Abstract
Background
Peripheral artery disease (PAD) is a common disease associated with atherosclerosis, leading to significant mortality and morbidity worldwide. Our study focuses on the association between insulin resistance (IR) and PAD, specifically investigating the triglyceride-glucose index (TyG) as a potential surrogate marker of IR in the context of PAD by pooling the existing studies on this topic.
Methods
Online databases, including PubMed, Embase, Scopus, and the Web of Science, were searched to find the studies comparing the TyG index in PAD vs. control, reporting the TyG index among PAD severities, and assessing the association of increase in TyG with PAD prevalence. Random-effect meta-analysis was performed to calculate the standardized mean difference (SMD) and 95% confidence interval (CI) for TyG level comparison and to calculate pooled odds ratio (ORs) for a 1-unit increase in TyG and higher vs. lower quartile/tertile of TyG association with PAD.
Results
In the final review, 22 studies comprising 73,168 cases were included. Random-effect meta-analysis showed that patients with PAD had significantly higher levels of the TyG index compared with controls (SMD 0.76, 95%CI 0.65–0.88, P < 0.001). Also, higher severities of PAD were associated with higher TyG levels (SMD 0.48, 95%CI 0.22–0.74, P = 0.0003). Additionally, a 1-unit increase in TyG was associated with a 60% increase in odds of PAD (OR 1.60, 95%CI 1.41–1.80, P < 0.001). Finally, the highest quartile (Q4) of TyG had significantly higher odds of PAD compared to Q1 (OR 1.94, 95%CI 1.49–2.54, P < 0.001).
Conclusion
Our meta-analysis has identified a significant association between TyG levels and PAD and its severity. These findings not only contribute to our understanding of the role of IR in PAD pathology but also offer clinicians an exact index for evaluating PAD risk and its complications. This could potentially lead to more effective prevention and management strategies in the future.
Similar content being viewed by others
Introduction
Peripheral artery disease (PAD) is a central clinical problem affecting about 1.52% of adults older than 40 years while reports estimate > 20% in adults aged 80 or more [1,2,3]. This athero-occlusive condition leads to hind limb ischemia, and despite progress in drug therapies and revascularization, the prognosis remains poor, with almost half of the patients requiring limb amputation [4]. In addition, PAD is often underdiagnosed, with most patients not receiving optimal treatments that are proven to ameliorate prognosis and reduce mortality [5]. To overcome this problem, a diagnostic test such as a sufficiently sensitive and specific blood test would be expected to improve the recognition and treatment of these individuals. However, since PAD shares common risk factors and pathogenetic mechanisms with other cardiovascular diseases, no specific biomarker has yet been identified and implemented in clinical practice [5].
However, in the last decade, particular attention has been focused on the association between diabetes and PAD. Indeed, as demonstrated by clinical and preclinical studies, diabetes in human and animal models of PAD correlates with worsened outcomes [6, 7]. Notably, insulin resistance (IR) is undoubtedly one of the most prominent pathophysiological factors in this association. IR is a typical hallmark of type 2 diabetes mellitus (T2DM), exerting direct effects on the vasculature [8, 9], and as demonstrated by a cross-sectional study by Pande et al. [10], IR is strongly and independently associated with PAD. Similar results were observed by Britton et al. [11] in a population-based study of older American adults (Cardiovascular Health Study).
Interestingly, the studies above assessed IR using the homeostatic model of insulin resistance (HOMA-IR). However, using this method in clinical practice presents several limitations, including costs, time of execution, and invasiveness, making it very challenging, especially for undeveloped countries [12]. Therefore, a more straightforward and low-cost estimating tool, like the triglyceride–glucose (TyG) index, has also been used to identify a relationship between IR and PAD, with results that are not conclusive yet.
A previous systematic review and meta-analysis have shown that a higher TyG index is associated with the incidence of arterial stiffness [13]. Several studies have assessed the correlation between TyG and PAD in terms of diagnosis and prognosis. However, these results have not been pooled and there has been no systematic review that investigated these studies. Based on this premise, as the TyG index, this study aimed to consolidate current evidence regarding the relationship between the TyG index and PAD and test its diagnostic and predictive value for adverse outcomes.
