Abstract
Background
Persons with schizophrenia are excluded from psychedelic-assisted therapy due to concerns about the risk of triggering or worsening psychosis. However, there is limited meta-analytic data on the risk of psychedelic-induced psychosis in individuals with pre-existing psychotic disorders.
Methods
We conducted a systematic review, meta-analysis, and overview of reviews to assess the incidence of psychedelic-induced psychosis and symptom exacerbation in schizophrenia. Our pre-registered protocol (CRD42023399591) covered: LSD, psilocybin, mescaline, DMT, and MDMA, using data from Embase, PubMed, PsyARTICLES, PsyINFO, and trial registries up to November 2023. A random-effects model was used to calculate psychosis incidence, with standardized assessments of study quality.
Results
From 131 publications, we analyzed 14 systematic reviews, 20 reviews, 35 randomized-controlled trials (RCTs), 10 case-control studies, 30 uncontrolled trials (UCTs), and 22 cohort studies, most of which were low quality. Meta-analysis of nine studies showed an incidence of psychedelic-induced psychosis at 0.002% in population studies, 0.2% in UCTs, and 0.6% in RCTs. In UCTs including individuals with schizophrenia, 3.8% developed long-lasting psychotic symptoms. Of those with psychedelic-induced psychosis, 13.1% later developed schizophrenia. Sensitivity analyses confirmed the results.
Conclusion
In summary, the reviewed evidence suggests that schizophrenia might not be a definite exclusion criterion for clinical trials exploring safety and efficacy of psychedelics for treatment-resistant depression and negative symptoms. However, given the low quality and limited number of studies, more high-quality research is needed, and a conservative approach is recommended until further data is available.
Similar content being viewed by others
Introduction
Supported by the U.S. Food and Drug Administration (FDA) and other funding agencies, the scientific research and interest in the therapeutic potential of psychedelics for treating mental health conditions is rapidly expanding [1]. Significant breakthroughs in psychedelic research provide valuable insights into the mechanisms of mental disorders and offer novel therapeutic strategies for treatment-resistant conditions in depression [2], substance use disorders [3], anxiety [4], and post-traumatic stress disorder (PTSD) [5]. However, specific patient populations have not been considered for psychedelic treatment approaches, such as patients with schizophrenia and bipolar disorders until recently [6], mainly due to concerns about psychedelic-induced acute or long-lasting psychotic symptoms [7]. It thus remains an open question whether psychedelic drugs could have a role in the treatment of schizophrenia, in particular for patients with prominent negative symptoms [8]. Focusing on these observations of psychedelic-induced psychotic experiences, the serotonin hypothesis of schizophrenia proposed that serotonin-2A overactivity could be involved in the pathophysiology of schizophrenia (Vollenweider et al. [9]). However, recent research shows that cannabis and illegal psychostimulant drugs are much more likely than serotoninergic psychedelics to induce psychotic symptoms in users (30-55%) [10, 11]. The serotonin hypothesis of schizophrenia suggests serotonergic psychosis typically results in a reversible psychotic syndrome lasting a few hours, which shares certain similarities with acute decompensation of psychotic symptoms in schizophrenia. However, this serotonergic psychosis is characterized primarily by visual hallucinations and shows no response to D2 blockers, distinguishing it from the dopamine and glutamatergic-related psychosis seen in schizophrenia (Bowers et al. [12]).
Prior to the advent of the serotonergic schizophrenia hypothesis, some researchers in the 50 s and 60 s used lysergic acid diethylamide (LSD) for stabilized patients with schizophrenia (Fink et al. [13]; Hoch et al. [14]).
However, concerns regarding ‘overdiagnosis’ in patients with schizophrenia has been reported which greatly limited these findings [15]. Other serotonergic drugs, such as antidepressant, have shown small effects in improving depressive and negative symptoms in patients with schizophrenia [16]. Therefore, the potential of serotonergic psychedelics to enhance neuroplasticity as seen in animal studies [17] could offer significant promise for patients with treatment-resistant depressive symptoms and negative symptoms [8, 18]; NCT05770375.
In contrast to these hypothetical benefits of psychedelics in the treatment of schizophrenia, the relationship between serotonergic psychedelics and exacerbations of psychotic episodes is unclear. Overall, Cohen suggests a low rate of prolonged psychotic reactions in LSD users (1.8 per 1000) [19]. Accordingly, Strassman proposed the distinction between acute panic reactions resolving spontaneously within a day, prolonged psychotic reactions lasting more than one day, and more complex chronic undifferentiated psychotic reactions [7]. A recent meta-analysis by Murrie and colleagues found a 26% transition rate to psychoses for hallucinogen users, which is lower than the rate associated with cannabis (34%) but not amphetamines (22%) [11, 20]. However, these results are based on only three studies, mainly driven by one study’s weight on phencyclidine (PCP) users. PCP blocks the uptake of dopamine and norepinephrine, leading to sympathomimetic effects, and is a NMDA receptor antagonist provoking dissociative symptoms, which is absent from usual doses of classic psychedelics [21]. Furthermore, recent randomized-controlled trials (RCTs), including patients with depression and PTSD, show the absence of risk of prolonged psychotic reaction following the use of MDMA, LSD, and psilocybin in selected populations free from any history of personal or family risk of psychotic symptoms [22]. Taken together, the effective risk of psychedelic-induced psychosis or worsening of pre-existing psychotic symptoms in schizophrenia -as well as in the early stages of the psychosis spectrum- remains incompletely understood. This uncertainty raises the question whether the current exclusion of patients with psychotic symptoms may be too restrictive and hamper progress in developing novel treatment approaches for negative symptoms of schizophrenia. To shed light on the existing evidence for the risk of psychedelic-induced psychosis and worsening symptoms of schizophrenia, we conducted an overview of reviews, a systematic review and meta-analysis. We focused on serotonergic psychedelic drugs of which some have shown promise as therapeutics in mental disorders and potential candidates for treatment in schizophrenia, including LSD, N,N-Dimethyltryptamine (DMT), mescaline, psilocybin and 3,4-Methylenedioxymethamphetamine (MDMA). Although MDMA has different mechanisms than classic psychedelics with additional action on dopaminergic, and GABAergic systems [23, 24], we decided to include MDMA considering the similar effects on the serotonin system and the therapeutic potential in PTSD [25]. Our primary aim was to summarize the literature regarding the current evidence of the risk for de novo psychedelic-induced psychosis, exacerbation of psychotic symptoms in pre-existing psychotic disorders, and development of schizophrenia after psychedelic-induced psychosis. Our secondary aim was to conduct a meta-analysis of the incidence of long-lasting (>48 h) psychotic symptoms following psychedelic use for each specific population and the risk of transition to schizophrenia.
Methods
Registration
This systematic review was conducted according to the Preferred Reported Items for Systematic Reviews and Meta-Analysis (PRISMA) (Supplementary Information 1). The review protocol was registered on PROSPERO in April 2023 (CRD 42023399591). The literature search was updated in November 2023.
Search strategy
A multi-step literature search was performed in other to conduct a joint umbrella review and a meta-analysis [26]. First, two reviewers (MS, AG) independently reviewed titles and abstracts using Rayyan, a research collaboration web platform for systematic reviews in PubMed, Embase, and PsycINFO using specific search terms (Supplementary Information 1). The only limits applied were human studies. In case of disagreement, full texts were analyzed until both reviewers reached a consensus. Second, two authors (MSa and AG) independently extracted articles using a predefined data extraction form. Third, snowball searches of reference lists were conducted by cross-referencing key papers and other relevant articles identified by the electronic searches, in particular in retrieved reviews.
Eligibility criteria, hierarchization of the quality of evidence, and data extraction
We included systematic reviews, reviews, guidelines, meta-analysis, RCTs, clinical trials, case-control studies, uncontrolled trials, prospective and retrospective cohorts, and population-survey/register studies that explored the link between psychosis or other psychiatric side-effects following serotoninergic psychedelic consumption (LSD, DMT, mescaline, psilocybin, MDMA). We decided to exclude both cannabis, salvia divinorum and PCP from our analysis, as their mechanisms of action are clearly distinct. Moreover, due to their specific neurobiological effects, cannabis and PCP are strongly linked to the occurrence of psychoses, and salvia divinorum has dissociative properties. Case reports and case series were excluded.
Considering the extensive history of psychedelic research and the expected significant heterogeneity in the quality of evidence, we employed a hierarchy of evidence to identify the ‘best evidence’ by examining all published studies from uncontrolled trials to reviews and guidelines. Firstly, given that the definition of ‘psychedelic (hallucinogen)-induced psychosis’ is not truly established, we considered Strassman’s landmark article on adverse reactions to psychedelics drugs [7], and the definition of substance-induced psychosis according to the DSM-5 to guide our inclusion criteria for identified studies and more recent reviews on the subject [27] (Supplementary Information 2). We distinguish between various groups of psychedelic users on the continuum ranging from ‘healthy’ individuals to patients with schizophrenia: individuals considered as ‘healthy individuals’, patients with other diagnoses (e.g., depression), patients with susceptibility for psychosis, patients with long-lasting psychotic symptoms, patients with a diagnosis of schizophrenia. Additionally, we attempted to assess the temporal relationship between prolonged psychotic reactions and the use of psychedelics whenever possible.
