Abstract
Dysfunctional glial cells play a pre-eminent role in schizophrenia pathophysiology. Post-mortem studies have provided evidence for significantly decreased glial cell numbers in different brain regions of individuals with schizophrenia. Reduced glial cell numbers are most pronounced in oligodendroglia, but reduced astrocyte cell densities have also been reported. This review highlights that oligo- and astroglial deficits are a key histopathological feature in schizophrenia, distinct from typical changes seen in neurodegenerative disorders. Significant deficits of oligodendrocytes in schizophrenia may arise in two ways: (i) demise of mature functionally compromised oligodendrocytes; and (ii) lack of mature oligodendrocytes due to failed maturation of progenitor cells. We also analyse in detail the controversy regarding deficits of astrocytes. Regardless of their origin, glial cell deficits have several pathophysiological consequences. Among these, myelination deficits due to a reduced number of oligodendrocytes may be the most important factor, resulting in the disconnectivity between neurons and different brain regions observed in schizophrenia. When glial cells die, it appears to be through degeneration, a process which is basically reversible. Thus, therapeutic interventions that (i) help rescue glial cells (ii) or improve their maturation might be a viable option. Since antipsychotic treatment alone does not seem to prevent glial cell loss or maturation deficits, there is intense search for new therapeutic options. Current proposals range from the application of antidepressants and other chemical agents as well as physical exercise to engrafting healthy glial cells into brains of schizophrenia patients.
Similar content being viewed by others
Introduction
Schizophrenia (SCZ) is a serious brain disorder that affects approximately 24 million people, or 1 in 300 people, worldwide [1]. The disease is mostly regarded as a neurodevelopmental disorder involving changes in brain circuitry that progress throughout life [2,3,4]. The aetiology of SCZ is still not completely understood, but appears to emerge from a complex interplay of polygenic risk and environmental factors [5]. After decades of research into the neurobiological basis of SCZ, it is becoming increasingly apparent that aberrant glial function is a major contributor to the pathophysiology of this disease [6,7,8,9,10,11].
Normal brain development involves neuronal proliferation, migration, synapse formation, arborisation (circuit formation) and myelination [4]. The first two processes mostly occur prenatally, while the latter two are crucial for brain maturation into early adulthood [4, 12]. The prefrontal cortex is the last brain region to mature, which involves the pruning of redundant synapses, synaptic plasticity and increased myelination throughout adolescence and early adulthood, during the period of prodrome and onset of psychosis [4, 12]. Glial cells play a crucial role in this process. Excessive synaptic pruning by microglial cells (MCs), reduced myelination and energy supply of axons by oligodendrocytes (OLs), and impaired synaptic plasticity, synaptic glutamate recycling and neuronal energy supply due to astrocyte (AC) dysfunction may lead to an excitatory-inhibitory imbalance and increased vulnerability (e.g. to psychosocial trauma/stress, illicit drugs, infections, autoimmunity) of prefrontal brain circuits at this stage of life (Table 1) [13,14,15,16,17].
Glial cell loss is a key feature of SCZ histopathology. Post-mortem findings replicated by several laboratories have provided evidence for significantly reduced glial cell densities in different brain regions of individuals with SCZ [6, 7, 18,19,20,21,22,23]. Notably, these changes have not only been observed in prefrontal brain, but prefrontal circuits are particularly sensitive during the typical age of onset of SCZ. Although the phenomenon of decreased glial cell densities is most pronounced in OLs, it is not restricted to this glial cell type, since reduced AC densities have also been reported [18, 24,25,26,27,28,29]. While MCs have also attracted increasing attention recently [30, 31], our review focuses exclusively on OLs and ACs for the sake of clarity and consistency.
In search of further explanations for OL deficits in SCZ, the development of these cells has been analysed [32,33,34,35]. The identification of developmental irregularities led to the hypothesis that a major reason for OL deficits might be a disruption in their maturation [32, 35,36,37,38,39,40]. If so, we should have to reconsider certain aspects of glial cell loss in SCZ concerning the sources, pathophysiological consequences and possible ways to prevent cell loss.
The present review aims at increasing our understanding of how lower cell numbers of OLs occur as part of the pathophysiology of SCZ. This could indicate either that OLs are being lost or that they are less because their formation or maturation is reduced by neurodevelopmental disruption. These considerations will be discussed in the broader context of AC and OL abnormalities. Finally, we will highlight potential novel therapeutic approaches targeting glial cell deficits in SCZ, as a means of slowing the progression of this disease and alleviating symptoms.
Search strategy
This review is based on a literature search including doctoral theses and patents in PubMed and Google Scholar databases from 1980 to 18 May 2024. The search terms were “schizophrenia” in combination with one or more of the following terms: “glia”, “gliosis”, “astrocytes”, “oligodendrocytes”, “oligodendrocyte progenitor”, “microglia”, “network disconnectivity”, “histopathology”, “glial cell loss”, “glial cell deficits”, “dysfunctional glia”, “myelin”, “protein expression”, “RNA expression”, “morphology”, “glial cell counting”, “GFAP”, “glial cell differentiation”, “glial cell maturation”, “atrophy”, “degeneration”, “necroptosis”, “apoptosis”, “phagocytosis”, “glial cell repair”, “medication”, “antipsychotic”, “neuroleptic”, “pathophysiological significance”, “therapy”, “aerobic exercise”, “glial cell transplantation”, “animal model”, and “cell culture”. No language restrictions were applied.
Oligodendrocytes (Ols)
Oligodendrogliopathy and myelin loss are core features of SCZ pathology
Although there has been some discrepancies in reporting, it is now assumed that the ratio between glia (ACs, OLs, MCs) and neurons in normal human brains is around 1:1 [41], and most reports state that OLs are the most abundant glial cell [42]. Based on localisation, Pío del Río Hortega subdivided OLs into four subtypes: (i) cells arranged in rows between nerve fibres, (ii) vascular satellite cells, (iii) Schwannoid OLs, and (iv) cells as satellites to neurons, juxtaposing neuronal perikarya [43, 44].
For decades, the importance of OLs for normal brain functioning has been underestimated. The main task of these cells is the proper myelination of many, but not all, neurites in the developing and adult brain, which is critical for rapid axonal conductance, correct information flow between neurons and metabolic supply [45]. Various lines of evidence converge to implicate OL and myelin deficits in SCZ. It has been shown that impairments in myelination of fibre tracts can lead to reduced white matter integrity in SCZ [46, 47]. This may lead to disturbed network connectivity, regarded as a hallmark of the disease [48, 49].
Histological and molecular profiling studies have revealed structural disarrangement and defects of the chemical makeup of myelin in SCZ. Myelin water imaging studies support evidence of abnormalities in white matter of people with SCZ [50]. Using light microscopy, less intense staining of deep white matter myelin has been observed in SCZ compared with controls [51]. At the electron microscopic level, a distorted organisation of myelin sheath lamellae has been found, which is characterised by reduced compactness, formation of concentric lamellar bodies, and inclusions between lamellae [52,53,54]. The demyelinating quaking mouse model of SCZ involves mice with a mutation in the QKI gene, leading to myelin deficiencies and SCZ-like symptoms. Remarkably, the myelin pathology found is nearly identical to what has been observed in post-mortem brains of individuals with SCZ [55]. This model links myelin abnormalities to SCZ, highlighting the importance of genes involved in myelin production and the impact of disrupted neural connectivity. There is also good evidence for multiple changes in the chemical composition of myelin [56, 57]. Proteomic and gene expression analyses have identified decreased concentrations of myelin-associated glycoprotein (MAG), quaking 1, 2´, 3´-cyclic nucleotide 3´-phosphodiesterase (CNP) messenger Ribonucleic Acid (mRNA) transcripts, transferrin, phospholipids and other components in white matter in SCZ [8, 56, 57]. Moreover, a subset of myelin-related genes has been implicated in SCZ by genetic linkage analysis [58, 59].
These myelin abnormalities have raised suspicions that the myelin-producing OLs themselves, including their development and function, are at least partly disrupted in SCZ [32]. Robust gene-expression evidence has also identified SCZ-associated alterations in OL profiles. Using a large SCZ genome-wide association study (GWAS), Goudriaan et al. found 29 OL genes strongly associated with SCZ, and the majority of these coded for myelin-shaping proteins [58]. Microarray, proteomic and immunocytochemical studies have shown that most OL proteins show decreased abundance in SCZ. Amongst these were CNP, MAG, erb-b2 receptor tyrosine kinase 3 (ErbB3), Oligodendrocyte transcription factor 2 (OLIG2), A disintegrin and metalloproteinase domain-containing protein 12 (ADAM12) and Neurite outgrowth inhibitor B (Nogo B) [60,61,62,63,64,65,66]. Only some were found to be up-regulated, including Neuregulin 1 (NRG1), erb-b2 receptor tyrosine kinase 4 (ErbB4), S100B, Neurite outgrowth inhibitor C (Nogo C), Disrupted In Schizophrenia 1 (DISC1) and prohibitin [66,67,68,69,70]. Uranova et al. showed that OLs from SCZ individuals had pronounced pathomorphological alterations (Fig.1). Follow up investigations found that OLs in various cortical and subcortical areas of the SCZ patients in this group had dystrophic and degenerative changes in structure. This sometimes featured swelling, reduction in the area of the nucleus and volume density of chromatin, and changes in the cytoplasm and cellular organelles, including reduction of the number of mitochondria and ribosomes, and accumulation of lipofuscin granules [52, 54, 71,72,73].
M Mitochondria, OL Oligodendrocyte, L Lipofuscin. R ribosomes (arrows); Scale bar = 2 μm. Reproduced with permission from Elsevier [73].
Reduced number of oligodendrocytes in schizophrenia
Findings of reduced OL numbers in white and grey matter areas have been replicated in histological studies of SCZ, mostly by morphometric estimation of cell numbers on Nissl-stained sections [7, 19,20,21,22,23, 54, 71,72,73,74,75,76] (although two studies did not find this [77, 78]). In studies, which reported losses, reductions were approximately 30% in certain brain regions [54, 73, 74]. Decreased OL densities were also found when applying CNP staining to detect mature OLs [74]. Interestingly, illness-associated OL losses were not restricted to parafascicular OLs as the main myelin producers, but also involved cortical perineuronal and pericapillary OLs [79,80,81,82]. All these OL subtypes are involved in synaptic plasticity through various mechanisms (Table 1): 1.) Parafascicular OLs are responsible for the myelination and energy supply of axons, modulating the speed and timing of action potentials, which is essential for synaptic strength and plasticity [45, 83, 84]. 2.) perineuronal OLs release neurotrophic factors and provide metabolic support that affects neuronal health and plasticity [43], and 3.) pericapillary OLs contribute to maintaining the neural environment and blood-brain barrier integrity, indirectly supporting synaptic plasticity [85].
