Abstract
Centrosome amplification, a hallmark of diverse malignancies, enables cancer cell survival through centrosome clustering during mitosis, presenting a promising therapeutic target for selective elimination of cancer cells with supernumerary centrosomes. While the regulatory mechanisms underlying centrosome clustering remain poorly understood, our study identifies LIM kinase 2 (LIMK2) as a critical regulator of this process, demonstrating cancer correlation with tumor progression. Mechanistically, LIMK2 phosphorylates mammalian sterile-20-like kinase 4 (MST4) at threonine 178 (T178), activating its kinase function. Activated MST4 subsequently binds and phosphorylates nucleophosmin 1 (NPM1) at T95, a modification essential for centrosome clustering and tumor cell proliferation. Genetic depletion of NPM1 disrupts centrosome clustering and suppresses malignant growth. In vivo studies revealed that LIMK2 knockout significantly attenuates 4-nitroquinoline-1-oxide (4NQO) induced esophageal tumorigenesis in murine models. Therapeutic targeting of LIMK2 through shRNA-mediated knock down or pharmacological inhibition (CRT0105950) suppresses centrosome clustering by preventing “pseudo-bipolar” spindle formation, inducing mitosis arrest. This centrosome de-clustering promotes multipolar spindle assembly, ultimately triggering apoptotic cell death. Notably, CRT0105950 treatment effectively suppressed cell-derived xenograft tumor growth. Our findings elucidate the pivotal role of the LIMK2/MST4/NPM1 pathway in cancer progression and establish a novel therapeutic paradigm for broad-spectrum anticancer intervention.
Similar content being viewed by others
Introduction
Centrosome amplification is a hallmark of cancer, associated with aggressive disease and poorer clinical outcomes [1,2,3]. Causes of centrosome amplification include cell–cell fusion [4], cytokinesis failure, centrosome overduplication [5], centrosome fragmentation, and mitotic slippage [6, 7]. Such amplification leads to abnormal chromosome segregation during mitosis, driving chromosome instability, aneuploidy, tumor progression, metastasis, and poor prognosis [1, 8,9,10]. Despite these challenges, cancer cells mitigate lethal consequences by clustering extra centrosomes into two poles to enable bipolar mitosis [11, 12]. However, the molecular mechanisms underlying centrosome clustering remain unclear, making it critical to elucidate these processes for the development of targeted cancer therapies.
LIM domain kinase 2 (LIMK2) is a serine/threonine kinase regulates actin filament dynamics and plays a critical role in the growth and invasion of castration-resistant prostate cancer (CRPC) [13,14,15,16], triple-negative breast cancer (TNBC) [17], lung squamous cell carcinoma (LUSC) [18], and glioblastoma (GBM) [19]. To date, NK3 homeobox 1 (NKX3.1) [14], cofilin [20], membrane type 1 matrix metalloproteinase (MT1-MMP) [21], Twist-related protein 1 (TWIST1) [15] and phosphatase and tensin homolog (PTEN) [16] are the only known substrates of LIMK2. However, its role in centrosome amplification and clustering has not been well studied.
NPM1 is a nucleolar phosphoprotein involved in proliferation, apoptosis, ribosome assembly, and centrosome duplication [22]. Phosphorylation at specific sites regulates its function, with recent studies showed that targeting NPM1 disrupts centrosome clustering, leading to multipolar mitosis and apoptosis [23]. Previous findings identified T95 and T199 as critical phosphorylation sites for NPM1’s centrosomal association [24, 25]. However, the kinase responsible for T95 phosphorylation was unknown. Our study addresses this gap by identifying MST4 as the kinase that phosphorylates NPM1 at T95, thereby promoting centrosome clustering. MST4, a member of the sterile20-like (STE) serine/threonine kinase family [26], is known to activate ERK, promoting cell growth, transformation, epithelial-mesenchymal transition, and metastasis [27]. However, its role in centrosome clustering had not been demonstrated until now.