Secondly, two authors (MSa, AG) independently categorized the quality of evidence and the risk of bias by classifying studies based on their type and evaluating their quality using specific assessment tools. We utilized the AMSTAR-2 scale for systematic reviews [28], the SANRA scale for reviews [29], the Cochrane Risk-Of-Bias tool for RCTs (RoB 2) [30], and Risk Of Bias In Non-randomized Studies of Interventions tool (ROBINS-I) for non-randomized studies [31]. To further enhance the hierarchy of evidence, we employed the Oxford Center of Evidence Based Medicine (OCEBM) level of evidence framework [32]. A distinguishing feature of the OCEBM is that the levels cover the entire range of clinical questions, such as the evidence for incidence, prognosis, therapeutic effects, rare harms, common harms, and usefulness of screening. To ease the reception of the hierarchy of evidence, we present in the results section uncontrolled studies, followed by cohort studies, case-control studies, and RCTs, for each drug and subgroup of the identified population. Two authors (MS, AG) independently extracted all data according to a preestablished data extraction form.
Statistical analysis
To conduct a meta-analysis of incidence, we employed a logit transformation to stabilize the variance. We selected a random-effects model to account for anticipated heterogeneity among the included studies, for each subgroup of populations or measure of exposure to the primary outcome [33]. To measure heterogeneity, we utilized the I2 statistic and the Q test [34]. The I2 values were categorized as indicating low, moderate, substantial, and considerable heterogeneity, representing <25%, <25–50%, <50–75%, and ≥75%, respectively. The analyses were performed using R software, specifically version 4.3.1, with the metafor package [35]. We opted for the logit transformation of proportions as it is more suitable for a wide range of incidence values and when proportions are close to 0 or 1 [36].
Results
Search results
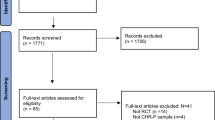
Among the 2170 records identified, we assessed 223 full-text records for eligibility and included 131 publications published from 1947 to 2023, encompassing 14 systematic reviews, 20 non-systematic reviews, and 96 individual studies. These studies were 35 RCTs, 10 case-controls studies, 30 uncontrolled trials (UCTs), and 22 cohort studies (retrospective or prospective cohorts) (Supplementary Table S1 and Fig. 1). Table 1 reflects the characteristics of included studies, and Table 2 of the reviews. The list of the 91 excluded studies is reported in supplementary Table S2.
a Incidence of psychedelic-induced psychosis according to the number of sessions across healthy individuals, patients with depression, and patients with schizophrenia. Of importance, RCTs, UCT and cohort studies are combined in this forest plot. b Incidence of psychedelic-induced psychosis according to the number of sessions, in healthy individuals only. Please note that Studerus et al. [75], Novak et al. [79] studies are RCTs. All other studies are retrospective cohort studies. When excluding these 2 RCTS, the incidence does not change. When considering only those 2 RCTS, the incidence is 0.4% (95%; CI 0.1–1.7)(I2 = 0%). c Lifetime occurrence of prolonged psychosis according to number of sessions considering only studies that included patients with schizophrenia.
Evidence from UCT
Evidence from UCT: psychedelic-induced psychosis in healthy individuals
Two UCTs utilizing LSD were found, published from 1956 to 2023 (Table 1.1 and Table 2). These studies noted that a few psychopathology-free individuals can exhibit paranoid symptoms with reduced insight starting at 100 µg LSD doses, confined to the session [37, 38]. The method of administration does not appear to affect the occurrence of these symptoms. Cohen suggest a 1.8% rate of prolonged psychotic reactions after LSD use in healthy individuals. However, this author speculates whether these affected individuals had preexisting psychopathology.
These studies presented a low level of evidence with critical risks of bias at the ROB2 scale and at most Level 2 ranking at the OCEBM (Table 1).
For DMT, authors also describe paranoid symptoms in a minority of participants [39]. More recently, DMT has been studied in healthy individuals, with no reported psychopathological complications (Table 1.2.1).
Concerning psilocybin, authors noted dose-dependent psychotic-like symptoms (intense anxiety) and hallucinations in healthy volunteers [40]. An open-label study found that up to 22% of patients exhibited mild paranoia and ideas of reference during the sessions [41]. Finally, one recent state-of-the-art open-label study proposed psilocybin-assisted therapy (one session, 25 mg) to patients with bipolar type II depression [6]. Authors found no side-effects at the given dose for selected patients, however patients with psychotic features were excluded. The cross-sectional nature of these studies limits the quality of evidence, with most studies showing significant risk at evidence levels 2–3 according to OCEBM criteria.
Evidence from UCT: use of psychedelics in patients with schizophrenia
We found eleven UCTs administering LSD sessions to adults with schizophrenia, including two studies involving treatment-resistant patients (Table 1.1.1). Oral LSD doses ranged from 30 to 500 μg for these stabilized patients. However, most studies did not mention whether patients were taking an antipsychotic medication. Given the publication dates spanning 1947 to 1971, it is likely that the majority underwent LSD sessions without concurrent medication -or only with D2 blockers- as no information on any medication was found in these articles.
Several studies indicate that individuals with schizophrenia exhibit ‘resistance’ to LSD effects in contrast to healthy volunteers [42,43,44,45]. Another study noted phenomenological aspects involving the intensification of preexisting symptomatology under LSD, frequently associated with heightened sexual motifs, increased psychomotor activity, and euphoria. Forrer and Goldner conducted a study administering LSD through intra-muscular injections to six patients across 42 sessions, revealing no notable distinction from the oral route [46]. In contrast, two studies employing intravenous LSD administration among patients with schizophrenia reported more distinct reactions [13, 47]. Indeed, in all studies, no long-lasting psychotic reactions were found, albeit in one study that administered intravenously LSD (0.5 µg/kg to 10 µg/kg) [13], after 1 to 3 sessions, 3 of 65 patients presented long-lasting psychotic reactions up a to a week after LSD sessions. Moreover, in another study with intravenous LSD, two patients presented marked fear during the session [47].
Hoch and colleagues in 1952 compared effects of LSD and mescaline in patients with schizophrenia [14], and found that patients with ‘pseudoneurotic’ and schizoaffective disorders improved (Table 1.6.1). A study involving ‘prepubertal children’ with schizophrenia [48] noted no side effects or significant emotional reactions to LSD at doses ranging from 50 to 150 μg. However, for both studies important methodological question arises regarding the accuracy of diagnoses. Additionally, we located four studies encompassing children with treatment-resistant severe autism accompanied by psychotic symptoms. LSD dosages varied between 40 and 400 µg. In nearly all these studies, short-term enhancements in speech and behavior, heightened emotional responsiveness, improved positive mood, and decreased compulsive ritualistic conduct were observed. All identified studies were rated with critical risk for bias on the ROB2 scale, yet with a level 2 OCEBM classification considering the specific population.
Evidence from cohort studies
Evidence from cohort studies: substance-induced psychosis in previously healthy individuals
For LSD, we retrieved four cohort studies [49,50,51,52] (Table 1.1.1). McGlothlin & Arnold conducted a 10-year follow-up of 247 healthy individuals who underwent LSD sessions. They found one instance of prolonged psychosis after three LSD sessions, resulting in a week-long hospitalization. Bowers conducted a follow-up study of up to 5.8 years in 15 patients with LSD-induced psychosis and found that approximately half of the patients presented good outcomes. Vardy & Kay tracked 29 patients diagnosed with LSD-induced psychosis for up to five years [52]. During the follow-up, authors could not distinguish these patients from those experiencing a first episode of psychosis. Niemi-Pynttäri and colleagues retrospectively analyzed Finland’s nationwide hospital discharge register, discovering a cumulative 24% risk of receiving a schizophrenia spectrum diagnosis for patients with initial hallucinogen-induced psychosis [50].
For DMT, we found a 13-year epidemiological survey in a Brazilian indigenous community where regular ayahuasca users ( > 50 uses per year) were studied [53]. Notably, almost no cases of persistent psychotic phenomena were reported (estimated at 1 in 50,000).
Similarly, in the case of psilocybin, a 4-year population survey among Navajo Indians who were frequent peyote users documented isolated incidents of enduring psychotic symptoms (estimated at 1 in 70,000) [54]. In 2017, Dos Santos and colleagues suggested that the occurrence of psychotic episodes linked to ayahuasca/DMT intake is rare, and these infrequent cases seem to be related to preexisting traits (e.g.; family history of psychosis, concomitant use of other drugs), prior and possibly concurrent substance abuse, and a lack of supervised settings [55].
For ecstasy, a prospective cohort study tracked patients diagnosed with ecstasy-induced psychotic disorders and estimated that 10% of these patients had previously experienced psychotic symptoms [56]. Various population studies were also identified. While most studies are of notably low quality, characterized by critical risk and Level 4 OCEBM ratings (Table 1.4.1), some higher-quality studies reported a 4.4% occurrence of non-affective psychosis diagnosis among psychedelic users [57,58,59] Intriguingly, lifetime psychedelic use was linked to a reduced likelihood of undergoing inpatient mental health treatments in the past year (adjusted odds ratio 0.7; p = 0.01). These cohorts’ studies presented moderate to critical ROB2 rating and were classified as level 2 OCEBM studies.
