Abstract
Elevated plasma levels of trimethylamine N-oxide (TMAO)—a compound derived from diet and the gut microbiome—have been widely studied for their association with diabetes risk and their potential role in disease pathophysiology and complications. However, clinical studies, both prospective and retrospective, have yielded conflicting results. For example, elevated levels of TMAO are frequently linked to an increased risk of cardiovascular and renal complications in individuals with diabetes. However, the robustness and independence of these associations differ across study populations and are influenced by the degree of adjustment for confounding risk factors. Considering insulin’s regulatory effect on FMO3 activity in liver cells, TMAO may serve as a marker of hepatic insulin resistance, which could partially explain its association with diabetes risk. The role of TMAO in diabetes pathology remains controversial; while some studies emphasize its detrimental impact on insulin sensitivity and the progression of diabetes-related complications, others suggest potential protective effects. Investigating the largely unexplored role of TMAO’s precursor, trimethylamine, may help elucidate these discrepancies. This review consolidates clinical and experimental findings to clarify TMAO’s complex mechanistic contributions to diabetes pathology.
Similar content being viewed by others
Introduction
The management of diabetes and its long-term complications has significantly improved over the past decade with the introduction of new therapeutic interventions aimed at optimizing blood glucose control and weight management, such as sodium-glucose co-transporter-2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP-1 RAs), alongside the promotion of healthier lifestyle. This has contributed to improved patient outcomes. However, diabetes remains a significant socioeconomic burden due to its rising prevalence. According to the International Diabetes Federation, nearly half a billion people worldwide are currently living with diabetes, and this figure is projected to increase by 51% by 2045 [1]. Therefore, continuous efforts are being made to fully uncover the underlying pathophysiological processes and to identify new intervention targets or markers for recognizing high-risk individuals.
Recent research on the role of gut microbiota in health and disease has grown rapidly. Studies have demonstrated associations between gut microbial dysbiosis and type 1 diabetes [2], type 2 diabetes [3], and insulin sensitivity [4]. However, the underlying mechanisms and causal relationships remain to be fully established.
Among the potential mediators of microbiota-host interactions, trimethylamine N-oxide (TMAO), a derivative of bacterial metabolism, has garnered significant scientific interest once it has been linked to increased cardiovascular risk [5]. Since then, numerous experimental and clinical studies have explored TMAO’s as a marker and mediator of pathological and/or adaptive processes. Similarly, research on the role of TMAO in the risk assessment of diabetic patients and its involvement in the pathological processes of diabetes and its complications is growing [6]. However, the data are somewhat conflicting, presenting challenges for the development of new diagnostic, prognostic, or treatment strategies in cardiovascular diseases whether using TMAO as a biomarker or a therapeutic target [7].
The purpose of this review is to summarize the available evidence on the role of TMAO in diabetes, encompassing both clinical and experimental studies. Relevant studies evaluating the association between TMAO and diabetes were searched in electronic databases up to June 2024.
TMAO metaorganismal pathway
TMAO is a common organic compound found in animals, plants and fungi [8]. In humans, most TMAO is produced in the liver through the oxygenation of trimethylamine (TMA) by flavin-containing monooxygenase-3 (FMO3). TMA is a toxic and odorous byproduct of gut bacterial metabolism of choline, L-carnitine, betaine, and phosphatidylcholine, which are abundant in red meat [9, 10]. Due to this connection, the TMA/TMAO pathway has been postulated as a potential link between red meat consumption and increased cardiovascular risk [10]. However, fish and other seafood, which are considered healthy, provide a direct source of TMAO, as marine animals accumulate large amount of this molecule [11]. Nonetheless, broad-spectrum antibiotic treatment has been shown to reduce TMAO concentrations in the blood of both humans and laboratory animals, supporting the idea that gut bacteria are the primary source of TMAO in the bloodstream. Plasma levels of TMAO typically range from 3 μmol/L in healthy individuals to 40 μmol/L in those with kidney failure [5, 10, 12]. Kidneys actively excrete TMAO [13], which may explain why kidney function is a major determinant of plasma TMAO concentration [14]. Relatively high inter- and intra-individual variations in plasma TMAO level are observed in humans [15], which may be attributed not only to kidney function but also to amount of dietary choline, carnitine, TMA, and TMAO, as well as metabolic activity of gut microbiota or FMO3.
Numerous experimental studies have been performed to determine whether TMAO promotes cardiovascular disease. TMAO supplementation or its dietary precursors have been shown to increase atherosclerotic plaque formation in mice [16, 17]. However, some studies contradict these findings, with Collins et al. even suggesting a protective effect of TMAO against atherosclerosis [18]. TMAO has also been shown to increase heart failure severity in mice, with some research indicating that it may induce cardiac fibrosis and disrupt heart energy metabolism [19,20,21]. Despite this, studies by Querio et al. [22] and others [23] do not support the damaging effects of TMAO on cardiomyocytes. Therefore, it remains unclear whether elevated TMAO levels in cardiovascular disease reflect its harmful nature or if they are merely a confounder. Alternatively, the increase in TMAO could be part of an adaptive response [24].
It is important to note that TMAO is an osmolyte—a small compound capable of maintaining cell volume and counteracting the protein-destabilizing effects of osmotic and hydrostatic stresses. Deep-sea animals utilize this property of TMAO [25], however, human tissues are subjected to high osmotic and hydrostatic pressures as well. For instance, cells in the kidney medulla function in an environment where osmolarity is three to five times higher than that of plasma. To produce concentrated urine, the kidneys accumulate sodium and urea, creating a high osmotic gradient in the medullary region. It has been reported that kidneys also accumulate TMAO, which may act as an osmotic agent and protect kidney cell proteins from disturbances caused by urea [26, 27].
Osmotic stress also plays a role in pathological conditions such as diabetes, where hyperglycemia can cause significant osmotic changes. In fact, diabetes-associated disturbances in osmoregulation are very likely to contribute to the development of cellular dysfunctions and late diabetic complications [28]. Research has demonstrated that diabetes alters the concentration of osmolytes, e.g. decreases taurine and increases sorbitol intracellular accumulation [28]. Arguably, changes in TMAO concentration observed in diabetes may also reflect these shifts in osmolyte levels. While it remains to be determined whether this is an adaptive response, TMAO has been shown to act as a chemical chaperone in diabetes, a topic that will be explored in more detail later.
Plasma and urine TMAO excretion is increased in diabetes
Although observational studies do not provide direct evidence of causal relationships, identifying biomarkers associated with disease across various patient cohorts is a crucial step in establishing new intervention targets or risk markers. Elevated TMAO levels in diabetes have been reported as early as 1998, when Messana et al. demonstrated higher TMAO concentrations in the urine of diabetic patients, independent of metabolic control, glucosuria, and HbA1c levels [29]. Since then, a number of studies have shown the association between plasma TMAO concentration and diabetes in various cohorts, including population-based [30] as well as different cardiovascular disease cohorts [31, 32]. In 2019, Zhuang et al. conducted a meta-analysis that included 12 studies with a total of 15,314 participants, revealing a dose-dependent association between circulating TMAO levels and diabetes [33]. The analysis included patients with stable coronary artery disease [34, 35], heart failure [36, 37], patients after acute coronary event [38], patients undergoing cardiovascular surgery [39] or coronary angiography [40], as well as population-based [30, 41] and several diabetes case-control studies [42,43,44]. Among these, only one study reported no difference in TMAO levels between diabetic and non-diabetic patients [35].
Subsequent studies have largely confirmed the meta-analysis findings, though some inconsistencies persist. Namely, TMAO levels were associated with diabetes in coronary heart disease patients [45], in obese population [46], in patients at a high risk of cardiovascular events [47], two large cohorts of general population [48, 49] and smaller case-control study [50]. Additionally, TMAO plasma concentrations were found to be higher in patients with type 2 diabetes compared to both healthy controls and patients with acute myocardial infarction [51]. In fact, despite the fact that TMAO was initially recognized with respect to atherosclerosis, there are studies showing no significant association between TMAO and atherosclerotic cardiovascular disease, and suggesting it is the presence or absence of diabetes that has a decisive effect on plasma TMAO levels [48, 52]. In this regard, Yu et al. directly revealed that a history of diabetes magnified the association between TMAO and coronary heart disease, with high TMAO levels associated with odds ratios of 6.21 and 1.56 in diabetic and non-diabetic subjects, respectively [53].