In this study, we investigated the LIMK2/MST4/NPM1 signaling pathway in centrosome clustering and tumor cell proliferation in various cancers. Centrosome clustering enables cancer cells to overcome centrosome amplification, maintain mitotic fidelity, and sustain proliferation. We identified that LIMK2 phosphorylates MST4 at tyrosine 178, enhancing its kinase activity. MST4, in turn, phosphorylates NPM1 at tyrosine 95, facilitating centrosome clustering and driving mitotic progression, ultimately promoting tumor cell proliferation. Importantly, disrupting LIMK2 function, either through genetic knock down or pharmacological inhibition, significantly impaired centrosome clustering, caused mitotic defects, induced mitotic arrest, and triggered apoptotic in esophageal squamous cell carcinoma (ESCC), stomach adenocarcinoma (STAD), liver hepatocellular carcinoma (LIHC), and lung squamous cell carcinoma (LUSC) cancer cells. These results highlight LIMK2 as a key regulator of centrosome dynamics and a promising therapeutic target.
Materials and methods
Reagents and antibodies
Active LIMK2 (#L05-10G, SignalChem, BC, Canada), active MST4 (#M59-10G, SignalChem), and inactive NPM1 Fusion protein (Ag7415, proteintech, Chicago, IL, USA) were used to do in vitro kinase assay. More detailed information about reagents and antibodies can be found in the “Supplementary materials and methods”.
Cell culture
KYSE30, KYSE70, KYSE140, KYSE150, KYSE410, KYSE450, KYSE510, NCI-H226, MGC803, NL20, and GES-1 were obtained from the Chinese Academy of Science Cell Bank (Shanghai, China). The detailed information is available in the “Supplementary materials and methods”.
Lentivirus construction and infection
Lentivirus vector pLKO.1 containing shRNA sequence targeted three genes (LIMK2, MST4, NPM1) were constructed. LIMK2 overexpression plasmid was constructed. shRNA sequences were designed as detailed in Supplementary Table 1. The detailed information is available in the “Supplementary materials and methods”.
Construction of point mutation plasmids
pcDNA3.1-3×Flag-MST4 (T178A), pcDNA3.1-3×HA-NPM1 (T95A and T95D) and pGEX-4T-1-GST-NPM1 (T95A and T199A) mutate plasmids was constructed. Detailed primers were described in Supplementary Table 2. The point mutation was constructed according to the instructions of Fast Multisite Mutagenesis System (FM201, TRANS, Beijing, China).
Western blot
Cell extracts were subjected to a Western blot. The detailed information is available in the “Supplementary materials and methods”.
Immunoprecipitation (IP)
Endogenous IP was performed using the Pierce Co-IP Kit (26149, Thermo Scientific™) according to the manufacturer’s instructions. The detailed information is available in the “Supplementary materials and methods”.
Hematoxylin-eosin (H&E) staining and immunohistochemistry (IHC) analysis
Tumor and normal tissue microarray sections were prepared by Shanghai Xinchao Biotech Company (Shanghai, China). Specimens were stained with antibodies against LIMK2 (1:50, ab45165, Abcam). The specific procedures according to manufacturer instructions (SV0002, BOSTER, Wuhan, Hubei, China). The detailed information is available in the “Supplementary materials and methods”.
Immunofluorescence staining
On the first day, 20,000 cells were seeded in a 24-well plate with a circular microscope cover glass (801007, NEST, Wuxi, Jiangsu, China). The detailed information is available in the “Supplementary materials and methods”.
In vitro kinase assay
For kinase assays using MST4 as a substrate, expressed in the pET-42b vector in E. coli, and then mixed with LIMK2 (L05-10G, Signalchem) in ATP-containing kinase buffer (K01-09-05, Signalchem) and incubated for 30 min at 30 °C. The detailed information is available in the “Supplementary materials and methods”.
Protein expression and purification
Full-length human NPM1 and mutated NPM1 were cloned into a pGEX-4T-1 expressing vector, which possesses a GST-tag. The detailed information is available in the “Supplementary materials and methods”.