Evidence from cohort studies: use of psychedelics in patients with schizophrenia
No cohort study on the use of psychedelics in patients with schizophrenia was identified.
Evidence from case-control studies
Evidence from case-control studies: substance-induced psychosis in healthy individuals
In 1968, Ungerleider suggested that up to one third of patients with LSD-induced psychosis might have schizophrenia [60]. In a separate case-control study, Hays & Tilley proposed that LSD-induced psychosis differs from schizophrenia, having fewer primary delusions and auditory hallucinations, more visual hallucinations, and less emotional blunting [61].
For MDMA, one case-control study proposed that patients with recent use of MDMA presented less blunted affect and more hostile behavior. Rare cases of psychosis with high levels of aggressiveness and violence are reported [62].
Evidence from case-control studies: use of psychedelics in patients with schizophrenia
One case-control study compared patients with schizophrenia and schizoaffective disorder using LSD to healthy individuals [63] (Table 1.1.2). Sloan and Doust’s [63] study reported the safety of 40–120 μg LSD doses and improved mood in unmedicated patients with schizophrenia. This study carried a critical risk for bias and a Level 2 OCEBM classification.
Evidence from RCTs
Evidence from RCTs: substance-induced psychosis in healthy individuals and patients with depression
For healthy individuals without personal or familial risk for psychosis, no long-lasting psychotic reactions are reported in experimental conditions, albeit important anxiety reactions linked to the ego-dissolution phenomena have been described [64,65,66]. Psychological support seems to be highly effective in mitigating such anxiety responses. One RCT administered psilocybin and LSD to healthy volunteers. Two volunteers presented a loss of their insight during the psychedelic session with paranoid thoughts (1.5 mcg/kg of LSD, and 114 mcg/kg of psilocybin) [67]. In another RCT, ten former morphine addicts received intra-muscular LSD (0.75–1.5 mcg/kg) or mescaline (2.5–5 mg/kg), without adverse reactions reported [68].
Eight RCTs administering MDMA to patients with PTSD and resistant PTSD found no long-lasting psychotic symptoms. However, up to 90% of participants experienced the emergence of dose-dependent anxiety (Table 1.5.2).
Moreover, we found eight RCTs administering doses ranging from 25(+12.5) mg to 125(+62.5) mg to PTSD patients (Table 1.5.2). Authors observed that a minority of patients experienced pronounced anxiety, correlating with dose [69], with anxiety reactions reported in up to 90% of cases at higher doses. Short-term and frequent insomnia reactions were also reported.
Except for some studies published before 1990, most presented a low risk of bias and a Level 1–2 OCEBM classification. Similarly, for DMT, no increase in psychotic reactions was found (Table 1.2.2).
For psilocybin, nine RCTs were identified with doses ranging from 3 to 50 mg/70 kg (Table 1.3.2), essentially for patients with depression and resistant depression (with no personal of familial history of psychosis). None of the recent studies published from 1999 to 2023 has reported enduring psychotic reactions, except for one study with a patient experiencing abnormal dream illusions [70], but without requiring antipsychotics. Nonetheless, a few authors note brief paranoia or heightened anxiety during DMT sessions, affecting fewer than 10% of patients [71, 72].
Evidence from RCTs: use of psychedelics in patients with schizophrenia
One RCT conducted in 1966 was identified [73]. Patients with chronic schizophrenia using LSD mostly improved with euphoric reactions followed by improved social behavior. For three weeks, they received between 100 and 300 mg LSD daily, followed by 100 to 300 mg of thioridazine a few hours later. After that only thioridazine continued for an additional period of three weeks. Considering the lack of information on randomization and information on the placebo used, this study presented critical risk of bias and a Level 2 OCEBM classification.
MRI-studies of psychedelic drug effects
We identified several MRI studies focusing on population of regular users of psychedelics, suggesting no specific harm of psychedelics on the brain. These findings might be of particular interest because they could provide potentially a putative therapeutic mechanism of how psychedelics might ameliorate negative symptoms in schizophrenia (Wolf et al. [8]). We detail these studies in the supplements (Supplementary Information 3).
Hallucinogen persisting perception disorder
Occurrence of Hallucinogen Persisting Perception Disorder (HPPD) (not considered a primary psychedelic-induced psychosis) has also been studied. HPPD was not associated with exacerbation of psychotic symptoms in individuals with schizophrenia (Supplementary Information 4).
Guidelines for safety and adverse events managements
We identified two guidelines on human hallucinogen research and on the abuse potential of medical psilocybin (Supplementary Table S7). We report in the supplement these resources, and several reviews, and cross-sectional study on selection of participants for psychedelic trials (Supplementary Table S7). In most studies, the assumption of contra-indications for inclusion of patients with schizophrenia, psychosis or bipolar disorder is mainly based on case reports.
Meta-analysis of the incidence of psychedelic-induced psychosis
We retrieved seven studies that reported lifetime incidence of psychedelic-induced psychosis, in healthy individuals [51, 67, 74, 75], patients with depression [22], or patients with schizophrenia [13, 14].
Furthermore, 10 studies also reported the risk of psychedelic-induced psychosis according to the number of psychedelic sessions (Baker, [76]; S. Cohen [37]; Fink, [13]; Hoch et al. [14]; Leuner, [77]; Malleson, [78]; McGlothlin & Arnold, [51]; Novak [79]; Perez et al. [22]; Studerus et al. [75]) and five studies reported incidences of psychedelic-induced psychosis in population studies [50, 53, 54, 80, 81].
We detail characteristics of all retained study for the meta-analysis in the supplements (Supplementary Table S8).
Meta-analysis of the incidence of psychedelic-induced psychosis according to the number of sessions, in healthy individuals, patients with depression, or patients with schizophrenia
A total of 7931 sessions of LSD and psilocybin were included from 10 low to high quality experimental studies, mostly involving LSD, published between 1960 and 2010: seven cohort studies (Baker [82]; S. Cohen [37]; Leuner [77]; Malleson [78]; McGlothlin & Arnold [51]; Novak [79]), one pool of eight high-quality RCTs in healthy individuals (n = 110) [75]; seven RCTs in patients with depression (n = 263) [22]; and two non-randomized controlled studies in patients with schizophrenia (n = 133) [13, 14]. One retrieved RCTs delivering LSD to patients with schizophrenia could not be included, as there were no clear cases or worsening for all 20 included patients in the intervention group [73].
The details of all retained studies in our meta-analysis is reported in the supplement (Supplementary Table S8).
When pooling all studies, the combined incidence of psychedelic-induced psychosis was 0.4% (95%CI 0.1–1), with notable heterogeneity (I2 = 75%; n = 7931) (Fig. 1A). The visual inspection of the funnel plot indicated that the observed heterogeneity was primarily attributed to the studies involving patients with schizophrenia (Supplementary Fig. S1).
We conducted a sensitivity analysis regarding this analysis, by including only the two studies with low risk of bias, that excluded patients with a history of psychosis (Studerus et al., 2010; Perez et al. [22]) (Supplementary Fig. S3). The combined incidence was however similar 0.4% (95%CI 0.1–1.6) in absence of heterogeneity (I2 = 0%; n = 490).
Furthermore, when excluding patients with psychiatric disorders, the incidence of psychedelic-induced psychosis in healthy participants dropped to 0.2% (95%CI 0.1–0.3; I2 = 0%; n = 8) (Fig. 1B). Moreover, the incidence of psychedelic-induced psychosis for patients with depression was not different from the incidence in healthy participants 0.4% (95%CI 0.1–2.8). When considering the subgroup of patients with schizophrenia, this incidence was higher 3.8% (95%CI 1.6–8.9; I2 = 0%; n = 2)(Fig. 1C), however, it is important to note that these studies were published more than half a century ago. These patients had from one to three sessions of LSD (10-120 µg oral LSD, and IV 0.5-10 µg/kg LSD), and in almost all cases no long-lasting psychotic symptoms were observed. One cross-sectional, web-based study was not included in our meta-analysis, considering the self-report nature of the data, in the absence of confirmed diagnoses (Evans et al. 2023). In this study, the incidence in unsupervised conditions of long-lasting psychotic symptoms induced by psychedelics was 5%.
Lifetime occurrence of prolonged psychosis
Lifetime occurrences of prolonged psychosis were documented in five studies (n = 622): one follow-up study involving healthy participants [51], two trials with patients with psychosis [13, 14], and two articles summarizing several recent clinical trials, including healthy individuals and patients with depression. The observed incidence of psychedelic-induced psychosis was 2% (95%CI 0.5–7.7; I2 = 0%) across a cohort of 622 patients (Fig. 2). With exclusion of the two lower quality studies including patients with schizophrenia (n = 563), the incidence dropped to 0.6% (95%CI 0.2–1.8; I2 = 0%) (Fig. 3). We conducted a sensitivity analysis, retaining two studies with low risk of bias (Studerus et al., 2010; Perez et al. [22]) (Supplementary Fig. S4), however results were unchanged 0.7% (95%CI 0.2–2.8; I2 = 0%).