However, some studies demonstrated that TMAO precursors like choline [54] or TMA [55], rather than TMAO itself, are more significantly associated with type 2 diabetes. Huo et al. showed that the TMAO/TMA ratio decreased gradually during the transition from healthy control through T2DM without microalbuminuria to T2DM with microalbuminuria [55]. In fact, virtually none of other above-mentioned studies measured the concentration of TMA so it may be speculated that TMA would turn out to be a better marker of diabetes than TMAO. Additionally, TMAO may not be a suitable marker for diabetes in certain populations, as demonstrated in HIV patients [56]. Last but not least, the vast majority of studies focus on type 2 diabetes, with much weaker evidence supporting an association between TMAO and other types of diabetes. In fact, to the best of our knowledge, currently there are no studies directly demonstrating TMAO elevation in patients with type 1 diabetes. Although some studies [32] included diabetes patients in general, according to definition of diabetes provided by WHO [57], it should be noted that type 2 diabetes accounts for more than 90% of all diabetes cases [58]. Consequently, the demonstrated associations may not be as significant for type 1 diabetes patients.
Several studies have also examined the correlation between TMAO and gestational diabetes mellitus (GDM). A study by Lin et al. found that TMAO levels in maternal and cord plasma were elevated in women with GDM. Intriguingly, the study suggested a protective role for TMAO in promoting placental and fetal development by inhibiting neutrophil extracellular trap (NET) formation [59]. Another study reported an association between TMAO levels in maternal blood, but not in cord plasma, with GDM [60]. Conversely, Barzilay et al. observed differences in TMAO concentrations between the GDM and control groups only in cord blood, not in maternal blood [61]. On the other hand, two other research groups found no association between TMAO and GDM [62, 63]. Furthermore, Huo et al. demonstrated that it was TMA that was linearly associated with GDM, whereas TMA precursors and TMAO were inversely associated with GDM. Interestingly, the authors suggested that the association between TMAO and the risk of GDM may be U-shaped [64].
Observational studies play a crucial role in identifying associations; however, they are subject to significant limitations. One major limitation is the presence of confounding factors, as variables such as dietary patterns, kidney function, age, and comorbidities can significantly influence TMAO levels. Additionally, the issue of reverse causality must be considered, wherein disease states may influence TMAO production or clearance, rather than TMAO being the causal factor in disease development. Furthermore, substantial heterogeneity across studies complicates the interpretation of results. Variability in study populations, methods used to measure TMAO, and definitions of health outcomes contribute to inconsistencies, making it challenging to draw definitive conclusions from observational research.
TMAO and risk of diabetes
With the growing, albeit inconsistent, evidence linking TMAO with diabetes, research is increasingly focused on evaluating TMAO’s potential as a risk biomarker for incident diabetes (see Table 1). Prospective cohort studies address many limitations of cross-sectional research and show temporal relationships between baseline TMAO levels and subsequent diabetes development. Regarding type 2 diabetes, two research groups identified a positive association between higher baseline TMAO levels and an increased risk of developing diabetes [65, 66]. In the study by Huang et al., both initial TMAO levels and long-term changes in TMAO were associated with incident type 2 diabetes [66]. However, three other studies found no such association [67, 68], although TMAO correlated with markers of insulin sensitivity (HOMA2-IR, fasting insulin). An analysis by Friedrich et al. suggests that sex differences may contribute to these discrepancies. They found that increased TMAO levels were associated with a higher risk of diabetes, but only in women, not in men [69]. Complicating matters further, Papandreou et al. reported a negative association, indicating that higher TMAO levels were linked with a lower risk of future diabetes [70]. This contradictory finding highlights the complexity of TMAO metabolism and suggests potential protective mechanisms that may operate under certain conditions. Also, high intra- and inter-individual variability of TMAO concentrations in diabetic patients may partially explain these inconsistent observations [71].
Similarly, studies evaluating the risk of developing GDM in pregnancy reported contradictory results [72, 73]. Moreover, Liu et al. found that it was actually TMA, rather than TMAO, that was exaggerating the risk of GDM [73], once again highlighting this direct TMAO precursor as a potentially more reliable biomarker candidate.
Importantly, the conflicting results surrounding TMAO provide no definitive insight into its causal role in diabetes pathogenesis. To address this, Jia et al. conducted a bidirectional Mendelian randomization analysis to explore the potential causal effect of TMAO on diabetes. Despite the limitations of Mendelian randomization like genetic pleiotropy, it can still offer a more robust estimate of causality than conventional observational studies. Utilizing summary data from genome-wide association studies, they found that genetically predicted higher TMAO levels were not associated with an increased risk of type 2 diabetes or other cardiometabolic diseases. Instead, they observed reverse causality, suggesting that it is diabetes and kidney disease that elevate TMAO levels [74]. Miao et al. proposed the mechanism of this reverse causality showing that insulin resistance upregulates the activity of FMO-3 and hence increases TMAO levels. Specifically, they demonstrated that one of insulin’s most significant effects on the mouse liver in vivo is to suppress FMO3 and TMAO, a function that is impaired in insulin-resistant states [75]. Therefore, TMAO may be an indicator of hepatic insulin resistance, which partially explains the TMAO-associated risk of cardiovascular events and diabetes. Although studies have often adjusted for obesity-related factors, such as body mass index and diabetes, these adjustments likely do not fully encompass the impact of hepatic insulin resistance [76]. The identification of TMAO as a potential biomarker of hepatic insulin resistance offers a novel perspective on its clinical utility, suggesting it may provide information about specific metabolic dysfunctions that complement traditional diabetes markers.
Adding to this already complex picture, it has been repeatedly reported that, unlike TMAO and choline [77], another TMAO precursor, betaine, is negatively correlated with diabetes risk [49, 61, 78]. This raises the interesting possibility that diabetes may alter gut microbiota metabolism of these compounds, potentially inhibiting the two-step oxidation of choline to betaine, consequently enhancing the formation of TMA from choline.
TMAO and cardiovascular risk in diabetic patients
Diabetes significantly increases the risk of atherosclerotic cardiovascular disease compared to the general population [79]. Given the growing data regarding linkage between TMAO and cardiovascular disease, the role of this metabolite in identifying high-risk individuals within the diabetic population warrants close examination. Substantial evidence point to TMAO as a very promising cardiovascular risk biomarker [80, 81]. However, in the context of diabetes, data has yielded rather mixed results, offering no clear consensus on this matter.
Table 2 summarizes the available studies exploring the relationship between TMAO and cardiovascular risk in individuals with diabetes. Several retrospective observational studies have demonstrated a correlation between elevated TMAO levels and an increased risk of various adverse cardiovascular outcomes in people with type 2 diabetes [38, 82, 83]. These findings have been corroborated by several prospective cohort studies [44, 84, 85]. Interestingly, some research suggests that diabetes may amplify the relationship between elevated TMAO levels and increased cardiovascular risk [38, 86]. Conversely, other studies have reported that this association does not hold true for individuals with a history of diabetes [87, 88]. In line with these latter findings, some studies conducted on diabetic cohorts have found no significant association between TMAO levels and cardiovascular risk [89,90,91,92]. For instance, Schrauben et al. demonstrated that neither plasma TMAO levels nor the fractional excretion of TMAO were linked to an increased incidence of atherosclerotic cardiovascular disease or heart failure [90].
A critical factor to consider is the strong association between plasma TMAO levels and kidney function, which has been well-documented in patients with type 2 diabetes [93, 94]. This association represents a significant confounding factor in TMAO-based risk assessment, as reduced kidney function is independently associated with both elevated TMAO levels and increased cardiovascular risk. Consequently, it is not surprising that many studies observe an attenuation or even a complete loss of the previously observed associations between TMAO and cardiovascular risk after adjusting for estimated glomerular filtration rate (eGFR). For example, in a large prospective cohort study, Wargny et al. initially found that TMAO was associated with an increased risk of heart failure requiring hospitalization. However, after adjusting for eGFR, the association became insignificant [95]. A similar observation was reported by Winther et al. in patients with type 1 diabetes, where the association between TMAO levels and mortality or cardiovascular events was abolished after accounting for baseline eGFR [96].
In summary, while elevated TMAO levels are frequently associated with heightened cardiovascular and renal risks in diabetic patients, the strength and independence of these associations vary depending on the study population and the adjustments made for other risk factors. Especially, stratification approaches that incorporate both TMAO levels and eGFR may provide more accurate risk prediction than either parameter alone.