Death of mature OLs is one possible reason for their reduced numbers in SCZ. Given the observed ultrastructural cellular dystrophic and degenerative changes of OLs in SCZ, degeneration with subsequent cell death could be a cause of this [81]. Findings of smaller OL size and reduced function may be a consequence of cell atrophy – a pathological process characterized by a reduction in cell size and function due to a loss of cell substance. Atrophic cells generally become smaller and their metabolic activity and functionality are significantly impaired, although they remain viable. This process is analogous to the proposed fate of astrocytes (ACs) in SCZ as discussed in the AC chapter [86,87,88,89]. Of note, both atrophy and degeneration are reversible in their early stages. Another possibility was suggested by Hu and colleagues who showed that sustained ErbB receptor activation in Plp-tTA (Proteolipid Protein promoter driving expression of the tetracycline transactivator) transgenic mice caused inflammatory demyelination and hypomyelination through necroptosis of mature OLs [33]. This may be relevant as the observed white matter abnormalities were reminiscent of histopathological characteristics found in SCZ brains, and it is known that in SCZ neuregulin-ErbB signalling in OLs is disturbed [90].
Necroptosis, another pathological process, is a type of regulated cell death triggered by death receptors and a pathological hallmark in multiple sclerosis [7, 91]. Whether or not necroptosis is relevant for OLs losses in SCZ remains to be established, although cell death of mature OLs is different from that of differentiating OLs. Importantly, not all OLs may be affected by the aforementioned pathological processes. Instead, dysfunctional OLs, some of which undergo degeneration, atrophy or necroptosis [7, 33, 86,87,88,89], can coexist with unaffected ones [32].
Recent findings show that cuprizone treatment (a standard approach to study mechanisms of OL death) initiated a caspase-3–dependent form of rapid cell death only in differentiating OLs, while mature OLs did not activate this pathway and exhibited delayed cell death [92]. It is also known that during normal brain development, MCs digest OL progenitor cells [93], raising the possibility that activated MCs might also phagocytose mature OLs in SCZ. However, Uranova et al. found no evidence for increased OL dystrophy in association with MC activation [54].
Theoretically, OL deficits may be a consequence of medication, since clozapine and haloperidol treatments have been shown to cause multiple molecular alterations in human cultured OLs [94, 95]. Moreover, antipsychotic treatment has been shown to enhance mitochondrial autophagy in OLs [96]. However, OL numbers were not found to be significantly reduced in macaque monkey brains after chronic exposure to antipsychotics [97]. Moreover, quetiapine was shown to enhance OL regeneration and myelin repair in the cuprizone-induced demyelination mouse model of multiple sclerosis and SCZ [98]. Thus, medication is unlikely to initiate OL death.
With regard to pathophysiological consequences of OL losses, demise of a single OL can lead to loss of myelin sheath for several internodes of 20-60 axons, amplifying the loss to multiple neurons [34]. This will disrupt axonal conductance and metabolic supply of the affected fibres. Thus, massive OL losses will leave high numbers of neurites either partially or completely unmyelinated, contributing to the well-documented, disturbed white matter integrity and network disconnectivity in SCZ [46]. This network disconnectivity might have effects on multiple brain functions, including higher order brain processes [99]. Based on the results of their stereological study, Falkai et al. proposed that the decreased number of OLs in the hippocampus may contribute to cognitive deficits in SCZ by impairing the connectivity of this brain structure [37]. Another stereological study identified reduced OL numbers in the prefrontal cortex [75]. This could also lead to negative effects on glutamate [100] and dopamine functions [101, 102].
Oligodendrocyte Progenitor Cells (OPCs)
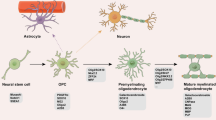
Another potential cause for lower mature OL numbers in SCZ could be a diminished formation or maturation of OPCs. Indeed, a decrease in the numerical density of clusters containing OPCs has been recently reported in SCZ [23]. OLs are generated from OPCs, which emerge from radial glia during development [40]. These continuously self-renewing cells constitute 5–8% of the total cell population in the central nervous system (CNS) [103]. In humans OL development starts at the beginning of the second trimester and continues into adulthood [104]. The first signs of myelination can be found in the fetal human brain gestational months 3-4 [105]. During their development human OPCs undergo successive symmetric divisions to exponentially increase the progenitor cell pool size [32, 106]. The steps of OL development can be characterised according to their migratory capacity, increase in morphologic complexity and expression of specific cell markers [107]. OL maturation can be divided into four main stages: OPC, pre-OL (or late OPC), immature (or pre-myelinating) OL and mature (or myelinating) OL [107]. Typical specific stage markers are platelet-derived growth factor receptor α, the proteoglycan NG2 (both expressed in OPCs [108]) and G-protein receptor (GPR)17 (expressed in pre-OLs [109]).
Emerging roles for reduced OPC numbers in SCZ
According to recent publications, many OL deficits in SCZ might result from failed OL maturation [32, 35,36,37,38,39,40, 110, 111]. This hypothesis assumes failure in some aspect of OPC differentiation in SCZ [34, 112]. Katsel and co-workers were the first to identify OL cell cycle abnormalities in SCZ [34]. The results of their microarray and quantitative real time PCR analyses provided evidence for gene expression changes of key regulators of G1/S cell cycle phase transition and genes central to OL differentiation in SCZ subjects. Their findings point to abnormal cell cycle re-entry in post-mitotic OLs. The same group also showed that OL and myelin deficits in SCZ involved failure of OPCs to exit the cell cycle for differentiation and maturation into OLs [76].
Other studies have provided further clues for possible cell cycle abnormalities in SCZ. Leucine rich repeat and Immunoglobin-like domain-containing protein 1 (LINGO-1), an identified negative regulator of OPC differentiation and myelination [32, 113] was found to be present at higher levels in brains of subjects with SCZ compared to non-psychiatric controls [114]. Also, we demonstrated increased expression of the cell cycle regulator protein prohibitin in white matter OLs in SCZ [69, 115]. Moreover, an increased expression of DISC1 in mature OLs (Fig. 2A) and enhanced expression of the DISC1-Δ3 variant in OPCs have been identified in SCZ [70, 110]. DISC1 is known to negatively regulate differentiation of OPCs into OLs [38, 110, 116, 117]. Furthermore, some but not all investigators found reduced expression of OLIG2, which promotes OL differentiation [118], in brains of SCZ patients (Fig. 2B) [35, 82, 110]. In addition, stress-induced immune activation of MC during development appears to contribute to defective differentiation competence of OPCs [9, 38, 111].
A White matter DISC1-immunoreactive OLs. The density of DISC1-expressing OLs was found to be enhanced in SCZ [70]. DISC1 negatively regulates differentiation of OPCs into OLs [38, 110, 116, 117]. B White matter OLIG2 expressing OLs. The density of OLIG2 immunopositive OLs was found to be reduced in SCZ [35, 82]. OLIG2 promotes OL differentiation [118].
Syed and co-workers showed that differentiation of cultured OPCs is inhibited by certain myelin proteins [119]. Theoretically, such an interaction may also take place in SCZ and illness-associated partial disintegration of myelin can lead to release of proteins that affect OPC function [52]. To investigate the proposed dysregulation of OL differentiation in SCZ, Mauney et al. compared the density of NG2- and OLIG2-immunoreactive cells between SCZ and normal control subjects [35]. They found that the number of cells expressing the specific late OPC marker NG2 was unchanged in SCZ, and concluded that late OPCs were numerically unaltered. That would mean that, despite potential developmental impairments, OPCs survived up to this stage but still needed to undergo maturation to myelinating OLs.
Unaltered numbers of NG2-expressing OPCs in SCZ were recently confirmed by other researchers [110]. However, it was also shown that these cells have an abnormal morphology, with hypertrophy, an elevated number of branches and greater branch lengths in SCZ patients [110]. Such morphological alterations might contribute to their inability to finish development (Fig. 3). However, under normal conditions only some of the NG2-expressing cells differentiate into mature OLs [120]. Second, it must be taken into account that in SCZ a significant portion of NG2 expressing cells successfully transform into myelinating OLs. This provides an explanation for the coexistence of “healthy” (myelin-generating mature OLs) and “sick” (non-generating mature OLs) [32]. However, some findings suggest OPC losses occur at earlier stages of differentiation. In multiple brain regions, studies have found significant reductions in density of OL clusters, which are typically composted of 2–9 OPCs at different stages of development [23, 121, 122]. This is in agreement with earlier observations of Hof et al. which found that spatial distribution of OLs exhibited a “less clustered arrangement” in SCZ [75]. Of note, analysis of Nissl-stained sections showed that immature OLs are spatially organised both within and outside clusters and are morphologically indistinguishable from mature OLs [81]. Thus, it cannot be excluded that OLs and OPCs were counted together in SCZ studies using this staining method. Thus, it is not clear to which extent losses of OPCs and/or of mature OLs, contribute to the reduced number of these cells in SCZ [36].
Schematic drawing based on the papers of Barateiro and Fernandes [107], Manuney et al. [35] and Yu et al. [110]. Figure generated with Biorender (www.biorender.com).
Using an animal model of multiple sclerosis (Plp-tTA transgenic mice), Hu et al. showed that long term over-activation of the ErbB receptor causes demyelination by driving necroptosis of mature OLs and apoptosis of OPCs [33]. However, as mentioned before, it is not known if results of such model experiments reflect the situation in SCZ. Concerning the influence of medication on OL differentiation and maturation, we are not aware of any papers which reported OL developmental abnormalities in drug-naïve patients with SCZ. Hence it cannot be said, if antipsychotic treatment initiates and/or advances disruption of OL maturation. Instead, most communications deal with positive effects of antipsychotics on OL development.
Antipsychotics have been shown to promote differentiation of OPCs by regulating the transcription factors OLIG1 and OLIG2 [118]. A study of the hypothalamus of olanzapine-treated mice identified an increase in numbers of new-born cells differentiating to the OL lineage but not the neuronal lineage [123]. The same research team showed that olanzapine stimulated proliferation but inhibited differentiation of cultured rat OL progenitors [124]. Haloperidol is capable of activating quiescent OPCs in the adult mouse brain [125], while quetiapine was found to modulate the OPC cell cycle and thereby facilitate OL differentiation [126, 127]. Finally, we described protective effects of haloperidol and clozapine on energy-deprived Oligodendrocyte Line 93 (OLN-93) OLs [128]. This is of relevance because metabolic and oxidative stress are known to impair OPC differentiation in some brain disorders including SCZ [129, 130].
The main pathophysiological outcome of a deficit of mature OLs in SCZ is hypo-myelination with the aforementioned negative consequences. However, there is evidence that OPCs also have important non-myelinating functions. For instance, they possess an immunomodulatory capacity [46] and are involved in regulation of interneuron migration [103]. The latter property is of particular interest because disturbed OPC differentiation and migration in SCZ might contribute to abnormalities in connectivity proposed to take place in the disease [131]. Various aspects of glial cell losses in SCZ are summarised in Table 2.