MTT, colony formation, and soft agar assays
For MTT, cells were seeded in a 96-well plate and treated with CRT0105950 at concentrations of 0, 2.5, 5, and 10 μM. The detailed information is available in the “Supplementary materials and methods”.
Protein digestion for LC–MS/MS analyses
Briefly, after Co-IP experiments, get interested gel bands and cut bands into pieces. The detailed information is available in the “Supplementary materials and methods”.
Cell cycle and cell apoptosis
For cell cycle analysis, 2 × 105 knock down cells were seeded into 6 cm dish and incubated 24 h. The detailed information is available in the “Supplementary materials and methods”.
In vivo cell-derived xenograft (CDX) animal studies
In our animal research, we employed 6–8 weeks old female BALB/c nude mice. All in vivo studies were conducted following a thorough ethical review and received approval from the Ethics Committee of the China-US (Henan) Hormel Cancer Institute (CUHCI2020038). The detailed information is available in the “Supplementary materials and methods”.
4-nitrochinoline-oxide (4NQO)-induced esophageal tumor model
LIMK2 fox heterozygous recombinant C57BL/6N mice were generated at the Mouse Clinical Institute, Illkirch, France (IR00002025/E8). And esophagus Cre mice (ED-L2-Cre) were obtained from Beijing Vitalstar Biotechnology. The detailed information is available in the “Supplementary materials and methods”.
Bimolecular fluorescence complementation (BiFC) assay
Human LIMK2 cDNA (2061 bp) and MST4 cDNA (1251 bp) were inserted into the BiFC vectors pBiFC-VN173 and pBiFC-VC155, respectively. The vectors were generously provided by Professor Yanan Jiang (Zhengzhou University, China). Recombinant plasmids were co-transfected into HEK293T. After 48 h, cells were photographed under a confocal microscope, whose excitation and emission wavelengths were 488 nm.
Statistical analysis
In this paper, GraphPad Prism 9.0 software (RRID: SCR_002798) was used for statistical analysis of the experimental data. All experimental data were showed as means ± SD. Statistical significance was determined using a two-tailed Student’s t test or one-way analysis of variance (ANOVA), as appropriate. p value less than 0.05 were considered statistically significant, with *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
LIMK2 mediates centrosome clustering in cancer cells with centrosome amplification
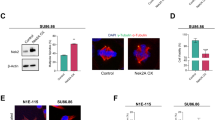
Centrosome amplification and clustering are hallmarks of nearly all cancer types. Firstly, we compared the centrosome numbers and clustering in normal versus cancerous cells. We used SHEE (immortalized esophageal epithelial cells), GES-1 (human gastric epithelial cells), HL7702 (normal human liver cells), and NL20 (human bronchial epithelial cells) as normal cell models, and KYSE150 (ESCC), MGC803 (STAD), HCCLM3 (LIHC), and NCI-H226 (LUSC) as cancer cell models. Our findings consistently showed that cancerous cells had abnormal centrosome numbers, unlike the normal cells, which typically had just two centrosomes. Moreover, only cancer cells exhibit the characteristic of centrosome clustering (Fig. 1A). To pinpoint the genes responsible for this, we thoroughly searched the MiCroKiTS database for human proteins. Our analysis identified 570 proteins related to centrosome function, including 66 kinases, with 19 of which are overexpressed in various cancers. Notably, the function of LIMK2 in centrosomes is still poorly understood (Fig. 1B). LIMK2 is highly expressed in most cancer tissues, according to the TIMER2.0 database (Fig. S1A). Additionally, mRNA levels were significantly elevated in Esophageal Carcinoma (ESCA), STAD, LIHC, and LUSC compared to normal tissues, as shown using the UALCAN tool (Fig. S1B). We overexpressed LIMK2 in normal esophageal epithelial cells (SHEE) and observed a significant increase in centrosome numbers, well beyond the typical two. As centrosomes amplified, cells showed extensive centrosome clustering. These results suggest that LIMK2 promotes both centrosome amplification and clustering (Fig. 1C, D).