TMAO and other diabetic complications
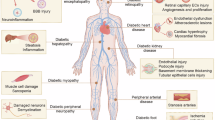
Recent research has also explored the relationship between TMAO and various diabetic complications and comorbidities beyond cardiovascular outcomes. TMAO levels have been found to be significantly higher in patients with diabetic retinopathy compared to those with nondiabetic retinopathy, healthy controls, and those with type 2 diabetes mellitus without retinopathy [97]. Additionally, elevated plasma TMAO levels have been associated not only with an increased risk of developing diabetic retinopathy but also with greater severity of the condition in patients with type 2 diabetes [98]. Although further research is needed to establish causality, a study by Xue et al. suggests that elevated TMAO in patients with proliferative diabetic retinopathy may exacerbate retinal dysfunction, as demonstrated in vitro using retinal cells [99].
Beyond retinopathy, TMAO has also been linked to other diabetic complications. For instance, higher TMAO levels have been associated with increased stroke severity [100] and mild cognitive impairment in the diabetic patients [101]. TMAO levels have also been correlated with osteoporotic complications, including low bone mineral density, osteoporosis, and osteoporotic fractures in patients with type 2 diabetes [102]. However, contrasting findings have been reported, with some studies suggesting that TMAO may protect against bone mineral density reduction during weight loss, independent of dietary interventions and baseline diabetes risk factors [103].
Figure 1 summarizes links between elevated TMAO levels and diabetic complications.
Impact of pharmacological treatments and lifestyle interventions on plasma TMAO Levels
The concentration of TMAO in the body can be influenced by various therapies and lifestyle changes. In the context of diabetes, special consideration should be given to drugs like metformin and SGLT2 inhibitors, which are key therapeutic agents in the treatment of this condition. Studies indicate that while metformin effectively lowers TMAO levels in mice [104, 105], it may conversely increase these levels in humans [106, 107]. In patients with newly diagnosed type 2 diabetes, treatment with DPP-4 inhibitor does not significantly affect gut microbiota composition, whereas metformin promotes bile acid production, which may enhance the microbiota’s capacity to produce TMAO precursors [106]. On the other hand, CCL4 inhibition leads to changes in gut microbiota and normalizes the lipid profile, ultimately reducing TMAO levels [108]. GLP-1 receptor agonists and SGLT2 inhibitors can also lower TMAO levels while simultaneously improving metabolic control [109, 110]. However, inhibiting SGLT2, when used after myocardial infarction, may paradoxically increase TMAO levels [111].
Lifestyle interventions, which are a key component of diabetes management, also appear to regulate TMAO levels. High-intensity exercise [50], and a vegan diet have been shown to lower TMAO levels, positively impacting cardiovascular health [50, 112, 113]. In contrast, a Western diet, rich in fats and meat, tends to increase this metabolite [106, 114, 115], although fish consumption was associated with significantly greater increases in circulating TMAO than consumption of eggs or beef [116]. Interestingly, some data suggest that Mediterranean diet, along with interventions aimed at increasing the intake of fish, vegetables, and whole grains, does not significantly affect TMAO levels [106, 115, 117, 118]. This suggests that TMAO cannot be regarded as a universal biomarker for cardiometabolic risk independent of dietary factors.
Given the frequent co-occurrence of obesity with type 2 diabetes, the impact of bariatric surgery on TMAO levels also deserves attention. One study indicated that vertical sleeve gastrectomy (VSG) results in reduced TMAO levels [119], while other studies have found that both VSG and Roux-en-Y gastric bypass (RYGB) significantly reduce body weight without necessarily lowering TMAO levels [106, 117]. In some cases, these surgeries even resulted in increased TMAO levels, particularly in patients with type 2 diabetes following RYGB surgery [120]. Other research suggests that TMAO levels may decrease in the long term, depending on the type of surgery and individual patient characteristics [112]. Additionally, there are potentially more effective alternatives to invasive treatments, such as phospholipase A2, group 1B (PLA2G1B) enzyme inactivation and the use of its inhibitors, which have been shown to effectively reduce TMAO levels and offer metabolic health benefits [119].
In conclusion, the varied effects of therapeutic and dietary interventions on TMAO levels underscore the importance of considering diet, lifestyle, and diabetes treatment context when evaluating TMAO’s role as a cardiometabolic risk marker.
The role of TMAO in diabetes – harmful or protective?
The association between TMAO and diabetes risk raises critical questions about its role in disease etiology. Specifically, it remains unclear whether TMAO actively contributes to the development of diabetes, functions as an adaptive response, or merely reflects underlying pathological processes. This ambiguity has fueled increasing scientific interest in investigating the impact of TMAO on glucose homeostasis.
Several studies suggest that the molecule may play a detrimental role in diabetes. For instance, TMAO treatment has been shown to worsen kidney function, inflammation, and fibrosis in rats with diabetic kidney disease [121].
Additionally, TMAO has been shown to exacerbate diabetic retinopathy by enhancing the proliferation, migration, and tube formation of retinal cells. It also disrupts vascular integrity and impairs tight junctions, further contributing to the progression of retinal vascular complications [122]. Gao et al. further demonstrated that TMAO impairs glucose tolerance, disrupts hepatic insulin signaling pathways, and induces inflammation in adipose tissue in mice fed a high-fat diet [123, 124]. Supporting these findings, a recent study by Kong et al. revealed that TMAO directly decreases glucose-stimulated insulin secretion (GSIS) in MIN6 cells and primary islets from both mice and humans. The study further demonstrated that prolonged exposure to a TMAO precursor, choline, induced significant β-cell dysfunction. This included endoplasmic reticulum (ER) stress, dedifferentiation, apoptosis, and the disruption of β-cell transcriptional identity, highlighting its potential role in impairing β-cell function. Interestingly, reducing TMAO levels by knocking down FMO3 improved β-cell GSIS, increased β-cell proportion, and enhanced glucose tolerance in both male db/db mice and mice fed a choline-enriched diet [125]. Regarding the potential mechanisms of TMAO’s action, it has been suggested that TMAO can directly bind to and activate protein kinase R-like endoplasmic reticulum kinase (PERK), an ER stress kinase, which subsequently induces Forkhead box protein O1 (FoxO1). Inhibition of FMO3, which reduces TMAO levels, has been shown to decrease PERK and FoxO1 activation, ultimately improving glucose tolerance [126]. Similarly, other studies have reported that TMAO induces the PERK-EIF2α-ER stress signaling axis in ex vivo slices and db/db mice [127] and that administration of a Chinese herb ameliorated diabetic cardiomyopathy in mice by reducing serum TMAO levels and suppressing the TMAO/PERK/FoxO1 signaling pathway [128].
Conversely, compelling evidence also suggests a protective role for TMAO in the context of diabetes. Notably, chronic subcutaneous administration of TMAO has been shown to enhance glucose tolerance in mice on a high-fat diet. Additionally, TMAO directly stimulates insulin secretion in isolated pancreatic islets, indicating potential benefits in glucose regulation [129]. While these findings appear to contradict previously mentioned studies, they align with earlier research by Bai et al. from 1998, which demonstrated that subcutaneous or intraperitoneal TMAO injections lower blood glucose levels [130]. These results also support the growing body of evidence that highlights TMAO’s role as a chemical chaperone, capable of reducing endoplasmic reticulum (ER) stress in β-cells. Specifically, TMAO has been found to alleviate ER stress in INS-1 cells [131] and to protect β-cells and primary islet function under diabetic glucolipotoxic conditions by restoring insulin production, insulin granule formation, and insulin secretion [132]. Moreover, TMAO has shown protective effects in liver cells by reducing ER stress and mitigating lipid-induced disruptions in insulin signaling [133]. As a chemical chaperone, TMAO also plays a crucial role in preventing protein misfolding and aggregation, which is particularly relevant in the pathogenesis of diabetes. For instance, TMAO has been demonstrated to counteract the formation of amyloid plaques from human islet amyloid polypeptide (amylin) [134, 135]. Misfolding and aggregation of amylin lead to amyloid fibril formation, causing β-cell dysfunction or death, which contributes to the development of type 2 diabetes [134]. TMAO significantly enhances the formation of properly folded and compact structures of amylin, thereby preventing its harmful aggregation [135]. Evidence also exists that challenges the notion of TMAO exacerbating diabetic complications. For instance, TMAO treatment has been found to mitigate oxidative-nitrative stress in diabetic peripheral neuropathy, suggesting a potential protective effect against certain diabetes-related complications [136] and diabetes-induced impairment in gap junctional communication among astrocytes [137]. Additionally, TMAO has been reported to ameliorate body mass loss and reproductive complications associated with diabetes in rats [138]. Finally, TMAO has been found to improve diabetic wound healing by inhibiting the formation of neutrophil extracellular traps (NETs) and promoting angiogenesis [139], similar to its already mentioned beneficial effects observed in placental and fetal development [59].