ASTROCYTES (ACs)
Astrogliopathy is important in SCZ pathology
ACs are the second most common glial cell type in the human brain. These cells are heterogeneous with regards to morphology, physiological properties and spatial organisation [24, 132]. ACs are known to change with age in properties such as morphology, functions, inflammatory responses, interactions with neurons, molecular profiles, and regional variability [133]. Being associated through intercellular channels termed connexins, ACs form a functional reticular network in the CNS known as the AC syncytium [134]. ACs play multiple roles in normal brain function, including supplying neurons and OLs with substrates for energy metabolism, control of extracellular water and electrolyte homeostasis, expression and release of “gliotransmitters” and neuromodulators, recycling of aminoacidergic neurotransmitters via the glutamate/GABA-glutamine cycle at the tripartite synapse, modulation of immune and inflammatory responses, synthesis of multiple trophic factors, contribution to mitochondria biogenesis, regulation of the blood-brain barrier, and promotion of synapse formation and elimination [135]. Since almost all of these processes are abnormal in SCZ [87, 136,137,138,139,140,141,142,143,144,145,146,147], functional impaiments in ACs have been implicated in SCZ pathology.
Using GWAS, Goudriaan et al. found 6 AC gene sets associated with SCZ [58]. Among the many identified genes were glial fibrillary acidic protein (GFAP), apolipoprotein E (APOE), and solute carrier family 1 A2 and A3 (SLC1A2 and SLC1A3) [58]. A number of studies have reported altered expression of mRNAs and encoded proteins in ACs of SCZ patients, although some of these findings have been contradictory, including those regarding expression of GFAP protein and mRNA [148,149,150,151,152], glutaminase and glutamine synthetase levels [153,154,155], abundance of enzymes and metabolites of the kynurenine pathway [140, 156], levels of the excitatory amino acid transporter proteins EAAT1 and EAAT2 [157], S100B abundance [67, 158], levels of ectonucleoside triphosphate diphosphohydrolase-1 and 2 mRNA [159], and number of DISC1 expressing ACs [160]. With regard to structural AC alterations, light microscopic studies have revealed swollen cathepsin-immunoreactive cortical ACs [161] and “gemistocytic” (i.e., swollen, reactive) GFAP positive ACs in SCZ brains [25, 26].
At the electron microscopic level, both dystrophic and swollen ACs were described by Uranova and co-workers [162, 163]. Another study found a significantly reduced number of mitochondria in hippocampal ACs in a subgroup of SCZ patients [164]. These structural alterations progressed with duration of illness [164]. Thus, astrogliopathy in SCZ may manifest itself in opposing morpho-functional changes, including atrophy with functional asthenia, and hypertrophy with elevated reactivity [86, 87, 89, 146, 151]. Hypertrophic ACs are thought to stand out by increased expression of GFAP, a hallmark of reactivity [146]. However, recent findings have shown that atrophic human brain ACs may also display increased GFAP expression [165], thus calling into question the usefulness of this cell marker to differentiate between atrophic and hypertrophic ACs. Whether or not hypertrophic ACs may pass into the state of atrophy has not been explored.
Astrocyte deficits in schizophrenia
We suggest that the best way to count ACs to improve sensitivity is using Cresyl violet (Nissl)-stained sections. Although this approach is methodically demanding and some expertise is needed to differentiate between ACs and OLs [166], it delivers reliable results [19,20,21,22]. Nissl staining should be preferred over the use of cell markers since the latter may show variable expression levels against a background of unchanged AC cell densities in certain conditions. Applying Nissl staining, normal densities of dentate gyrus ACs were found in SZ [20], while a significantly decreased density of DISC1 immunoreactive ACs was found in this brain region in cases of the same cohort [160]. Using Nissl stained sections, Pakkenberg observed a significant decrease of glial cell densities in nucleus mediodorsalis thalami and nucleus accumbens [6], and Benes et al. saw a tendency towards lower glial cell densities in several neocortical areas in SCZ [167]. Unfortunately, both groups did not differentiate between ACs and OLs. Along similar lines, no alterations in AC densities were found in the neocortex, hippocampus and dentate gyrus of SCZ subjects after Nissl staining [19,20,21,22, 168].
Research carried out with the AC marker GFAP have yielded inconsistent results. A number of papers reported significantly decreased densities of GFAP-immunolabelled AC profiles in various cortical and subcortical brain regions in SCZ and in schizophrenia suicide completers [18, 24, 26, 27, 154, 169], while other studies found either no difference [164, 170, 171] or increased densities of GFAP immunopositive cells [149, 151, 172, 173]. From these contradictory data, illness-associated AC losses cannot be a widespread phenomenon. Nonetheless, reduced GFAP cell densities as reported by 6 independent research groups might speak for subtle, regionally circumscribed AC losses in a subset of SCZ cases, assuming that the observed reductions actually reflect cell losses [18, 24, 26, 27, 154, 169]. However, it should be noted that cell density measurements may be biased due to tissue shrinkage after fixation and staining. Additionally, changes in cell density can occur without any change in the actual number of cells, which may indicate a functional impairment.
The above findings raise several questions: (i) which AC subpopulations might be affected? (ii) is there an influence of antipsychotic treatment on AC demise? and (iii) how important are AC losses as part of a wide-spread astrogliopathy? Concerning the first question, it is likely that a small portion of compromised ACs die by atrophy and not by degeneration [86, 88, 169]. Since some ACs are GFAP-negative [174], it cannot be said with certainty from classical histopathological approaches, which subtype of ACs is most affected. As atrophy is a reversible process, there may exist ways to rescue these cells. Importantly, although several lines of evidence suggest dysregulated AC differentiation in childhood onset and adult onset SCZ [9, 175,176,177,178], there is no evidence of cell loss during the disturbed AC maturation process (in contrast to OL maturation in SCZ; see above). However, definitive proof of the absence of AC progenitor deficits is still lacking, since there are still no quantitative studies using appropriate markers for these cells.
Investigations on the possible influence of antipsychotic treatment on AC cell densities have mostly dealt with post-mortem brains of chronic SCZ patients who had received antipsychotic medication over varying numbers of years. Therefore, it cannot be ruled out that AC losses are a consequence of long-term medication. In support of this assumption are findings of haloperidol-induced cytotoxicity in human ACs [179, 180], reduction of AC (but not OL) densities in macaque monkey brains after chronic exposure to antipsychotics [97], and effects of chronic antipsychotic medication on AC marker expression in rat brains [181]. However, recent MRI studies have demonstrated significantly reduced concentrations of myo-inositol in the prefrontal cortex at an early, state of acute SCZ, prior to medication. This suggests that dysregulation of ACs may be an initial, treatment-independent event [182]. Whether or not long-term treatment is involved in the worsening of the functional situation of compromised ACs, remains an open question.
One question that remains is whether AC losses affect SCZ pathology? Rats injected with the AC specific toxin L-α-aminoadipate in the medial prefrontal cortex showed attentional set-shifting and a decline of learning and working memory [183]. However, histological analysis of rat brain sections revealed massive AC loss in the targeted region as opposed to a slight to moderate decrease found in SCZ. In view of the high impact of functionally compromised ACs on the pathophysiology of SCZ, it is conceivable that the absence of a small number of ACs has only a minimal influence [184]. However, when AC losses appear at “strategic places” (for example, a 32% reduction in the GFAP-area fraction in layer V of the dorsolateral prefrontal cortex [18]), they could disrupt neuron-glia interactions in this layer, and thus have a dysfunctional effect on prefronto-striatal circuits in SCZ [18]. Notably, these late maturating circuits are probably more vulnerable towards glial hypofunction at the typical adolescent or young adult age of onset of SCZ [4].
A summary of the key OL and AC biomarkers identified in SCZ is given in Table 3.
Therapeutic opportunities
A comparison of key histological features, clinical implications and mechanistic insights between SCZ and neurodegenerative diseases is given in Table 4. These differences suggest that therapeutic interventions which help to rescue glial cell deficits may be a viable approach as novel SCZ treatments.
Effects of psychiatric medications on OLs, OPCs or ACs
First- and second-generation antipsychotics have been shown to modulate OLs [94] and ACs [181, 185]. Some animal and cell culture studies found detrimental effects of chronic treatment on OLs, OPCs or ACs, which resulted in reduced cell count or function [96, 97, 124, 180]. On the other hand, there have been indications for glioprotective effects, particularly in the cases of quetiapine, olanzapine and clozapine, on the proliferation of OPCs, myelin repair and maturation of OLs [95, 98, 118, 123,124,125,126,127,128, 180].
AC express receptors for neurotransmitters such as dopamine, GABA, glutamate and serotonin, which are targets for antipsychotics [186]. Additionally, ACs produce the enzyme glutathione synthase and are pivotal in the synthesis and regulation of glutathione, a critical antioxidant involved in reducing oxidative stress; they are capable of modulating inflammation pathways, mitochondrial function, and glutamate metabolism [180].
Some studies have indicated that antipsychotics may also have protective effects on cells through induction of neurotrophic factors. Shao et al. tested both atypical (quetiapine and clozapine) and typical (haloperidol) antipsychotics for their ability to induce secretion of the glial cell line–derived neurotrophic factor (GDNF) from C6 glioma cells [187]. They found that all three drugs induced GDNF release from an unchanged number of cells.
After analysing findings from several post-mortem studies on depressed subjects Banasr and colleagues concluded that structural alterations in depression result from atrophy and loss of neurons and glial cells that can be blocked or even reversed by antidepressant treatment [188]. Since symptoms of depression are common in SCZ, many patients receive antidepressants in addition to antipsychotics [189]. Unfortunately, there are no studies which compare glial cells losses in SCZ patients with and without antidepressant treatment.
Taken together, these findings suggest that glioprotection is likely to be an additional mechanism of action to the known modulation of synaptic neurotransmission for some antipsychotics. Due to lack of information about glial cell deficits in brains of untreated SCZ patients, it is difficult to say whether or not medication helps to mitigate glial abnormalities or even prevent glial cell losses. The availability of literature on post-mortem SCZ brains and glia from before 1950, when chlorpromazine was discovered, could help to resolve this issue. However, the substantiated finding that, despite antipsychotic treatment, SCZ patients may suffer from deficits of OLs, OPCs and ACs is a clear indication that antipsychotic medication is not enough to fully prevent glial cell losses.