Incidence rate of psychedelic-induced psychosis in population studies
Two studies reported the rate of psychedelic-induced psychosis in population studies (n = 123,800) [53, 54]. One study reported the use of ayahuasca and the other one of peyote in ritual ceremonies. Pooling those two studies of psychedelics users in indigenous populations, we obtained an incidence of 0.002% (95%CI 0-0.006; I2 = 0%) (Fig. 4).
Rate of conversion from substance-induced psychotic disorder to schizophrenia
Three studies reported the rate of conversion from psychedelic-induced psychotic disorder (SIPD) to schizophrenia in three population follow-up studies of patients that used hallucinogens as their main drugs of use (n = 353) [50, 80, 81]. The incidence of conversion from SIPD to schizophrenia was 13.1% (95%CI 9.4-17.9) over a mean period of 10.5 years pin the presence of a low heterogeneity (I2 = 24%) (Fig. 5).
Discussion
The present study is the first comprehensive systematic review and meta-analysis investigating the incidence of psychedelic-induced psychosis in the general population, indigenous population, and individuals with mental disorders, including schizophrenia. This study provides a wide-ranging overview and hierarchy of evidence to address the question of the risk of psychedelic-induced psychosis across different populations and psychedelics.
Our findings from population-based studies indicate a very small incidence of psychedelic-induced psychosis in both the general population and indigenous population that frequently use psychedelics. Recent RCTs involving patients with depression reported that such enduring side effects occur in less than 2% of cases. However, it is important to note that these trials excluded patients at risk for psychosis and therefore do not directly inform on the risk of triggering psychotic symptoms in these excluded and potentially vulnerable subgroups of patients.
In contrast, instances of psychedelic-induced psychotic reactions predominantly occurred in individuals who obtained psychedelics from illicit sources or used new psychoactive substances [83]. Notably, several UCTs further report a low incidence of psychedelic-induced psychosis in patients with schizophrenia or with autism spectrum disorders (less than 4%). Notably, these studies were conducted during the 1970s and presented with high risks of bias, which limits their credibility. Moreover, we observed an incidence rate of 13% of patients transitioning from psychedelic-induced psychosis to schizophrenia. This pooled rate was obtained from just three good quality studies, which contrasts with the higher rate of 26% reported by Murrie and colleagues [20], who included also one PCP study in their analysis.
In summary, our incidence findings suggest that most of the evidence around the risk of psychosis associated with serotoninergic psychedelics is of low quality, yet that use of serotonergic psychedelics rarely induced psychosis in the general population -when excluding patients with psychotic features- while the risk of transition to schizophrenia is considerable in those who develop psychedelic-induced psychosis. It is important to highlight that a significant portion of the available data derives from lower-quality studies and definitive conclusions cannot be drawn from the existing evidence.
Phenomenology of serotoninergic psychedelics trip
According to Hoch et al. there seems to be a low incidence of auditory hallucinations but a significant occurrence of visual hallucinations in patients with schizophrenia experimenting with mescaline and LSD [14]. Langs & Barr noted that LSD primarily induces visual hallucinations, whereas patients with schizophrenia display more delusional thinking [84]. Wießner and colleagues suggest the connection between the psychosis model and therapeutic model could rest in mystical experiences [85].
Among reviewed works, Strassman’s 1984 article on adverse reactions to psychedelic drugs distinguishes various reactions—from acute, time-limited panic responses during administration, to brief and transient psychotic fluctuations as well as psychoses lasting days, to recurrent flashbacks and persistent undifferentiated psychotic and treatment-resistant cases [7]. Strassman proposed that the development of LSD-induced psychosis is multifactorial, involving factors such as family history, personality traits, and exposure to multiple drugs [7]. In identified reviews, Paparelli and colleagues suggested that stimulants and THC are more likely to induce paranoid beliefs, particularly with repeated use, while LSD is more closely associated with visual hallucinations [86].
More recently, Orsolini and colleagues advise against ayahuasca use in patients with bipolar disorder or a psychotic disorder due to an elevated risk of manic episodes (based on two case reports) and psychotic onset [87]. A web-based survey on experience with ‘magic-mushroom’ consumption also found that a third of patients with self-reported bipolar disorder diagnosis presented hypomanic symptoms in the week following drug ingestion [88], raising more questions on the risk of manic switch than of exacerbation of psychotic symptoms. This finding is strengthened with a recent longitudinal study by Honk and colleagues reporting from 1.3 to 6.5% and 2 to 12% of psychotic symptoms in individuals that had a lifetime use of psychedelics, and a psychedelic use in the last 2 months, respectively. We were not able to include this study in our meta-analysis considering how results were reported, nevertheless, the authors conclude that among individuals with a personal or family history of bipolar disorders, psychedelic use was associated with an increase in psychotic symptoms, and on the contrary were decreased in individuals with a personal (but not family) history of psychotic disorders. Authors propose that while psychedelic use attenuates (or does not affect) the risk for psychosis in individuals with a personal (or family) history of psychotic disorders, it increases the risk for mania with psychotic features in individuals with a personal or family history of bipolar disorder [89].
Dose-response findings
The determination of the ‘optimal safe dose’ is a critical consideration in addressing the issue of psychedelic-induced psychosis. While each patient may have their own personalized optimal dosage, a recent study identified near-maximum effective doses of psilocybin, referred to as ED95, for depression scores. The ED95 doses were 8.92, 24.68, and 36.08 mg/70 kg for patients with secondary depression, primary depression, and both subgroups, respectively [22].
Moreover, we identified several studies that reported that individuals with schizophrenia often exhibit a subpar response to typical moderate dosages of psilocybin that are well-tolerated by healthy subjects. The question of whether exceeding the conventional dosage is warranted, particularly for patients burdened with severe negative symptoms and limited positive symptoms, originates from the findings of Perez and colleagues, who found that patients with treatment-resistant depression require higher doses of psilocybin (36.08 mg/kg). Conversely, patients prone to significant positive symptoms may benefit from commencing their treatment with lower doses to mitigate the risk of long-lasting psychotic reactions. In addition, dose-dependent risk of anxiety symptoms have been reported up to 90% of individuals with anxiety disorders at doses of 125 mg MDMA [90]. Given that anxiety disorders are a frequently co-occurring comorbidity in patients with psychosis, dose-dependent risk should be considered for multiple symptom dimension beyond acute exacerbation of positive symptoms.
Identifying the “optimal safe dose” for add-on psychedelic treatment in patients with psychosis also concerns a critical consideration of the potential interacting effects with concomitant antipsychotic medication. Many second-generation antipsychotics have high affinity to 5HT2A receptors and act as antagonists, therefore maybe attenuating the therapeutic effects of psychedelic drugs. In contrast, antipsychotics with high affinity to D2 receptors, such as haloperidol -at a given dose-, might have no effect or potential even worsen some psychedelic effects (Vollenweider et al. [9]; Vollenweider & Kometer [91]). Thus, the effects of simultaneous treatment with antipsychotics and psychedelics needs further investigation.
In addition, other elements, such as the purity of the substance, or the setting might also be relevant to consider. Many authors propose that psychedelic-induced psychosis appears to be more prevalent in recreational or uncontrolled settings involving black-market substances. For instance, synthetic cathinones are marketed as cheap substitutes for other stimulants, such as amphetamines and cocaine, as is the case for products sold as Molly.
Neurobiological mechanism of potential therapeutic effects
Individualized doses of psilocybin could be a therapeutic option for patients presenting with treatment-resistant/predominant depressive symptoms or negative symptoms [18, 92].
Consequently, the enhancement of neuroplasticity as well as the psychedelic experience could both carry therapeutic effects [93]. Timmermann et al. [94] suggest that the long-term benefits of these psychedelics could be due to enhanced neuroplasticity [94]. Neuroplasticity might be mainly promoted by activation of intracellular 5-HT2A receptors in animal studies [95, 96] and/or, by direct binding of BDNF receptor Trkb that may allow for a relatively widespread rewiring of neuronal networks [97]. In a recent publication, Heresco-Levy & Lerer proposed that intricate serotonergic-glutamatergic interactions, including ionotropic glutamate receptors, tropomyosin receptor kinase B (TrkB), and the mammalian target of rapamycin (mTOR), play a pivotal role in neuroplasticity induced by serotonergic psychedelics [98]. Integrating these lines of research and their own preliminary data ([98], the authors hypothesize that the administration of a psychedelic and an NMDAR modulator simultaneously may enhance the therapeutic efficacy of each treatment and facilitate dose adjustments and enhanced safety [98].
In addition, to enhanced neuroplasticity, it has been proposed that the profound ‘mystical’ experiences induced by psychedelics could be a key factor in their immediate antidepressant effects [99, 100]. However, whether these experiences are related to increased neuroplasticity in humans remains an open question.