What might account for the discrepancies observed in research on the role of TMAO? We propose three potential explanations.
Firstly, in accordance with a fundamental principle of toxicology, the toxicity of a substance is determined by its dose. It is plausible that TMAO functions as an adaptive, protective mechanism up to a certain concentration, beyond which its harmful effects become apparent. For instance, in the aforementioned study by Krueger et al., TMAO at a concentration of 40 µM demonstrated protective effects on islet function. In contrast, β-cells exposed to higher concentrations of 80 and 160 µM exhibited impaired mitochondrial viability and reduced glucose-stimulated insulin secretion (GSIS) [132]. Accordingly, many studies showing its negative effect have used rather supraphysiological doses of TMAO [121,122,123,124]. Conversely, Chen et al. suggest that TMAO reduces endoplasmic reticulum stress only at very high (pharmacological) concentrations, such as 300 mM, whereas pathophysiological concentrations (50 mM) do not produce the same effect [126]. All above suggests that TMAO has concentration-dependent biphasic effects.
Secondly, a recent study by Li et al. suggests that the divergent effects of TMAO may be influenced by the route of administration. Specifically, they demonstrated that oral administration of TMAO, but not intraperitoneal injection, resulted in impaired glucose tolerance, elevated lipid levels, and chronic inflammation in adipose tissue [140]. Oral TMAO administration may confound results due to gut microbial conversion to TMA, whereas subcutaneous/intraperitoneal routes isolate TMAO-specific effects. Indeed, virtually all studies showing damaging effect of TMAO have used oral administration of TMAO or its precursors [121,122,123,124,125], while after intraperitoneal or subcutaneous administration only the protective effects were observed [129, 130]. Li et al. propose that after oral gavage, TMAO is converted by gut microbiota into TMA, which is the truly harmful compound. Their findings indicate that TMA, rather than TMAO, may impair glucose tolerance [140]. Arguably, it is a decrease in TMA that is responsible for various positive effects of dimethylbutanol (DMB, an inhibitor of choline to TMA conversion in the gut), such as improved glucose tolerance and insulin sensitivity [141] and decreased the vulnerability of diabetic rats to atrial fibrillation [142], even though many researchers attribute these effects to reduced TMAO levels. Although TMA is increasingly recognized as a damaging agent [23, 143,144,145], its role in cardiometabolic pathology remains largely unexplored.
Lastly, it has been suggested that FMO3, the enzyme responsible for converting TMA into TMAO, might exert harmful effects independently of TMAO levels [76], as it may directly boost hepatic lipogenesis and gluconeogenesis [146], and support the expression of FoxO1, a key node responsible for the insulin-resistant state [75]. However, other studies indicate the protective effect of FMO3, such as reducing lipid-induced endoplasmic reticulum stress in the liver, independent of insulin signaling pathways [147, 148].
In summary, while some studies suggest that TMAO exacerbates insulin resistance and accelerates disease progression, others propose that it may have protective effects. This discrepancy leaves a key question unresolved: Is TMAO itself pathogenic, or does it simply serve as a biomarker reflecting underlying metabolic dysfunction? It is plausible that elevated TMAO levels primarily indicate hepatic insulin resistance, with their concentrations largely influenced by renal function and dietary intake, rather than playing a direct causal role in the pathogenesis of diabetes.
The possible mechanisms regarding TMAO role in diabetes are summarized in Fig. 2.
Details in the text. ER stress endoplasmic reticulum stress, FoxO1 Forkhead box protein O1, FMO3 Flavin-containing monooxygenase 3, GDM gestational diabetes mellitus, NET neutrophil extracellular traps, PERK protein kinase R-like endoplasmic reticulum kinase, TMA trimethylamine, TMAO trimethylamine N-oxide.
Clinical applications
Despite extensive research, the immediate clinical utility of TMAO in diabetes management remains limited due to several factors. The substantial variability in TMAO levels—affected by diet, kidney function, and microbial metabolism—complicates the establishment of standardized reference ranges. Additionally, the bidirectional relationship between TMAO and metabolic dysfunction suggests that elevated levels may reflect a consequence, rather than a cause, of diabetes, reducing its potential as an intervention target.
However, emerging evidence highlights potential applications in specific clinical contexts. In patients with established diabetes, TMAO may serve as a complementary biomarker for hepatic insulin resistance, offering insights not captured by traditional glycemic measures. Furthermore, its association with diabetic complications suggests it could identify high-risk individuals for intensified monitoring and intervention, such as those with elevated TMAO and impaired kidney function who may be at increased risk for cardiovascular complications.
The impact of therapeutic interventions on TMAO metabolism also warrants attention. Research indicates that common diabetes medications—such as metformin, GLP-1 receptor agonists, and SGLT2 inhibitors—affect TMAO levels differently. Understanding these drug-specific effects may help optimize therapeutic strategies.
Several promising research directions may enhance TMAO’s clinical utility. Comprehensive metabolic profiling, simultaneously measuring TMAO, TMA, and related precursors (choline, carnitine, betaine), could improve risk stratification compared to isolated TMAO assessment. The varying associations of these metabolites with diabetes risk suggest that a metabolic signature approach may outperform single biomarker strategies.
Longitudinal studies assessing changes in TMAO levels could clarify whether these fluctuations predict disease progression or therapeutic response, offering more insight than static measurements. Integration with emerging biomarkers of insulin resistance and β-cell function may further refine predictive accuracy.
Finally, the role of TMAO in different diabetes phenotypes warrants further exploration. While most research has focused on type 2 diabetes, data on type 1 and monogenic forms are limited. Understanding whether TMAO associations vary by diabetes subtype could improve risk stratification and uncover distinct pathophysiological mechanisms. Additionally, investigating genetic variants in TMA-TMAO pathway enzymes may identify high-risk subpopulations for targeted intervention.
Perspectives
Given that patients with diabetes often have elevated levels of TMAO in their blood, the scientific community has been driven by a “gut feeling,” prompting numerous studies to explore the association between TMAO and diabetes risk, as well as its direct effects on diabetes pathophysiology and complications. However, the results have been somewhat conflicting. It is plausible that TMAO is merely an indicator of hepatic insulin resistance, largely influenced by kidney function and diet. The role of TMAO in diabetes pathology remains controversial. Some studies suggest it worsens insulin tolerance and diabetes complications, while others indicate potential protective effects. At the core of the issue lies a fundamental question: What is the true harmful agent—TMAO, its precursor TMA, or the liver enzyme FMO3 itself? This critical question emphasizes the need for further research, particularly regarding the direct effects of TMA on glucose homeostasis.
Currently, there is insufficient evidence to classify TMAO as a direct pathogenic factor in diabetes. However, TMAO shows strong potential as a complementary biomarker for clinical risk stratification, as it may reflect aspects of metabolic dysfunction that are not captured by traditional markers like blood glucose levels.
As research into the role of TMAO in diabetes advances, it underscores the complex relationship between gut microbiota and host metabolism. With improved analytical methods and broader access to metabolic profiling, TMAO and related metabolites could become valuable tools for more personalized approaches to assessing diabetes risk, subtyping, and monitoring treatment responses.
References
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107843.
Abdellatif AM, Sarvetnick NE. Current understanding of the role of gut dysbiosis in type 1 diabetes. J diabetes. 2019;11:632–44.
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60.
Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–81.
Tang WW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–84.
Du L, Li Q, Yi H, Kuang T, Tang Y, Fan G. Gut microbiota-derived metabolites as key actors in type 2 diabetes mellitus. Biomed Pharmacother. 2022;149:112839.
Nowiński A, Ufnal M. Trimethylamine N-oxide: a harmful, protective or diagnostic marker in lifestyle diseases?. Nutrition. 2018;46:7–12.
Ufnal M, Zadlo A, Ostaszewski R. TMAO: a small molecule of great expectations. Nutrition. 2015;31:1317–23.
Zeisel SH, Youssef M, Hensey S. Conversion of dietary choline to trimethylamine and dimethylamine in rats: dose-response relationship. J Nutr. 1989;119:800–4.
Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85.
Niizeki N, Tanimoto S. Trimethylamine N-Oxide (TMAO) in Seafoods: Shared Mechanisms Between Fish and Humans for Forming Gut-Microbial TMAO: Overview of Animal TMAO-Yield Potential. Food Rev Int. 2024;40:3471–86.
Bain MA, Faull R, Fornasini G, Milne RW, Evans AM. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant. 2006;21:1300–4.
Teft WA, Morse BL, Leake BF, Wilson A, Mansell SE, Hegele RA, et al. Identification and characterization of trimethylamine-N-oxide uptake and efflux transporters. Mol Pharmaceutics. 2017;14:310–8.
Missailidis C, Hällqvist J, Qureshi AR, Barany P, Heimbürger O, Lindholm B, et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PloS One. 2016;11:e0141738.
Kühn T, Rohrmann S, Sookthai D, Johnson T, Katzke V, Kaaks R, et al. Intra-individual variation of plasma trimethylamine-N-oxide (TMAO), betaine and choline over 1 year. Clin Chem Lab Med. 2017;55:261–8.
Geng J, Yang C, Wang B, Zhang X, Hu T, Gu Y, et al. Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomed Pharmacother. 2018;97:941–7.
Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63.
Collins HL, Drazul-Schrader D, Sulpizio AC, Koster PD, Williamson Y, Adelman SJ, et al. L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE−/− transgenic mice expressing CETP. Atherosclerosis. 2016;244:29–37.
Yang W, Zhang S, Zhu J, Jiang H, Jia D, Ou T, et al. Gut microbe-derived metabolite trimethylamine N-oxide accelerates fibroblast-myofibroblast differentiation and induces cardiac fibrosis. J Mol Cell Cardiol. 2019;134:119–30.
Li Z, Wu Z, Yan J, Liu H, Liu Q, Deng Y, et al. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Laboratory Investig. 2019;99:346–57.
Makrecka-Kuka M, Volska K, Antone U, Vilskersts R, Grinberga S, Bandere D, et al. Trimethylamine N-oxide impairs pyruvate and fatty acid oxidation in cardiac mitochondria. Toxicol Lett. 2017;267:32–8.
Querio G, Antoniotti S, Levi R, Gallo MP. Trimethylamine N-oxide does not impact viability, ROS production, and mitochondrial membrane potential of adult rat cardiomyocytes. Int J Mol Sci. 2019;20:3045.
Jaworska K, Hering D, Mosieniak G, Bielak-Zmijewska A, Pilz M, Konwerski M, et al. TMA, a forgotten uremic toxin, but not TMAO, is involved in cardiovascular pathology. Toxins. 2019;11:490.
Ufnal M, Nowiński A. Is increased plasma TMAO a compensatory response to hydrostatic and osmotic stress in cardiovascular diseases?. Med Hypotheses. 2019;130:109271.
Yancey PH, Blake WR, Conley J. Unusual organic osmolytes in deep-sea animals: adaptations to hydrostatic pressure and other perturbants. Comparative Biochem Physiol Part A Mol Integr Physiol. 2002;133:667–76.
Wang A, Bolen D. A naturally occurring protective system in urea-rich cells: mechanism of osmolyte protection of proteins against urea denaturation. Biochemistry. 1997;36:9101–8.
Yancey P, Rhea M, Kemp K, Bailey DM. Trimethylamine oxide, betaine and other osmolytes in deep-sea animals: depth trends and effects on enzymes under hydrostatic pressure. Cell Mol Biol. 2004;50:371–6.
Hansen SH. The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev. 2001;17:330–46.
Messana I, Forni F, Ferrari F, Rossi C, Giardina B, Zuppi C. Proton nuclear magnetic resonance spectral profiles of urine in type II diabetic patients. Clin Chem. 1998;44:1529–34.
Gruppen EG, Garcia E, Connelly MA, Jeyarajah EJ, Otvos JD, Bakker SJ, et al. TMAO is associated with mortality: impact of modestly impaired renal function. Sci Rep. 2017;7:13781.
Sanchez-Alcoholado L, Castellano-Castillo D, Jordán-Martínez L, Moreno-Indias I, Cardila-Cruz P, Elena D, et al. Role of gut microbiota on cardio-metabolic parameters and immunity in coronary artery disease patients with and without type-2 diabetes mellitus. Front Microbiol. 2017;8:1936.
Dambrova M, Latkovskis G, Kuka J, Strele I, Konrade I, Grinberga S, et al. Diabetes is associated with higher trimethylamine N-oxide plasma levels. Exper Clin Endocrinol diabetes. 2016;124:251–6.
Zhuang R, Ge X, Han L, Yu P, Gong X, Meng Q, et al. Gut microbe–generated metabolite trimethylamine N-oxide and the risk of diabetes: a systematic review and dose-response meta-analysis. Obesity Rev. 2019;20:883–94.
Senthong V, Wang Z, Li XS, Fan Y, Wu Y, Wilson Tang W, et al. Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE-like patient cohort. J Am Heart Assoc. 2016;5:e002816.
Liu X, Xie Z, Sun M, Wang X, Li J, Cui J, et al. Plasma trimethylamine N-oxide is associated with vulnerable plaque characteristics in CAD patients as assessed by optical coherence tomography. Int J Cardiol. 2018;265:18–23.
Suzuki T, Heaney LM, Bhandari SS, Jones DJ, Ng LL. Trimethylamine N-oxide and prognosis in acute heart failure. Heart. 2016;102:841–8.
Tang WW, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–14.
Lever M, George PM, Slow S, Bellamy D, Young JM, Ho M, et al. Betaine and trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: an observational study. PloS One. 2014;9:e114969.
Mafune A, Iwamoto T, Tsutsumi Y, Nakashima A, Yamamoto I, Yokoyama K, et al. Associations among serum trimethylamine-N-oxide (TMAO) levels, kidney function and infarcted coronary artery number in patients undergoing cardiovascular surgery: a cross-sectional study. Clin Exp Nephrol. 2016;20:731–9.
Mueller DM, Allenspach M, Othman A, Saely CH, Muendlein A, Vonbank A, et al. Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis. 2015;243:638–44.
Afzaal M, Saeed F, Shah YA, Hussain M, Rabail R, Socol CT, et al. Human gut microbiota in health and disease: Unveiling the relationship. Front Microbiol. 2022;13:999001.
Obeid R, Awwad HM, Rabagny Y, Graeber S, Herrmann W, Geisel J. Plasma trimethylamine N-oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am J Clin Nutr. 2016;103:703–11.
Shan Z, Sun T, Huang H, Chen S, Chen L, Luo C, et al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am J Clin Nutr. 2017;106:888–94.
Tang WW, Wang Z, Li XS, Fan Y, Li DS, Wu Y, et al. Increased trimethylamine N-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem. 2017;63:297–306.
Dong Z, Liang Z, Guo M, Hu S, Shen Z, Hai X. The association between plasma levels of trimethylamine N-oxide and the risk of coronary heart disease in Chinese patients with or without type 2 diabetes mellitus. Dis Markers. 2018;2018:1578320.
Fikri AM, Smyth R, Kumar V, Al-Abadla Z, Abusnana S, Munday MR. Pre-diagnostic biomarkers of type 2 diabetes identified in the UAE’s obese national population using targeted metabolomics. Sci Rep. 2020;10:17616.
Senthong V, Kiatchoosakun S, Wongvipaporn C, Phetcharaburanin J, Tatsanavivat P, Sritara P, et al. Gut microbiota-generated metabolite, trimethylamine-N-oxide, and subclinical myocardial damage: a multicenter study from Thailand. Sci Rep. 2021;11:14963.
Ringel C, Dittrich J, Gaudl A, Schellong P, Beuchel CF, Baber R, et al. Association of plasma trimethylamine N-oxide levels with atherosclerotic cardiovascular disease and factors of the metabolic syndrome. Atherosclerosis. 2021;335:62–7.
Zeng Q, Zhao M, Wang F, Li Y, Li H, Zheng J, et al. Integrating choline and specific intestinal microbiota to classify type 2 diabetes in adults: a machine learning based metagenomics study. Front Endocrinol. 2022;13:906310.
Kalagi NA, Thota RN, Stojanovski E, Alburikan KA, Garg ML. Association between plasma trimethylamine N-oxide levels and type 2 diabetes: a case control study. Nutrients. 2022;14:2093.