Methods
Protocol and guidelines
The study was registered in PROSPERO (the International Prospective Register for Systematic Reviews) under the registration number (CRD42024548468) and was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [14].
Search strategy and study selection
A thorough systematic search was conducted in international databases including PubMed, Scopus, Web of Science, and Embase using a combination of Medical Subject Headings (MeSH) terms and other keywords to identify all studies published up until the 24th of April 2024 (Supplementary Table 1).
We included studies that (1) evaluated the relationship of TyG index and PAD through quartiles and tertiles and/or by 1-unit increase, (2) measured TyG index in patients with PAD (and a control group), and (3) compared TyG levels based on PAD severity. The following studies were excluded: (1) Review studies, (2) conference abstracts, (3) case reports, (4) non-English language publications, (5) studies not reporting TyG levels, and (6) studies with non-PAD population.
TyG index, as a measure of IR, is calculated from TG and FPG as follows:
The records were merged and duplicates were removed using the EndNote software. Subsequently, two reviewers (AS and AK) separately screened the titles and abstracts of all identified studies before full-text screening relevant records to determine their eligibility. Disagreements between the reviewers were resolved through a third reviewer (AHB).
Data extraction and quality assessment
Data extraction was performed by two independent authors (AS and AK) and any disagreements were resolved through mutual consensus. The following information was extracted and charted: first author’s name, publication year, country of origin, study design, study population, sample size, mean age, male percentage, main findings of each study, and the odds ratios (ORs) with their 95% confidence (CI).
The Newcastle-Ottawa Scale (NOS) was used to assess the quality of the included studies [15]. The three main domains assessed by the NOS include selection, comparability, and outcome. On this scale, a score of ≥ 7 is considered high quality.
Statistical analysis
The statistical analyses were performed in R version 4.3.0 (R Core Team [2021], Vienna, Austria). For comparison of the TyG index in patients with PAD and healthy controls, standardized mean difference (SMD) and its 95% CI were calculated through random-effect meta-analysis. Odds ratios (ORs) and 95% CIs for a 1-unit increase in TyG index association with PAD were also assessed by random-effect meta-analysis. Similarly, the highest vs. lowest quartile/tertile of TyG comparison was made using meta-analysis.
When the median and interquartile range were reported, the mean and standard deviation were calculated using methods developed by Luo and Wan [16, 17]. The bias-corrected Hedges’ g SMD was used to compare the mean TyG index in patients with PAD and controls and groups with different severity levels. We used the I2 statistic to evaluate heterogeneity. I2 > 50% reflected significant heterogeneity. A sensitivity analysis using the leave-one-out method was performed to assess each study’s effects on the overall pooled effect size. For investigation of publication bias, Egger’s test [18] and visual inspection of the funnel plot by trim-and-fill method were used. P < 0.05 was considered statistically significant throughout the analyses.
Results
Literature search and included study characteristics
The initial search led to a total of 247 records (51 results from PubMed, 62 from Embase, 53 from the Web of Science, and 81 from Scopus). After removing duplicates (n = 141), 65 studies were excluded for title and abstract reasons. Other 19 articles were excluded after full-text screening. A complete PRISMA flowchart and description of the search process and screening are shown in Fig. 1.
At the end of this screening process, 22 studies were finally included and analyzed [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. Table 1 illustrates the features of these studies as well as their populations’ baseline characteristics. Studies were conducted between 2018 and 2024, and the leading country was China, with 13 studies [23, 24, 27, 30, 32,33,34,35,36,37,38,39,40], followed by Turkey [19, 21, 25, 31], the United States [22, 28], Taiwan [20], Japan [29], and South Korea [26] with a total population composed of 73,168 cases. Among the studies, three [30, 31] examined patients with PAD and compared them with healthy controls [19], two compared different severities of PAD in terms of the TyG index [21, 25], and the others were population-based studies that included four diabetic populations [20, 30, 32, 34] and two hypertensive ones [27, 35]. Based on the NOS criteria for quality assessment of observational studies, all studies had high quality, as shown in Supplementary Table 2.