Therapeutic potential of serotoninergic psychedelics in patients with schizophrenia
In the reviewed trials with healthy participants and non-psychotic disorders, individuals with a personal or familial history of psychotic features were systematically excluded. This exclusion criterion poses a challenge in interpreting results, which cannot be applied to patients exhibiting a clinical or familial risk for psychosis. Consequently, the same cautious approach should be extended to populations at risk for substance-induced psychosis stemming from various substances, warranting their exclusion from clinical trials involving psychedelics. However, cohort studies specifically investigating the safety and efficacy of these substances in such populations under strictly controlled conditions would be of high interest.
Based on the few existing low-quality studies, there is no clear evidence for high incidence rates of psychedelic-induced psychosis in antipsychotic treated patients. Further high-quality studies are required to either confirm or refute the observed low incidence rates in the present study. Furthermore, the current findings may prompt a re-evaluation of the existing contraindications, which currently bar patients with schizophrenia who may be eligible for participation in research studies testing psychedelic-assisted therapy. In this regard, individuals with chronic and stable schizophrenia, particularly those experiencing predominant depressive and/or negative symptoms and continuous antipsychotic medication, may qualify for psychedelic-assisted therapy.
In fact, an ascending-dose tolerability study involving MDMA for patients with schizophrenia has already started (NCT05770375). Future trials are already starting for other specific populations, such as individuals with autism spectrum disorders (NCT05651126) or comorbid personality disorders (NCT05399498). Nevertheless, ethical considerations underscore the imperative that individuals within the schizophrenia-spectrum disorder, possessing a history of aggressive or suicidal behavior during psychotic episodes, should not undergo exposure to this therapeutic intervention.
Limitations
This work has several limitations. While we included a significant number of studies, there was considerable variation in their type and quality. Overall quality of studies was low and only few studies (n = 9) could be included in the meta-analysis, hence the presented findings should be interpreted with caution. Moreover, studies primarily focused on different types of serotonergic psychedelics. Diagnoses across the 70-year span show evolving standards, affecting methodological consistency (e.g., study methods, settings, psychological support). Most studies were classified as having a critical risk of bias according to the ROBINS scale, with an OCEBM level of 2–3. Current RCTs that present a low risk of bias propose that psychedelic-induced psychosis is exceptional in controlled settings, including patients not presenting with psychotic symptoms or risk. Although most results were coherent and obtained low to moderate heterogeneity, several factors could potentially impact our results. Notably, population characteristics before the onset of any psychiatric issues are often not reported, as is the cumulative exposure to drugs and the overall quality of the drugs considered. For instance, illicitly manufactured LSD may be contaminated with substances that have atropine-like effects, potentially influencing the occurrence of psychotic symptoms. Additionally, the definition of ‘psychedelics-induced psychosis’ lacks firm establishment, with current authors often using SIPD DSM-5 criteria, requiring a temporal association between drug exposure and persistent psychotic symptoms. Furthermore, another important issue is the effective blinding in RCTs involving psychedelics due to the inherent alterations in consciousness that these compounds induce synergistic effects between the current psychological therapy and expectancy effects of the psychedelic trips are also difficult to control [101]. One solution to this limitation could be to include active placebos or active comparator study designs in future studies [102].
Conclusion
The incidence of serotonergic psychedelics-induced psychosis was relatively low in the general population, but a considerably increased risk of transition to schizophrenia emerged after psychedelic-induced psychosis. Although the evidence base is currently comprised of only low-quality studies, the current findings warrant further studies to clarify whether the categorical exclusion of individuals living with schizophrenia is indeed necessary for studies involving the administration of psychedelic substances. Moreover, studies developing and validating prediction models of psychedelic-induced psychosis, and transition from psychedelic-induced psychosis to schizophrenia are needed. In conclusion, high-quality research is required to clarify the risk-benefit ratio of psychedelic treatments in individuals with schizophrenia and a conservative approach is recommended until further data is available.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Data files). The data and the analysis R code that generated the results and figures is available online with the metafor package.
Code availability
Code used for these analyses are available from the corresponding author upon reasonable request.
References
Solmi M, Chen C, Daure C, Buot A, Ljuslin M, Verroust V, et al. A century of research on psychedelics: A scientometric analysis on trends and knowledge maps of hallucinogens, entactogens, entheogens and dissociative drugs. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol. 2022;64:44–60.
Goodwin GM, Aaronson ST, Alvarez O, Arden PC, Baker A, Bennett JC, et al. Single-dose psilocybin for a treatment-resistant episode of major depression. N Engl J Med. 2022;387:1637–48.
van der Meer PB, Fuentes JJ, Kaptein AA, Schoones JW, de Waal MM, Goudriaan AE, et al. Therapeutic effect of psilocybin in addiction: a systematic review. Front Psychiatry. 2023;14:1134454.
Feulner L, Sermchaiwong T, Rodland N, Galarneau D. Efficacy and safety of psychedelics in treating anxiety disorders. Ochsner J. 2023;23:315–28.
Khan AJ, Bradley E, O’Donovan A, Woolley J. Psilocybin for trauma-related disorders. Curr Top Behav Neurosci. 2022;56:319–32.
Aaronson ST, van der Vaart A, Miller T, LaPratt J, Swartz K, Shoultz A, et al. Single-dose synthetic psilocybin with psychotherapy for treatment-resistant bipolar type II major depressive episodes: a nonrandomized controlled trial. JAMA Psychiatry. 2023. https://doi.org/10.1001/jamapsychiatry.2023.4685.
Strassman RJ. Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis. 1984;172:577–95.
Wolf G, Singh S, Blakolmer K, Lerer L, Lifschytz T, Heresco-Levy U, et al. Could psychedelic drugs have a role in the treatment of schizophrenia? Rationale and strategy for safe implementation. Mol Psychiatry. 2023;28:44–58.
Vollenweider F, Vollenweider-Scherpenhuyzen M, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1999;9:3897–902.
Sabé M, Zhao N, Kaiser S. A systematic review and meta-analysis of the prevalence of cocaine-induced psychosis in cocaine users. Prog Neuro-Psychopharmacology Biol Psychiatry. 2021;109:110263.
Solmi M, De Toffol M, Kim JY, Choi MJ, Stubbs B, Thompson T, et al. Balancing risks and benefits of cannabis use: umbrella review of meta-analyses of randomised controlled trials and observational studies. BMJ. 2023;382:e072348.
Bowers MB Jr, Mazure CM, Nelson JC, Jatlow PI. Psychotogenic drug use and neuroleptic response. Schizophr Bull. 1990;16:81–5.
Fink M, Simeon J, Haque W, Itil T. Prolonged adverse reactions to LSD in psychotic subjects. Arch Gen Psychiatry. 1966;15:450–4.
Hoch PH, Cattell JP, Pennes HH. Effects of Mescaline and Lysergic Acid (d-LSD-25). Am J Psychiatry. 1952;108:579–84.
Kramer M. Cross-national study of diagnosis of the mental disorders: origin of the problem. Am J Psychiatry. 1969;125:1–11.
Helfer B, Samara MT, Huhn M, Klupp E, Leucht C, Zhu Y, et al. Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry. 2016;173:876–86.
Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018;23:3170–82.
Arnovitz MD, Spitzberg AJ, Davani AJ, Vadhan NP, Holland J, Kane JM, et al. MDMA for the treatment of negative symptoms in schizophrenia. J Clin Med. 2022;11:3255.
Cohen S. LSD: the varieties of psychotic experience. J Psychoactive Drugs. 1985;17:291–6.
Murrie B, Lappin J, Large M, Sara G. Transition of substance-induced, brief, and atypical psychoses to schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2020;46:505–16.
Seeman P, Guan H-C. Phencyclidine and glutamate agonist LY379268 stimulate dopamine D2High receptors: D2 basis for schizophrenia. Synapse. 2008;62:819–28.
Perez N, Langlest F, Mallet L, De Pieri M, Sentissi O, Thorens G, et al. Psilocybin-assisted therapy for depression: a systematic review and dose-response meta-analysis of human studies. Eur Neuropsychopharmacol. 2023;76:61–76.
Mustafa NS, Bakar NHA, Mohamad N, Adnan LHM, Fauzi NFAM, Thoarlim A, et al. MDMA and the brain: a short review on the role of neurotransmitters in neurotoxicity. Basic Clin Neurosci. 2020;11:381–8.
Liechti ME, Vollenweider FX. Which neuroreceptors mediate the subjective effects of MDMA in humans? A summary of mechanistic studies. Hum Psychopharmacol. 2001;16:589–98.
Mitchell JM, Ot’alora G M, van der Kolk B, Shannon S, Bogenschutz M, Gelfand Y, et al. MDMA-assisted therapy for moderate to severe PTSD: a randomized, placebo-controlled phase 3 trial. Nat Med. 2023;29:2473–80.
Belbasis L, Bellou V, Ioannidis JPA. Conducting umbrella reviews. BMJ Med. 2022;1:e000071.
Fiorentini A, Cantù F, Crisanti C, Cereda G, Oldani L, Brambilla P. Substance-induced psychoses: an updated literature review. Front Psychiatry. 2021;12:694863.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Baethge C, Goldbeck-Wood S, Mertens S. SANRA—a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;4:5.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
OCEBM. The Oxford Levels of Evidence 2. OCEBM Levels Evid Work Gr. 2011. 2011.
DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–14.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. 2010;36:1–48.
Barker TH, Migliavaca CB, Stein C, Colpani V, Falavigna M, Aromataris E, et al. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol. 2021;21:189.
Cohen S. Lysergic acid diethylamide: side effects and complications. J Nerv Ment Dis. 1960;130:30–40.
Bercel NA, Travis LEEE, Olinger LB, Dreikurs E, Polos MG. Model psychoses induced by LSD-25 in normals: I. Psychophysiological investigations, with special reference to the mechanism of the paranoid reaction. AMA Arch Neurol Psychiatry. 1956;75:588–611.
Gillin JC, Kaplan J, Stillman R, Wyatt RJ. The psychedelic model of schizophrenia: the case of N,N -dimethyltryptamine. Am J Psychiatry. 1976;133:203–8.
Rümmele W, Gnirss F. Untersuchungen mit Psilocybin, einer psychotropen Subtanz aus Psilocybe Mexicana. Schweiz Arch Neurol Psychiatr. 1961;87:365–85.
Anderson BT, Danforth A, Daroff PR, Stauffer C, Ekman E, Agin-Liebes G, et al. Psilocybin-assisted group therapy for demoralized older long-term AIDS survivor men: An open-label safety and feasibility pilot study. EClinicalMedicine. 2020;27:100538.
Stoll WA. Lysergsäure-Diäthylamid, ein Phantastikum aus der Mutterkorngruppe. Schweiz Arch Neurol Psychiatr. 1947;60:279–323.
Condrau G. Klinische erfahrungen an geisteskranken mit lysergsäure-diäthylamid. Acta Psychiatr Scand. 1949;24:9–32.
Belsanti R. Modificazioni neuro-psico-biochimiche indotte dalla dietilamide dell’acido lisergico in schizofrenici e frenastenici. Acta Neurol (Napoli). 1952;7:25.
Anastasopoulos G, Photiades H. Effects of LSD-25 on relatives of schizophrenic patients. J Ment Sci. 1962;108:95–8.
Forrer GR, Goldner RD. Experimental physiological studies with lysergic acid diethylamide (LSD-25). AMA Arch Neurol Psychiatry. 1951;65:581–8.
Liddell DW, Weil-Malherbe H. The effects of methedrine and of lysergic acid diethylamide on mental processes and on the blood andrenaline level. J Neurol Neurosurg Psychiatry. 1953;16:7–13.
Bender L, Faretra G, Cobrinik L. LSD and UML treatment of hospitalized disturbed children. Recent Adv Biol Psychiatry. 1963;5:84–92.
Bowers MBJ. Psychoses precipitated by psychotomimetic drugs. A follow-up study. Arch Gen Psychiatry. 1977;34:832–5.
Niemi-Pynttäri JA, Sund R, Putkonen H, Vorma H, Wahlbeck K, Pirkola SP. Substance-induced psychoses converting into schizophrenia. J Clin Psychiatry. 2013;74:e94–e99.
McGlothlin WH, Arnold DO. LSD revisited: a ten-year follow-up of medical LSD use. Arch Gen Psychiatry. 1971;24:35–49.
Vardy MM, Kay SR. LSD psychosis or LSD-induced schizophrenia?: a multimethod inquiry. Arch Gen Psychiatry. 1983;40:877–83.
Lima FAS, Tófoli LF, Labate B, Jungaberle H. An epidemiological surveillance system by the UDV: mental health recommendations concerning the religous use of hoasca 2011.
Bergman RL. Navajo peyote use: Its apparent safety. Am J Psychiatry. 1971;128:695–9.
Dos Santos R, Bouso JC, Hallak J. Ayahuasca, dimethyltryptamine, and psychosis: A systematic review of human studies. Ther Adv Psychopharmacol. 2017;7:1–17.
Landabaso MA, Iraurgi I, Jiménez-Lerma JM, Calle R, Sanz J, Gutiérrez-Fraile M. Ecstasy-induced psychotic disorder: six-month follow-up study. Eur Addict Res. 2002;8:133–40.
Krebs TS, Johansen P-Ø. Psychedelics and mental health: a population study. PLoS One. 2013;8:e63972.
Baylé FJ, Misdrahi D, Llorca PM, Lançon C, Olivier V, Quintin P, et al. [Acute schizophrenia concept and definition: investigation of a French psychiatrist population]. Encephale. 2005;31:10–7.
Johansen P-Ø, Krebs T. Psychedelics not linked to mental health problems or suicidal behavior: A population study. J Psychopharmacol. 2015;29.
Ungerleider JT, Fisher DD, Fuller M, Caldwell A. The ‘bad trip’–the etiology of the adverse LSD reaction. Am J Psychiatry. 1968;124:1483–90.
Hays P, Tilley JR. The differences between LSD psychosis and schizophrenia. Can Psychiatr Assoc J. 1973;18:331–3.
Rugani F, Bacciardi S, Rovai L, Pacini M, Maremmani AGI, Deltito J, et al. Symptomatological features of patients with and without Ecstasy use during their first psychotic episode. Int J Environ Res Public Health. 2012;9:2283–92.
Sloane B, Doust LW. Psychophysiological investigations in experimental psychoses: results of the exhibition of d-lysergic acid diethylamide to psychiatric patients. J Ment Sci. 1954;100:129–44.
Schmid Y, Enzler F, Gasser P, Grouzmann E, Preller K, Vollenweider F, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78:544–53.
Preller K, Burt J, Ji JL, Schleifer C, Adkinson B, Staempfli P, et al. Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. Elife. 2018;7. https://doi.org/10.7554/eLife.35082.
Wiessner I, Falchi M, Palhano-Fontes F, Feilding A, Ribeiro S, Tófoli L. LSD, madness and healing: Mystical experiences as possible link between psychosis model and therapy model. Psychol Med. 2021;53:1–15.
Isbell H. Comparison of the reactions induced by psilocybin and LSD-25 in man. Psychopharmacologia. 1959;1:29–38.
Wolbach AB, Miner EJ, Isbell H. Comparison of psilocin with psilocybin, mescaline and LSD-25. Psychopharmacologia. 1962;3:219–23.
Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Doblin R. The safety and efficacy of {+/-}3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. J Psychopharmacol. 2011;25:439–52.
Carhart-Harris R, Giribaldi B, Watts R, Baker-Jones M, Murphy-Beiner A, Murphy R, et al. Trial of psilocybin versus escitalopram for depression. N Engl J Med. 2021;384:1402–11.
Davis AK, Barrett FS, May DG, Cosimano MP, Sepeda ND, Johnson MW, et al. Effects of psilocybin-assisted therapy on major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78:481–9.
Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology (Berl). 2004;172:145–56.
Shirvaikar RV, Kelkar YW. Therapeutic trial of lysergic Acid diethylamide (LSD) and thioridazine in chronic schizophrenia. Neurol India. 1966;14:97–101.
Carstairs SD, Cantrell FL. Peyote and mescaline exposures: a 12-year review of a statewide poison center database. Clin Toxicol (Phila). 2010;48:350–3.
Studerus E, Kometer M, Hasler F, Vollenweider FX. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. J Psychopharmacol. 2011;25:1434–52.
Baker E LSD psychotherapy LSD psycho-exploration: three reports. In: Bobbs-Merrill, editor. use LSD Psychother. Alcohol., Indianapolis: Abramson HA; 1967. p. 191–207.
Leuner H Present state of psycholytic therapy and its possibilities. In: AH, Abramson, editors. 2nd Int. Conf. Use LSD Psychother. Alcohol., Amityville: The Bobbs-Merrill Co. Inc. 1965.
Malleson N. Acute Adverse Reactions to Lsd in Clinical and Experimental use in the United Kingdom. Br J Psychiatry. 1971;118:229–30.
Novak SJ. LSD before Leary. Sidney Cohen’s critique of 1950s psychedelic drug research. Isis; an Int Rev Devoted to Hist Sci Its Cult Influ. 1997;88:87–110.
Starzer MSK, Nordentoft M, Hjorthøj C. Rates and predictors of conversion to schizophrenia or bipolar disorder following substance-induced psychosis. Am J Psychiatry. 2018;175:343–50.
Rognli EB, Heiberg IH, Jacobsen BK, Høye A, Bramness JG. Transition from substance-induced psychosis to schizophrenia spectrum disorder or bipolar disorder. Am J Psychiatry. 2023;180:437–44.
Baker EF. The use of Lysergic acid diethylamide (LSD) in psychotherapy. Can Med Assoc J. 1964;91:1200–2.
Graddy R, Buresh ME, Rastegar DA. New and Emerging Illicit Psychoactive Substances. Med Clin North Am. 2018;102:697–714.
Langs RJ, Barr HL Lysergic acid diethylamide (lsd-25) and schizophrenic reactions. J Nerv Ment Dis. 1968;147.
Wießner I, Falchi M, Palhano-Fontes F, Feilding A, Ribeiro S, Tófoli LF. LSD, madness and healing: Mystical experiences as possible link between psychosis model and therapy model. Psychol Med. 2023;53:1151–65.