Stem cell research and cell therapy
As mentioned above, OLs and ACs probably die by pathological processes which are basically reversible. Hence, compounds which target these pathways should be investigated for successful glioprotection. Of potential relevance to SCZ, a recent review focused on use of induced pluripotent stem cells (iPSCs) to increase our understanding of the involvement of ACs, MCs and OLs in the pathology of neurodegenerative diseases [190]. The studies involve using these cells in the production of cellular and organoid cultures, as well as intracranial transplantation to potentially provide useful disease models and insights into new drug targets and therapies. iPSCs can self-renew and differentiate into a variety of cells. These can be used to replace damaged or dead cells in animal models of various diseases and there has also been some success in human studies. In a patient with idiopathic Parkinson’s disease, iPSCs derived from the patient were differentiated into midbrain dopaminergic progenitor cells and implanted into the putamen, which led to improvement in symptoms over 18-24 months [191]. Although the original focus of research was on generation of neuronal cells, iPSCs can also be differentiated into glial cells and thus represent a potential future treatment strategy for SCZ. It is possible that iPSCs could also be used to implant functional OLs leading to an improvement in connectivity in SCZ.
Remyelinating agents
Studies on the promotion of remyelination in diseases such as multiple sclerosis could also be applicable in SCZ. Münzel, and Williams, reviewed existing drugs that promote remyelination by OLs to enhance white matter and brain connectivity [192]. Clinical trials are either planned or underway for this purpose with the testing of compounds such as alemtuzumab, dimethyl fumarate, LINGO-1 antibodies, and IgM monoclonal antibody 22. Other potential remyelinating compounds described include clemastine, GSK239512, opicinumab, GNbAC1, simvastatin, biotin, quetiapine, fumarate, and domperidone. LINGO-1 is a negative regulator of OPC differentiation and remyelination [193], and reagents which block this pathway could improve OPC differentiation efficiency. IgM22 could also have a positive effect on white matter integrity in SCZ through its association with anti-apoptotic signalling in pre-myelinating OLs [194]. Notch-1 is also a negative regulator of remyelination [195] and Wnt pathway signalling appears to delay oligodentrocyte maturation [196]. In addition, the application of sonic hedgehog (Shh) and a retinoid X receptor (RXR) agonist resulted in increased OPC proliferation and differentiation in animal experiments [197, 198]. Finally, D-aspartate administration was shown to stimulate OPC maturation and accelerate myelin recovery during remyelination, which improved motor coordination [199].
Anti-inflammatory agents
Compounds that possess anti-inflammatory properties, such as nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and minocycline, have been investigated for their potential use in neuropsychiatric disorders [200]. Use of minocycline as an adjunctive treatment in SCZ is thought to have an overall beneficial effect [201], which may include protective roles for glial cells. In cell culture minocycline was found to reduce OL damage caused by MC activation [202] and to protect OPCs against injury caused by oxygen-glucose deprivation [203].
Antioxidant drugs and natural products
Numerous studies have found increased oxidative stress and redox imbalance in SCZ, caused by both genetic predisposition and environmental influences, such as immune dysfunction. In addition to neuronal damage caused by oxidative stress, damage to OLs can also occur. OLs are vulnerable to oxidative stress and glutathione deficiency can lead to arrest of OPC proliferation and apoptosis, resulting in hypomyelination. In addition, death of OLs or OPCs can also increase neuroinflammation and oxidative stress. On the other hand, reduction of oxidative stress could lead to an improvement of white matter integrity in SCZ patients. The effectiveness of some antioxidant drugs and natural substances has already been investigated [204], including N-acetylcysteine (NAC), ginkgo biloba, selegiline, allopurinol, polyunsaturated fatty acids (PUFAs) and vitamins E and C. In a meta-analysis, administration of the antioxidant and free radical-scavenging NAC led to significant improvement in psychotic symptoms and cognition after 24 weeks [205]. In addition, substances that act on redox-regulated transcription factors have also been proposed. For example, application of the Nrf2 transcription factor activator sulforaphane led to an increase in blood and brain glutathione (GSH) levels in healthy adults which could lead to improved cognition in patients with SCZ [206, 207]. Since typical antipsychotics such as haloperidol presumably lead to increased oxidative stress in ACs, NAC has been proposed as adjunctive treatment [180, 208].
Schmitz et al. emphasized the potential of natural substances such as curcumin, isoflavones, and sulforaphane, endogenous mammalian compounds including lipoic acid and guanosine (a guanine-based purine) and resveratrol, which supposedly have glioprotective effects [180]. Their protective effects on glial cells include anti-inflammatory properties partly mediated by inhibition of MC activation, antioxidant effects with improvement of mitochondrial function, influence on glutamate metabolism and survival and differentiation of OLs [209]. Relevant metabolic pathways that could be targeted include NFκB inhibition and Nrf2/HO-1 activation. Resveratrol may influence various AC functions such as inflammatory response, glutamate homeostasis, and antioxidant defense and trophic factor release. In addition, this natural compound may have glioprotective effects on OLs and MCs. There are initial clinical experiences with resveratrol add-on therapy in the treatment of negative symptoms in patients with stable SCZ [210].
Physical exercise
A simple and potentially effective way to positively influence the fate of glial cells in SCZ is long-term physical activity [37, 39]. The rationale for this comes from studies which found that OLs in developing and adult rodent brains are highly responsive to physical exercise. For example, NG2-expressing cell proliferation increases in exercising juvenile rats [211]. Importantly, there is evidence that aerobic exercise has an impact on glial cells in humans. Papiol et al. [39] and Falkai et al. [37] investigated SCZ patients and healthy controls who performed aerobic exercise, as well as patients who only played table soccer. MRI-based assessments were carried out at baseline and after 3 months. It was found that the polygenic burden associated with OPCs and radial glia significantly influenced the volume changes between baseline and 3 months in hippocampus subfields in SCZ patients performing aerobic exercise. Also, aerobic exercise training led to a volume increase in the hippocampal subfield CA4, together with improved cognition in individuals with SCZ [39]. Analysis of cell-type specific SCZ polygenic risk scores showed that exercise-induced volume increase significantly correlated with OPCs. Moreover, higher OPC-or radial glia-associated genetic risk burden was associated with a smaller volume increase, or even a decrease, during exercise. The authors of both communications propose that aerobic exercise training combined with the histamine blocker clemastine or other pro-myelinating drugs might help to promote regeneration of myelin plasticity in SCZ. However, further studies are required to determine whether or not physical activity of SCZ patients prevents glial cell death.
Conclusions and future perspectives
In this review, we have highlighted the point that macro-glial networks are disrupted in SCZ, distinct from the changes seen in neurodegenerative conditions which appear to arise mainly from neuronal deficits (Table 4). We described two different ways that might lead to drastically reduced OL numbers in SCZ: (i) death of mature, myelinating OLs by atrophy and/or degeneration; and (ii) deficits arising from failure of OPCs to complete their development into mature OLs. In contrast, minor AC deficits can be found in SCZ and these cells are thought to die by atrophy. Glial cell deficits in SCZ bring about many serious functional consequences, with myelination deficits and, thereby, disrupted neuronal connectivity being an obvious critical outcome. Since antipsychotic treatment alone is not able to prevent glial cell losses, there is urgent need to identify new therapeutic options that effectively target these pathways. Given the distinction from neurodegenerative disorders, the proposed approaches include the application of antidepressants and other chemical compounds, remyelinating agents, natural products, physical exercise and engrafting healthy glial cells into brains of SCZ patients. A better understanding of gliopathologies in SCZ could lead to development of additional tools in the investigation of this psychiatric illness, with a focus on diagnostics, new treatments and outcome assessments.
References
World Health Organization. Mental disorders. 2024. https://www.who.int/news-room/fact-sheets/detail/mental-disorders. Accessed 29 Jun 2024.
Stone WS, Phillips MR, Yang LH, Kegeles LS, Susser ES, Lieberman JA. Neurodegenerative model of schizophrenia: Growing evidence to support a revisit. Schizophrenia Res. 2022;243:154–62.
Wawrzczak-Bargieła A, Bilecki W, Maćkowiak M. Epigenetic Targets in Schizophrenia Development and Therapy. Brain Sci. 2023;13:426.
Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–93.
Peedicayil J. Genome-Environment Interactions and Psychiatric Disorders. Biomedicines. 2023;11:1209.
Pakkenberg B. Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen psychiatry. 1990;47:1023–8.
Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophrenia Res. 2004;67:269–75.
Bernstein H-G, Steiner J, Guest PC, Dobrowolny H, Bogerts B. Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophrenia Res. 2015;161:4–18.
Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7:272–81.
Liu S-H, Du Y, Chen L, Cheng Y. Glial Cell Abnormalities in Major Psychiatric Diseases: A Systematic Review of Postmortem Brain Studies. Mol Neurobiol. 2022;59:1665–92.
Jenkins AK, Ketchesin KD, Becker-Krail DD, McClung CA. Molecular Rhythmicity in Glia: Importance for Brain Health and Relevance to Psychiatric Disease. Biological Psychiatry. 2024;96:909–18.
Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–57.
Allen NJ, Barres BA. Neuroscience: Glia - more than just brain glue. Nature. 2009;457:675–7.
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705.
Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–9.
Li Q. Astrocytes: The Rising Stars that Regulate Synaptic Plasticity and Long-Term Memory Formation. In Synaptic tagging and capture: from synapses to behaviour. Springer International PU; 2024, pp. Springer, Cham (Switzerland) 309–20.
Pallarés-Moratalla C, Bergers G. The ins and outs of microglial cells in brain health and disease. Front Immunol. 2024;15:1305087.
Rajkowska G, Miguel-Hidalgo JJ, Makkos Z, Meltzer H, Overholser J, Stockmeier C. Layer-specific reductions in GFAP-reactive astroglia in the dorsolateral prefrontal cortex in schizophrenia. Schizophrenia Res. 2002;57:127–38.
Schmitt A, Steyskal C, Bernstein H-G, Schneider-Axmann T, Parlapani E, Schaeffer EL, et al. Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta neuropathologica. 2009;117:395–407.
Falkai P, Malchow B, Wetzestein K, Nowastowski V, Bernstein H-G, Steiner J, et al. Decreased Oligodendrocyte and Neuron Number in Anterior Hippocampal Areas and the Entire Hippocampus in Schizophrenia: A Stereological Postmortem Study. Schizophr Bull. 2016;42:S4–12.
Falkai P, Raabe F, Bogerts B, Schneider-Axmann T, Malchow B, Tatsch L, et al. Association between altered hippocampal oligodendrocyte number and neuronal circuit structures in schizophrenia: a postmortem analysis. Eur Arch Psychiatry Clin Neurosci. 2020;270:413–24.
Schmitt A, Tatsch L, Vollhardt A, Schneider-Axmann T, Raabe FJ, Roell L et al. Decreased Oligodendrocyte Number in Hippocampal Subfield CA4 in Schizophrenia: A Replication Study. Cells. 2022;11:3242.
Kolomeets NS, Uranova NA. Snizhenie chislennoi plotnosti oligodendrotsitov i klasterov oligodendrotsitov v golovke khvostatogo yadra pri shizofrenii. Zh nevrologii i psikhiatrii Im S S Korsakova. 2023;123:103–10.