Tang Y, Zou Y, Cui J, Ma X, Zhang L, Yu S, et al. Analysis of two intestinal bacterial metabolites (trimethylamine N-oxide and phenylacetylglutamine) in human serum samples of patients with T2DM and AMI using a liquid chromatography tandem mass spectrometry method. Clinica Chim Acta. 2022;536:162–8.
Liang H, Yu A, Wang Z, Zhang N, Wang Q, Gao H, et al. Atherosclerotic patients with diabetes mellitus may break through the threshold of healthy TMAO levels formed by long-term statins therapy. Heliyon. 2023;9:e13657.
Yu D, Shu XO, Rivera ES, Zhang X, Cai Q, Calcutt MW, et al. Urinary levels of trimethylamine-N-oxide and incident coronary heart disease: a prospective investigation among urban Chinese adults. J Am Heart Assoc. 2019;8:e010606.
Qi S, Liu L, He S, Wang L, Li J, Sun X. Trimethylamine N-Oxide and related metabolites in the serum and risk of type 2 diabetes in the Chinese population: a case-control study. Diabetes Metab Syndr Obes. 2023;16:547–55.
Huo L, Li H, Zhu M, Liu Y, Ren L, Hu J, et al. Enhanced trimethylamine metabolism and gut dysbiosis in type 2 diabetes mellitus with microalbumin. Front Endocrinol. 2023;14:1257457.
Hove-Skovsgaard M, Gaardbo JC, Kolte L, Winding K, Seljeflot I, Svardal A, et al. HIV-infected persons with type 2 diabetes show evidence of endothelial dysfunction and increased inflammation. BMC Infect Dis. 2017;17:1–8.
Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Med. 1998;15:539–53.
Liu J, Ren Z-H, Qiang H, Wu J, Shen M, Zhang L, et al. Trends in the incidence of diabetes mellitus: results from the Global Burden of Disease Study 2017 and implications for diabetes mellitus prevention. BMC Public Health. 2020;20:1–12.
Lin X, Zhang Y, He X, Chen Y, Chen N, Liu J, et al. The choline metabolite TMAO inhibits NETosis and promotes placental development in GDM of humans and mice. Diabetes. 2021;70:2250–63.
McArthur KL, Zhang M, Hong X, Wang G, Buckley JP, Wang X, et al. Trimethylamine N-oxide and its precursors are associated with gestational diabetes mellitus and pre-eclampsia in the boston birth cohort. Current Dev Nutr. 2022;6:nzac108.
Barzilay E, Moon A, Plumptre L, Masih SP, Sohn K-J, Visentin CE, et al. Fetal one-carbon nutrient concentrations may be affected by gestational diabetes. Nutr Res. 2018;55:57–64.
Gao Y, Chen H, Li J, Ren S, Yang Z, Zhou Y, et al. Alterations of gut microbiota-derived metabolites in gestational diabetes mellitus and clinical significance. J Clin Lab Anal. 2022;36:e24333.
Zhou Z, Yao Y, Sun Y, Wang X, Huang S, Hou J, et al. Serum betaine and dimethylglycine in mid-pregnancy and the risk of gestational diabetes mellitus: a case-control study. Endocrine. 2024;85:1–11.
Huo X, Li J, Cao Y-F, Li S-N, Shao P, Leng J, et al. Trimethylamine N-oxide metabolites in early pregnancy and risk of gestational diabetes: a nested case-control study. J Clin Endocrinol Metab. 2019;104:5529–39.
Li S-y, Chen S, Lu X-t, Fang A-p, Chen Y-m, Huang R-z, et al. Serum trimethylamine-N-oxide is associated with incident type 2 diabetes in middle-aged and older adults: a prospective cohort study. J Transl Med. 2022;20:374.
Huang Y, Wu Y, Zhang Y, Bai H, Peng R, Ruan W, et al. Dynamic changes in gut microbiota-derived metabolite trimethylamine-N-oxide and risk of type 2 diabetes mellitus: potential for dietary changes in diabetes prevention. Nutrients. 2024;16:1711.
Svingen GF, Schartum-Hansen H, Pedersen ER, Ueland PM, Tell GS, Mellgren G, et al. Prospective associations of systemic and urinary choline metabolites with incident type 2 diabetes. Clinical Chem. 2016;62:755–65.
Lemaitre RN, Jensen PN, Wang Z, Fretts AM, McKnight B, Nemet I, et al. Association of trimethylamine N-oxide and related metabolites in plasma and incident type 2 diabetes: the cardiovascular health study. JAMA Netw open. 2021;4:e2122844-e.
Friedrich N, Budde K, Suhre K, Völker U, John U, Felix SB, et al. Sex differences in urine metabolites related with risk of diabetes using NMR spectroscopy: results of the study of health in pomerania. Metabolomics. 2015;11:1405–15.
Papandreou C, Bulló M, Zheng Y, Ruiz-Canela M, Yu E, Guasch-Ferré M, et al. Plasma trimethylamine-N-oxide and related metabolites are associated with type 2 diabetes risk in the Prevención con Dieta Mediterránea (PREDIMED) trial. Am J Clin Nutr. 2018;108:163–73.
McEntyre CJ, Lever M, Chambers ST, George PM, Slow S, Elmslie JL, et al. Variation of betaine, N, N-dimethylglycine, choline, glycerophosphorylcholine, taurine and trimethylamine-N-oxide in the plasma and urine of overweight people with type 2 diabetes over a two-year period. Ann Clin Biochem. 2015;52:352–60.
Li P, Zhong C, Li S, Sun T, Huang H, Chen X, et al. Plasma concentration of trimethylamine-N-oxide and risk of gestational diabetes mellitus. Am J Clin Nutr. 2018;108:603–10.
Liu J, Li J, Yang K, Leng J, Li W, Yang W, et al. Ceramides and their interactive effects with trimethylamine-N-oxide metabolites on risk of gestational diabetes: a nested case-control study. Diabetes Res Clin Pract. 2021;171:108606.
Jia J, Dou P, Gao M, Kong X, Li C, Liu Z, et al. Assessment of causal direction between gut microbiota–dependent metabolites and cardiometabolic health: a bidirectional Mendelian randomization analysis. Diabetes. 2019;68:1747–55.
Miao J, Ling AV, Manthena PV, Gearing ME, Graham MJ, Crooke RM, et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6:6498.
DiNicolantonio JJ, McCarty M, OKeefe J. Association of moderately elevated trimethylamine N-oxide with cardiovascular risk: is TMAO serving as a marker for hepatic insulin resistance. Arch Dis Childhood; 2019;6:e000890.
Zhou L, Li X, Li S, Wen X, Peng Y, Zhao L. Relationship between dietary choline intake and diabetes mellitus in the National Health and Nutrition Examination Survey 2007-2010. J Diabetes. 2021;13:554–61.
Sawicki CM, Pacheco LS, Rivas-Tumanyan S, Cao Z, Haslam DE, Liang L, et al. Association of gut microbiota-related metabolites and type 2 diabetes in two Puerto Rican cohorts. Nutrients. 2024;16:959.
Wong ND, Sattar N. Cardiovascular risk in diabetes mellitus: epidemiology, assessment and prevention. Nat Rev Cardiol. 2023;20:685–95.
Li D, Lu Y, Yuan S, Cai X, He Y, Chen J, et al. Gut microbiota–derived metabolite trimethylamine-N-oxide and multiple health outcomes: an umbrella review and updated meta-analysis. Am J Clin Nutr. 2022;116:230–43.
Han J-M, Guo L, Chen X-H, Xie Q, Song X-Y, Ma Y-L. Relationship between trimethylamine N-oxide and the risk of hypertension in patients with cardiovascular disease: a meta-analysis and dose-response relationship analysis. Medicine. 2024;103:e36784.
Flores-Guerrero JL, van Dijk PR, Connelly MA, Garcia E, Bilo HJ, Navis G, et al. Circulating trimethylamine N-oxide is associated with increased risk of cardiovascular mortality in type-2 diabetes: Results from a Dutch Diabetes Cohort (ZODIAC-59). J Clin Med. 2021;10:2269.
Sapa H, Gutiérrez OM, Shlipak MG, Katz R, Ix JH, Sarnak MJ, et al. Association of uremic solutes with cardiovascular death in diabetic kidney disease. Am J Kidney Dis. 2022;80:502–12.e1.