Paparelli A, Di Forti M, Morrison PD, Murray RM. Drug-induced psychosis: how to avoid star gazing in schizophrenia research by looking at more obvious sources of light. Front Behav Neurosci. 2011;5:1.
Orsolini L, Chiappini S, Papanti D, Latini R, Volpe U, Fornaro M, et al. How does ayahuasca work from a psychiatric perspective? Pros and cons of the entheogenic therapy. Hum Psychopharmacol. 2020;35:e2728.
Morton E, Sakai K, Ashtari A, Pleet M, Michalak EE, Woolley J. Risks and benefits of psilocybin use in people with bipolar disorder: An international web-based survey on experiences of ‘magic mushroom’ consumption. J Psychopharmacol. 2023;37:49–60.
Honk L, Stenfors CUD, Goldberg SB, Hendricks PS, Osika W, Dourron HM, et al. Longitudinal associations between psychedelic use and psychotic symptoms in the United States and United Kingdom. J Affect Disord. 2024. https://doi.org/10.1016/j.jad.2024.01.197.
Mithoefer MC, Feduccia AA, Jerome L, Mithoefer A, Wagner M, Walsh Z, et al. MDMA-assisted psychotherapy for treatment of PTSD: study design and rationale for phase 3 trials based on pooled analysis of six phase 2 randomized controlled trials. Psychopharmacology (Berl). 2019;236:2735–45.
Vollenweider FX, Kometer M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci. 2010;11:642–51.
Hirschfeld T, Schmidt TT. Dose-response relationships of psilocybin-induced subjective experiences in humans. J Psychopharmacol. 2021;35:384–97.
Gattuso JJ, Perkins D, Ruffell S, Lawrence AJ, Hoyer D, Jacobson LH, et al. Default Mode Network Modulation by Psychedelics: A Systematic Review. Int J Neuropsychopharmacol. 2023;26:155–88.
Timmermann C, Bauer PR, Gosseries O, Vanhaudenhuyse A, Vollenweider F, Laureys S, et al. A neurophenomenological approach to non-ordinary states of consciousness: hypnosis, meditation, and psychedelics. Trends Cogn Sci. 2023;27:139–59.
Vargas MV, Dunlap LE, Dong C, Carter SJ, Tombari RJ, Jami SA, et al. Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science. 2023;379:700–6.
de Vos CMH, Mason NL, Kuypers KPC. Psychedelics and neuroplasticity: a systematic review unraveling the biological underpinnings of psychedelics. Front Psychiatry. 2021;12:72606.
Moliner R, Girych M, Brunello CA, Kovaleva V, Biojone C, Enkavi G, et al. Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat Neurosci. 2023;26:1032–41.
Heresco-Levy U, Lerer B. Synergistic psychedelic - NMDAR modulator treatment for neuropsychiatric disorders. Mol Psychiatry. 2024;29:146–52.
Ko K, Knight G, Rucker JJ, Cleare AJ. Psychedelics, mystical experience, and therapeutic efficacy: a systematic review. Front Psychiatry. 2022;13:917199.
Winkelman MJ. The mechanisms of psychedelic visionary experiences: hypotheses from evolutionary psychology. Front Neurosci. 2017;11:539.
Dworkin RH, McDermott MP, Nayak SM, Strain EC. Psychedelics and psychotherapy: is the whole greater than the sum of its parts? Clin Pharmacol Ther. 2023;114:1166–9.
Wen A, Singhal N, Jones BDM, Zeifman RJ, Mehta S, Shenasa MA, et al. A systematic review of study design and placebo controls in psychedelic research. Psychedelic Med. 2023;2:15–24.
Busch A, Johnson W. L.S.D. 25 as an aid in psychotherapy; preliminary report of a new drug. Dis Nerv Syst. 1950;11:241–3.
De Giacomo U. La catatonie toxique expérimentale. Acta Neurol (Napoli). 1951;7:5–10.
Mayer-Gross W. Experimental psychoses and other mental abnormalities produced by drugs. Br Med J. 1951;2:317–21.
Katzenelbogen S, Fang AD. Narcosynthesis effects of sodium amytal, methedrine and L.S.D-25. Dis Nerv Syst. 1953;14:85–8.
Cholden LS, Kurland A, Savage C. Clinical reactions and tolerance to LSD in chronic schizophrenia. J Nerv Ment Dis. 1955;122.
Abramson HA. The use of LSD in psychotherapy: transactions of a conference on D-lysergic acid diethylamide (LSD-25). April 22, 23 and 24, 1959, Princeton, NJ: Josiah Macy, Jr. Foundation; 1960.
Freedman AM, Ebin EVAV, Wilson EA. Autistic schizophrenic children: an experiment in the use of D-Lysergic acid diethylamide (LSD-25). Arch Gen Psychiatry. 1962;6:203–13.
Fischer G, Castile D. An investigation to determine the therapeutic effectiveness of LSD-25 and psilocybin on hospitalized severely emotionally disturbed children. Purdue University Archives and Special Collections; 1963.
Bender L, Goldschmidt L, Sankar DVS, Freedman AM. Treatment of autistic schizophrenic children with LSD-25 and UML-491 BT—recent advances in biological psychiatry: Volume IV: The Proceedings of the Sixteenth Annual Convention and Scientific Program of the Society of Biological Psychiatry, Atlantic City. In: Wortis J, editor. Springer US; 1962. p. 170–9. https://doi.org/10.1007/978-1-4684-8306-2_17.
Bender L. D-lysergic acid in the treatment of the biological features of childhood schizophrenia. Dis Nerv Syst. 1966;7:43–6.
Blumenfield M, Glickman L. Ten months experience with LSD users admitted to county psychiatric receiving hospital. N Y State J Med. 1967;67:1849–53.
Dewhurst K, Hatrick JA. Differential diagnosis and treatment of lysergic acid diethylamide induced psychosis. Practitioner. 1972;209:327–32.
Roy A. LSD and onset of schizophrenia. Can J Psychiatry. 1981;26:64–5.
Smart RG, Storm T, Baker EF, Solursh L. A controlled study of lysergide in the treatment of alcoholism. 1. The effects on drinking behavior. Q J Stud Alcohol. 1966;27:469–82.
Lev-Ran S, Feingold D, Frenkel A, Lerner AG. Clinical characteristics of individuals with schizophrenia and hallucinogen persisting perception disorder: a preliminary investigation. J Dual Diagn. 2014;10:79–83.
Lev-Ran S, Feingold D, Rudinski D, Katz S, Arturo LG. Schizophrenia and hallucinogen persisting perception disorder: a clinical investigation. Am J Addict. 2015;24:197–9.
Tomsovic M, Edwards RV. Lysergide treatment of schizophrenic and nonschizophrenic alcoholics: a controlled evaluation. Q J Stud Alcohol. 1970;31:932–49.
Gasser P, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. 2014;202:513–20.
Schmid Y, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78:544–53.
Jiménez-Garrido DF, et al. Effects of ayahuasca on mental health and quality of life in naïve users: a longitudinal and cross-sectional study combination. Sci Rep. 2020;10:4075.
Bickel P, Dittrich A, Schopf J. Effekte von N,N-dimethyltryptamin (DMT) auf psychotizismus-tests. Pharmacopsychiatry. 1977;10:10–14.
Bouso JC, et al. Long-term use of psychedelic drugs is associated with differences in brain structure and personality in humans. Eur Neuropsychopharmacol. 2015;25:483–92.
Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51:98–108.
Dos Santos RG, et al. Ayahuasca improves self-perception of speech performance in subjects with social anxiety disorder: a pilot, proof-of-concept, randomized, placebo-controlled trial. J Clin Psychopharmacol. 2021;41:540–50.
vollenweider, F. X. et al. Positron emission tomography and fluorodeoxyglucose sturies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacol. 1997;16;357–72.
Garcia-Romeu A, Griffiths RR, Johnson MW. Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr Drug Abus Rev. 2014;7:157–64.
Grob CS, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry. 2011;68:71–8.
Griffiths RR, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30:1181–97.
Ross S, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30:1165–80.
Bogenschutz MP, et al. Percentage of heavy drinking days following psilocybin-assisted psychotherapy vs placebo in the treatment of adult patients with alcohol use disorder: a randomized clinical trial. JAMA Psychiatry. 2022;79:953–62.
von Rotz R, et al. Single-dose psilocybin-assisted therapy in major depressive disorder: A placebo-controlled, double-blind, randomised clinical trial. EClinicalMedicine. 2023;56:101809.
Bouso JC, Doblin R, Farré M, Alcázar MA, Gómez-Jarabo G. MDMA-assisted psychotherapy using low doses in a small sample of women with chronic posttraumatic stress disorder. J Psychoact Drugs. 2008;40:225–36.
Oehen P, Traber R, Widmer V, Schnyder U. A randomized, controlled pilot study of MDMA (± 3,4-Methylenedioxymethamphetamine)-assisted psychotherapy for treatment of resistant, chronic Post-Traumatic Stress Disorder (PTSD). J Psychopharmacol. 2013;27:40–52.
