Abstract
Wnt/β-catenin signalling is important for normal hematopoietic stem/progenitor cell (HSPC) biology and heavily implicated in acute and chronic myeloid leukaemia (AML and CML). The central mediator β-catenin is an attractive therapeutic target in myeloid neoplasms however its targeting has been hampered by a poor characterisation of its molecular interactions in haematopoietic cells, which will differ from its network in solid tissues. Our previous β-catenin interactome study identified the significant enrichment of RNA-binding proteins (RBP) implying post-transcriptional roles for β-catenin in myeloid cells. To identify β-catenin interacting mRNAs we performed β-catenin RNA-immunoprecipitation coupled to RNA-sequencing (RIP-seq) and identified significantly enriched Wnt signalling pathway transcripts. Using β-catenin cross-linking immunoprecipitation (CLIP) we demonstrated a limited capacity for β-catenin to bind RNA directly, implying dependence on other RBPs. β-Catenin was found to interact with Musashi-2 (MSI2) in both myeloid cell lines and primary AML patient samples, where expression was significantly correlated. MSI2 knockdown reduced Wnt signalling output (TCF/LEF activity), through suppression of LEF-1 expression and nuclear localisation. Through both RIP and CLIP we demonstrate MSI2 binds LEF1 mRNA in a partly β-catenin dependent fashion, and may impact the post-transcriptional control of LEF-1 expression. Finally, we show that MSI2-mediated expansion of human HSPCs could be partly driven through LEF1 regulation. This is the first study to experimentally demonstrate functional crosstalk between MSI2 and Wnt signalling in human cells, and indicates potential novel post-transcriptional roles for β-catenin in a haematological context.
Similar content being viewed by others
Introduction
Canonical Wnt signalling is an evolutionarily conserved pathway, critical in normal development and disease [1]. In normal haematopoiesis, the central mediator β-catenin regulates the self-renewal [2] and differentiation [3,4,5] of haematopoietic stem/progenitor cells (HSPC), where its expression is tightly regulated to ensure the correct balance between HSPC maintenance and development [6, 7]. However its dysregulation is observed across numerous haematological malignancies, including myeloid leukaemias. Overexpression of β-catenin occurs in up to 80% of acute myeloid leukaemia (AML) cases [8], through a multitude of mechanisms [9], that result in hyperactive Wnt signalling [10] and adverse patient survival [11]. Deregulated Wnt signalling contributes to the development of several AML subtypes including normal karyotype [12], FLT3-mutant [13], del(5q)+ [14], myelodysplastic syndrome (MDS)-related [15], CBF-mutated [16], and PML-RARα+ [17]. Mouse models have also shown β-catenin regulates the emergence, maintenance, and drug resistance of leukaemia stem cells in both AML [18,19,20,21,22,23] and chronic myeloid leukaemia (CML) [24, 25]. This, coupled with the observation that only very low levels of Wnt/β-catenin signalling sustain normal haematopoiesis [6], makes the pathway an attractive therapeutic target. Yet despite this promise, pharmacological attempts to disrupt β-catenin have achieved limited success to date, hampered by a poor understanding of its molecular interactions in a haematopoietic context.
In solid tissues, β-catenin has important cell adhesion functions through interaction with cadherins in adherens junctions [26]. However, such tight cell-cell adhesion is not prominent in a fluid tissue like blood, meaning free β-catenin likely forms alternative interactions in haematopoietic cells. Indeed, our previous evaluation of β-catenin localisation in myeloid cells has not indicated significant plasma cell membrane localisation [27]. To address this knowledge gap and identify new approaches for targeting β-catenin in haematological malignancy, we characterised β-catenin’s interaction network in myeloid cells and discovered a plethora of novel protein partners [8]. Of note was the significant enrichment of RNA-binding proteins (RBP) [9]. RBPs are a diverse family of proteins that bind and regulate the stability, splicing, transport and translation of target RNAs, and are heavily implicated in normal hematopoiesis and leukaemia [28]. Such interactions imply that β-catenin could have a hitherto uncharacterised but important role in post-transcriptional gene expression in haematopoietic cells.
In this study, we show for the first time that β-catenin associates with a Wnt signalling mRNA network mediated through interactions with canonical RBPs like MSI2. We demonstrate Musashi-2 (MSI2) interacts with β-catenin and influences Wnt signalling output through modulation of the Wnt transcription factor LEF-1, which could be an important axis for controlling the growth and survival of human HSPCs.
Method
Primary samples
Bone marrow, peripheral blood or leukapheresis samples from patients diagnosed with AML (Clinical information in Supplementary Table S1) were collected in accordance with the Declaration of Helsinki and with approval of University Hospitals Bristol NHS Trust and London Brent Research Ethics Committee. Human cord blood (CB) was obtained following informed consent from healthy mothers at full-term undergoing elective caesarian sections at the Royal Sussex County Hospital, with approval from University Hospitals Sussex (UHS) NHS Foundation trust, the East of England—Essex Research Ethics Committee and HRA and Health and Care Research Wales (18/EE/0403). From all primary samples mononuclear cells (MNC) were isolated via density gradient separation using Ficoll-Hypaque (Merck Millipore) and only samples with ≥80% viability were included in the study. The CD34+ fraction was derived as previously described [29] from cryopreserved cord blood MNC preparations and enriched to >85% purity using MiniMACS (Miltenyi Biotec) according to the manufacturer’s instructions.
Cell culture and drug treatments
The myeloid cell lines K562, HL60, HEL, U937, PLB-985, NOMO1, OCI-AML3, EOLI, ML-1, THP-1, KU812 (The European Collection of Authenticated Cell Cultures) and OCI-AML2, MV4-11, KG-1, KG1a SET2, NB4 and MONOMAC6 (Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures GmbH) were confirmed mycoplasma-free and authenticated via short-tandem repeat (STR) analysis prior to general culture as previously [30]. β-Catenin was stabilised using the GSK-3β inhibitor CHIR99021 (Merck-Millipore) as previously described [8], whilst transcription was inhibited via 2 µg/ml Actinomycin D (Merck-Millipore) at the stated timepoints. Purified human CB CD34+ HSPC were isolated and cultured at 5 × 105/mL in StemSpan SFEMII (StemCell Technologies) supplemented with human stem cell factor (150 ng/mL), human FLT3-ligand (150 ng/mL), and human thrombopoietin (TPO; 20 ng/mL; Proteintech Group). HSPC number and subsequent fold expansion in liquid culture were ascertained by manual cell counting in combination with trypan blue (Merck-Millipore) exclusion assays.
RNA immunoprecipitation (RIP)
RIP analyses were performed using the MagnaRIP kit (Merck-Millipore) according to manufacturer’s guidelines. Briefly, 2 × 106 cells were treated overnight with CHIR99021 or DMSO at 37 °C with 5% CO2. Cells were collected and washed in phosphate-buffered saline (PBS) followed by resuspension in lysis buffer, incubation on ice and overnight storage −80 °C. Antibody-bead complexes were prepared following manufacturers guidelines with 5 μg mouse IgG (Becton Dickinson), rabbit IgG (Merck Millipore), β-catenin (Clone 14, Becton Dickinson), HuR (Clone 3A2, Invitrogen), MSI2 (EP1305Y, Abcam, Oxford, UK) or LIN28B (Cell Signalling Technology) and incubated at room temperature for 30 min with rotation. The RIP lysates were thawed and incubated with antibody-bead complexes with rotation at 4 °C overnight followed by washing of unbound antibody.
Each immunoprecipitation was resuspended in proteinase K buffer and shaken at 55 °C for 30 m to remove contaminating protein and followed by addition of phenol: chloroform: isoamyl alcohol in a 25:24:1 (Thermofisher Scientific) to separate phases. Aqueous phase was removed to a new eppendorf followed by the addition of ethanol, salt and enhancer to precipitate RNA at −80 °C overnight. All tubes were centrifuged, followed by washing in 80% ethanol and then air-drying of pellet for 10 min at room temperature after a final centrifugation. The RNA pellet was resuspended in 20 μl of nuclease-free water and the tubes kept at −80 °C ahead of sample assessment by an mRNA bioanalyzer Pico Series II (Agilent).