Croyal M, Saulnier P-J, Aguesse A, Gand E, Ragot S, Roussel R, et al. Plasma trimethylamine N-oxide and risk of cardiovascular events in patients with type 2 diabetes. J Clin Endocrinol Metab. 2020;105:2371–80.
Yu P-S, Wu P-H, Hung W-W, Lin M-Y, Zhen Y-Y, Hung W-C, et al. Association between trimethylamine N-oxide and adverse kidney outcomes and overall mortality in type 2 diabetes mellitus. J Clin Endocrinol Metabol. 2024;109:dgae009.
Eyileten C, Jarosz-Popek J, Jakubik D, Gasecka A, Wolska M, Ufnal M, et al. Plasma trimethylamine-N-oxide is an independent predictor of long-term cardiovascular mortality in patients undergoing percutaneous coronary intervention for acute coronary syndrome. Front Cardiovasc Med. 2021;8:728724.
Bao M, Li H, Li J. Circulating trimethylamine N-oxide is correlated with high coronary artery atherosclerotic burden in individuals with newly diagnosed coronary heart disease. BMC Cardiovasc Disord. 2024;24:265.
Chen G, He L, Dou X, Liu T. Association of trimethylamine-N-oxide levels with risk of cardiovascular disease and mortality among elderly subjects: A systematic review and meta-analysis. Cardiorenal Med. 2022;12:39–54.
Cardona A, O’Brien A, Bernier MC, Somogyi A, Wysocki VH, Smart S, et al. Trimethylamine N-oxide and incident atherosclerotic events in high-risk individuals with diabetes: an ACCORD trial post hoc analysis. BMJ Open Diabetes Res Care. 2019;7:e000718.
Schrauben SJ, Sapa H, Xie D, Zhang X, Anderson AH, Shlipak MG, et al. Association of urine and plasma ADMA with atherosclerotic risk in DKD cardiovascular disease risk in diabetic kidney disease: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Nephrol Dial Transplant. 2023;38:2809–15.
Yu N, Gu N, Wang Y, Zhou B, Lu D, Li J, et al. The association of plasma trimethylamine N-oxide with coronary atherosclerotic burden in patients with type 2 diabetes among a Chinese north population. Diabetes Metab Syndr Obes. 2022;15:69–78.
Winther SA, Øllgaard JC, Hansen TW, Von Scholten BJ, Reinhard H, Ahluwalia TS, et al. Plasma trimethylamine N-oxide and its metabolic precursors and risk of mortality, cardiovascular and renal disease in individuals with type 2-diabetes and albuminuria. PLoS One. 2021;16:e0244402.
Kalagi NA, Thota RN, Stojanovski E, Alburikan KA, Garg ML. Plasma trimethylamine N-oxide levels are associated with poor kidney function in people with type 2 diabetes. Nutrients. 2023;15:812.
Huang Y, Zhu Z, Huang Z, Zhou J. Elevated serum trimethylamine oxide levels as potential biomarker for diabetic kidney disease. Endocrine Connections. 2023;12:e220542.
Wargny M, Croyal M, Ragot S, Gand E, Jacobi D, Trochu J-N, et al. Nutritional biomarkers and heart failure requiring hospitalization in patients with type 2 diabetes: the SURDIAGENE cohort. Cardiovasc Diabetol. 2022;21:101.
Winther SA, Øllgaard JC, Tofte N, Tarnow L, Wang Z, Ahluwalia TS, et al. Utility of plasma concentration of trimethylamine N-oxide in predicting cardiovascular and renal complications in individuals with type 1 diabetes. Diabetes care. 2019;42:1512–20.
Yakar B, Onalan E, Kaymaz T, Donder E, Gursu MF. The role of trimethylamine-N-oxide level in the diagnosis of diabetic retinopathy and the differential diagnosis of diabetic and nondiabetic retinopathy. Arquivos Brasileiros de Oftalmologia. 2022;87:e2021–0527.
Liu W, Wang C, Xia Y, Xia W, Liu G, Ren C, et al. Elevated plasma trimethylamine-N-oxide levels are associated with diabetic retinopathy. Acta Diabetologica. 2021;58:221–9.
Xue L, Huang L, Tian Y, Cao X, Song Y. Trimethylamine-N-Oxide Promotes High-Glucose-Induced Dysfunction and NLRP3 Inflammasome Activation in Retinal Microvascular Endothelial Cells. J Ophthalmol. 2023;2023:8224752.
Li Z, Hui J, Li S, Cao T, Zhang J, Mao X, et al. Trimethylamine N-oxide predicts stroke severity in diabetic patients with acute ischaemic stroke and is related to glycemic variability. Eur J Neurol. 2023;30:3478–86.
Xu N, Wan J, Wang C, Liu J, Qian C, Tan H. Increased serum trimethylamine n-oxide level in type 2 diabetic patients with mild cognitive impairment. Diabetes Metab Syndr Obes. 2022;15:2197–205.
Yuan Y, Gan C, Wang M, Zou J, Wang Z, Li S, et al. Association of serum trimethylamine N-oxide levels and bone mineral density in type 2 diabetes mellitus. Endocrine. 2024;84:1–11.
Zhou T, Heianza Y, Chen Y, Li X, Sun D, DiDonato JA, et al. Circulating gut microbiota metabolite trimethylamine N-oxide (TMAO) and changes in bone density in response to weight loss diets: the POUNDS lost trial. Diabetes Care. 2019;42:1365–71.
Kuka J, Videja M, Makrecka-Kuka M, Liepins J, Grinberga S, Sevostjanovs E, et al. Metformin decreases bacterial trimethylamine production and trimethylamine N-oxide levels in db/db mice. Sci Rep. 2020;10:14555.
Su C, Li X, Yang Y, Du Y, Zhang X, Wang L, et al. Metformin alleviates choline diet-induced TMAO elevation in C57BL/6J mice by influencing gut-microbiota composition and functionality. Nutr Diabetes. 2021;11:27.
Canyelles M, Pérez A, Junza A, Miñambres I, Yanes O, Sardà H, et al. Divergent effects of glycemic control and bariatric surgery on circulating concentrations of TMAO in newly diagnosed T2D patients and morbidly obese. Diagnostics. 2022;12:2783.
Huo T, Cai S, Lu X, Sha Y, Yu M, Li F. Metabonomic study of biochemical changes in the serum of type 2 diabetes mellitus patients after the treatment of metformin hydrochloride. J Pharm Biomed Anal. 2009;49:976–82.
Chang TT, Chen JW. Direct CCL4 inhibition modulates gut microbiota, reduces circulating trimethylamine N-Oxide, and improves glucose and lipid metabolism in high-fat-diet-induced diabetes mellitus. J Inflamm Res. 2021;14:6237–50.
Shyshkan-Shyshova KO, Zinych OV, Кushnareva NM, Кovalchuk AV, Prybyla ОV. The effects of incretin mimetics on the level of the microbial metabolite trimethylamine-N-oxide, a marker of cardiovascular risk in type 2 diabetic patients. Int J Endocrinol. 2023;19:523–8.
Wang L, Wang Y, Xu H, Li W. Effect of dapagliflozin on ferroptosis through the gut microbiota metabolite TMAO during myocardial ischemia-reperfusion injury in diabetes mellitus rats. Sci Rep. 2024;14:13851.
Aziz F, Tripolt NJ, Pferschy PN, Kolesnik E, Mangge H, Curcic P, et al. Alterations in trimethylamine-N-oxide in response to Empagliflozin therapy: a secondary analysis of the EMMY trial. Cardiovasc Diabetol. 2023;22:184.
Kwee LC, Ilkayeva O, Muehlbauer MJ, Bihlmeyer N, Wolfe B, Purnell JQ, et al. Metabolites and diabetes remission after weight loss. Nutr Diabetes. 2021;11:10.
Argyridou S, Davies MJ, Biddle GJH, Bernieh D, Suzuki T, Dawkins NP, et al. Evaluation of an 8-Week Vegan Diet on Plasma Trimethylamine-N-Oxide and Postchallenge Glucose in Adults with Dysglycemia or Obesity. J Nutr. 2021;151:1844–53.
Heianza Y, Sun D, Li X, DiDonato JA, Bray GA, Sacks FM, et al. Gut microbiota metabolites, amino acid metabolites and improvements in insulin sensitivity and glucose metabolism: the POUNDS Lost trial. Gut. 2019;68:263–70.