A Immunofluorescence images of normal cells and cancer cells. Centrosome status was observed by staining for Pericentrin (orange), Centrin 1 (green), α-Tubulin (red), and DNA (DAPI, blue). Scale bar is 5 μm. B Overlap map of MiCroKITs database and kinase proteins. C, D Images of SHEE and Flag-LIMK2 overexpressed SHEE cells stained for the Pericentrin (orange), Centrin 1 (green), α-Tubulin (red), and DNA (DAPI, blue). Scale bar is 5 μm. Quantification of centrosome number in indicated cells. Statistical significance were shown 62.2% of the cells exhibit centrosome clustering. E Western blot of LIMK2 and GAPDH (loading control) of KYSE150 (ESCC), MGC803 (STAD), HCCLM3 (LIHC), and NCI-H226 (LUSC) cells treated with LIMK2 shRNA (n = 3, independent experiments). F Colony number were conducted in KYSE150, MGC803, HCCLM3, and NCI-H226 cells treated with LIMK2 shRNA. (n = 3, independent experiments). Colony number was analyzed using Image J software, with cell size larger than 20 defined as valid clones. Colony numbers are as follows: KYSE150 (Mock: 176.7 ± 6.1, shLIMK2#3: 54.0 ± 8.7, shLIMK2#10: 65.7 ± 8.1), MGC803 (Mock: 33.0 ± 1.7, shLIMK2#3: 5.0 ± 2.0, shLIMK2#10: 6.3 ± 2.9), HCCLM3 (Mock: 49.3 ± 5.5, shLIMK2#3: 6.3 ± 4.2, shLIMK2#10: 13.3 ± 4.0), and NCI-H226 (Mock: 34.3 ± 8.1, shLIMK2#3: 5.7 ± 0.6, shLIMK2#10: 9.3 ± 4.5). G Images of LIMK2 shRNA-treated KYSE150, MGC803, HCCLM3, and NCI-H226 cells stained for the Pericentrin (orange), Centrin 1 (green), α-Tubulin (red), and DNA (DAPI, blue). Scale bar is 5 μm. Left panels: representative images. Right panels: quantification of multipolar mitosis in indicated cells (n = 3, independent experiments). Each experiment involves more than 80 metaphase cells. H Flow cytometry analysis Annexin V/PI staining in indicated cells (n = 3, independent experiments). I Western blot analysis of the mitotic arrest marker p-H3 (S10) and apoptosis marker cleaved PARP in indicated cells (n = 3, independent experiments). All data were shown as means ± SD and were analyzed with the two-tailed Student’s t test.*p < 0.05, **p < 0.01, ***p < 0.001.
To elucidate the biological roles of LIMK2 in human malignancies, we established four representative cancer models: ESCC, STAD, LIHC, and LUSC. Our experiments demonstrated that lentivirus-mediated knock down of LIMK2 in cancer cells resulted in a marked reduction in colony formation (Fig. 1E, F). To experimentally demonstrate the role of LIMK2 in centrosome clustering, we found that LIMK2 knock down significantly increased the percentage of multipolar cells with scattered centrosomes and subsequently induced mitotic arrest (Fig. 1G). To demonstrate the direct effects of LIMK2 on mitotic progression, cancer cells exhibited strong mitotic arrest after LIMK2 knock down, as evidenced by increased p-Histone H3 (Ser10) levels, a marker of mitosis arrest (Fig. 1I), demonstrating the key role of LIMK2 in mitotic progression. Flow cytometry showed higher apoptosis in LIMK2 knock down cells (Fig. 1H), and elevated cleaved-PARP levels, a marker of apoptosis, correlated with the formation of multipolar spindles (Fig. 1I). To elucidate the functional role of LIMK2, the cell cycle was analyzed in LIMK2 knock down cells. Knock down of LIMK2 induced cell cycle arrest at G2/M phase (Fig. S1C). Furthermore, we examined the expression of proteins associated with the G2/M phase of the cell cycle, LIMK2 silencing reduced the expression of CyclinB1 compared with the control (Fig. S1D). Together, these results indicate that LIMK2 is crucial for centrosome amplification and clustering. Its depletion disrupts centrosome clustering, induces cell cycle arrest at G2/M phase, and simultaneously promotes the formation of multipolar spindles, induces mitotic arrest and triggers apoptosis.