RIP sequencing analysis
RIP samples were initially checked using the Qubit HS RNA (Invitrogen) and Tapestation 4200 High Sensitivity RNA Screen tapes (Agilent) for quality and quantity. RNA libraries were prepared using the Illumina Stranded Total RNA kit (Illumina). The rRNA depletion step was excluded, and 7.5 µl of each sample was mixed with EPH3 followed by denaturation. This was then taken forward for cDNA synthesis and library preparation using the Illumina Stranded Total RNA protocol. The index addition was done using 2.5 µl of RSB, RNA index anchors and LIGX. 15 cycles of enrichment PCR were performed and libraries checked using the Qubit BR dsDNA (Invitrogen) and Tapestation 4200 D1000 screen tapes. Libraries were pooled using 280 ng of each, and the final pooled checked using the Qubit BR dsDNA, Tapestation 4200 D1000 screen tapes and NEBNext Library Quant kit for Illumina (New England Biolabs). The pool was sequenced on the NextSeq2000 using the P2 100 cycle X-Leap chemistry kit in a single end configuration. 550pM and 2% PhiX were used to load the cartridge, and the Illumina dark cycle chemistry for the P2 flow cells also used. Base calling was performed on instrument using the onboard BCLConvert. Raw FASTQ files were trimmed using Cutadapt (version 4.6) to remove adapters identified during the quality control process [31]. The trimmed reads were then mapped to the human genome using HISAT2 (version 2.2.1) with default parameters [32]. The human genome index file used for alignment was downloaded from the HISAT2 website. SAMtools (version 1.21) was used to convert SAM files generated by HISAT2 into BAM files for read counting [33]. Reads were then counted using featureCounts (version 2.0.6) with a human annotation file from Ensembl, applying the parameters: “--primary -C -t exon -g gene_id”. The raw read counts were imported into R to identify differentially enriched RNAs between basal (DMSO) and stabilised β-catenin (CHIR99021) conditions. Differential enrichment analysis, including fold change calculation and statistical analysis, was conducted with the DESeq2 package (version 1.44.0) [34] in R. Data manipulation and visualisation were performed using the tidyverse (version 2.0.0) [35] and ComplexHeatmap (version 2.20.0) [36] packages.
Cross-linking immunoprecipitation (CLIP)
4 × 107 cells were harvested, washed twice with room-temperature PBS and split across four 60 mm × 15 mm cell culture dishes (Corning) prior to UV crosslinking. Crosslinking was performed using three rounds of 100 mJ/cm² UV-254 nm irradiation, with agitation between each round. Following UV treatment, cells were recombined and centrifuged before disposal of supernatant and cell pellets snap-frozen in liquid nitrogen. Lysates were resuspended in 500 µL lysis buffer as previously [27], and sonicated for 10 cycles of alternate 30 seconds of sonication with 30-s rests. Sonicated lysates were centrifuged to pellet debris followed by quantitation of lysates using the DC protein assay (BioRad) as previously described [27], to ensure equal protein input for downstream RT-qPCR. Four units of Turbo DNase (Ambion, Thermofisher) were added to each sample and incubated at 37 °C for 3 min followed by cooling on ice for 3 min. 8 µg β-catenin or matched murine IgG antibody (Becton Dickinson), MSI2 (EP1305Y, Abcam) or LIN28B, and matched Rabbit IgG antibody (Cell Signalling Technology) was conjugated to beads as previously described [27], but using CLIP lysis buffer (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1% Igepal CA-630, 1% sodium deoxycholate) without protease inhibitors. Lysates were incubated with antibody-bead complexes for a minimum of 1 h at 4 °C with rotation with 150 µL original lysate retained for input samples, after which unbound antibody discarded from beads using washes in CLIP lysis buffer. Antibody-bead complexes were resuspended in 250 µL Proteinase K elution buffer (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1% Igepal CA-630, 1% sodium deoxycholate) with 20 mg/mL proteinase K solution (ThermoScientific) added to each sample, followed by incubation at 65 °C for 1 h prior to magnetic bead separation and supernatant transferred to fresh tubes. To each sample, 750 µL TRIzol LS reagent (Invitrogen) was added before storage at −80 °C for RNA extraction. RNA extraction, purification, and quantification were performed as above.
RNA extraction, clean up and quantitation
RNA was extracted using the Zymo RNA mini-prep kit (Cambridge Bioscience), cleaned using RNeasy MinElute Clean-up kit (Qiagen), or by DNase treatment using the DNA-free removal kit (Thermofisher), all via manufacturer guidelines [37]. RNA was quantified using a Nanodrop 2000 spectrophotometer (Thermofisher) blanked against nuclease-free water.
RT-qPCR
Purified RNA was converted to cDNA using the high-capacity RNA-to-cDNA kit (Thermofisher) or the one-step RT-qPCR mix (APTO-GEN, Raynham, Massachusetts, USA). All primers used in this study are listed in Supplementary Table S2 and were diluted to 10 μM with qRT-PCR programmes used as per manufacturers guidelines (Merck-Millipore). RT-qPCR was performed on a QuantStudio™ 3 Real-Time PCR System (ThermoFisher Scientific) in conjunction with associated software. Relative gene expression was determined first by subtracting the CT value of housekeeping genes (ACTB, GAPDH or RNA18SN1) from the CT value of genes of interest to generate ΔCT. The ΔΔCT was then calculated by subtracting the average control ΔCT from the ΔCT of each condition and fold change calculated through 2-ΔΔCT.
To account for differences in RNA sample preparation, RIP RNA CT values were normalised to the corresponding input RNA fraction CT value for the same qPCR assay as per manufacturer’s guidelines. Briefly, ΔCT values were calculated by subtracting the CT value of the input fraction from the CT value of the RIP fraction for each target gene, ensuring variability in RNA input or preparation was accounted for. In contrast, for CLIP analysis, normalisation of CLIP RNA to the input was not required, as sample concentrations were matched at the protein level prior to Co-IP.
Co-immunoprecipitation (Co-IP)
Cross-linking and Co-IP was executed as previously [8]. Briefly, 5 μg of MSI2 (EP1305Y, Abcam), HuR (Clone 3A2, Invitrogen), β-catenin (Clone 14, Becton Dickinson), mouse IgG or rabbit IgG was complexed with Protein G Dynabeads (Invitrogen), and incubated with 1 mg of whole cell lysate overnight in Co-IP lysis buffer (1 x Cell Signalling Technology lysis buffer, plus 10% glycerol and 0.5 mM dithiothreitol) at 4 °C or with the addition of 20 μg/ml RNase A (Thermofisher Scientific). Following washing of antibody-bead complexes five times in Co-IP lysis buffer, each sample was diluted in 2x Laemmli buffer (ThermoFisher Scientific) plus Co-IP lysis buffer and heated to 95 °C for 5 min prior to immunoblotting.
Immunoblotting
Immunoblotting was performed as previously [8], using antibodies to β-catenin, MSI2, LEF-1, GAPDH (Proteintech, Manchester, UK), Lamin A/C (Merck Millipore, New Jersey, United States) and α-tubulin (Merck Millipore) already outlined in above sections. Densitometric analysis of β-catenin, MSI2 and LEF-1 expression was performed using ImageJ software v1.53t (National Institute of Health, Bethesda, Maryland, USA) normalising to the respective loading control density present within each respective sample (whole cell = GAPDH, nuclear fractions = Lamin A/C).
Lentivirus preparation
K562 and KU812 cells were lentivirally transduced with the β-catenin-activated reporter (BAR) or mutant ‘found unresponsive’ control (fuBAR) system as previously [8]. The lentiviral expression plasmids used for transgene expression in this study are listed in Supplementary Table S3 and were used alongside their appropriate matched controls.
Flow cytometry
For Wnt reporter assessment, 2 × 105 BAR/fuBAR-containing cells were treated overnight with either DMSO or CHIR99021. TCF/LEF reporter activity (Venus Yellow Fluorescent Protein [YFP] intensity) was evaluated by flow cytometric analysis using a CytoFLEX Flow cytometer (Beckman Coulter, California, USA) in conjunction with FlowJo software Version 10.10 (Tree Star Inc., Ashland, OR USA) acquiring a minimum of 2 × 104 debris excluded events. For immunophenotyping, up to 5 × 104 CB-derived CD34+ HSPC were seeded into wells of 96-well plate in 100 µl staining buffer (1xPBS, 0.5% BSA) and stained with 10 µg/mL antibodies to CD34-PE (Clone 581, Biolegend) and CD45-PerCPCy5.5 (Clone QA17A19, Biolegend) or the equivalent concentration-, manufacturer- and isotype-matched control antibodies (Clone MOPC-21, Biolegend).
Nuclear/cytoplasmic fractionation
Nuclear/cytoplasmic fractionation was performed as previously [8].
Statistics
Spearman Rank correlation was assessed using Prism Version 10.3.1 (GraphPad Software, LLC), alongside statistical tests including one-sample or students t-test, using a threshold of p < 0.05. Unless otherwise stated all tests were performed in biological triplicates with data representing the mean ± 1 standard deviation (s.d).