TyG levels in patients with PAD vs control
Three studies assessed TyG levels in patients with PAD and compared them with healthy controls [19, 30, 31]. Caliskan et al. [19], in a single-center, observational, retrospective study, analyzed 440 patients (211 PAD and 229 healthy controls) evaluated with color Doppler ultrasonography. Interestingly, the presence of T2DM was higher in the PAD group than in the control group (p < 0.001). In line with this observation, the TyG index was significantly higher in the PAD group than in healthy controls (9.19 ± 0.57 vs. 8.80 ± 0.59; P < 0.001). In addition, these authors demonstrated that the TyG index was an independent parameter of PAD (OR = 1.111, 95% CI = 1.083–1.139; p < 0.001).
For their part, Ning et al. [30] assessed the TyG index in 1040 patients with diabetes, of which 168 were diagnosed with lower extremity arterial disease (LEAD). These authors observed that the TyG index was higher in the LEAD group compared to the control group (9.94 ± 0.78 vs. 9.36 ± 0.70, P < 0.001). In addition, the TyG index was independently associated with LEAD risk in patients with diabetes (OR = 3.92, 95% CI = 2.92–5.26; P < 0.001).
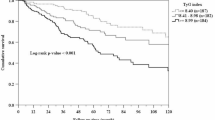
Finally, Pala et al. [31], in a retrospective study, analyzed a population of 296 patients diagnosed with PAD divided into two groups according to the Rutherford Classification (Group 1 (category 0 to 3) and Group 2 (category 4 to 6). Those in group 2 who developed chronic limb-threatening ischemia (CLTI) and had a higher TyG index compared to those in group 1 (9.27 ± 0.31 vs. 9.00 ± 0.34, P < 0.001).
Notably, the meta-analysis performed on these studies showed that patients with PAD had statistically significantly higher levels of the TyG index (SMD 0.76, 95% CI 0.65 to 0.88, P < 0.0001). There was no heterogeneity in this analysis (I2: 0%). The forest plot for this analysis is shown in Fig. 2.
TyG levels in different severities of PAD
Next, we assessed two studies to compare PAD severities regarding the TyG index [21, 25]. In a retrospective observational comparative study, Cora and colleagues [21] analyzed 200 patients divided into two groups (group 1=moderate and group 2=severe) with PAD grouped based on Global Limb Anatomic Staging System (GLASS) criteria. Patients in group 2 had significantly higher TyG index than in group 1 (9.21 ± 0.61 vs. 8.96 ± 0.54, P = 0.040).
Similarly, Karaduman et al. [25] examined the TyG index in 71 patients with PAD. The patients were divided into two groups according to the angiographically detected lesions and the TransAtlantic InterSociety Consensus-II (TASC-II) classification (Group 1 = TASC-II grade A–B and Group 2 = TASC-II grade C–D). Importantly, these authors observed that patients in Group 2 had a higher TyG index than those in Group 1. Finally, a meta-analysis of these studies revealed that the TyG index was associated with the severity of PAD (SMD 0.48, 95% CI 0.22 to 0.74, P = 0.0003, Fig. 3).
Change in risk of PAD by one unit increase in TyG
Seven studies evaluated the association between one unit increase in the TyG index and the risk of PAD using brachial-ankle pulse wave velocity (baPWV) [23, 24, 27, 32, 34, 38, 39]. Of note, the meta-analysis of these studies revealed that one unit increase in the TyG index was associated with a 60% higher risk of PAD (OR 1.60, 95% CI 1.41 to 1.80, P < 0.0001, Fig. 4). This analysis had 69% heterogeneity (P < 0.01). None of the studies had a significant effect on the overall effect size (Supplementary Fig. 1). Finally, Egger’s test did not indicate the presence of publication bias (P = 0.55). However, the funnel plot showed asymmetry and the addition of two studies led to an OR of 1.49 (95% CI 1.29 to 1.71) (Supplementary Fig. 2).
Change in risk of PAD by TyG tertiles
Three studies compared the risk of PAD (high baPWV) in the TyG tertiles [24, 34, 38]. We performed a meta-analysis comparing the risk of PAD between the third and first tertiles of the TyG index in the study population. Our pooled analysis demonstrated that patients in the third tertile of the TyG index had a 72% higher risk of PAD compared to the reference tertile (OR 1.72, 95% CI 1.42 to 2.07, P < 0.0001, Fig. 5). This analysis was not associated with heterogeneity between studies (I2 = 0, P = 0.53).