Steffek AE, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH. Cortical expression of glial fibrillary acidic protein and glutamine synthetase is decreased in schizophrenia. Schizophrenia Res. 2008;103:71–82.
Williams MR, Harb H, Pearce RKB, Hirsch SR, Maier M. Oligodendrocyte density is changed in the basolateral amygdala in schizophrenia but not depression. Schizophrenia Res. 2013;147:402–3.
Williams MR, Hampton T, Pearce RKB, Hirsch SR, Ansorge O, Thom M, et al. Astrocyte decrease in the subgenual cingulate and callosal genu in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2013;263:41–52.
Williams MR, Galvin K, O’Sullivan B, MacDonald CD, Ching EWK, Turkheimer F, et al. Neuropathological changes in the substantia nigra in schizophrenia but not depression. Eur Arch Psychiatry Clin Neurosci. 2014;264:285–96.
Uranova NA, Vikhreva OV, Rakhmanova VI. Abnormal microglial reactivity in gray matter of the prefrontal cortex in schizophrenia. Asian J Psychiatry. 2021;63:102752.
Vikhreva OV, Uranova NA. Reaktivnost’ mikroglii v prefrontal’noi kore pri raznykh tipakh techeniya shizofrenii. Zh nevrologii i psikhiatrii Im S S Korsakova. 2021;121:77–83.
van Kesteren, Gremmels CFMG, Witte H, de LD, Hol EM, van Gool AR, et al. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry. 2017;7:e1075.
Snijders GJLJ, van Zuiden W, Sneeboer MAM, van Berdenis Berlekom A, van der Geest AT, Schnieder T, et al. A loss of mature microglial markers without immune activation in schizophrenia. Glia. 2021;69:1251–67.
Fessel J. Abnormal oligodendrocyte function in schizophrenia explains the long latent interval in some patients. Transl Psychiatry. 2022;12:120.
Hu X, Xiao G, He L, Niu X, Li H, Lou T, et al. Sustained ErbB Activation Causes Demyelination and Hypomyelination by Driving Necroptosis of Mature Oligodendrocytes and Apoptosis of Oligodendrocyte Precursor Cells. J Neurosci. 2021;41:9872–90.
Katsel P, Davis KL, Li C, Tan W, Greenstein E, Kleiner Hoffman LB, et al. Abnormal indices of cell cycle activity in schizophrenia and their potential association with oligodendrocytes. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2008;33:2993–3009.
Mauney SA, Pietersen CY, Sonntag K-C, Woo T-UW. Differentiation of oligodendrocyte precursors is impaired in the prefrontal cortex in schizophrenia. Schizophrenia Res. 2015;169:374–80.
Raabe FJ, Slapakova L, Rossner MJ, Cantuti-Castelvetri L, Simons M, Falkai PG et al. Oligodendrocytes as A New Therapeutic Target in Schizophrenia: From Histopathological Findings to Neuron-Oligodendrocyte Interaction. Cells. 2019;8:1496.
Falkai P, Rossner MJ, Raabe FJ, Wagner E, Keeser D, Maurus I, et al. Disturbed Oligodendroglial Maturation Causes Cognitive Dysfunction in Schizophrenia: A New Hypothesis. Schizophr Bull. 2023;49:1614–24.
Kolomeets NS. Oligodendrocyte Progenitors in Schizophrenia: The Role in Pathogenesis and Potential Treatment Target. ПСИХИАТРИЯ. 2024;21:46–64.
Papiol S, Keeser D, Hasan A, Schneider-Axmann T, Raabe F, Degenhardt F, et al. Polygenic burden associated to oligodendrocyte precursor cells and radial glia influences the hippocampal volume changes induced by aerobic exercise in schizophrenia patients. Transl Psychiatry. 2019;9:284.
Yi C, Verkhratsky A, Niu J. Pathological potential of oligodendrocyte precursor cells: terra incognita. Trends Neurosci. 2023;46:581–96.
Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti REL, Leite REP, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–41.
von Bartheld CS, Bahney J, Herculano-Houzel S. The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J Comp Neurol. 2016;524:3865–95.
Bernstein H-G, Keilhoff G, Dobrowolny H, Guest PC, Steiner J. Perineuronal oligodendrocytes in health and disease: the journey so far. Rev Neurosci. 2019;31:89–99.
Osanai Y, Yamazaki R, Shinohara Y, Ohno N. Heterogeneity and regulation of oligodendrocyte morphology. Front cell Dev Biol. 2022;10:1030486.
Looser ZJ, Faik Z, Ravotto L, Zanker HS, Jung RB, Werner HB, et al. Oligodendrocyte-axon metabolic coupling is mediated by extracellular K+ and maintains axonal health. Nat Neurosci. 2024;27:433–48.
Kuhn S, Gritti L, Crooks D, Dombrowski Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells. 2019;8:1424.
Zhao J, Huang C-C, Zhang Y, Liu Y, Tsai S-J, Lin C-P, et al. Structure-function coupling in white matter uncovers the abnormal brain connectivity in Schizophrenia. Transl Psychiatry. 2023;13:214.
Cassoli JS, Guest PC, Malchow B, Schmitt A, Falkai P, Martins-de-Souza D. Disturbed macro-connectivity in schizophrenia linked to oligodendrocyte dysfunction: from structural findings to molecules. npj Schizophr. 2015;1:15034.
Valdés-Tovar M, Rodríguez-Ramírez AM, Rodríguez-Cárdenas L, Sotelo-Ramírez CE, Camarena B, Sanabrais-Jiménez MA, et al. Insights into myelin dysfunction in schizophrenia and bipolar disorder. World J Psychiatry. 2022;12:264–85.
MacKay AL, Laule C. Magnetic Resonance of Myelin Water: An in vivo Marker for Myelin. Brain Plasticity. 2016;2:71–91.
Regenold WT, Phatak P, Marano CM, Gearhart L, Viens CH, Hisley KC. Myelin staining of deep white matter in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and unipolar major depression. Psychiatry Res. 2007;151:179–88.
Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, et al. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597–610.
Uranova NA, Vostrikov VM, Vikhreva OV, Zimina IS, Kolomeets NS, Orlovskaya DD. The role of oligodendrocyte pathology in schizophrenia. Int J Neuropsychopharmacol. 2007;10:537–45.
Uranova NA, Vikhreva OV, Rakhmanova VI, Orlovskaya DD. Ultrastructural pathology of oligodendrocytes adjacent to microglia in prefrontal white matter in schizophrenia. npj Schizophr. 2018;4:26.
Rosenbluth J, Bobrowski-Khoury N. Structural bases for central nervous system malfunction in the quaking mouse: dysmyelination in a potential model of schizophrenia. J Neurosci Res. 2013;91:374–81.
Martins-de-Souza D. Proteome and transcriptome analysis suggests oligodendrocyte dysfunction in schizophrenia. J Psychiatr Res. 2010;44:149–56.
Martins-de-Souza D, Guest PC, Reis-de-Oliveira G, Schmitt A, Falkai P, Turck CW. An overview of the human brain myelin proteome and differences associated with schizophrenia. World J Biol Psychiatry Off J World Federation Societies Biol Psychiatry. 2021;22:271–87.
Goudriaan A, de Leeuw C, Ripke S, Hultman CM, Sklar P, Sullivan PF, et al. Specific glial functions contribute to schizophrenia susceptibility. Schizophrenia Bull. 2014;40:925–35.
Karoutzou G, Emrich HM, Dietrich DE. The myelin-pathogenesis puzzle in schizophrenia: a literature review. Mol Psychiatry. 2008;13:245–60.
Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA. 2001;98:4746–51.
Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805.
Aston C, Jiang L, Sokolov BP. Microarray analysis of postmortem temporal cortex from patients with schizophrenia. J Neurosci Res. 2004;77:858–66.
Schoeneck L, Movebach J, Meyer-Lotz G, Keilhoff G, Mawrin C, Dobrowolny H et al. Dyregulated expression of the oligodendroglial transcriptionfactor Olig 2 in schizophrenia and depression. Pharmacopsychiatry. 2009;42:2227–42.
Farkas N, Lendeckel U, Dobrowolny H, Funke S, Steiner J, Keilhoff G, et al. Reduced density of ADAM 12-immunoreactive oligodendrocytes in the anterior cingulate white matter of patients with schizophrenia. World J Biol Psychiatry Off J World Federation Societies Biol Psychiatry. 2010;11:556–66.
Matthews PR, Eastwood SL, Harrison PJ. Reduced myelin basic protein and actin-related gene expression in visual cortex in schizophrenia. PloS One. 2012;7:e38211.
Novak G, Tallerico T. Nogo A, B and C expression in schizophrenia, depression and bipolar frontal cortex, and correlation of Nogo expression with CAA/TATC polymorphism in 3’-UTR. Brain Res. 2006;1120:161–71.
Steiner J, Bernstein H-G, Bielau H, Farkas N, Winter J, Dobrowolny H, et al. S100B-immunopositive glia is elevated in paranoid as compared to residual schizophrenia: a morphometric study. J Psychiatr Res. 2008;42:868–76.
Chong VZ, Thompson M, Beltaifa S, Webster MJ, Law AJ, Weickert CS. Elevated neuregulin-1 and ErbB4 protein in the prefrontal cortex of schizophrenic patients. Schizophrenia Res. 2008;100:270–80.
Bernstein H-G, Smalla K-H, Dürrschmidt D, Keilhoff G, Dobrowolny H, Steiner J, et al. Increased density of prohibitin-immunoreactive oligodendrocytes in the dorsolateral prefrontal white matter of subjects with schizophrenia suggests extraneuronal roles for the protein in the disease. Neuromolecular Med. 2012;14:270–80.
Bernstein H-G, Jauch E, Dobrowolny H, Mawrin C, Steiner J, Bogerts B. Increased density of DISC1-immunoreactive oligodendroglial cells in fronto-parietal white matter of patients with paranoid schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2016;266:495–504.
Uranova NA, Orlovskaia DD, Vikhreva OV, Zimina IS, Rakhmanova VI. Morfometricheskaia otsenka ul’trastrukturnykh plasticheskikh perestroek v mozge pri éndogennykh psikhozakh (reaktsii oligodendroglii). Vestnik Rossiiskoi akademii meditsinskikh nauk. 2001:42–8.
Kolomeets NS, Uranova NA. Pathology of oligodendroglia and myelinated fibers of the hippocampus in schizophrenia (an ultrastructural and morphometric study). Zh Nevrologii i Psikhiatrii Im S S Korsakova. 2008;108:52–60.
Vikhreva OV, Rakhmanova VI, Orlovskaya DD, Uranova NA. Ultrastructural alterations of oligodendrocytes in prefrontal white matter in schizophrenia: A post-mortem morphometric study. Schizophrenia Res. 2016;177:28–36.