Results
β-Catenin RIP-seq reveals enrichment with Wnt signalling mRNAs
Our previous β-catenin interactome study in myeloid cells revealed the significant enrichment of RBPs [8, 9], indicating a putative novel role for β-catenin in post-transcriptional processes in haematological cells. To first confirm whether β-catenin associates with RNA in myeloid cells we performed β-catenin RIP and examined RNA concentration and size using a Bioanalyser system. We first confirmed the efficiency of our RIP approach with K562 cells using a well-known RBP; HuR (Fig. 1A, also found to interact with β-catenin: Supplemental Fig. S1) [38], which consistently enriched with a known mRNA binding partner ACTB as detected by RT-qPCR (Fig. 1B). Using this assay we next successfully enriched β-catenin via RIP from K562 cells under basal (DMSO) or Wnt signalling stimulated (5 μM GSK3β inhibitor CHIR99021) conditions (Fig. 1C) and showed visible RNA enrichment versus IgG-RIP (Fig. 1D).
A Immunoblot showing HuR levels in HuR RIP performed from K562 cells. B Graph summarising the fold enrichment ACTB mRNA (known HuR binding target) [82] obtained from RT-qPCR analysis of IgG or HuR RIP analysis from K562 cells (n = 3) C Representative immunoblot showing β-catenin level obtained from β-catenin RIPs performed from K562 cells (±CHIR99021), ID = immunodepleted lysate. D Agilent 2100 Bioanalyzer gel showing RNA isolated from IgG or β-catenin RIP performed from K562 cells (±CHIR99021).
Following successful β-catenin RIP we next coupled this to RNA-sequencing to identify β-catenin-associated transcripts (Fig. 2A, B). We opted to focus on mRNA interaction in this study (rather than lncRNA) given the existing association between β-catenin and mRNAs [38,39,40,41]. K562 and HEL cells were selected for β-catenin RIP analyses because we have demonstrated them to be Wnt signalling responsive, and we used these myeloid lines previously to identify RBP enrichment in the β-catenin interactome which is of direct relevance for this study [8]. Overall we identified 582 and 1179 mRNAs that were enriched (FDR p < 0.05) between basal and stabilised β-catenin conditions, in K562 and HEL cells respectively (raw β-catenin RIP-seq data publicly available at EMBL-EBI ArrayExpress under reference E-MTAB-14675, and processed data available as Supplementary Data File 1). Of these altered transcripts, 392 were unique to K562 cells, 989 unique to HEL cells and 190 common to both cell types (Fig. 2C). Gene ontology (GO) analysis of shared mRNAs between both cell lines revealed Wnt signalling transcripts (encoding regulatory components of the pathway) as amongst the most significantly enriched from β-catenin RIP-seq (Fig. 2D), as well as transcripts implicated in metabolic reprogramming and glycolysis (Warburg effect). Using β-catenin RIP-RT-qPCR (versus IgG RIP-RT-qPCR) we validated enrichment of several Wnt signalling mRNAs in K562 and HEL cells including AMER1, BCL9L, AXIN2 and LEF1 (Fig. 2E, F). Taken together, these data show that β-catenin interacts with an mRNA network significantly enriched for Wnt signalling transcripts in myeloid cells.
Volcano plots showing the fold change in mRNA abundance detected within β-catenin RIP performed from DMSO (basal Wnt signalling) versus CHIR99021 (activated Wnt signalling) treated A K562 or B HEL cells (n = 3). Red dots represent enriched (Padj < 0.05) mRNAs whilst blue dots highlight Wnt signalling mRNAs and black dots highlight metabolic mRNAs. C Venn diagram illustrating the unique and shared mRNAs partners identified from β-catenin RIP performed from CHIR99021 versus DMSO treated K562 or HEL cells. D Gene ontology (GO) analysis using the human Molecular Signatures Database (Elsevier Pathway Collection) via Enrichr for pathways represented amongst the most significantly and commonly enriched mRNAs (Padj < 0.05), obtained in β-catenin RIP from K562 and HEL cells ±CHIR99021 with adjusted −Log10 P values annotated. Summary graphs showing the fold enrichment of selected Wnt signalling mRNAs isolated from IgG or β-catenin RIP-RT-qPCR performed in E K562 and F HEL cells. Data represents mean ± 1 s.d (n = 3). Statistical analysis is denoted by *p < 0.05 and **p < 0.01 as deduced from a one-sample t-test.
β-Catenin has weak capacity for RNA binding in myeloid cells
β-Catenin lacks any type of RNA-binding motif typically present in canonical RBPs such as RRMs (RNA-recognition motif), K-homology (KH), cold shock (CS) or zinc finger (ZF) domains [42]. It does however contain intrinsically disordered regions (IDR), which comprise β-catenin’s N- and C-termini (Supplementary Fig. S2) [43], which are commonly implicated in RNA binding by non-canonical RBPs [44]. Furthermore, Armadillo domains are one of the most frequently implicated structures in non-canonical RNA binding and β-catenin contains 12 such repeating domains in its central region which predict RNA binding (Supplemental Figure S3) [45]. β-Catenin has been reported to directly bind the 3′-UTR of mRNAs [38, 39], and has recently been shown to bind double-stranded RNA [46]. Therefore, to assess the RNA binding capacity of β-catenin in the haematopoietic system we performed β-catenin CLIP, a more stringent method of RBP:RNA capture. After first confirming efficient β-catenin CLIP (Fig. 3A), we once again performed β-catenin RIP and consistently isolated a significantly higher concentration of RNA versus IgG RIP (Fig. 3B, C). However, using a β-catenin CLIP approach we obtained no significantly different RNA enrichment over the IgG CLIP control (Fig. 3B, C). Using LIN28B as a positive control given its well-established status as an RBP, we observed visible RNA enrichment over the IgG control under both conditions as expected (Fig. 3B), suggesting β-catenin does not directly bind RNA strongly in this context.
A Representative immunoblot showing β-catenin level in IgG or β-catenin CLIP performed from K562 cells using 1 h or 16 h primary antibody incubation, ID = immunodepleted lysate. B Representative Agilent 2100 Bioanalyzer gel showing RNA isolated from IgG, β-catenin or LIN28B RIP and CLIP performed in K562 cells. C Summary graph indicating the RNA concentration isolated from IgG or β-catenin RIP and CLIP. Data represent mean ± SEM (n = 3 for CLIP, n = 8 for RIP). Statistical analysis is denoted by *p < 0.05 as deduced from a student’s t test.
MSI2 interacts with β-catenin in myeloid cells
We anticipated that β-catenin might network with Wnt signalling mRNAs indirectly through other canonical RBPs and returned to our previously characterised β-catenin interactome [8, 9]. MSI2 was identified as a putative β-catenin interacting RBP in myeloid cells (Fig. 4A) [8], and colorectal SW620 cells suggesting this interaction could also occur beyond haematopoietic tissue (Supplementary Fig. S4). MSI2 was selected for further study from our original β-catenin interactome on the basis that the interaction was detected in at least two cell lines (reaching statistical significance in at least one subcellular fraction), had a low presence (<10%) in the contaminant repository for affinity purification (CRAP)ome database (present in 44/716 datasets indicating a low contaminant risk) [47], and has previously documented roles in myeloid leukaemia and the regulation of HSPC [48, 49]. To confirm the interaction, we performed reciprocal MSI2 Co-IP from K562 and HEL cells and confirmed consistent β-catenin enrichment under both basal and Wnt signalling activated conditions (Fig. 4B, D). Furthermore, since MSI2 is an RBP [48], and β-catenin has also been shown to bind RNA [38, 39], we assessed whether the β-catenin:MSI2 interaction could be a consequence of RNA co-occupancy. However, after repeating MSI2 co-IP ± RNase A, and confirming complete digestion of RNA (Supplementary Fig. S5) [27], the MSI2:β-catenin protein interaction remained, suggesting these proteins are complexed irrespective of RNA presence (Fig. 4C, E).
A Scatter plots showing MSI2 detection in β-catenin interactomes performed in cytosolic fractions of K562 and HEL cells. Vertical dashed red line indicates the threshold for 2-fold change in protein binding at log2 (=1) relative to IgG Co-IP. The horizontal red line represents threshold for p < 0.05 on log10 scale (=1.3). Highlighted red dots indicate enriched interactions (p < 0.05), green labels highlight position of MSI2, and blue labels highlight position of β-catenin bait. Representative immunoblots showing the level of β-catenin protein present in MSI2 Co-IPs derived from B K562 - RNase A, C K562 + 20 µg/mL RNaseA, D HEL - RNase A and E HEL + 20 µg/mL RNaseA whole cell lysates, ±5µM CHIR99021 overnight. ID immunodepleted lysate.
Using AlphaFold3 [50], we generated predictions for the structures of β-catenin in complex with known interacting partners (Supplementary Fig. S6). Whilst AlphaFold3 is able to predict with good confidence (predicted interface TM-score (iptm) of 0.66 and 0.65, respectively) models of the β-catenin-LEF-1 (Supplementary Fig. S6A, B) and β-catenin-E-cadherin complex, it was not able to predict an accurate model of the β-catenin-MSI2 complex (iptm of 0.18). To further investigate the predicted interaction, we used PDBePISA [51], to analyse the protein-protein interface. Despite a sizeable buried hydrophobic interface, the complexation significance score of 1 suggested that the interface is not significant for assembly formation. Overall, the analysis suggests that the nature of the β-catenin:MSI2 interaction may be indirect, possibly as part of a larger complex.