Change in risk of PAD by TyG quartiles
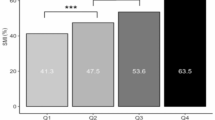
Six studies compared the risk of PAD (high baPWV) in the TyG quartiles [26, 27, 29, 33, 39, 40]. In our meta-analysis, we compared the risk of PAD between the fourth and first quartiles of the TyG index in the study population. In the pooled analysis, we observed that patients in the fourth quartile of the TyG index had a 94% higher PAD risk than the reference quartile (OR 1.94, 95% CI 1.49 to 2.54, P < 0.0001, Fig. 6). This analysis was associated with high heterogeneity between studies (I2 = 85%, P < 0.01). While there was no significant effect of each individual study on the overall effect size by leave-one-out analysis (Supplementary Fig. 3), the funnel plot showed asymmetry for publication bias (Supplementary Fig. 4). The addition of four studies led to higher odds of PAD in Q4 vs. Q1 (OR 1.41, 95% CI 1.00 to 1.97, P = 0.049). However, Egger’s test was not significant for this analysis (P = 0.107).
Diagnostic ability of TyG index for PAD
Two studies explored the diagnostic ability of the TyG index in PAD [19, 23]. In their research, Caliskan et al. [1] found that the TyG index had acceptable predictive ability for PAD (sensitivity: 57.8%, specificity: 70%, AUC: 0.689). In addition, Guo and colleagues’ study found that AUC for predicting the TyG index was 0.708 in women and 0.580 in men [23].
Discussion
This study was the first to systematically examine the potential association between the TyG index and PAD. Accordingly, we performed a comprehensive analysis of 22 out of 247 studies that were exhaustively searched and identified on four leading online databases (Pubmed, Embase, Scopus, and Web of Science), ensuring the inclusion of the most relevant and reliable data. Notably, among the essential findings resulting from our analysis, we report three main results. The first is that a higher TyG index is associated with PAD. Secondly, we revealed that the TyG index was considerably higher in patients with severe PAD compared with those with moderate PAD. Hence, this suggests that the TyG index is a valuable marker of PAD severity. Finally, our analysis revealed that an increase in the TyG index was associated with a more increased risk of PAD, as higher TyG quartiles had elevated rates of PAD.
Diabetes is a worldwide health problem characterized by elevated blood glucose levels induced by defects in insulin production, IR, or both [37, 38]. Consequently, diabetes and IR contribute to developing vascular disorders, including PAD, impairing vasodilation, elasticity of the arterial walls (arterial stiffness), and increasing intima-media thickness and vascular calcification [39]. Importantly, these IR-related vascular dysfunctions are also present in asymptomatic PAD subjects and remain highly undetected, significantly limiting adequate treatments and contributing to overall mortality [40]. Therefore, assessing IR may positively affect PAD patients in terms of therapeutic choice, survival, and morbidity. Among the tools used to evaluate IR, the TyG index remains the best choice because of its low costs, execution time, and safety. In addition, compared to other methods, like HOMA-IR, the TyG index had higher sensitivity in predicting prediabetes/diabetes diagnosis [41] and is more strongly associated with arterial stiffness and atherosclerosis diseases [30]. Therefore, based on this premise, different studies have assessed the association between the TyG index and PAD, and the results from these studies still need to be unified.
Among several methods of IR assessment, the traditional HOMA-IR has been used as a valuable and reliable marker [41]. However, due to difficulties in measuring insulin levels, especially in developing countries with limited resources, the availability of this method of IR assessment has been questioned [41]. On the other hand, TyG index calculation is more financially reasonable and easily available through common laboratory measurements, as it has shown promising results in the identification of IR among normal populations [42, 43]. In line, this index has outperformed HOMA-IR in several studies [43,44,45]. Hence, given the importance of PAD and its assessment, and considering the availability of TyG measurement, its use is suggested in these conditions.
