Abstract
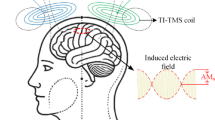
The efficacy of transcranial magnetic stimulation (TMS) for depression may depend on targeting specific brain circuits. However, this has not been tested for TMS with the H1 coil, a widely used device believed to target more broadly and deeply than TMS with figure-of-8 coils. This study examined whether targeting a specific brain circuit with H1 coil TMS treatment may impact depressive symptom improvement. We retrospectively analyzed data from 97 patients at McLean Hospital, who received at least 19 TMS sessions and had incidentally completed an anatomical brain MRI. We modeled each patient’s electric (E-)field using SimNIBS and estimated the connectivity of the E-field using a normative connectome (n = 1000), which was correlated with depression improvement as measured by the Quick Inventory of Depressive Symptomatology. H1 E-fields improving depression were preferentially connected to a distinct brain circuit, validated with leave-one-out cross-validation (p = 0.0005). This circuit was significantly similar to a predefined causal depression circuit (spatial r = 0.59, p = 0.04) derived from TMS, deep brain stimulation, and lesion studies. E-fields with greater connectivity similarity to this circuit led to greater symptom improvement (r = 0.41, p < 0.001). Post-hoc analyses revealed that more posterior coil positioning increases H1 E-field overlap with the depression circuit, with high overlap at scalp locations 3–6 cm anterior to the motor hotspot. Thus, H1 coil stimulation sites that improve depression converge on a common causal depression circuit. Prospective studies are needed to validate these findings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data will be shared upon presenting a research proposal to the corresponding author, and signing an institutional data sharing agreement.
References
Brunoni AR, Chaimani A, Moffa AH, Razza LB, Gattaz WF, Daskalakis ZJ, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiatry. 2017;74:143–52.
Cao X, Deng C, Su X, Guo Y. Response and remission rates following high-frequency vs. low-frequency repetitive transcranial magnetic stimulation (rTMS) over right DLPFC for treating major depressive disorder (MDD): a meta-analysis of randomized, double-blind trials. Front Psychiatry. 2018;9:413.
Sackeim HA, Aaronson ST, Carpenter LL, Hutton TM, Mina M, Pages K, et al. Clinical outcomes in a large registry of patients with major depressive disorder treated with Transcranial Magnetic Stimulation. J Affect Disord. 2020;277:65–74.
Tendler A, Goerigk S, Zibman S, Ouaknine S, Harmelech T, Pell GS, et al. Deep TMS H1 Coil treatment for depression: Results from a large post marketing data analysis. Psychiatry Res. 2023;324:115179.
Fitzgerald PB, Hoy K, Daskalakis ZJ, Kulkarni J. A randomized trial of the anti‐depressant effects of low‐and high‐frequency transcranial magnetic stimulation in treatment‐resistant depression. Depress Anxiety. 2009;26:229–34.
Siddiqi SH, Taylor SF, Cooke D, Pascual-Leone A, George MS, Fox MD. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry. 2020;177:435–46.
Siddiqi SH, Weigand A, Pascual-Leone A, Fox MD. Identification of personalized transcranial magnetic stimulation targets based on subgenual cingulate connectivity: an independent replication. Biol Psychiatry. 2021;90:e55–6.
Williams NR, Sudheimer KD, Bentzley BS, Pannu J, Stimpson KH, Duvio D, et al. High-dose spaced theta-burst TMS as a rapid-acting antidepressant in highly refractory depression. Brain. 2018;141:e18–e18.
Cole EJ, Phillips AL, Bentzley BS, Stimpson KH, Nejad R, Barmak F, et al. Stanford neuromodulation therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179:132–41.
Deng ZD, Lisanby SH, Peterchev AV. Electric field depth–focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul. 2013;6:1–13.
Levkovitz Y, Isserles M, Padberg F, Lisanby SH, Bystritsky A, Xia G, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry. 2015;14:64–73.
Data on file. Brainsway. [Internet]. Available from: https://www.brainsway.com/how-does-it-work/build-deep-vs-traditional-tms/.
Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603.
Weigand A, Horn A, Caballero R, Cooke D, Stern AP, Taylor SF, et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatry. 2018;84:28–37.
Jing Y, Zhao N, Deng X, Feng Z, Huang G, Meng M, et al. Pregenual or subgenual anterior cingulate cortex as potential effective region for brain stimulation of depression. Brain Behav. 2020;10:e01591.
Siddiqi SH, Schaper FL, Horn A, Hsu J, Padmanabhan JL, Brodtmann A, et al. Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nat Hum Behav. 2021;5:1707–16.
Argyelan M, Deng ZD, Ousdal OT, Oltedal L, Angulo B, Baradits M, et al. Electroconvulsive therapy-induced volumetric brain changes converge on a common causal circuit in depression. Mol Psychiatry. 2024;29:229–37.
Zibman S, Pell GS, Barnea-Ygael N, Roth Y, Zangen A. Application of transcranial magnetic stimulation for major depression: coil design and neuroanatomical variability considerations. Eur Neuropsychopharmacol. 2021;45:73–88.
Parazzini M, Fiocchi S, Chiaramello E, Roth Y, Zangen A, Ravazzani P. Electric field estimation of deep transcranial magnetic stimulation clinically used for the treatment of neuropsychiatric disorders in anatomical head models. Med Eng Phys. 2017;43:30–8.
Trivedi MH, Rush A, Ibrahim H, Carmody T, Biggs M, Suppes T, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34:73–82.