β-Catenin interacts with MSI2 in myeloid cell lines and primary AML cells
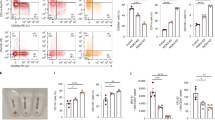
To assess the potential scope of cooperation between MSI2 and β-catenin across myeloid cells we assessed MSI2 expression across a panel of 18 myeloid cell lines. Two thirds of the myeloid cell lines assessed exhibited abundant co-expression of both β-catenin and MSI2 (Fig. 5A) which correlated through densitometric analysis (p = 0.03; Fig. 5B). We were unable to link this correlation with any specific cell line feature like morphology or genotype. To explore the clinical relevance of the β-catenin:MSI2 association, we screened 20 AML patients for protein expression of MSI2 and β-catenin (patient clinical details provided in Supplementary Table S1). Like myeloid cell lines, MSI2 was frequently co-expressed with β-catenin to a similar frequency observed in myeloid cell lines (12/20 samples [60%]; Fig. 5C), with an extensive correlation (p < 0.001) between the two proteins from densitometric analysis (Fig. 5D). There was no obvious association of this correlation with any particular clinical characteristic, although such a small cohort would likely lack sufficient statistical power to confidently link with a clinical parameter. To examine if the MSI2:β-catenin interaction could be detected in patient cells, we performed an MSI2 Co-IP from a primary AML sample containing abundant levels of both proteins and where ample cellular material existed (AML patient #10) and confirmed MSI2 interaction with β-catenin, but not with GAPDH ruling out non-specific binding (Fig. 5E). Collectively, these data indicate the β-catenin:MSI2 interaction could occur frequently across primary AML samples and could be clinically relevant given the association of both proteins with inferior survival in AML [11, 52, 53].
A Immunoblot of 18 myeloid leukaemia cell lines showing the relative level of β-catenin and MSI2 protein, with GAPDH used to assess protein loading. B Summary scatter plot showing the correlation (Spearman Rank R = 0.6, P < 0.03) between relative β-catenin and MSI2 protein expression across 18 myeloid cell lines as deduced from densitometry (normalised to GAPDH expression present within each cell line, AU = arbitrary units). C Immunoblot screen of 20 primary AML patient samples showing the relative level of β-catenin and MSI2 protein (light and dark exposures). * Denotes samples co-overexpressing both β-catenin and MSI2 relative to levels in CB MNC and CB CD34+ enriched fraction (pooled from five independent CB samples). X = Void sample containing no protein as deduced from negative GAPDH detection. D Summary scatter plot showing the correlation (Spearman Rank R = 0.79, P < 0.0001) between relative β-catenin and MSI2 protein expression across 20 primary AML patient samples as deduced from densitometry (normalised to GAPDH expression present in each sample, AU = arbitrary units). E Immunoblot showing the level of β-catenin and GAPDH protein present in an MSI2 Co-IP performed from primary AML patient sample #10 of sample screen.
MSI2 impacts Wnt signalling output through modulation of LEF-1
To evaluate if MSI2 could bind or regulate Wnt mRNAs we assessed its net impact on Wnt signalling output using the BAR system as done previously [8, 27, 30]. MSI2 knockdown in HEL cells diminished Wnt signalling output and LEF-1 expression (Supplementary Fig. S7), however we were unable to use this cell line for subsequent experiments since only one MSI2 shRNA variant remained viable following lentiviral transduction and selection. Depletion of MSI2 using two unique shRNAs had little impact on β-catenin level but did cause a concomitant decrease in expression of the Wnt transcription factor LEF-1 (Fig. 6A) in K562 and KU812 cells. As could be expected from diminished LEF-1, this resulted in diminished Wnt signalling output from both K562 and KU812 cells exhibiting MSI2 knockdown (Fig. 6B–D). We also demonstrated that ectopic MSI2 expression increased nuclear LEF-1 expression and TCF/LEF activity in K562 cells in Wnt signalling activated conditions (Supplementary Fig. S8).
A Immunoblots showing MSI2, β-catenin and LEF1 level in K562 and KU812 cells harbouring MSI2 shRNA or non-targeting shRNA controls. GAPDH indicates protein loading. B Representative flow cytometric histograms showing intensity of the TCF-dependent expression of Venus Yellow Fluorescent Protein (YFP) from the β-catenin activated reporter (BAR) reporter, or negative control ‘found unresponsive’ BAR (fuBAR; containing mutated promoter binding sites) in K562 and KU812 cells ± MSI2 shRNA following treatment with 5 μM CHIR99021 overnight. The fuBAR (dashed), non-targeting control shRNA (grey filled), and two MSI2 shRNAs (blue or red) histograms are shown. Summary graphs showing the median fluorescence intensity (MFI) generated from the BAR/fuBAR in C K562 and D KU812 cells ±MSI2 shRNA with ±5 μM CHIR99021. E Immunoblots showing total β-catenin, LEF-1, and MSI2 subcellular localisation in K562 and KU812 cells lentivirally transduced with two different MSI2 shRNAs ±5 μM CHIR99021. Lamin A/C and α-tubulin indicate the purity/loading of the nuclear (N) and cytosol (C) fractions respectively. Densitometric quantitation of LEF-1 (relative to nuclear Lamin A/C, AU = arbitrary units) present in nuclear fractions of F K562 and G KU812 cells ± MSI2 shRNA with ±5 μM CHIR99021. Summary graph showing the fold change in LEF1 and TCF7L2 mRNA expression as assessed by RT-qPCR in (H) K562 and (I) KU812 cells expressing MSI2 shRNA relative to non-targeting control shRNA (represented by dotted line at y = 1). Fold change is relative to matched respective controls (black dashed line) and overall expression was normalised to the housekeeping gene β-actin (ACTB). Data represent mean ± 1 s.d (n = 3). Statistical analysis is denoted by *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 as deduced from a student’s t test or one-sample t-test for RT-qPCR data.
To understand the basis for reduced TCF/LEF output in MSI2-deficient cells, we performed nuclear/cytoplasmic fractionation and examined the expression/localisation of Wnt signalling components. Again, we observed limited impact of MSI2 knockdown on β-catenin level or nuclear localisation in response to Wnt signalling activation but did note reduced LEF-1 expression and nuclear localisation in K562 and KU812 cells, which was more pronounced in Wnt signalling active versus basal states (Fig. 6E, F, G). To understand the basis for reduced LEF-1 expression upon MSI2 knockdown we examined mRNA levels using qRT-PCR and observed significantly reduced LEF1 (but not that of another critical Wnt effector; TCF7L2) expression in MSI2-deficient K562 and KU812 cells (Fig. 6H, I). Collectively these data indicate that MSI2 can influence Wnt signalling activity through modulation of mRNA and protein levels of the Wnt effector LEF-1.
MSI2 binds LEF1 mRNA and influences its stability
Given the role of MSI2 as an RBP and regulator of post-transcriptional gene expression [48], we next assessed whether LEF1 could be an mRNA partner for MSI2. After first confirming efficient MSI2 RIP in K562 cells (Fig. 7A), and enrichment of known mRNA partners MYB and MYC [54], we observed enriched LEF1 (and TCF7L2) mRNA versus control IgG RIP (Fig. 7B). Similarly, using an MSI2 CLIP approach we observed enrichment of LEF1 transcript (and MYC and MYB) versus IgG CLIP (Fig. 7C) indicating that LEF1 could be a direct mRNA binding target of MSI2. Given β-catenin immunoprecipitates with LEF1 mRNA through both RIP-seq and RIP-qRT-PCR (Fig. 2) but shows limited capacity for direct RNA binding (Fig. 3), we hypothesised that β-catenin may impact binding of canonical RBPs like MSI2 to LEF1 mRNA. To interrogate this, we generated β-catenin depleted K562 cells using two unique shRNAs and re-assessed MSI2 binding to LEF1 mRNA using MSI2 RIP-RT-qPCR and MSI2-CLIP-RT-qPCR. Firstly, we confirmed β-catenin depletion had no impact on overall MSI2 protein level (Fig. 7D). Total LEF1 mRNA expression was mostly unchanged in β-catenin depleted K562 RIP and CLIP inputs except for an increase observed in the RIP input of β-catenin shRNA#2 (Fig. 7E, F). However, we observed attenuated of LEF1 enrichment upon β-catenin knockdown through both MSI2-RIP (Fig. 7G) and MSI2-CLIP (Fig. 7H)
A Immunoblot showing MSI2 level in MSI2 RIP performed from K562 cells. ID= immunodepleted lysate. Summary graphs showing the fold enrichment of TCF7L2, MYC, MYB, and LEF1 mRNA in MSI2 B RIP and C CLIP performed from K562 cells. Fold enrichment is relative to matched IgG RIPs (dashed black line). D Immunoblots showing MSI2 and β-catenin levels in K562 cell lines harbouring β-catenin shRNAs versus non-targeting shRNA control. GAPDH indicates protein loading. Summary graphs showing the fold change in LEF1 mRNA expression in input K562 cells harbouring β-catenin shRNA versus non-targeting shRNA control (dashed black line) used for E MSI2 RIP and F) MSI2 CLIP. Summary graphs showing the fold enrichment of LEF1 mRNA (versus IgG RIP) in MSI2 G) RIP and H CLIP generated from K562 cells ± β-catenin shRNAs. I Line graph summarising the fold change in LEF1 mRNA expression (relative to T = 0 h for each respective cell line) in K562 cells harbouring MSI2 shRNA (red) versus non-targeting shRNA control (black) treated with 2 µg/ml ActD for indicated times. J Immunoblot showing the levels of MSI2, β-catenin, and LEF-1 protein in K562 cells ±MSI2 shRNA with treatment of 2 µg/ml ActD for indicated timepoints. Data represents mean ± 1 s.d (n = 3). Statistical analysis is denoted by *p < 0.05 and **p < 0.01 as deduced from a student’s t-test.
