global brain communication. Proc Natl Acad Sci USA. 2012;109:11372–7.
Zhang R, Shao R, Xu G, Lu W, Zheng W, Miao Q, et al. Aberrant brain structural-functional connectivity coupling in euthymic bipolar disorder. Hum Brain Mapp. 2019;40:3452–63.
Dahan L, Rampon C, Florian C. Age-related memory decline, dysfunction of the hippocampus and therapeutic opportunities. Prog Neuropsychopharmacol Biol Psychiatry. 2020;102:109943.
Wennberg AMV, Wu MN, Rosenberg PB, Spira AP. Sleep disturbance, cognitive decline, and dementia: a review. Semin Neurol. 2017;37:395–406.
Lee MH, Yun CH, Min A, Hwang YH, Lee SK, Kim DY, et al. Altered structural brain network resulting from white matter injury in obstructive sleep apnea. Sleep. 2019;42:zsz120.
Park B, Palomares JA, Woo MA, Kang DW, Macey PM, Yan-Go FL, et al. Disrupted functional brain network organization in patients with obstructive sleep apnea. Brain Behav. 2016;6:e00441.
Byun JI, Jahng GH, Ryu CW, Park S, Lee KH, Hong SO, et al. Altered intrinsic brain functional network dynamics in moderate-to-severe obstructive sleep apnea. Sleep Med. 2023;101:550–7.
Suarez LE, Markello RD, Betzel RF, Misic B. Linking structure and function in macroscale brain networks. Trends Cogn Sci. 2020;24:302–15.
Avena-Koenigsberger A, Misic B, Sporns O. Communication dynamics in complex brain networks. Nat Rev Neurosci. 2017;19:17–33.
Preti MG, Van De Ville D. Decoupling of brain function from structure reveals regional behavioral specialization in humans. Nat Commun. 2019;10:4747.
Zanirati ShettyAK. G. The interstitial system of the brain in health and disease. Aging Dis. 2020;11:200.
Wang M, Ding F, Deng S, Guo X, Wang W, Iliff JJ, et al. Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J Neurosci. 2017;37:2870–7.
Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34:16180–93.
Wang Y, Huang C, Guo Q, Chu H. Aquaporin-4 and cognitive disorders. Aging Dis. 2022;13:61–72.
McKenzie S, Eichenbaum H. New approach illuminates how memory systems switch. Trends Cogn Sci. 2012;16:102–3.
Kylkilahti TM, Berends E, Ramos M, Shanbhag NC, Töger J, Markenroth Bloch K, et al. Achieving brain clearance and preventing neurodegenerative diseases—A glymphatic perspective. J Cereb Blood Flow Metab. 2021;41:2137–49.
Leong RL, Chee MW. Understanding the need for sleep to improve cognition. Annu Rev Psychol. 2023;74:27–57.
Drummond SP, Paulus MP, Tapert SF. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J Sleep Res. 2006;15:261–5.
Chai Y, Fang Z, Yang FN, Xu S, Deng Y, Raine A, et al. Two nights of recovery sleep restores hippocampal connectivity but not episodic memory after total sleep deprivation. Sci Rep. 2020;10:8774.
Wang J, Zhou Y, Zhang K, Ran W, Zhu X, Zhong W, et al. Glymphatic function plays a protective role in ageing-related cognitive decline. Age Ageing. 2023;52:afad107.
Salehpour F, Khademi M, Bragin DE, DiDuro JO. Photobiomodulation therapy and the glymphatic system: promising applications for augmenting the brain lymphatic drainage system. Int J Mol Sci. 2022;23:2975.
He XF, Liu DX, Zhang Q, Liang FY, Dai GY, Zeng JS, et al. Voluntary exercise promotes glymphatic clearance of amyloid beta and reduces the activation of astrocytes and microglia in aged mice. Front Mol Neurosci. 2017;10:144.
Liu X, Hao J, Yao E, Cao J, Zheng X, Yao D, et al. Polyunsaturated fatty acid supplement alleviates depression-incident cognitive dysfunction by protecting the cerebrovascular and glymphatic systems. Brain Behav Immun. 2020;89:357–70.
Nguchu BA, Zhao J, Wang Y, de Dieu Uwisengeyimana J, Wang X, Qiu B, et al. Altered glymphatic system in middle-aged cART-treated patients with HIV: a diffusion tensor imaging study. Front Neurol. 2022;13:819594.
McKnight CD, Trujillo P, Lopez AM, Petersen K, Considine C, Lin YC, et al. Diffusion along perivascular spaces reveals evidence supportive of glymphatic function impairment in Parkinson disease. Parkinsonism Relat Disord. 2021;89:98–104.
Tian Y, Cai X, Zhou Y, Jin A, Wang S, Yang Y, et al. Impaired glymphatic system as evidenced by low diffusivity along perivascular spaces is associated with cerebral small vessel disease: a population-based study. Stroke Vasc Neurol. 2023;8:e002191.
Acknowledgements
The authors acknowledge the data collection, data management, and information technology services, based on the Structured Research and Medical Informatics Cloud (SRMIC) platform system, provided by the Clinical Trial Center, Chang Gung Memorial Hospital, Linkou, Taiwan, with support by the Ministry of Health and Welfare under Grant No. MOHW110-TDU-B-212-124005. We thank the Genomic and Proteomic Core Laboratory of Chang Gung Memorial Hospital for the assistance in conducting the experiment.
Funding
This study was supported by grant CORPG3J0371 from Chang Gung Memorial Hospital and The University of Hong Kong May Endowed Professorship.
Author information
Authors and Affiliations
Contributions
Conceptualization: Tatia M.C. Lee, Shwu-Hua Lee, Ji-Tseng Fang; Methodology: Tatia M.C. Lee, Shwu-Hua Lee, Ji-Tseng Fang, Junji Ma; Data collection: Shwu-Hua Lee, Ji-Tseng Fang, Geng-Hao Liu, Ning-Hung Chen, Cheng Hong Toh, Jung-Lung Hsu, Kuan-Yi Wu, Chih-Mao Huang, Chih-Ming Lin; Data analysis, Tatia M.C. Lee, Junji Ma, Menglu Chen, Mengxia Gao; Writing—Original Draft, Junji Ma; Writing—Reviews & Editing, Tatia M.C. Lee, Shwu-Hua Lee, Ji-Tseng Fang, Junji Ma, Menglu Chen, Mengxia Gao; Supervision, Tatia M.C. Lee, Shwu-Hua Lee, Ji-Tseng Fang; Funding Acquisition, Tatia M.C. Lee, Shwu-Hua Lee. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the institutional review board of Chang Gung Medical Foundation, Taiwan (IRB No. 201900702A3; Registration number ClinicalTrials.gov: NCT04207502), and all methods in this study were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all the participants. The experimental procedure followed the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, J., Chen, M., Liu, GH. et al. Effects of sleep on the glymphatic functioning and multimodal human brain network affecting memory in older adults. Mol Psychiatry 30, 1717–1729 (2025). https://doi.org/10.1038/s41380-024-02778-0
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41380-024-02778-0