Danforth AL, et al. Reduction in social anxiety after MDMA-assisted psychotherapy with autistic adults: a randomized, double-blind, placebo-controlled pilot study. Psychopharmacol. (Berl). 2018;235:3137–48.
Ot’alora GM, et al. 3,4-Methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: a randomized phase 2 controlled trial. J Psychopharmacol. 2018;32:1295–307.
Jerome L, et al. Long-term follow-up outcomes of MDMA-assisted psychotherapy for treatment of PTSD: a longitudinal pooled analysis of six phase 2 trials. Psychopharmacol. (Berl). 2020;237:2485–97.
Mitchell JM, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27:1025–33.
Ponte L, et al. Sleep quality improvements after MDMA-assisted psychotherapy for the treatment of posttraumatic stress disorder. J Trauma Stress. 2021;34:851–63.
Brewerton TD, Gavidia I, Suro G, Perlman MM. Eating disorder patients with and without PTSD treated in residential care: discharge and 6-month follow-up results. J Eat Disord. 2023;11:48.
Nicholas CR, et al. The effects of MDMA-assisted therapy on alcohol and substance use in a phase 3 trial for treatment of severe PTSD. Drug Alcohol Depend. 2022;233:109356.
Pacey I. Randomized, double-blind, controlled of MDMA-assisted psychotherapy in 12 subjects with PTSD. NCT01958593. Lykos Therapeutics. 2017. https://clinicaltrials.gov/study/NCT01958593.
Wolfson PE, et al. MDMA-assisted psychotherapy for treatment of anxiety and other psychological distress related to life-threatening illnesses: a randomized pilot study. Sci Rep. 2020;10:20442.
Hollister LEOE, Shelton J, Krieger G. A controlled comparison of lysergic acid diethylamide (LSD) and dextroamphetamine in alcoholics. Am J Psychiatry. 1969;125:1352–57.
Breakey WR, Goodell H, Lorenz PC, McHugh PR. Hallucinogenic drugs as precipitants of schizophrenia. Psychol Med. 1974;4:255–61.
Boutros NN, Bowers MBJ. Chronic substance-induced psychotic disorders: state of the literature. J Neuropsychiatry Clin Neurosci. 1996;8:262–9.
Shoval G, et al. Substance use, suicidality, and adolescent-onset schizophrenia: an Israeli 10-year retrospective study. J Child Adolesc Psychopharmacol. 2006;16:767–75.
Hendricks PS, Clark CB, Johnson MW, Fontaine KR, Cropsey KL. Hallucinogen use predicts reduced recidivism among substance-involved offenders under community corrections supervision. J Psychopharmacol. 2014;28:62–6.
Hendricks PS, Thorne CB, Clark CB, Coombs DW, Johnson MW. Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. J Psychopharmacol. 2015;29:280–8.
Vallersnes OM, et al. Psychosis associated with acute recreational drug toxicity: a European case series. BMC Psychiatry. 2016;16:293.
Evans J, et al. Extended difficulties following the use of psychedelic drugs: a mixed methods study. PLoS ONE. 2023;18:e0293349.
Simonsson O, et al. Longitudinal associations between psychedelic use and unusual visual experiences in the United States and the United Kingdom. J. Psychopharmacol. 2023. https://doi.org/10.1177/02698811231218931.
Smart RG, Bateman K. Unfavourable reactions to LSD: a review and analysis of the available case reports. Can Med Assoc J. 1967;97:1214–21.
Leuner H. Present state of psycholytic therapy and its possibilities. In: Abramson AH, editor. 2nd International Conference on the Use of LSD in Psychotherapy and Alcoholism. Amityville, N.Y.: The Bobbs-Merrill Co. Inc.; 1965.
Panhuysen LH. Undesirable side effects of LSD administration. Ned Tijdschr Geneeskd. 1970;114:723–7.
De Gregorio D, Comai S, Posa L, Gobbi G. d-lysergic acid diethylamide (LSD) as a model of psychosis: mechanism of action and pharmacology. Int J Mol Sci. 2016;17.
Jacob MS, Presti DE. Endogenous psychoactive tryptamines reconsidered: an anxiolytic role for dimethyltryptamine. Med Hypotheses. 2005;64:930–7.
Grammenos D, Barker SA. On the transmethylation hypothesis: stress, N,N-dimethyltryptamine, and positive symptoms of psychosis. J Neural Transm. 2015;122:733–9.
Gable RS. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction. 2007;102:24–34.
Skryabin VY, Vinnikova M, Nenastieva A, Alekseyuk V. Hallucinogen persisting perception disorder: A literature review and three case reports. J Addict Dis. 2018;37:268–78.
McGuire P. Long term psychiatric and cognitive effects of MDMA use. Toxicol Lett. 2000;112–113:153–6.
Soar K, Turner JJD, Parrott AC. Psychiatric disorders in Ecstasy (MDMA) users: a literature review focusing on personal predisposition and drug history. Hum Psychopharmacol. 2001;16:641–5.
Smith KW, Sicignano DJ, Hernandez AV, White CM. MDMA-assisted psychotherapy for treatment of posttraumatic stress disorder: a systematic review with meta-analysis. J Clin Pharmacol. 2022;62:463–71.
Mogar RE, Aldrich RW. The use of psychedelic agents with autistic schizophrenic children. Behav Neuropsychiatry. 1969;1:44–50.
Glass GS. Psychedelic drugs, stress, and the ego. J Nerv Ment Dis. 1973;156:232–41.
McCabe OL. Psychedelic drug crises: toxicity and therapeutics. J Psychedelic Drugs. 1977;9:107–21.
Hermele M, Ran Y, Lee PA, Wen X-G. Properties of an algebraic spin liquid on the kagome lattice. Phys Rev B. 2008;77:224413.
Trope A, et al. Psychedelic-assisted group therapy: a systematic review. J Psychoact Drugs. 2019;51:174–88.
Lerner AG, et al. Flashback and Hallucinogen Persisting Perception Disorder: clinical aspects and pharmacological treatment approach. Isr J Psychiatry Relat Sci. 2002;39:92–9.
Litjens RPW, Brunt TM, Alderliefste G-J, Westerink RHS. Hallucinogen persisting perception disorder and the serotonergic system: a comprehensive review including new MDMA-related clinical cases. Eur Neuropsychopharmacol. 2014;24:1309–23.
Halpern JH, Pope HGJ. Hallucinogen persisting perception disorder: what do we know after 50 years. Drug Alcohol Depend. 2003;69:109–19.
Orsolini L, et al. The ‘Endless Trip’ among the NPS users: psychopathology and psychopharmacology in the hallucinogen-persisting perception disorder. a systematic review. Front Psychiatry. 2017;8:240.
Martinotti G, et al. Hallucinogen persisting perception disorder: etiology, clinical features, and therapeutic perspectives. Brain Sci. 2018;8.
Doyle MA, et al. Hallucinogen persisting perceptual disorder: a scoping review covering frequency, risk factors, prevention, and treatment. Expert Opin Drug Saf. 2022;21:733–43.
Funding
Open access funding provided by University of Geneva.
Author information
Authors and Affiliations
Contributions
MiS and MK designed the study and wrote the protocol. MiS, AG and MK and performed the literature search and extracted the data. MiS undertook the statistical analysis. MiS and MK wrote the manuscript. AS, AG, MP, LM, LC, HR, LP, FS, GT, DZ, KP, KB, SL, CUC, MaS and SK contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
CUC has been a consultant and/or advisor to or has received honoraria from: AbbVie, Acadia, Adock Ingram, Alkermes, Allergan, Angelini, Aristo, Biogen, Boehringer-Ingelheim, Bristol-Meyers Squibb, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Delpor, Denovo, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Jamjoom Pharma, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Neurelis, Newron, Noven, Novo Nordisk, Otsuka, Pharmabrain, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Sage, Seqirus, SK Life Science, Sumitomo Pharma America, Sunovion, Sun Pharma, Supernus, Tabuk, Takeda, Teva, Tolmar, Vertex, and Viatris. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Compass Pathways, Denovo, Lundbeck, Relmada, Reviva, Rovi, Supernus, and Teva. He has received grant support from Janssen and Takeda. He received royalties from UpToDate and is also a stock option holder of Cardio Diagnostics, Kuleon Biosciences, LB Pharma, Mindpax, and Quantic. KHP is currently an employee of Boehringer Ingelheim GmBH & CO KG, Chief Scientist and on the Board of Directors of the Heffter Research Institute, and scientific advisor for the MIND Foundation. MaS has received honoraria/has been a consultant for AbbVie, Angelini, Lundbeck and Otsuka. SK has received advisory board honoraria from Boehringer Ingelheim and royalties for cognitive testing and training software from Schufried. All other authors report no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sabé, M., Sulstarova, A., Glangetas, A. et al. Reconsidering evidence for psychedelic-induced psychosis: an overview of reviews, a systematic review, and meta-analysis of human studies. Mol Psychiatry 30, 1223–1255 (2025). https://doi.org/10.1038/s41380-024-02800-5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41380-024-02800-5