Finally, given MSI2’s prominent roles in post-transcription, we next assessed whether its interaction with LEF1 mRNA could influence its stability following the global inhibition of transcription by actinomycin D (ActD) treatment. Using the K562 variant exhibiting the most complete MSI2 knockdown (shRNA#2; Fig. 6A) LEF1 mRNA demonstrated decreased stability compared with the non-targeting shRNA (relative to their respective 0 hr timepoint levels; Fig. 7I). This corresponded to a markedly accelerated depletion of LEF-1 protein at 4 and 8 h ActD treatment compared with non-targeting shRNA controls (Fig. 7J), which is particularly notable given the stable half-life we typically observe for the LEF-1 peptide (Supplementary Fig. S9). Taken together, these data suggest that β-catenin promotes MSI2 binding to LEF1, and MSI2 can modulate the subsequent stability of LEF1 mRNA through a post-transcriptional mechanism.
MSI2 regulation of LEF-1 may represent an important regulatory axis in human HSPC growth and survival
MSI2 and LEF1 have individually documented roles in the maintenance and function of HSPC [49, 55,56,57,58]. Previously, MSI2 has been shown to promote HSPC expansion and we hypothesised that some of this activity could be mediated through LEF-1. To interrogate this hypothesis human CB-derived CD34+ HSPC were isolated and lentivirally transduced with ectopic MSI2 expression (Fig. 8A) alongside either non-targeting, or LEF-1 shRNAs (Fig. 8B). LEF1 target shRNA sequences were prior tested for their efficiency in depleting LEF-1 expression in K562 cells and observed to be highly efficient (Supplementary Fig. S10). From day 4 onwards (3 days post-lentiviral transduction) we observed a modest but consistent increase in the expansion rates of HSPCs expressing ectopic MSI2 versus control cultures, which was higher (32% ± 2 increase) at day 8 of liquid culture (Fig. 8C) in keeping with previous reports of MSI2-mediated HSPC expansion [58]. A higher percentage of CD34+ events was also observed in MSI2 overexpressing cultures versus controls at this timepoint (Fig. 8D). Increases in the expansion rates and CD34 positivity of MSI2 overexpressing cultures were curtailed in the presence of LEF-1 shRNA (Fig. 8C, D). Primary HSPC cultures harbouring LEF-1 shRNA exhibited consistently reduced growth rates which were lower with shRNA#2 at days 8, 11 and 13 of liquid culture (Fig. 8D). Indeed, the growth repression mediated by LEF-1 extended beyond suppressing MSI2-induced expansion, implying independently important roles for LEF-1 in human HSPC biology. These data are consistent with our previous study showing LEF-1 can impact the proliferation of myeloid leukaemia cells [8], and implies that part of MSI2’s capacity to expand human HSPCs could be mediated through LEF-1 (Fig. 8E).
A Immunoblot showing the level of MSI2 in day 4 primary CB-derived HSPC cultures following 3 days post lentiviral transduction with empty vector (EV) or human MSI2. GAPDH indicates protein loading. B Representative day 4 flow cytometric histogram plots showing percentage GFP+ events in primary HSPC cultures lentivirally transduced with ectopic MSI2 ± LEF-1 or non-targeting (NT) shRNA, versus matched untransduced cells. C Line graph showing the fold-expansion of HSPC cultures following lentiviral transduction with ectopic MSI2 ± LEF-1 shRNAs. D Bar graph showing the relative percentage of CD34+ events in day 8 HSPC liquid cultures following lentiviral transduction with ectopic MSI2 ± LEF-1 shRNAs. All data represent mean ± 1 s.d (n = 3, each replicate pooled from multiple donors). Statistical analysis is denoted by *p < 0.05 and **p < 0.01 as deduced from a student’s t test. E Graphical summary depicting how the β-catenin:MSI2 axis could regulate LEF-1 expression and subsequent growth/survival of human HSPC.
Discussion
Wnt signalling is a critical pathway regulating human HSPCs and is frequently implicated in haematological malignancy. The central mediator of the pathway, β-catenin, has been a longstanding therapeutic target in leukaemia but attempts to therapeutically target the molecule have achieved limited success to date—curtailed by a poor understanding of its molecular interactions in haematopoietic cells. In solid tissues, β-catenin has a vital role in cell adhesion within membrane complexes where it facilitates tight cell-cell adhesion, however such complexes are not a prominent feature of blood cells meaning free β-catenin likely serves alternative functions in this context. Following on from our previous studies showing that β-catenin interacts with vast RBP networks in myeloid cells [8, 9], we now demonstrate for the first time that β-catenin may contribute towards post-transcriptional regulation in haematopoietic cells.
To identify transcripts over which β-catenin may have post-transcriptional influence in myeloid cells we initially performed β-catenin RIPseq after first confirming β-catenin pulled down with RNA. We identified hundreds of enriched transcripts with β-catenin under basal versus Wnt signalling activated conditions across K562 and HEL cells. From both K562 and HEL β-catenin RIPseq we identified the Wnt signalling pathway itself as one of the top pathways/processes enriched from common β-catenin-associated mRNAs. The existence of feedback loops within Wnt signalling has been known for some time with both positive and negative regulators known to be transcriptional targets of the pathway such as LEF1 [59], LGR5 [60], FZD7 [61], and AXIN2 [62], RNF43 [63], DKK1 [64], respectively. However, these were thought to be regulated in a predominantly transcriptional fashion. Our data raise the prospect that β-catenin may also influence feedback loops in Wnt signalling through post-transcriptional mechanisms including both established Wnt target genes, but also Wnt signalling mRNAs that are not transcriptional targets of the pathway including AMER1, BCL9L, TCF7, DVL1/2/3 and CSNK1E.
The concept that β-catenin could post-transcriptionally govern Wnt signalling is gaining traction with a recent report showing that is it recruited to the translation initiation complex in the dentate gyrus of mice [65]. Patil et al. demonstrated that following eIF4E phosphorylation, β-catenin was substantially recruited to the eIF4E cap complex following long-term potentiation where it preferentially augmented the translation of Wnt signalling mRNAs including Wnt4, Lrp5, Fzd2, Fzd4 and Dvl2. This follows from another study reporting β-catenin recruitment to the messenger ribonucleoprotein and translational pre‐initiation complex through the fragile X mental retardation protein (FMRP), where it repressed translation [66]. Such a post-transcriptional role for free cytoplasmic/nuclear β-catenin might be more prominent in a fluid tissue like blood where its other well-characterised role in cell adhesion may not be so relevant. However, trying to disentangle β-catenin’s transcriptional influences from its post-transcriptional effects is technically challenging, especially since data from T-cell acute lymphoblastic leukaemia cells indicate β-catenin-dependent transcriptional signatures include genes involved with RNA biosynthesis and processing [67].
The capacity for β-catenin to bind RNA directly remains unclear. Previously, β-catenin has been shown to bind selective mRNAs including COX2 through the 3′UTR AU-rich elements in concert with RBPs such as HuR [38, 39], and recently has been shown to bind double-stranded RNA [46]. Numerous reports also exist of β-catenin binding and modulating the stability/splicing of numerous transcripts including CA9, SNAI1, IL6 [40], Cadherin 11 [41], and an oestrogen receptor-β (ER-β) variant [68]. Armadillo domains have also been identified as one of the most frequent domain types associated with RNA binding amongst non-canonical RBPs [45], and β-catenin has 12 such repeating structures in its central domain. However, β-catenin has no recognised canonical RNA binding motif, and we observed little RNA pull down through a more stringent CLIP assessment in myeloid cells. Therefore, we hypothesise that β-catenin’s interactions with RNA in this context occur indirectly through binding other canonical RBPs and there were many identified in our original β-catenin interactome study [8, 9]. Thus far, we have confirmed interactions with RBPs such as WT1 [27], HuR (Supplementary Fig. S1), LIN28B and TOE1 (unpublished) and all of these β-catenin:RBP interactions occur irrespective of RNA presence (Fig. 4B, C), suggesting β-catenin complexes with RBPs, rather than associates though co-occupancy of RNA. This follows from many other reported interactions of β-catenin with RBPs including FUS [69], HuR [38, 40], and FMRP [66], and suggests a wider role for β-catenin in RNA:RBP complexes.