Collectively, our study comprehensively analyzed all these studies and corroborated the importance of assessing the TyG index in the clinical assessment of PAD. In addition, it supports the valuable predictive role in patients at risk of PAD development. In clinical settings, since TG and FPG are routinely measured for patients with high cardiovascular risk, including those with PAD, the calculation of TyG and considering it in the assessment of prognosis in patients with PAD could be easy and reliable if confirmed in future studies.
Strengths and limitations
The fundamental strength of the present study lies in the large number of included studies and cases that can provide substantial evidence and insights for further research. Meta-analysis of continuous increase in TyG and TyG quartiles is another strength of the current review. However, five main limitations need to be acknowledged in this study. First, results may be influenced by study populations and sample sizes. Second, our research did not consider all metabolic conditions that could affect the results. Third, using different TyG index cutoffs and quartiles in various studies may introduce bias. Fourth, most existing studies are cross-sectional, which limits their ability to establish causality. Finally, the few studies included in the meta-analyses of PAD vs. control and PAD severities limit our findings and warrant additional studies. These limitations contribute to the mixed findings and emphasize the necessity for further research to clarify the relationship between the TyG index and PAD.
Conclusion
To date, there is no ideal specific marker for PAD, neither for risk stratification nor for diagnosis. In the last decades, even though the direct role of IR in PAD has not been fully established, a linkage between these conditions has been proven. In this context, we reported the clinical utility of assessing the TyG index, an easy-to-dose IR marker and a valuable index of several IR-related disorders. For instance, we have previously documented the association of this index with several IR-related CV and non-CV disorders, supporting its valuable diagnostic and prognostic role [12, 46,47,48,49,50]. In line with this notion, the present study’s findings provide evidence for a significant association between this index and PAD and demonstrate the usefulness of this index as a predictive marker of PAD incidence. Therefore, our study supports the importance of including the TyG index assessment in the clinical setting to identify individuals at risk for PAD and CVDs in general, in which IR plays a pathogenic role.
Data availability
All data included in this study are shown in this article or supplementary information, any further request is available by contacting the corresponding author.
References
Song P, Fang Z, Wang H, Cai Y, Rahimi K, Zhu Y, et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Health. 2020;8:e721–e9.
Shu J, Santulli G. Update on peripheral artery disease: epidemiology and evidence-based facts. Atherosclerosis. 2018;275:379–81.
GBD 2019 Peripheral Artery Disease Collaborators Global burden of peripheral artery disease and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Glob Health. 2023;11(10):e1553–e65.
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45:S5–67. Suppl S
Cooke JP, Wilson AM. Biomarkers of peripheral arterial disease. J Am Coll Cardiol. 2010;55:2017–23.
Singh MV, Dokun AO. Diabetes mellitus in peripheral artery disease: beyond a risk factor. Front Cardiovasc Med. 2023;10:1148040.
Dokun AO, Chen L, Lanjewar SS, Lye RJ, Annex BH. Glycaemic control improves perfusion recovery and VEGFR2 protein expression in diabetic mice following experimental PAD. Cardiovasc Res. 2014;101:364–72.
Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–32.
Lüscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part II. Circulation. 2003;108:1655–61.
Pande RL, Perlstein TS, Beckman JA, Creager MA. Association of insulin resistance and inflammation with peripheral arterial disease: the National Health and Nutrition Examination Survey, 1999 to 2004. Circulation. 2008;118:33–41.
Britton KA, Mukamal KJ, Ix JH, Siscovick DS, Newman AB, de Boer IH, et al. Insulin resistance and incident peripheral artery disease in the Cardiovascular Health Study. Vasc Med. 2012;17:85–93.
Khalaji A, Behnoush AH, Khanmohammadi S, Ghanbari Mardasi K, Sharifkashani S, Sahebkar A, et al. Triglyceride-glucose index and heart failure: a systematic review and meta-analysis. Cardiovasc Diabetol. 2023;22:244.
Zhong H, Shao Y, Guo G, Zhan Y, Liu B, Shao M, et al. Association between the triglyceride-glucose index and arterial stiffness: A meta-analysis. Medicine. 2023;102:e33194.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford; (2000).
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–805.
Egger M, Smith Davey, Schneider G, Minder M. C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–34.