Hof PR, Haroutunian V, Copland C, Davis KL, Buxbaum JD. Molecular and cellular evidence for an oligodendrocyte abnormality in schizophrenia. Neurochem Res. 2002;27:1193–1200.
Hof PR, Haroutunian V, Friedrich VL, Byne W, Buitron C, Perl DP, et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53:1075–85.
Kerns D, Vong GS, Barley K, Dracheva S, Katsel P, Casaccia P, et al. Gene expression abnormalities and oligodendrocyte deficits in the internal capsule in schizophrenia. Schizophrenia Res. 2010;120:150–8.
Segal D, Schmitz C, Hof PR. Spatial distribution and density of oligodendrocytes in the cingulum bundle are unaltered in schizophrenia. Acta Neuropathologica. 2009;117:385–94.
Höistad M, Heinsen H, Wicinski B, Schmitz C, Hof PR. Stereological assessment of the dorsal anterior cingulate cortex in schizophrenia: absence of changes in neuronal and glial densities. Neuropathol Appl Neurobiol. 2013;39:348–61.
Vostrikov V, Orlovskaya D, Uranova N. Deficit of pericapillary oligodendrocytes in the prefrontal cortex in schizophrenia. World J Biol Psychiatry. 2008;9:34–42.
Vostrikov VM, Uranova NA, Orlovskaya DD. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophrenia Res. 2007;94:273–80.
Kolomeets NS, Uranova NA. Reduced oligodendrocyte density in layer 5 of the prefrontal cortex in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2019;269:379–86.
Kolomeets NS, Uranova NA. Reduced number of satellite oligodendrocytes of pyramidal neurons in layer 5 of the prefrontal cortex in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2022;272:947–55.
Monje M. Myelin Plasticity and Nervous System Function. Annu Rev Neurosci. 2018;41:61–76.
Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–21.
Kimura I, Dohgu S, Takata F, Matsumoto J, Watanabe T, Iwao T, et al. Oligodendrocytes upregulate blood-brain barrier function through mechanisms other than the PDGF-BB/PDGFRα pathway in the barrier-tightening effect of oligodendrocyte progenitor cells. Neurosci Lett. 2020;715:134594.
Verkhratsky A, Rodríguez JJ, Steardo L. Astrogliopathology: a central element of neuropsychiatric diseases? Neuroscientist Rev J Bringing Neurobiol Neurol Psychiatry. 2014;20:576–88.
Verkhratsky A, Parpura V. Astrogliopathology in neurological, neurodevelopmental and psychiatric disorders. Neurobiol Dis. 2016;85:254–61.
Verkhratsky A, Steardo L, Peng L, Parpura V. Astroglia, Glutamatergic Transmission and Psychiatric Diseases. Adv Neurobiol. 2016;13:307–26.
Verkhratsky A, Nedergaard M. Physiology of Astroglia. Physiological Rev. 2018;98:239–389.
Prats C, Fatjó-Vilas M, Penzol MJ, Kebir O, Pina-Camacho L, Demontis D, et al. Association and epistatic analysis of white matter related genes across the continuum schizophrenia and autism spectrum disorders: The joint effect of NRG1-ErbB genes. World J Biol Psychiatry Off J World Federation Societies Biol Psychiatry. 2022;23:208–18.
Liu X, Xie X, Ren Y, Shao Z, Zhang N, Li L, et al. The role of necroptosis in disease and treatment. MedComm. 2021;2:730–55.
Chapman TW, Kamen Y, Piedra ET, Hill RA. Oligodendrocyte Maturation Alters the Cell Death Mechanisms That Cause Demyelination. J Neurosci. 2024;44:e1794232024.
Irfan M, Evonuk KS, De Silva TM. Microglia phagocytose oligodendrocyte progenitor cells and synapses during early postnatal development: implications for white versus gray matter maturation. FEBS J. 2022;289:2110–27.
Brandão-Teles C, de Almeida V, Cassoli JS, Martins-de-Souza D. Biochemical Pathways Triggered by Antipsychotics in Human corrected Oligodendrocytes: Potential of Discovering New Treatment Targets. Front Pharmacol. 2019;10:186.
Seabra G, de Almeida V, Reis-de-Oliveira G, Crunfli F, Antunes ASLM, Martins-de-Souza D. Ubiquitin-proteasome system, lipid metabolism and DNA damage repair are triggered by antipsychotic medication in human oligodendrocytes: implications in schizophrenia. Sci Rep. 2020;10:12655.
Bernstein H-G, Keilhoff G, Dobrowolny H, Steiner J. Enhanced mitochondrial autophagy (mitophagy) in oligodendrocytes might play a role in white matter pathology in schizophrenia. Med Hypotheses. 2020;134:109443.
Konopaske GT, Dorph-Petersen K-A, Sweet RA, Pierri JN, Zhang W, Sampson AR, et al. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol Psychiatry. 2008;63:759–65.
Zhang Y, Zhang H, Wang L, Jiang W, Xu H, Xiao L, et al. Quetiapine enhances oligodendrocyte regeneration and myelin repair after cuprizone-induced demyelination. Schizophrenia Res. 2012;138:8–17.
Khelfaoui H, Ibaceta-Gonzalez C, Angulo MC. Functional myelin in cognition and neurodevelopmental disorders. Cell Mol Life Sci CMLS. 2024;81:181.
Gallo V, Ghiani CA. Glutamate receptors in glia: new cells, new inputs and new functions. Trends Pharmacol Sci. 2000;21:252–8.
Nikulina EM, Skrinskaya JA, Avgustinovich DF, Popova NK. Dopaminergic brain system in the quaking mutant mouse. Pharmacol Biochem Behav. 1995;50:333–7.
Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol. 2011;93:13–24.
Fang L-P, Bai X. Oligodendrocyte precursor cells: the multitaskers in the brain. Pflugers Archiv. Eur J Physiol. 2023;475:1035–44.
Jakovcevski I, Filipovic R, Mo Z, Rakic S, Zecevic N. Oligodendrocyte development and the onset of myelination in the human fetal brain. Front Neuroanat. 2009;3:5.
Deoni SCL, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, et al. Mapping infant brain myelination with magnetic resonance imaging. J Neurosci. 2011;31:784–91.
Huang W, Bhaduri A, Velmeshev D, Wang S, Wang L, Rottkamp CA, et al. Origins and Proliferative States of Human Oligodendrocyte Precursor Cells. Cell. 2020;182:594–608.e11.
Barateiro A, Fernandes A. Temporal oligodendrocyte lineage progression: in vitro models of proliferation, differentiation and myelination. Biochim Biophys Acta. 2014;1843:1917–29.
Nishiyama A, Lin X-H, Giese N, Heldin C-H, Stallcup WB. Interaction between NG2 proteoglycan and PDGF ?-receptor on O2A progenitor cells is required for optimal response to PDGF. J Neurosci Res. 1996;43:315–30.
Boda E, Viganò F, Rosa P, Fumagalli M, Labat-Gest V, Tempia F, et al. The GPR17 receptor in NG2 expressing cells: focus on in vivo cell maturation and participation in acute trauma and chronic damage. Glia. 2011;59:1958–73.
Yu G, Su Y, Guo C, Yi C, Yu B, Chen H, et al. Pathological oligodendrocyte precursor cells revealed in human schizophrenic brains and trigger schizophrenia-like behaviors and synaptic defects in genetic animal model. Mol Psychiatry. 2022;27:5154–66.
Rivera AD, Normanton JR, Butt AM, Azim K. The Genomic Intersection of Oligodendrocyte Dynamics in Schizophrenia and Aging Unravels Novel Pathological Mechanisms and Therapeutic Potentials. Int J Mol Sci. 2024;25:4452.
Bernstein H-G, Stürze E, Bogerts B. Cell cycle disturbances in schizophrenia: The journey so far. Acta Clin Croatica. 2010;49:33–5.
Jepson S, Vought B, Gross CH, Gan L, Austen D, Frantz JD, et al. LINGO-1, a transmembrane signaling protein, inhibits oligodendrocyte differentiation and myelination through intercellular self-interactions. J Biol Chem. 2012;287:22184–95.
Fernandez-Enright F, Andrews JL, Newell KA, Pantelis C, Huang XF. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry. 2014;4:e348.
Roskams AJ, Friedman V, Wood CM, Walker L, Owens GA, Stewart DA, et al. Cell cycle activity and expression of prohibitin mRNA. J Cell Physiol. 1993;157:289–95.
Hattori T, Shimizu S, Koyama Y, Emoto H, Matsumoto Y, Kumamoto N, et al. DISC1 (disrupted-in-schizophrenia-1) regulates differentiation of oligodendrocytes. PloS One. 2014;9:e88506.
Pieczonka K, Khazaei M, Fehlings MG. Promoting the Differentiation of Neural Progenitor Cells into Oligodendrocytes through the Induction of Olig2 Expression: A Transcriptomic Study Using RNA-seq Analysis. Cells. 2023;12:1252.
Fang F, Zhang H, Zhang Y, Xu H, Huang Q, Adilijiang A, et al. Antipsychotics promote the differentiation of oligodendrocyte progenitor cells by regulating oligodendrocyte lineage transcription factors 1 and 2. Life Sci. 2013;93:429–34.
Syed YA, Baer AS, Lubec G, Hoeger H, Widhalm G, Kotter MR. Inhibition of oligodendrocyte precursor cell differentiation by myelin-associated proteins. Neurosurgical Focus. 2008;24:E5.
Sánchez-González R, Bribián A, López-Mascaraque L. Cell Fate Potential of NG2 Progenitors. Sci Rep. 2020;10:9876.
Vostrikov VM, Uranova NA. Reduced density of oligodendrocytes and oligodendrocyte clusters in the caudate nucleus in major psychiatric illnesses. Schizophrenia Res. 2020;215:211–6.
Kolomeets NS, Vostrikov VM, Uranova NA. Narusheniia klasterizatsii oligodendrotsitov v supra- i infragranuliarnykh sloiakh polia 10 prefrontal’noĭ kory pri shizofrenii. Zh Nevrologii i Psikhiatrii Im S S Korsakova. 2019;119:62–8.
Yamauchi T, Tatsumi K, Makinodan M, Kimoto S, Toritsuka M, Okuda H, et al. Olanzapine increases cell mitotic activity and oligodendrocyte-lineage cells in the hypothalamus. Neurochemistry Int. 2010;57:565–71.
Kimoto S, Okuda A, Toritsuka M, Yamauchi T, Makinodan M, Okuda H, et al. Olanzapine stimulates proliferation but inhibits differentiation in rat oligodendrocyte precursor cell cultures. Prog Neuro Psychopharmacol Biol Psychiatry. 2011;35:1950–6.
Wang H, Xu H, Niu J, Mei F, Li X, Kong J, et al. Haloperidol activates quiescent oligodendroglia precursor cells in the adult mouse brain. Schizophrenia Res. 2010;119:164–74.