This is the first study to report an interaction between β-catenin and MSI2. An elegant study from Vu et al. also assessed MSI2 protein interactions in K562 cells but β-catenin was not detected in their mass spectrometry (MS) dataset [70]. This can be partly explained by the different Co-IP approaches taken between the two studies. In the Vu et al. study, the authors performed a HA Co-IP to precipitate ectopically expressed HA-tagged MSI2. In contrast, our study initially performed a Co-IP of endogenous β-catenin using a β-catenin validated antibody to first identify the β-catenin:MSI2 interaction by MS, and then confirmed the interaction through a reciprocal Co-IP of endogenous MSI2 using a validated MSI2 antibody. Interestingly, many of the MSI2-interacting proteins from the Vu et al. study were also present in our original β-catenin interactome, including SYNCRIP, CAPRIN1, HNRNPR, MYBBP1A and HNRNPA3, however their high presence in the CRAPome (>10%) [47] meant they were not prioritised for experimental follow up. It would however be of great interest to examine whether β-catenin is present in wider MSI2 ribonucleoprotein complexes.
We were also the first to report functional crosstalk between Wnt/β-catenin signalling and MSI2 in myeloid cells [71]. A subsequent report from the Sheng group adopted a deletion of the chromosome 5q (del (5q)) murine model of MDS which has previously demonstrated a dependency on the negative Wnt regulator and tumour suppressor APC for MDS development [15]. The authors show that MSI2 may also impact Wnt signalling function in murine HSC, however there are no established measurements of Wnt signalling activity (e.g. TCF/LEF activity, Wnt target gene assessment, β-catenin stabilisation/nuclear localision dynamics) performed in this study [72]. We found the principle connection between these two proteins appeared to be the Wnt transcription factor LEF-1 which was suppressed with MSI2 knockdown across multiple cell lines. This aligns with a previous report of lower LEF-1 (and TCF4) levels (protein and mRNA) upon MSI2 depletion in SMMC-7721 hepatocellular carcinoma cells [73]. A Wnt gene signature was also observed in response to MSI2 loss in four human leukaemia cell lines in a study by Kharas et al., which were reciprocally upregulated upon ectopic MSI2 expression in murine HSC [55]. Given the well-documented importance of MSI2 to HSPCs [49, 74, 75], we reasoned that regulation of LEF1 might be a mechanism utilised by MSI2 to control HSPC growth and survival. Indeed, LEF1 knockdown perturbed MSI2-mediated expansion of HSPCs, however, we cannot rule out LEF1 acting independently of any MSI2-mediated regulation since it is also a key regulator of the HSPC compartment [57, 76].
Finally, this study partly examined the mechanism by which MSI2 could regulate LEF1 expression and found that MSI2 bound LEF1 mRNA through both RIP and CLIP approaches. This is in agreement with an elegant study by the Pillai group who also performed MSI2 iCLIP in K562 cells and we noted that LEF1 was a high-confidence mRNA partner in their dataset [77]. Interestingly, they found only 2.6% of 4000 high-confidence MSI2 mRNA partners (mainly via 3′-UTR) are subsequently subject to translational regulation by MSI2. Our study also suggests that perhaps mRNA stability, rather than translation, is the key mechanism regulating LEF1 expression, concordant with MSI2’s previously documented roles in modulating mRNA stability [78,79,80,81]. We observed the degree of MSI2:LEF1 binding could be impacted by β-catenin level (with no consistent or substantial overall change to total LEF1 or MSI2 levels) alluding to a wider role for β-catenin augmenting RBP:RNA complexes in myeloid cells. How β-catenin influences RBP:RNA complexes remains unknown and could represent a novel function for β-catenin in haematopoietic cells. In its well-characterised transcriptional role it recruits TCF/LEF transcription factors and transcriptional co-activators (CBP, p300) to the DNA promoters of Wnt target genes, and perhaps β-catenin performs similar scaffolding functions for facilitating specific RBP:RNA interactions, particularly those implicating Wnt signalling mRNAs. However, trying to resolve its well characterised transcriptional effects, from any potential post-transcriptional influences, will be technically challenging, especially since both nascent RNA and active RNA polymerase II have been recently been observed within β-catenin condensates [43]. Further work is now required to define β-catenin’s post-transcriptional versus transcriptional roles in a haematological context.
References
Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–99. https://doi.org/10.1016/j.cell.2017.05.016.
Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–14.
Danek P, Kardosova M, Janeckova L, Karkoulia E, Vanickova K, Fabisik M, et al. beta-Catenin-TCF/LEF signaling promotes steady-state and emergency granulopoiesis via G-CSF receptor upregulation. Blood. 2020;136:2574–87. https://doi.org/10.1182/blood.2019004664.
Brown AL, Salerno DG, Sadras T, Engler GA, Kok CH, Wilkinson CR, et al. The GM-CSF receptor utilizes beta-catenin and Tcf4 to specify macrophage lineage differentiation. Differentiation. 2012;83:47–59. https://doi.org/10.1016/j.diff.2011.08.003.
Shooshtarizadeh P, Helness A, Vadnais C, Brouwer N, Beauchemin H, Chen R, et al. Gfi1b regulates the level of Wnt/beta-catenin signaling in hematopoietic stem cells and megakaryocytes. Nat Commun. 2019;10:1270. https://doi.org/10.1038/s41467-019-09273-z.
Luis TC, Ichii M, Brugman MH, Kincade P, Staal FJ. Wnt signaling strength regulates normal hematopoiesis and its deregulation is involved in leukemia development. Leukemia. 2012;26:414–21. https://doi.org/10.1038/leu.2011.387.
Luis, Naber TC, Roozen BA, Brugman PP, de Haas MH, Ghazvini EF, et al. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell. 2011;9:345–56.
Morgan RG, Ridsdale J, Payne M, Heesom KJ, Wilson MC, Davidson A, et al. LEF-1 drives aberrant beta-catenin nuclear localization in myeloid leukemia cells. Haematologica. 2019;104:1365–77. https://doi.org/10.3324/haematol.2018.202846.
Wagstaff M, Coke B, Hodgkiss GR, and Morgan RG Targeting beta-catenin in acute myeloid leukaemia: past, present, and future perspectives. Biosci Rep. 2022; https://doi.org/10.1042/BSR20211841.
Simon M, Grandage VL, Linch DC, Khwaja A. Constitutive activation of the Wnt/beta-catenin signalling pathway in acute myeloid leukaemia. Oncogene. 2005;24:2410–20.
Ysebaert L, Chicanne G, Demur C, De TF, Prade-Houdellier N, Ruidavets JB, et al. Expression of beta-catenin by acute myeloid leukemia cells predicts enhanced clonogenic capacities and poor prognosis. Leukemia. 2006;20:1211–6.
Griffiths EA, Golding MC, Srivastava P, Povinelli BJ, James SR, Ford LA, et al. Pharmacological targeting of beta-catenin in normal karyotype acute myeloid leukemia blasts. Haematologica. 2015;100:e49–52. https://doi.org/10.3324/haematol.2014.113118.
Jiang X, Mak PY, Mu H, Tao W, Mak DH, Kornblau S, et al. Disruption of Wnt/beta-catenin exerts antileukemia activity and synergizes with FLT3 inhibition in FLT3-mutant acute myeloid leukemia. Clin Cancer Res. 2018;24:2417–29. https://doi.org/10.1158/1078-0432.CCR-17-1556.
Li L, Sheng Y, Li W, Hu C, Mittal N, Tohyama K, et al. beta-Catenin is a candidate therapeutic target for myeloid neoplasms with del(5q). Cancer Res. 2017;77:4116–26. https://doi.org/10.1158/0008-5472.CAN-17-0202.
Stoddart A, Wang J, Hu C, Fernald AA, Davis EM, Cheng JX, et al. Inhibition of WNT signaling in the bone marrow niche prevents the development of MDS in the Apc(del/+) MDS mouse model. Blood. 2017;129:2959–70. https://doi.org/10.1182/blood-2016-08-736454.
Tonks A, Pearn L, Musson M, Gilkes A, Mills KI, Burnett AK, et al. Transcriptional dysregulation mediated by RUNX1-RUNX1T1 in normal human progenitor cells and in acute myeloid leukaemia. Leukemia. 2007;21:2495–505.