Caliskan S, Boyuk F. Is Triglyceride-Glucose Index a Valuable Parameter in Peripheral Artery Disease? Cureus. 2023;15:e35532.
Chiu H, Tsai HJ, Huang JC, Wu PY, Hsu WH, Lee MY, et al. Associations between triglyceride-glucose index and micro-and macro-angiopathies in type 2 diabetes mellitus. Nutrients. 2020;12:328.
Çora AR, Çelik E. Relationship between peripheral arterial disease severity determined by the Glass classification and triglyceride-glucose index; novel association and novel classification system. Investig Clin. 2022;63:363–75.
Gao JW, Hao QY, Gao M, Zhang K, Li XZ, Wang JF, et al. Triglyceride-glucose index in the development of peripheral artery disease: findings from the Atherosclerosis Risk in Communities (ARIC) Study. Cardiovasc Diabetol. 2021;20:126.
Guo W, Zhu W, Wu J, Li X, Lu J, Qin P, et al. Triglyceride Glucose Index is associated with arterial stiffness and 10-Year cardiovascular disease risk in a Chinese population. Front Cardiovasc Med. 2021;8:585776.
Ji W, Gao L, Sun P, Jia J, Li J, Wang X, et al. Association of the triglyceride-glucose index and vascular target organ damage in a Beijing community-based population. Front Cardiovasc Med. 2022;9:948402.
Duran Karaduman B, Ayhan H, Keleş T, Bozkurt E. The triglyceride-glucose index predicts peripheral artery disease complexity. Turk J Med Sci. 2020;50:1217–22.
Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, et al. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17:41.
Li M, Zhan A, Huang X, Hu L, Zhou W, Wang T, et al. Positive association between triglyceride glucose index and arterial stiffness in hypertensive patients: the China H-type Hypertension Registry Study. Cardiovasc Diabetol. 2020;19:139.
Liu Y, Chang L, Wu M, Xu B, Kang L. Triglyceride glucose index was associated with the risk of peripheral artery disease. Angiology. 2022;73:655–9.
Nakagomi A, Sunami Y, Kawasaki Y, Fujisawa T, Kobayashi Y. Sex difference in the association between surrogate markers of insulin resistance and arterial stiffness. J Diabetes Complicat. 2020;34:107442.
Ning P, Zeng J, Feng Q, Chen M, Cao H. Triglyceride-Glucose Index as a predictor of lower extremity arterial disease in patients with diabetes: a hospitalized population retrospective study. Ann Vasc Surg. 2024;98:173–81.
Pala AA, Urcun YS. Relationship of triglyceride-glucose index with chronic limb-threatening ischemia in lower extremity peripheral artery disease. Vascular. 2022;30:455–62.
Pan Y, Zhong S, Zhou K, Tian Z, Chen F, Liu Z, et al. Association between diabetes complications and the triglyceride-glucose index in hospitalized patients with Type 2 diabetes. J Diabetes Res. 2021;2021:8757996.
Su Y, Wang S, Sun J, Zhang Y, Ma S, Li M, et al. Triglyceride Glucose Index associated with arterial stiffness in Chinese community-dwelling elderly. Front Cardiovasc Med. 2021;8:737899.
Wang S, Shi J, Peng Y, Fang Q, Mu Q, Gu W, et al. Stronger association of triglyceride glucose index than the HOMA-IR with arterial stiffness in patients with type 2 diabetes: a real-world single-centre study. Cardiovasc Diabetol. 2021;20:82.
Wu Z, Zhou D, Liu Y, Li Z, Wang J, Han Z, et al. Association of TyG index and TG/HDL-C ratio with arterial stiffness progression in a non-normotensive population. Cardiovasc Diabetol. 2021;20:134.
Wu S, Xu L, Wu M, Chen S, Wang Y, Tian Y. Association between triglyceride-glucose index and risk of arterial stiffness: a cohort study. Cardiovasc Diabetol. 2021;20:146.
Yan Y, Wang D, Sun Y, Ma Q, Wang K, Liao Y, et al. Triglyceride-glucose index trajectory and arterial stiffness: results from Hanzhong Adolescent Hypertension Cohort Study. Cardiovasc Diabetol. 2022;21:33.