LIMK2 promotes the growth of ESCC in vitro and in vivo
Our study found that LIMK2 plays a significant role in centrosome clustering in ESCC (Fig. 1G). However, the mechanism by which LIMK2 regulates centrosome clustering remains unclear. This study focuses on ESCC to explore the molecular mechanisms of LIMK2. Analysis of 107 ESCC samples showed significantly higher LIMK2 expression in cancer tissues than in adjacent normal tissues (Fig. 2A, B), as well as in 65 paired cancerous and adjacent normal tissues (Fig. 2C). Data from TCGA also showed increased LIMK2 mRNA in esophageal adenocarcinoma (ECA) and ESCC compared with normal tissues (Fig. 2D). TCGA database showed that higher LIMK2 expression predicted poor survival in ESCC patients (Fig. 2E). The western blot shows that of the 7 ESCC cell lines tested, 2 have lower, 2 have equivalent and 3 have higher LIMK2 expression than the SHEE cells (Fig. 2F). Targeting LIMK2 with shRNA in ESCC cells with high LIMK2 expression (KYSE150 and KYSE450) significantly reduced cell viability and suppressed colony formation (Fig. 2G–I). We selected KYSE410 and KYSE510 cell lines with low LIMK2 expression and artificially overexpressed LIMK2. Stable LIMK2 overexpression significantly increased colony formation in an anchorage-independent assay (Fig. 2J, K). To further explore whether LIMK2 could promote tumor growth in vivo, KYSE450 cells based CDX animal experiment was carried out. As expected, knock down of LIMK2 significantly decreased tumor volume and tumor weight (Fig. 2L–N).
A Representative images of IHC staining of LIMK2 in ESCC tissue microarray. Magnification ×40 and ×100. Scale bars respectively represents 250 µm and 50 µm. B Expression of LIMK2 in normal and tumor ESCC tissues (Adjacent = 66, Cancer = 107). C Expression of LIMK2 in paired normal and tumor ESCC tissues (n = 65). D Expression level of LIMK2 among ECA and ESCA from TCGA database. E Relationship between LIMK2 expression and the overall survival of patients from TCGA database. F Western blot analysis of LIMK2 expression in SHEE cells and seven human ESCC cell lines. GAPDH served as a control. G Western blot analysis of LIMK2 in knock down cells. GAPDH served as a control. H Indicated cells were subjected to soft agar assays, and the relative colony numbers were quantified (n = 3, independent experiments). I MTT assay showed the proliferation ability of LIMK2-shRNA treated cells (n = 3, independent experiments) J Western blot analysis of LIMK2 overexpressed cells. GAPDH served as a control. K Indicated cells were subjected to soft agar assays and the relative colony numbers were quantified (n = 3, independent experiments). L KYSE450 with or without LIMK2 knock down cells were injected into nude mice (n = 10). M, N Tumor growth curves and tumor weight were evaluated between shLIMK2 and Mock group. O Representative images of esophageal at necropsy. P, Q Tumor numbers and weight to length ratio were showed (WT + Vehicle, n = 6; WT + 4NQO, n = 9; LIMK2-CKO + Vehicle, n = 6; LIMK2-CKO + 4NQO, n = 9). All data were shown as means ± SD and were analyzed with the two-tailed Student’s t test.*p < 0.05, **p < 0.01, ***p < 0.001.