Muller-Tidow C, Steffen B, Cauvet T, Tickenbrock L, Ji P, Diederichs S, et al. Translocation products in acute myeloid leukemia activate the Wnt signaling pathway in hematopoietic cells. Mol Cell Biol. 2004;24:2890–904.
Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, et al. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327:1650–3.
Yeung J, Esposito MT, Gandillet A, Zeisig BB, Griessinger E, Bonnet D, et al. beta-Catenin mediates the establishment and drug resistance of MLL leukemic stem cells. Cancer Cell. 2010;18:606–18.
Dietrich PA, Yang C, Leung HH, Lynch JR, Gonzales E, Liu B, et al. GPR84 sustains aberrant beta-catenin signaling in leukemic stem cells for maintenance of MLL leukemogenesis. Blood. 2014;124:3284–94. https://doi.org/10.1182/blood-2013-10-532523.
Fong CY, Gilan O, Lam EY, Rubin AF, Ftouni S, Tyler D, et al. BET inhibitor resistance emerges from leukaemia stem cells. Nature. 2015;525:538–42. https://doi.org/10.1038/nature14888.
Perry JM, Tao F, Roy A, Lin T, He XC, Chen S, et al. Overcoming Wnt-beta-catenin dependent anticancer therapy resistance in leukaemia stem cells. Nat Cell Biol. 2020;22:689–700. https://doi.org/10.1038/s41556-020-0507-y.
Sakoda T, Kikushige Y, Miyamoto T, Irifune H, Harada T, Hatakeyama K, et al. TIM-3 signaling hijacks the canonical Wnt/beta-catenin pathway to maintain cancer stemness in acute myeloid leukemia. Blood Adv. 2023;7:2053–65. https://doi.org/10.1182/bloodadvances.2022008405.
Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–41.
Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–67.
Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. https://doi.org/10.1016/j.cell.2005.09.020.
Wagstaff M, Tsaponina O, Caalim G, Greenfield H, Milton-Harris L, Mancini EJ, et al. Crosstalk between beta-catenin and WT1 signaling activity in acute myeloid leukemia. Haematologica. 2023;108:283–9. https://doi.org/10.3324/haematol.2021.280294.
Hodson DJ, Screen M, Turner M. RNA-binding proteins in hematopoiesis and hematological malignancy. Blood. 2019;133:2365–73. https://doi.org/10.1182/blood-2018-10-839985.
Olaitan S, Wagstaff M, Sevim O, Akieme R, Toska V, Humphreys C, et al. Assessment of mononuclear cell populations derived from human umbilical cord blood. Eur J Haematol. 2022;109:787–8. https://doi.org/10.1111/ejh.13846.
Morgan RG, Pearn L, Liddiard K, Pumford SL, Burnett AK, Tonks A, et al. gamma-Catenin is overexpressed in acute myeloid leukemia and promotes the stabilization and nuclear localization of beta-catenin. Leukemia. 2013;27:336–43. https://doi.org/10.1038/leu.2012.221.
Martin M Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:3. https://doi.org/10.14806/ej.17.1.200.
Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37:907–15. https://doi.org/10.1038/s41587-019-0201-4.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–9. https://doi.org/10.1093/bioinformatics/btp352.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. https://doi.org/10.1186/s13059-014-0550-8.
Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4:1686. https://doi.org/10.21105/joss.01686.
Gu ZG, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32:2847–9. https://doi.org/10.1093/bioinformatics/btw313.
Van de Wetering M, Castrop J, Korinek V, Clevers H. Extensive alternative splicing and dual promoter usage generate Tcf-1 protein isoforms with differential transcription control properties. Mol Cell Biol. 1996;16:745–52.
Kim I, Kwak H, Lee HK, Hyun S, Jeong S. beta-Catenin recognizes a specific RNA motif in the cyclooxygenase-2 mRNA 3’-UTR and interacts with HuR in colon cancer cells. Nucleic Acids Res. 2012;40:6863–72. https://doi.org/10.1093/nar/gks331.
Lee HK, Jeong S. Beta-catenin stabilizes cyclooxygenase-2 mRNA by interacting with AU-rich elements of 3’-UTR. Nucleic Acids Res. 2006;34:5705–14. https://doi.org/10.1093/nar/gkl698.
D’Uva G, Bertoni S, Lauriola M, De Carolis S, Pacilli A, D’Anello L, et al. Beta-catenin/HuR post-transcriptional machinery governs cancer stem cell features in response to hypoxia. PloS One. 2013;8:e80742. https://doi.org/10.1371/journal.pone.0080742.
Farina AK, Bong YS, Feltes CM, Byers SW. Post-transcriptional regulation of cadherin-11 expression by GSK-3 and beta-catenin in prostate and breast cancer cells. PloS One. 2009;4:e4797. https://doi.org/10.1371/journal.pone.0004797.
Zhao Y, Mir C, Garcia-Mayea Y, Paciucci R, Kondoh H, LLeonart ME. RNA-binding proteins: underestimated contributors in tumorigenesis. Semin Cancer Biol. 2022;86:431–44. https://doi.org/10.1016/j.semcancer.2022.01.010.
Gui T, Fleming C, Manzato C, Bourgeois B, Sirati N, Heuer J, et al. Targeted perturbation of signaling-driven condensates. Mol Cell. 2023;83:4141–4157 e4111. https://doi.org/10.1016/j.molcel.2023.10.023.
Calabretta S, Richard S. Emerging roles of disordered sequences in RNA-binding proteins. Trends Biochem Sci. 2015;40:662–72. https://doi.org/10.1016/j.tibs.2015.08.012.
Moore S, Jarvelin AI, Davis I, Bond GL, Castello A. Expanding horizons: new roles for non-canonical RNA-binding proteins in cancer. Curr Opin Genet Dev. 2018;48:112–20. https://doi.org/10.1016/j.gde.2017.11.006.
Yang Z, Zhou J, Li Z, Guo J, Fang L, Xiao X, et al. Identification of whole-cell dsRNA-binding proteins by phase separation. RNA Biol. 2024;21:32–45. https://doi.org/10.1080/15476286.2024.2386498.
Mellacheruvu D, Wright Z, Couzens AL, Lambert JP, St-Denis NA, Li T, et al. The CRAPome: a contaminant repository for affinity purification-mass spectrometry data. Nat Methods. 2013;10:730–6. https://doi.org/10.1038/nmeth.2557.
Kharas MG, Lengner CJ. Stem cells, cancer, and MUSASHI in blood and guts. Trends Cancer. 2017;3:347–56. https://doi.org/10.1016/j.trecan.2017.03.007.
Hope KJ, Cellot S, Ting SB, MacRae T, Mayotte N, Iscove NN, et al. An RNAi screen identifies Msi2 and Prox1 as having opposite roles in the regulation of hematopoietic stem cell activity. Cell Stem Cell. 2010;7:101–13. https://doi.org/10.1016/j.stem.2010.06.007.
Abramson J, Adler J, Dunger J, Evans R, Green T, Pritzel A, et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature. 2024;630:493–500. https://doi.org/10.1038/s41586-024-07487-w.
Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–97. https://doi.org/10.1016/j.jmb.2007.05.022.
Byers RJ, Currie T, Tholouli E, Rodig SJ, Kutok JL. MSI2 protein expression predicts unfavorable outcomes in acute myeloid leukemia. Blood. 2011;118:2857–67. https://doi.org/10.1182/blood-2011-04-346767.
Thol F, Winschel C, Sonntag AK, Damm F, Wagner K, Chaturvedi A, et al. Prognostic significance of expression levels of stem cell regulators MSI2 and NUMB in acute myeloid leukemia. Ann Hematol. 2013;92:315–23. https://doi.org/10.1007/s00277-012-1637-5.
Nguyen DTT, Lu Y, Chu KL, Yang X, Park SM, Choo ZN, et al. HyperTRIBE uncovers increased MUSASHI-2 RNA binding activity and differential regulation in leukemic stem cells. Nat Commun. 2020;11:2026. https://doi.org/10.1038/s41467-020-15814-8.
Kharas MG, Lengner CJ, Al-Shahrour F, Bullinger L, Ball B, Zaidi S, et al. Musashi-2 regulates normal hematopoiesis and promotes aggressive myeloid leukemia. Nat Med. 2010;16:903–8. https://doi.org/10.1038/nm.2187.
Edmaier KE, Stahnke K, Vegi N, Mulaw M, Ihme S, Scheffold A, et al. Expression of the lymphoid enhancer factor 1 is required for normal hematopoietic stem and progenitor cell function. Leukemia. 2014;28:227–30. https://doi.org/10.1038/leu.2013.238.
Feder K, Edmaier-Schroger K, Rawat VPS, Kirsten N, Metzeler K, Kraus JM, et al. Differences in expression and function of LEF1 isoforms in normal versus leukemic hematopoiesis. Leukemia. 2020;34:1027–37. https://doi.org/10.1038/s41375-019-0635-1.