Xiao L, Xu H, Zhang Y, Wei Z, He J, Jiang W, et al. Quetiapine facilitates oligodendrocyte development and prevents mice from myelin breakdown and behavioral changes. Mol Psychiatry. 2008;13:697–708.
Mi G, Wang Y, Ye E, Gao Y, Liu Q, Chen P, et al. The antipsychotic drug quetiapine stimulates oligodendrocyte differentiation by modulating the cell cycle. Neurochemistry Int. 2018;118:242–51.
Steiner J, Sarnyai Z, Westphal S, Gos T, Bernstein H-G, Bogerts B, et al. Protective effects of haloperidol and clozapine on energy-deprived OLN-93 oligodendrocytes. Eur Arch Psychiatry Clin Neurosci. 2011;261:477–82.
Spaas J, van Veggel L, Schepers M, Tiane A, van Horssen J, Wilson DM, et al. Oxidative stress and impaired oligodendrocyte precursor cell differentiation in neurological disorders. Cell Mol Life Sci CMLS. 2021;78:4615–37.
Maas DA, Vallès A, Martens GJM. Oxidative stress, prefrontal cortex hypomyelination and cognitive symptoms in schizophrenia. Transl Psychiatry. 2017;7:e1171.
Toudji I, Toumi A, Chamberland É, Rossignol E. Interneuron odyssey: molecular mechanisms of tangential migration. Front Neural Circuits. 2023;17:1256455.
Sofroniew MV. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 2020;41:758–70.
Palmer AL, Ousman SS. Astrocytes and Aging. Front Aging Neurosci. 2018;10:337.
Zhou M, Zhong S, Verkhratsky A. Astrocyte syncytium: from neonatal genesis to aging degeneration. Neural Regeneration Res. 2024;19:395–6.
Kim Y, Park J, Choi YK. The Role of Astrocytes in the Central Nervous System Focused on BK Channel and Heme Oxygenase Metabolites: A Review. Antioxidants. 2019;8:121.
Burbaeva GS, Boksha IS, Turishcheva MS, Vorobyeva EA, Savushkina OK, Tereshkina EB. Glutamine synthetase and glutamate dehydrogenase in the prefrontal cortex of patients with schizophrenia. Prog Neuro Psychopharmacol Biol Psychiatry. 2003;27:675–80.
Mitterauer B. Nonfunctional glial proteins in tripartite synapses: a pathophysiological model of schizophrenia. Neuroscientist Rev J Bringing Neurobiol Neurol Psychiatry. 2005;11:192–8.
Habl, Zink G, Petroianu M, Bauer G, Schneider-Axmann M, Wilmsdorff T, et al. Increased D-amino acid oxidase expression in the bilateral hippocampal CA4 of schizophrenic patients: a post-mortem study. J Neural Transm. 2009;116:1657–65.
Uranova NA, Zimina IS, Vikhreva OV, Krukov NO, Rachmanova VI, Orlovskaya DD. Ultrastructural damage of capillaries in the neocortex in schizophrenia. World J Biol Psychiatry Off J World Federation Societies Biol Psychiatry. 2010;11:567–78.
Steiner J, Bogerts B, Sarnyai Z, Walter M, Gos T, Bernstein H-G, et al. Bridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: Potential role of glial NMDA receptor modulators and impaired blood-brain barrier integrity. World J Biol Psychiatry Off J World Federation Societies Biol Psychiatry. 2012;13:482–92.
Poels EMP, Kegeles LS, Kantrowitz JT, Javitt DC, Lieberman JA, Abi-Dargham A, et al. Glutamatergic abnormalities in schizophrenia: a review of proton MRS findings. Schizophrenia Res. 2014;152:325–32.
Bryll A, Skrzypek J, Krzyściak W, Szelągowska M, Śmierciak N, Kozicz T et al. Oxidative-Antioxidant Imbalance and Impaired Glucose Metabolism in Schizophrenia. Biomolecules. 2020;10:384.
Roberts RC. Mitochondrial dysfunction in schizophrenia: With a focus on postmortem studies. Mitochondrion. 2021;56:91–101.
Oliveira Figueiredo EC, de, Calì C, Petrelli F, Bezzi P. Emerging evidence for astrocyte dysfunction in schizophrenia. Glia. 2022;70:1585–604.
de Simone G, Mazza B, Vellucci L, Barone A, Ciccarelli M, et al. Schizophrenia Synaptic Pathology and Antipsychotic Treatment in the Framework of Oxidative and Mitochondrial Dysfunction: Translational Highlights for the Clinics and Treatment. Antioxidants. 2023;12:975.
Laricchiuta D, Papi M, Decandia D, Panuccio A, Cutuli D, Peciccia M, et al. The role of glial cells in mental illness: a systematic review on astroglia and microglia as potential players in schizophrenia and its cognitive and emotional aspects. Front Cell Neurosci. 2024;18:1358450.
Stanca S, Rossetti M, Bokulic Panichi L, Bongioanni P. The Cellular Dysfunction of the Brain-Blood Barrier from Endothelial Cells to Astrocytes: The Pathway towards Neurotransmitter Impairment in Schizophrenia. Int J Mol Sci. 2024;25:1250.
Webster MJ, O’Grady J, Kleinman JE, Weickert CS. Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia. Neuroscience. 2005;133:453–61.
Toro CT, Hallak JEC, Dunham JS, Deakin JFW. Glial fibrillary acidic protein and glutamine synthetase in subregions of prefrontal cortex in schizophrenia and mood disorder. Neurosci Lett. 2006;404:276–81.
Feresten AH, Barakauskas V, Ypsilanti A, Barr AM, Beasley CL. Increased expression of glial fibrillary acidic protein in prefrontal cortex in psychotic illness. Schizophrenia Res. 2013;150:252–7.
Catts VS, Wong J, Fillman SG, Fung SJ, Shannon Weickert C. Increased expression of astrocyte markers in schizophrenia: Association with neuroinflammation. Aust NZ J Psychiatry. 2014;48:722–34.
Rodrigues-Amorim, Rivera-Baltanás D, del Carmen Vallejo-Curto T, Rodriguez-Jamardo M, las Heras C, de E, et al. Plasma β-III tubulin, neurofilament light chain and glial fibrillary acidic protein are associated with neurodegeneration and progression in schizophrenia. Sci Rep. 2020;10:14271.
Bruneau EG, McCullumsmith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Increased expression of glutaminase and glutamine synthetase mRNA in the thalamus in schizophrenia. Schizophrenia Res. 2005;75:27–34.
Katsel P, Byne W, Roussos P, Tan W, Siever L, Haroutunian V. Astrocyte and glutamate markers in the superficial, deep, and white matter layers of the anterior cingulate gyrus in schizophrenia. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2011;36:1171–7.
Bernstein H-G, Tausch A, Wagner R, Steiner J, Seeleke P, Walter M. et al. Disruption of glutamate-glutamine-GABA cycle significantly impacts on suicidal behaviour: survey of the literature and own findings on glutamine synthetase. CNS Neurological Disord Drug Targets. 2013;12:900–13.
Miüller N, Schwarz MJ. The immunological basis of glutamatergic disturbance in schizophrenia: towards an integrated view. J Neural Transm Suppl. 2007;72:269–80.
McCullumsmith RE, O’Donovan SM, Drummond JB, Benesh FS, Simmons M, Roberts R, et al. Cell-specific abnormalities of glutamate transporters in schizophrenia: sick astrocytes and compensating relay neurons? Mol Psychiatry. 2016;21:823–30.
Rothermundt M, Ohrmann P, Abel S, Siegmund A, Pedersen A, Ponath G, et al. Glial cell activation in a subgroup of patients with schizophrenia indicated by increased S100B serum concentrations and elevated myo-inositol. Prog Neuro Psychopharmacol Biol Psychiatry. 2007;31:361–4.
O’Donovan SM, Sullivan C, Koene R, Devine E, Hasselfeld K, Moody CL, et al. Cell-subtype-specific changes in adenosine pathways in schizophrenia. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2018;43:1667–74.
Bernstein H-G, Dobrowolny H, Keilhoff G, Bogerts B, Steiner J. Reduced Density of DISC1 Expressing Astrocytes in the Dentate Gyrus but not in the Subventricular Zone in Schizophrenia. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2018;43:457–8.
Bernstein HG, Kirschke H, Wiederanders B, Khudoerkov RM, Hinz W, Rinne A. Lysosomal proteinases as putative diagnostic tools in human neuropathology: Alzheimer disease (AD) and schizophrenia. Acta Histochemica Supplementband. 1992;42:19–24.
Oĭfa AI, Uranova NA. Elektronno-mikroskopicheskiĭ analiz tsitoarkhitektonicheskikh narusheniĭ v kore golovnogo mozga pri shizofrenii. Zh Nevropatologii i Psikhiatrii Im S S Korsakova. 1991;91:48–52.
Uranova NA, Casanova MF, DeVaughn NM, Orlovskaya DD, Denisov DV. Ultrastructural alterations of synaptic contacts and astrocytes in postmortem caudate nucleus of schizophrenic patients. Schizophrenia Res. 1996;22:81–3.
Kolomeets NS, Uranova N. Ultrastructural abnormalities of astrocytes in the hippocampus in schizophrenia and duration of illness: a postortem morphometric study. World J Biol Psychiatry Off J World Federation Societies Biol Psychiatry. 2010;11:282–92.
Popov A, Brazhe N, Morozova K, Yashin K, Bychkov M, Nosova O, et al. Mitochondrial malfunction and atrophy of astrocytes in the aged human cerebral cortex. Nat Commun. 2023;14:8380.
Schnieder TP, Dwork AJ. Searching for neuropathology: gliosis in schizophrenia. Biol Psychiatry. 2011;69:134–9.
Benes FM, Davidson J, Bird ED. Quantitative cytoarchitectural studies of the cerebral cortex of schizophrenics. Arch Gen psychiatry. 1986;43:31–5.
Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55:215–24.
Hercher C, Chopra V, Beasley CL. Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J Psychiatry Neurosci. 2014;39:376–85.
Falkai P, Honer WG, David S, Bogerts B, Majtenyi C, Bayer TA. No evidence for astrogliosis in brains of schizophrenic patients. A post-mortem study. Neuropathol Appl Neurobiol. 1999;25:48–53.
Damadzic R, Bigelow LB, Krimer LS, Goldenson DA, Saunders RC, Kleinman JE, et al. A quantitative immunohistochemical study of astrocytes in the entorhinal cortex in schizophrenia, bipolar disorder and major depression: absence of significant astrocytosis. Brain Res Bull. 2001;55:611–8.
Stevens CD, Altshuler LL, Bogerts B, Falkai P. Quantitative study of gliosis in schizophrenia and Huntington’s chorea. Biol Psychiatry. 1988;24:697–700.
Trépanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry. 2016;21:1009–26.
Xu J. New Insights into GFAP Negative Astrocytes in Calbindin D28k Immunoreactive Astrocytes. Brain Sci. 2018;8:143.