Costabile G, Vetrani C, Bozzetto L, Giacco R, Bresciani L, Del Rio D, et al. Plasma TMAO increase after healthy diets: results from 2 randomized controlled trials with dietary fish, polyphenols, and whole-grain cereals. Am J Clin Nutr. 2021;114:1342–50.
Cho CE, Taesuwan S, Malysheva OV, Bender E, Tulchinsky NF, Yan J, et al. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res. 2017;61:1600324.
Troseid M, Hov JR, Nestvold TK, Thoresen H, Berge RK, Svardal A, et al. Major Increase in Microbiota-Dependent Proatherogenic Metabolite TMAO One Year After Bariatric Surgery. Metab Syndr Relat Disord. 2016;14:197–201.
Krishnan S, Gertz ER, Adams SH, Newman JW, Pedersen TL, Keim NL, et al. Effects of a diet based on the Dietary Guidelines on vascular health and TMAO in women with cardiometabolic risk factors. Nutr Metab Cardiovasc Dis. 2022;32:210–9.
Cash JG, Konaniah E, Hegde N, Kuhel DG, Watanabe M, Romick-Rosendale L, et al. Therapeutic reduction of lysophospholipids in the digestive tract recapitulates the metabolic benefits of bariatric surgery and promotes diabetes remission. Mol Metab. 2018;16:55–64.
Lee SJ, Park YS, Kim YJ, Han SU, Hwang GS, Han Y, et al. Changes in trimethylamine-N-oxide levels in obese patients following laparoscopic Roux-en-Y gastric bypass or sleeve gastrectomy in a Korean obesity surgical treatment study (KOBESS). J Clin Med. 2021;10:5091.
Fang Q, Zheng B, Liu N, Liu J, Liu W, Huang X, et al. Trimethylamine N-oxide exacerbates renal inflammation and fibrosis in rats with diabetic kidney disease. Front Physiol. 2021;12:682482.
Jiang J-Y, Liu W-M, Zhang Q-P, Ren H, Yao Q-Y, Liu G-Q, et al. Trimethylamine N-oxide aggravates vascular permeability and endothelial cell dysfunction under diabetic condition: in vitro and in vivo study. Int J Ophthalmol. 2024;17:25.
Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118:476–81.
Gao X, Xu J, Jiang C, Zhang Y, Xue Y, Li Z, et al. Fish oil ameliorates trimethylamine N-oxide-exacerbated glucose intolerance in high-fat diet-fed mice. Food Funct. 2015;6:1117–25.
Kong L, Zhao Q, Jiang X, Hu J, Jiang Q, Sheng L, et al. Trimethylamine N-oxide impairs β-cell function and glucose tolerance. Nat Commun. 2024;15:2526.
Chen S, Henderson A, Petriello MC, Romano KA, Gearing M, Miao J, et al. Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction. Cell Metab. 2019;30:1141–51.e5.
Govindarajulu M, Pinky PD, Steinke I, Bloemer J, Ramesh S, Kariharan T, et al. Gut metabolite TMAO induces synaptic plasticity deficits by promoting endoplasmic reticulum stress. Front Mol Neurosci. 2020;13:138.
Huang Y-l, Xiang Q, Zou J-j, Wu Y, Yu R. Zuogui Jiangtang Shuxin formula Ameliorates diabetic cardiomyopathy mice via modulating gut-heart axis. Front Endocrinol. 2023;14:1106812.
Dumas M-E, Rothwell AR, Hoyles L, Aranias T, Chilloux J, Calderari S, et al. Microbial-host co-metabolites are prodromal markers predicting phenotypic heterogeneity in behavior, obesity, and impaired glucose tolerance. Cell Rep. 2017;20:136–48.
Bai C, Biwersi J, Verkman A, Matthay MA. A mouse model to test the in vivo efficacy of chemical chaperones. J Pharmacol Toxicological methods. 1998;40:39–45.
Akerfeldt MC, Howes J, Chan JY, Stevens VA, Boubenna N, McGuire HM, et al. Cytokine-induced β-cell death is independent of endoplasmic reticulum stress signaling. Diabetes. 2008;57:3034–44.
Krueger ES, Beales JL, Russon KB, Elison WS, Davis JR, Hansen JM, et al. Gut metabolite trimethylamine n-oxide protects INS-1 β-cell and rat islet function under diabetic glucolipotoxic conditions. Biomolecules. 2021;11:1892.
Achard CS, Laybutt DR. Lipid-induced endoplasmic reticulum stress in liver cells results in two distinct outcomes: adaptation with enhanced insulin signaling or insulin resistance. Endocrinology. 2012;153:2164–77.
Khan A, Jahan I, Nayeem SM. In silico studies of the human IAPP in the presence of osmolytes. J Mol Model. 2022;28:188.
Kumari A, Sharma R, Somvanshi P, Grover A. Assessing the role of osmolytes on the conformational harmony of islet amyloid polypeptide. Int J Biol Macromolecules. 2020;164:2569–82.
Lupachyk S, Watcho P, Stavniichuk R, Shevalye H, Obrosova IG. Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy. Diabetes. 2013;62:944–52.
Gandhi GK, Ball KK, Cruz NF, Dienel GA. Hyperglycaemia and diabetes impair gap junctional communication among astrocytes. ASN Neuro. 2010;2:AN20090048.
Wankeu-Nya M, Watcho P, Deeh Defo PB, Ngadjui E, Nguelefack TB, Kamtchouing P, et al. Aqueous and ethanol extracts of Dracaena arborea (Wild) Link (Dracaenaceae) alleviate reproductive complications of diabetes mellitus in rats. Andrologia. 2019;51:e13381.
Li Z, Li L, Yue M, Peng Q, Pu X, Zhou Y. Tracing immunological interaction in trimethylamine N-oxide hydrogel-derived zwitterionic microenvironment during promoted diabetic wound regeneration. Adv Mater. 2024;36:2402738.
Li XY, Yu ZL, Zhao YC, Wang DD, Xue CH, Zhang TT, et al. Gut microbiota metabolite TMA may mediate the effects of TMAO on glucose and lipid metabolism in C57BL/6J mice. Mol Nutr Food Res. 2024;68:2300443.
Lanz M, Janeiro MH, Milagro FI, Puerta E, Ludwig IA, Pineda-Lucena A, et al. Trimethylamine N-oxide (TMAO) drives insulin resistance and cognitive deficiencies in a senescence accelerated mouse model. Mechanisms Ageing Dev. 2022;204:111668.
Jiang W-Y, Huo J-Y, Wang S-C, Cheng Y-D, Lyu Y-T, Jiang Z-X, et al. Trimethylamine N-oxide facilitates the progression of atrial fibrillation in rats with type 2 diabetes by aggravating cardiac inflammation and connexin remodeling. J Physiol Biochem. 2022;78:855–67.
Andraos S, Jones B, Lange K, Clifford SA, Thorstensen EB, Kerr JA, et al. Trimethylamine N-oxide (TMAO) is not associated with cardiometabolic phenotypes and inflammatory markers in children and adults. Current Dev Nutr. 2021;5:nzaa179.
Jaworska K, Bielinska K, Gawrys-Kopczynska M, Ufnal M. TMA (trimethylamine), but not its oxide TMAO (trimethylamine-oxide), exerts haemodynamic effects: implications for interpretation of cardiovascular actions of gut microbiome. Cardiovasc Res. 2019;115:1948–9.
Restini CBA, Fink GD, Watts SW. Vascular reactivity stimulated by TMA and TMAO: Are perivascular adipose tissue and endothelium involved?. Pharmacological Res. 2021;163:105273.
Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis [S]. J lipid Res. 2015;56:22–37.
Liao BM, McManus SA, Hughes WE, Schmitz-Peiffer C. Flavin-containing monooxygenase 3 reduces endoplasmic reticulum stress in lipid-treated hepatocytes. Mol Endocrinol. 2016;30:417–28.
Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, et al. The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015;10:326–38.
Funding
The work was supported by the National Science Centre, Poland grant no: UMO-2020/37/B/NZ5/00366.
Author information
Authors and Affiliations
Contributions
K.J. designed the study, performed a literature research and drafted the manuscript. M.K. contributed to drafting the manuscript and creating tables. M.U. provided important revisions to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jaworska, K., Kuś, M. & Ufnal, M. TMAO and diabetes: from the gut feeling to the heart of the problem. Nutr. Diabetes 15, 21 (2025). https://doi.org/10.1038/s41387-025-00377-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41387-025-00377-8