Rentas S, Holzapfel N, Belew MS, Pratt G, Voisin V, Wilhelm BT, et al. Musashi-2 attenuates AHR signalling to expand human haematopoietic stem cells. Nature. 2016;532:508–11. https://doi.org/10.1038/nature17665.
Hovanes K, Li TW, Munguia JE, Truong T, Milovanovic T, Lawrence Marsh J, et al. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet. 2001;28:53–57. https://doi.org/10.1038/88264.
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. https://doi.org/10.1038/nature06196.
Willert J, Epping M, Pollack JR, Brown PO, Nusse R. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol. 2002;2:8. https://doi.org/10.1186/1471-213x-2-8.
Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, et al. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta-catenin signaling is activated in human colon tumors. Proc Natl Acad Sci USA. 2001;98:14973–8. https://doi.org/10.1073/pnas.261574498.
Takahashi N, Yamaguchi K, Ikenoue T, Fujii T, Furukawa Y. Identification of two Wnt-responsive elements in the intron of RING finger protein 43 (RNF43) gene. PloS One. 2014;9:e86582. https://doi.org/10.1371/journal.pone.0086582.
Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, et al. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520–6. https://doi.org/10.1038/sj.onc.1207892.
Patil S, Chalkiadaki K, Mergiya TF, Krimbacher K, Amorim IS, Akerkar S, et al. eIF4E phosphorylation recruits beta-catenin to mRNA cap and promotes Wnt pathway translation in dentate gyrus LTP maintenance. iScience. 2023;26:106649. https://doi.org/10.1016/j.isci.2023.106649.
Ehyai S, Miyake T, Williams D, Vinayak J, Bayfield MA, McDermott, JC FMRP recruitment of beta-catenin to the translation pre-initiation complex represses translation. EMBO Rep. 2018;19. https://doi.org/10.15252/embr.201745536.
Garcia-Hernandez V, Arambilet D, Guillen Y, Lobo-Jarne T, Maqueda M, Gekas C, et al. beta-Catenin activity induces an RNA biosynthesis program promoting therapy resistance in T-cell acute lymphoblastic leukemia. EMBO Mol Med. 2023;15:e16554. https://doi.org/10.15252/emmm.202216554.
Lee HK, Choi YS, Park YA, Jeong S. Modulation of oncogenic transcription and alternative splicing by beta-catenin and an RNA aptamer in colon cancer cells. Cancer Res. 2006;66:10560–6. https://doi.org/10.1158/0008-5472.CAN-06-2526.
Sato S, Idogawa M, Honda K, Fujii G, Kawashima H, Takekuma K, et al. beta-catenin interacts with the FUS proto-oncogene product and regulates pre-mRNA splicing. Gastroenterology. 2005;129:1225–36. https://doi.org/10.1053/j.gastro.2005.07.025.
Vu LP, Prieto C, Amin EM, Chhangawala S, Krivtsov A, Calvo-Vidal MN, et al. Functional screen of MSI2 interactors identifies an essential role for SYNCRIP in myeloid leukemia stem cells. Nat Genet. 2017;49:866–75. https://doi.org/10.1038/ng.3854.
Wagstaff M, Nguyen D, Blair A, Towler B, Newbury S, and Morgan, R A β-catenin:MSI2 axis regulates the expression of LEF1 and subsequent human haematopoietic stem/progenitor cell proliferation. bioRxiv, 2024;2024.2001.2028.577638. https://doi.org/10.1101/2024.01.28.577638.
Zhang H, Guo R, Li Z, Ma R, Xu S, Yin L, et al. MSI2 mediates WNT/beta-Catenin pathway function in hematopoietic stem cells. Leukemia. 2024; https://doi.org/10.1038/s41375-024-02447-9.
Wang MH, Qin SY, Zhang SG, Li GX, Yu ZH, Wang K, et al. Musashi-2 promotes hepatitis B-virus related hepatocellular carcinoma progression via the Wnt/beta-catenin pathway. Am J Cancer Res. 2015;5:1089–1100.
de Andres-Aguayo L, Varas F, Kallin EM, Infante JF, Wurst W, Floss T, et al. Musashi 2 is a regulator of the HSC compartment identified by a retroviral insertion screen and knockout mice. Blood. 2011;118:554–64. https://doi.org/10.1182/blood-2010-12-322081.
Park SM, Deering RP, Lu Y, Tivnan P, Lianoglou S, Al-Shahrour F, et al. Musashi-2 controls cell fate, lineage bias, and TGF-beta signaling in HSCs. J Exp Med. 2014;211:71–87. https://doi.org/10.1084/jem.20130736.
Yu S, Li F, Xing S, Zhao T, Peng W, Xue HH. Hematopoietic and leukemic stem cells have distinct dependence on Tcf1 and Lef1 transcription factors. J Biol Chem. 2016;291:11148–60. https://doi.org/10.1074/jbc.M116.717801.
Karmakar S, Ramirez O, Paul KV, Gupta AK, Kumari V, Botti V, et al. Integrative genome-wide analysis reveals EIF3A as a key downstream regulator of translational repressor protein Musashi 2 (MSI2). NAR Cancer. 2022;4:zcac015. https://doi.org/10.1093/narcan/zcac015.
Zhao J, Zhang Y, Liu XS, Zhu FM, Xie F, Jiang CY, et al. RNA-binding protein Musashi2 stabilizing androgen receptor drives prostate cancer progression. Cancer Sci. 2020;111:369–82. https://doi.org/10.1111/cas.14280.
Li M, Li AQ, Zhou SL, Lv H, Wei P, Yang WT. RNA-binding protein MSI2 isoforms expression and regulation in progression of triple-negative breast cancer. J Exp Clin Cancer Res. 2020;39:92. https://doi.org/10.1186/s13046-020-01587-x.
Bennett CG, Riemondy K, Chapnick DA, Bunker E, Liu X, Kuersten S, et al. Genome-wide analysis of Musashi-2 targets reveals novel functions in governing epithelial cell migration. Nucleic Acids Res. 2016;44:3788–3800. https://doi.org/10.1093/nar/gkw207.
Duggimpudi S, Kloetgen A, Maney SK, Munch PC, Hezaveh K, Shaykhalishahi H, et al. Transcriptome-wide analysis uncovers the targets of the RNA-binding protein MSI2 and effects of MSI2’s RNA-binding activity on IL-6 signaling. J Biol Chem. 2018;293:15359–69. https://doi.org/10.1074/jbc.RA118.002243.
Dormoy-Raclet V, Menard I, Clair E, Kurban G, Mazroui R, Di Marco S, et al. The RNA-binding protein HuR promotes cell migration and cell invasion by stabilizing the beta-actin mRNA in a U-rich-element-dependent manner. Mol Cell Biol. 2007;27:5365–80. https://doi.org/10.1128/MCB.00113-07.
Acknowledgements
This work was funded by the Kay Kendall Leukaemia Fund (KKL1051/KKL1446), Leukaemia & Myeloma Research UK (4-5/06.21R) and the Children’s Cancer & Leukaemia Group (CCLGA 2023 16 Morgan). We are thankful for additional funding from the Republic of Türkiye National Ministry of Education (Sevim), Sussex Cancer Fund (Park) and British Society for Haematology (Park). We are grateful to the Leeds Genomics facility for executing RNA sequencing experiments. Kind thanks to Dr Paraskevi Diamanti (University of Bristol) for AML patient sample collection and Lizzy Hoole (Institute for Child Life & Health, UHBristol & Weston NHS Foundation Trust) for supplying clinical data. Thanks to the midwives and clinical research nurses at University Hospitals Sussex (UHS) NHS trust including Raquel Akieme, Valentina Toska, Lorraine Shah-Goodwin, Denise Skinner, Elohor Uwadiogbu and Caroline Humphreys, for the collection of human umbilical cord blood. We are indebted to the patients and their families who gave consent for their cells to be used for our research.
Author information
Authors and Affiliations
Contributions
MW performed experiments, analysed data, co-wrote the manuscript and managed the laboratory. OS completed RIP-seq analyses, AG performed CLIP analyses, MR generated library preps and ran RNA sequencing whilst HP generated cell lines and assisted EJM with AlphaFold modelling. DTTN provided experimental advice, whilst TC and AB sourced primary clinical samples. LC, SN and BT provided RNA experimental guidance and reagents. RGM performed experiments, analysed data, co-wrote the manuscript, secured funding and directed the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wagstaff, M., Sevim, O., Goff, A. et al. β-Catenin interacts with canonical RBPs including MSI2 to associate with a Wnt signalling mRNA network in myeloid leukaemia cells. Oncogene 44, 2490–2503 (2025). https://doi.org/10.1038/s41388-025-03415-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03415-y