Windrem MS, Osipovitch M, Liu Z, Bates J, Chandler-Militello D, Zou L, et al. Human iPSC Glial Mouse Chimeras Reveal Glial Contributions to Schizophrenia. Cell Stem Cell. 2017;21:195–208.e6.
Liu Z, Osipovitch M, Benraiss A, Huynh NPT, Foti R, Bates J, et al. Dysregulated Glial Differentiation in Schizophrenia May Be Relieved by Suppression of SMAD4- and REST-Dependent Signaling. Cell Rep. 2019;27:3832–.e6.
Akkouh IA, Hribkova H, Grabiec M, Budinska E, Szabo A, Kasparek T, et al. Derivation and Molecular Characterization of a Morphological Subpopulation of Human iPSC Astrocytes Reveal a Potential Role in Schizophrenia and Clozapine Response. Schizophr Bull. 2022;48:190–8.
Koskuvi M, Lehtonen Š, Trontti K, Keuters M, Wu Y-C, Koivisto H, et al. Contribution of astrocytes to familial risk and clinical manifestation of schizophrenia. Glia. 2022;70:650–60.
Hsu S-S, Liang W-Z. Ca2+ signaling as a mechanism of haloperidol-induced cytotoxicity in human astrocytes and assessing the protective role of a Ca2+ chelator. Naunyn-Schmiedeberg’s. Arch Pharmacol. 2020;393:2117–27.
Schmitz I, da Silva A, Bobermin LD, Gonçalves C-A, Steiner J, Quincozes-Santos A. The Janus face of antipsychotics in glial cells: Focus on glioprotection. Exp Biol Med. 2023;248:2120–30.
Zhang X, Wolfinger A, Wu X, Alnafisah R, Imami A, Hamoud A-R et al. Gene Enrichment Analysis of Astrocyte Subtypes in Psychiatric Disorders and Psychotropic Medication Datasets. Cells. 2022;11:3315.
Jeon P, Mackinley M, Théberge J, Palaniyappan L. The trajectory of putative astroglial dysfunction in first episode schizophrenia: a longitudinal 7-Tesla MRS study. Sci Rep. 2021;11:22333.
Lima A, Sardinha VM, Oliveira AF, Reis M, Mota C, Silva MA, et al. Astrocyte pathology in the prefrontal cortex impairs the cognitive function of rats. Mol Psychiatry. 2014;19:834–41.
Vivi E, Di Benedetto B. Brain stars take the lead during critical periods of early postnatal brain development: relevance of astrocytes in health and mental disorders. Mol Psychiatry. 2024;29:2821–33.
Martins-de-Souza D, Lebar M, Turck CW. Proteome analyses of cultured astrocytes treated with MK-801 and clozapine: similarities with schizophrenia. Eur Arch psychiatry Clin Neurosci. 2011;261:217–28.
Ben Achour S, Pascual O. Glia: the many ways to modulate synaptic plasticity. Neurochemistry Int. 2010;57:440–5.
Shao Z, Dyck LE, Wang H, Li X-M. Antipsychotic drugs cause glial cell line-derived neurotrophic factor secretion from C6 glioma cells. J Psychiatry Neurosci. 2006;31:32–7.
Banasr M, Dwyer JM, Duman RS. Cell atrophy and loss in depression: reversal by antidepressant treatment. Curr Opin cell Biol. 2011;23:730–7.
Mao Y-M, Zhang M-D. Augmentation with antidepressants in schizophrenia treatment: benefit or risk. Neuropsychiatr Dis Treat. 2015;11:701–13.
Albert K, Niskanen J, Kälvälä S, Lehtonen Š. Utilising Induced Pluripotent Stem Cells in Neurodegenerative Disease Research: Focus on Glia. Int J Mol Sci. 2021;22:4334.
Schweitzer JS, Song B, Herrington TM, Park T-Y, Lee N, Ko S, et al. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N Engl J Med. 2020;382:1926–32.
Münzel EJ, Williams A. Promoting remyelination in multiple sclerosis-recent advances. Drugs. 2013;73:2017–29.
Mi S, Miller RH, Tang W, Lee X, Hu B, Wu W, et al. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann Neurol. 2009;65:304–15.
Howe CL, Bieber AJ, Warrington AE, Pease LR, Rodriguez M. Antiapoptotic signaling by a remyelination-promoting human antimyelin antibody. Neurobiol Dis. 2004;15:120–31.
Zhang Y, Argaw AT, Gurfein BT, Zameer A, Snyder BJ, Ge C, et al. Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proc Natl Acad Sci USA. 2009;106:19162–7.
Fancy SPJ, Baranzini SE, Zhao C, Yuk D-I, Irvine K-A, Kaing S, et al. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–85.
Loulier K, Ruat M, Traiffort E. Increase of proliferating oligodendroglial progenitors in the adult mouse brain upon Sonic hedgehog delivery in the lateral ventricle. J Neurochem. 2006;98:530–42.
Huang JK, Jarjour AA, Nait Oumesmar B, Kerninon C, Williams A, Krezel W, et al. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci. 2011;14:45–53.
Rosa V de, Secondo A, Pannaccione A, Ciccone R, Formisano L, et al. D-Aspartate treatment attenuates myelin damage and stimulates myelin repair. EMBO Mol Med. 2019;11:e9278.
Messina A, Concerto C, Rodolico A, Petralia A, Caraci F, Signorelli MS. Is It Time for a Paradigm Shift in the Treatment of Schizophrenia? The Use of Inflammation-Reducing and Neuroprotective Drugs-A Review. Brain Sci. 2023;13:957.
Panizzutti B, Skvarc D, Lin S, Croce S, Meehan A, Bortolasci CC et al. Minocycline as Treatment for Psychiatric and Neurological Conditions: A Systematic Review and Meta-Analysis. Int J Mol Sci. 2023;24:5250.
Seki Y, Kato TA, Monji A, Mizoguchi Y, Horikawa H, Sato-Kasai M, et al. Pretreatment of aripiprazole and minocycline, but not haloperidol, suppresses oligodendrocyte damage from interferon-γ-stimulated microglia in co-culture model. Schizophrenia Res. 2013;151:20–8.
Schmitz T, Endesfelder S, Chew L-J, Zaak I, Bührer C. Minocycline protects oligodendroglial precursor cells against injury caused by oxygen-glucose deprivation. J Neurosci Res. 2012;90:933–44.
Magalhães PVS, Dean O, Andreazza AC, Berk M, Kapczinski F. Antioxidant treatments for schizophrenia. Cochrane Database Syst Rev. 2016;2:CD008919.
Yolland CO, Hanratty D, Neill E, Rossell SL, Berk M, Dean OM, et al. Meta-analysis of randomised controlled trials with N-acetylcysteine in the treatment of schizophrenia. Aust NZ J Psychiatry. 2020;54:453–66.
Sedlak TW, Nucifora LG, Koga M, Shaffer LS, Higgs C, Tanaka T, et al. Sulforaphane Augments Glutathione and Influences Brain Metabolites in Human Subjects: A Clinical Pilot Study. Mol Neuropsychiatry. 2018;3:214–22.
Shiina A, Kanahara N, Sasaki T, Oda Y, Hashimoto T, Hasegawa T, et al. An Open Study of Sulforaphane-rich Broccoli Sprout Extract in Patients with Schizophrenia. Clin Psychopharmacol Neurosci Off Sci J Korean Coll Neuropsychopharmacol. 2015;13:62–7.
Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia-a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–8.
Quincozes-Santos A, Santos CL, de Souza Almeida RR, da Silva A, Thomaz NK, et al. Gliotoxicity and Glioprotection: the Dual Role of Glial Cells. Mol Neurobiol. 2021;58:6577–92.
Samaei A, Moradi K, Bagheri S, Ashraf-Ganjouei A, Alikhani R, Mousavi SB, et al. Resveratrol Adjunct Therapy for Negative Symptoms in Patients With Stable Schizophrenia: A Double-Blind, Randomized Placebo-Controlled Trial. Int J Neuropsychopharmacol. 2020;23:775–82.
Tomlinson L, Huang PH, Colognato H. Prefrontal cortex NG2 glia undergo a developmental switch in their responsiveness to exercise. Developmental Neurobiol. 2018;78:687–700.
Vieira R, Mariani JN, Huynh NPT, Stephensen HJT, Solly R, Tate A, et al. Young glial progenitor cells competitively replace aged and diseased human glia in the adult chimeric mouse brain. Nat Biotechnol. 2024;42:719–30.
Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–44.
Kam T-I, Hinkle JT, Dawson TM, Dawson VL. Microglia and astrocyte dysfunction in parkinson’s disease. Neurobiol Dis. 2020;144:105028.
Kertesz A, Munoz DG. Frontotemporal dementia. Med Clin North Am. 2002;86:501–18.
The Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry. 1994;57:416–8.
Atri A. The Alzheimer’s Disease Clinical Spectrum: Diagnosis and Management. Med Clin North Am. 2019;103:263–93.
Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397:2284–303.
Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet. 2015;386:1672–82.
Acknowledgements
We thank Prof. Dr. Ted Abel (Department of Neuroscience and Pharmacology, Carver College of Medicine / Iowa Neuroscience Institute, University of Iowa, IA, USA) for reading the fist manuscript draft and for his scientific advice. Moreover, we are grateful to Prof. Dr. Christian Mawrin (Department of Neuropathology, Otto-von-Guericke-University Magdeburg, Magdeburg, Germany) for reviewing Table 4 and for his expert advice on the neuropathological features of neurodegeneration.
Funding
This work was supported by a doctoral scholarship awarded to MN from the Medical Faculty of the Otto-von-Guericke-University Magdeburg, Magdeburg, Germany (Application no. 531; Funding period: April 1 to October 31, 2024). Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
HGB designed the concept and wrote the first manuscript draft; MN, VV and HD contributed to the review of literature. MN and PCG, supplemented the therapy outlook section and edited the manuscript. VV and HD created the Figures. TNJ and JS contributed to the writing and editing of the manuscript. JS supervised the project. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bernstein, HG., Nussbaumer, M., Vasilevska, V. et al. Glial cell deficits are a key feature of schizophrenia: implications for neuronal circuit maintenance and histological differentiation from classical neurodegeneration. Mol Psychiatry 30, 1102–1116 (2025). https://doi.org/10.1038/s41380-024-02861-6
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41380-024-02861-6
This article is cited by
-
Combining phenomics with transcriptomics reveals cell-type-specific morphological and molecular signatures of the 22q11.2 deletion
Nature Communications (2025)
-
A single dose of haloperidol decanoate induces short-term hippocampal neuroinflammation: focus on the glial response
Pharmacological Reports (2025)
-
Cellular Diversity Underpins Cortical Organization and Disease Vulnerability in the Human Brain
Neuroscience Bulletin (2025)