Roth Y, Munasifi F, Harvey SA, Grammer G, Hanlon CA, Tendler A. Never Too Late: Safety and Efficacy of Deep TMS for Late-Life Depression. J Clin Med. 2024;13:816.
McClintock SM, Reti IM, Carpenter LL, McDonald WM, Dubin M, Taylor SF, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. 2017;79:3651.
Iglesias JE, Billot B, Balbastre Y, Magdamo C, Arnold SE, Das S, et al. SynthSR: A public AI tool to turn heterogeneous clinical brain scans into high-resolution T1-weighted images for 3D morphometry. Sci Adv. 2023;9:eadd3607.
Iglesias JE, Billot B, Balbastre Y, Tabari A, Conklin J, González RG, et al. Joint super-resolution and synthesis of 1 mm isotropic MP-RAGE volumes from clinical MRI exams with scans of different orientation, resolution and contrast. Neuroimage. 2021;237:118206.
Worbs T, Rumi B, Madsen KH, Thielscher A. Personalized electric field simulations of deformable large TMS coils based on automatic position and shape optimization. bioRxiv. 2024;2024–12.
Puonti O, Van Leemput K, Saturnino GB, Siebner HR, Madsen KH, Thielscher A. Accurate and robust whole-head segmentation from magnetic resonance images for individualized head modeling. Neuroimage. 2020;219:117044.
Johnson KA, Baig M, Ramsey D, Lisanby SH, Avery D, McDonald WM, et al. Prefrontal rTMS for treating depression: location and intensity results from the OPT-TMS multi-site clinical trial. Brain Stimul. 2013;6:108–17.
Ginou A, Roth Y, Zangen A. Comparison of superficial TMS and deep TMS for major depression. Brain Stimul Basic Transl Clin Res Neuromodulation. 2014;7:e19.
Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–97.
Cameron IM, Cunningham L, Crawford J, Eagles J, Eisen S, Lawton K, et al. Psychometric properties of the BASIS-24©(behaviour and symptom identification scale–revised) mental health outcome measure. Int J Psychiatry Clin Pr. 2007;11:36–43.
Lynch CJ, Elbau IG, Ng TH, Wolk D, Zhu S, Ayaz A, et al. Automated optimization of TMS coil placement for personalized functional network engagement. Neuron. 2022;110:3263–77.
Fitzgerald PB. Targeting repetitive transcranial magnetic stimulation in depression: do we really know what we are stimulating and how best to do it? Brain Stimul. 2021;14:730–6.
Carmi L, Alyagon U, Barnea-Ygael N, Zohar J, Dar R, Zangen A. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11:158–65.
Wang Q, Akram H, Muthuraman M, Gonzalez-Escamilla G, Sheth SA, Oxenford S, et al. Normative vs. patient-specific brain connectivity in deep brain stimulation. NeuroImage. 2021;224:117307.
Joutsa J, Moussawi K, Siddiqi SH, Abdolahi A, Drew W, Cohen AL, et al. Brain lesions disrupting addiction map to a common human brain circuit. Nat Med. 2022;28:1249–55.
Cash RF, Zalesky A. Personalized and circuit-based transcranial magnetic stimulation: Evidence, controversies, and opportunities. Biol Psychiatry. 2024;95:510–22.
Pages K, Aaronson ST, Carpenter LL, Hutton T, Rush AJ, Sackeim HA. Comparison of the Patient Health Questionnaire-9 and Quick Inventory of Depressive Symptomatology-Self Report in assessing the antidepressant effects of Transcranial Magnetic Stimulation: Sensitivity to change. Transcranial Magn Stimul. 2024;1:100075.
Funding
The current study was supported by an investigator-initiated grant to SHS from BrainsWay Ltd (Jerusalem, Israel), and the NIH (grant nos. K23MH121657 and R21MH126271).
Author information
Authors and Affiliations
Contributions
SB, GSP, SZ, SHS: conception and design of the work, the analyses, and the interpretation of the results. SB: writing of the manuscript, analysis of the data. NC, SP, SF, JRP: acquisition of the data, analysis of the data. GSP, SZ, SHS, JCB: critical feedback for intellectual content to the manuscript. All authors have approved the manuscript in its final form.
Corresponding author
Ethics declarations
Competing interests
GP and SZ are employed by and have a financial interest in BrainsWay. JCB receives financial support as an editorial stipend from Elsevier. JCB effort is supported by the Brain & Behavior Research Foundation Young Investigator Grant, (#31748), the Cindy & Paul Gamble Fund, the Marlene Zuckerman Fund, the McLean Hospital Center of Excellence in Depression and Anxiety Disorders, and the Department of Defense Advanced Research Projects Agency (HR00112320037). The views, opinions and/or findings expressed are those of the author and should not be interpreted as representing the official views or policies of the Department of Defense or the U.S. Government. SHS serves as a consultant for Kaizen Brain Center, Acacia Mental Health, and Magnus Medical. SHS owns stock in BrainsWay Inc (publicly traded) and Magnus Medical (not publicly traded). SHS owns independent intellectual property on the use of brain network mapping to target neuromodulation. SHS effort is supported by the NIH (grant nos. K23MH121657 and R21MH126271), the Brain and Behavior Research Foundation Young Investigator Grant, the Baszucki Brain Research Fund and the Department of Veterans Affairs (grant no. I01CX002293). The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baldi, S., Chiulli, N., Palm, S. et al. Targeting depression circuitry with H1 coil Transcranial Magnetic Stimulation: a retrospective circuit mapping study. Neuropsychopharmacol. 50, 1674–1682 (2025). https://doi.org/10.1038/s41386-025-02157-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-025-02157-5