To investigate whether LIMK2 deficiency could impede the development or progression of esophageal cancer in a 4NQO-induced model, we utilized two mouse types: wild-type (WT) and LIMK2 conditional knockout (CKO). Construct LIMK2 conditional knockout mice utilizing the established protocols of genetic engineering (Fig. S2A). We identified the genotypes using PCR, distinguishing between LIMK2 heterozygous, homozygous, and those with or without the Cre gene (Fig. S2B, C). The mice were then grouped and treated with either water or 4NQO, as outlined in our experimental plan (Fig. S2D). Mice treated with 4NQO experienced significant weight loss compared to those given water (Fig. S2E), and all 4NQO-treated mice developed esophageal tumors, while no tumors were observed in the water group. Notably, tumor formation was reduced in LIMK2 CKO mice compared to WT controls (Fig. 2O, P), and the tumor weight-to-length ratio was also lower in the CKO group (Fig. 2Q). Following 4NQO treatment, WT mice exhibited more severe precancerous changes, with some progressing to cancer, whereas CKO mice displayed milder alterations (Fig. S2F). Additionally, Ki67 levels were significantly elevated in tissues with greater malignancy (Fig. S2F, G). These findings suggest that LIMK2 acts as a promoter of cancer development and growth, and its absence can mitigate tumor progression in esophageal cancer, both in vitro and in vivo.
LIMK2 interacts with MST4 and phosphorylates it at T178 residue
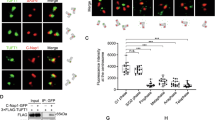
To uncover the oncogenic function of LIMK2 and elucidate the underlying molecular mechanism, we proceeded to investigate potential substrates in ESCC by using co-immunoprecipitation (Co-IP) assay, followed LC-MS/MS analysis. We found that MST4 as a potential interactor, confirmed by unique peptides (Fig. 3A). We then conducted Co-IP experiments in ESCC and HEK293T extracts validated the physical interaction between LIMK2 and MST4 (Fig. 3B, C). BiFC assay further confirmed this direct interaction in HEK293T cells (Fig. 3D). Immunofluorescence in KYSE150 and KYSE450 cells showed that LIMK2 and MST4 co-localizing in both the cytoplasm and nucleus (Fig. 3E). To delineate the binding region, MST4 was truncated into the kinase domain (1–274aa) and C-terminal region (275–416aa) (Fig. 3F). Co-transfection of full-length HA-tagged LIMK2 and MST4 truncations in HEK293T cells revealed that the kinase domain (1–274aa) is critical for LIMK2 interaction, while the C-terminal region is dispensable (Fig. 3G). Since LIMK2 is a serine/threonine kinase, we assessed whether it phosphorylates MST4 using an [γ-32P] ATP-labeled in vitro kinase assay. An autoradiography kinase assay confirmed that LIMK2 phosphorylates MST4 (Fig. 3H). This finding was further validated by an in vitro kinase assay using a pan p-Ser/Thr antibody (Fig. 3I). Given that the 1–274aa region mediates LIMK2 interaction, we hypothesized it contains the phosphorylation site (Fig. 3J). T178 site is known to activate its kinase activity [26, 28], was tested by incubating LIMK2 with either wild-type (WT) MST4 or a T178A mutant. Phosphorylation levels at p-Ser/Thr were significantly lower in the T178A mutant compared to WT (Fig. 3K), confirming T178 as a key site targeted by LIMK2. LIMK2 depletion in KYSE450 cells also reduced MST4 T178 phosphorylation (Fig. 3L). These results demonstrate that LIMK2 phosphorylates MST4 at T178, thereby enhancing its kinase activity, and identify a mechanism through which LIMK2 promotes ESCC progression.