Yang X, Gao Z, Huang X, Zhang M, Chen Z. The correlation of atherosclerosis and triglyceride glucose index: a secondary analysis of a national cross-sectional study of Japanese. BMC Cardiovasc Disord. 2022;22:250.
Zhang W, Huo W, Hu H, Li T, Yuan L, Zhang J, et al. Dose-response associations of triglyceride to high-density lipoprotein cholesterol ratio and triglyceride-glucose index with arterial stiffness risk. Lipids Health Dis. 2024;23:115.
Zhao S, Yu S, Chi C, Fan X, Tang J, Ji H, et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai Study. Cardiovasc Diabetol. 2019;18:95.
Li X, Wang J, Niu L, Tan Z, Ma J, He L, et al. Prevalence estimates of the insulin resistance and associated prevalence of heart failure among United Status adults. BMC Cardiovasc Disord. 2023;23:294.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304.
Vasques AC, Novaes FS, de Oliveira Mda S, Souza JR, Yamanaka A, Pareja JC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93:e98–e100.
Son D-H, Lee HS, Lee Y-J, Lee J-H, Han J-H. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr, Metab Cardiovasc Dis. 2022;32:596–604.
Luo P, Cao Y, Li P, Li W, Song Z, Fu Z, et al. TyG Index Performs Better Than HOMA-IR in Chinese Type 2 Diabetes Mellitus with a BMI < 35 kg/m2: A Hyperglycemic Clamp Validated Study. Medicine. 2022;58:876.
Azarboo A, Behnoush AH, Vaziri Z, Daneshvar MS, Taghvaei A, Jalali A, et al. Assessing the association between triglyceride-glucose index and atrial fibrillation: a systematic review and meta-analysis. Eur J Med Res. 2024;29:118.
Yang Y, Huang X, Wang Y, Leng L, Xu J, Feng L, et al. The impact of triglyceride-glucose index on ischemic stroke: a systematic review and meta-analysis. Cardiovasc Diabetol. 2023;22:2.
Behnoush AH, Mousavi A, Ghondaghsaz E, Shojaei S, Cannavo A, Khalaji A. The importance of assessing the triglyceride-glucose index (TyG) in patients with depression: A systematic review. Neurosci Biobehav Rev. 2024;159:105582.
Liang S, Wang C, Zhang J, Liu Z, Bai Y, Chen Z, et al. Triglyceride-glucose index and coronary artery disease: a systematic review and meta-analysis of risk, severity, and prognosis. Cardiovasc Diabetol. 2023;22:170.
Behnoush AH, Khalaji A, Ghondaghsaz E, Masrour M, Shokri Varniab Z, Khalaji S, et al. Triglyceride-glucose index and obstructive sleep apnea: a systematic review and meta-analysis. Lipids Health Dis. 2024;23:4.
Author information
Authors and Affiliations
Contributions
AS, AC, and AHB: Writing - original draft/ Conceptualization/ Formal analysis/ Visualization, AKA and ZS: Writing - original draft/Writing - review & editing, AK: Writing - original draft/ Supervision/ Writing - review & editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Samavarchitehrani, A., Cannavo, A., Behnoush, A.H. et al. Investigating the association between the triglyceride-glucose index and peripheral artery disease: a systematic review and meta-analysis. Nutr. Diabetes 14, 80 (2024). https://doi.org/10.1038/s41387-024-00341-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41387-024-00341-y
This article is cited by
-
Triglyceride-glucose-related indices and risk of cardiovascular disease and mortality in individuals with cardiovascular–kidney–metabolic (CKM) syndrome stages 0–3: a prospective cohort study of 282,920 participants in the UK Biobank
Cardiovascular Diabetology (2025)
-
Exploring the obesity paradox in chronic respiratory disease: the mediating effect of triglyceride-glucose index on mortality
npj Primary Care Respiratory Medicine (2025)
-
The association between triglyceride glucose index and the risk of cardiovascular disease in obstructive sleep apnea
Sleep and Breathing (2025)
-
The triglyceride-glucose index, ventricular arrhythmias and major cardiovascular events in patients at high risk of sudden cardiac death
Cardiovascular Diabetology (2024)