A Venn diagram showing the overlapping of mass spectrometry results. e.g., yellow region represents proteins specifically pulled down by the LIMK2 antibody in KYSE140 cells, blue region represents proteins specifically pulled down by the IgG antibody in KYSE140 cells, while the mixed-color region indicates common proteins identified across these two groups. Mass spectrometry analysis revealed that MST4 was specifically detected only in LIMK2 antibody-treated KYSE140 and KYSE150 cell lines, as indicated by the arrows in the figure. B, C Co-IP assays were performed to analyze the direct binding between LIMK2 and MST4. D BiFC assay showing physical interaction of LIMK2 with MST4 in HEK293T. Scale bar is 200 μm (n = 3, independent experiments). E IF analyses of cells using anti-LIMK2 and anti-MST4 antibodies. LIMK2 is shown in red, MST4 is green, DAPI is blue. Scale bar is 20 µm. F Schematic diagram of wild type MST4 and its deletion mutants. G HEK293T cells were transfected with wild type MST4 and its different deletion mutants together with HA tagged LIMK2, cell lysates were immunoprecipitated with Flag Anti-DYKDDDDK G1 Affinity Resin, and co-precipitated HA-LIMK2 was detected by western blot. H In vitro kinase assay was performed in the presence of [γ-32P] ATP, and the phosphorylation signal was visualized by autoradiography. I In vitro kinase assay of the MST4 by LIMK2. The phosphorylated protein was detected using a pan p-Ser/Thr antibody. J HEK293T were transfected with 1–274aa of Flag tagged MST4 plasmid and in vitro kinase assay of the MST4 (1–274aa) by LIMK2. The phosphorylated protein was detected using a pan p-Ser/Thr antibody. K Active LIMK2 incubated with MST4 (T178A) and subjected to western blot. L KYSE450 cells were transfected with control shRNA and shRNA targeting LIMK2. The levels of p-MST4 (T178) and MST4 were assessed by Western blot. GAPDH was used as loading control.
MST4 mediates the oncogenic effect of LIMK2 in ESCC
The role of MST4 in human ESCC remains poorly understood. Our experiments demonstrated that MST4 knock down inhibited cell growth and colony formation (Fig. 4A–D), while MST4 overexpression elicited the opposite effect (Fig. 4E–G). Analysis of the TCGA database further revealed elevated MST4 mRNA expression across most cancer types (Fig. S3A). To determine whether MST4 mediates the oncogenic effects of LIMK2 in ESCC, rescue experiments were performed in KYSE150 and KYSE450 cells (Fig. 4H). LIMK2 overexpression significantly enhanced cell proliferation and colony formation, but these effects were markedly reduced upon MST4 knock down (Fig. 4I, J). The role of MST4, a substrate of LIMK2, in centrosome clustering was further explored. Our findings revealed that MST4 knock down led to an increase in the proportion of multipolar cells exhibiting dispersed centrosomes (Fig. S3B). These observations underscore the importance of MST4 as a pivotal mediator in LIMK2-driven oncogenesis and its regulatory role in centrosome clustering within ESCC cells.
A Western blot of MST4 and GAPDH (loading control) of cells treated with MST4 shRNA. B MTT assay was measured for indicated cells (n = 3, independent experiments). C Resulting cells were subjected to soft agar assays and the relative colony numbers were quantified (n = 3, independent experiments). D Colony number were conducted in KYSE150 and KYSE450 cells treated with MST4 shRNA. (n = 3, independent experiments). Colony number was analyzed using Image J software, with cell size larger than 20 defined as valid clones. Colony numbers are as follows: KYSE150 (Mock: 55.7 ± 2.9, shMST4#2: 15.3 ± 2.1, shMST4#3: 5.3 ± 2.5), and KYSE450 (Mock: 66.3 ± 11.9, shMST4#2: 4.7 ± 2.1, shMST4#3: 5.3 ± 2.3). E Western blot assay of MST4 and Flag in indicated cells. GAPDH served as loading control. F MTT assay was measured for MST4-overexpressed cells (n = 3, independent experiments). G Colony formation assay in MST4-overexpressed cells (n = 3, independent experiments). Colony number was analyzed using Image J software, with cell size larger than 20 defined as valid clones. Colony numbers are as follows: KYSE30 (Vector: 10.3 ± 1.2, OE-MST4: 19.0 ± 2.6) and KYSE410 (Vector: 74.7 ± 6.7, OE-MST4: 101.7 ± 4.6). H Cells with LIMK2 overexpression and MST4 knock down was established, and the expression of Flag-LIMK2 and MST4 was detected by Western blot. GAPDH served as loading control. I Colony formation was performed for the indicated cells (n = 3, independent experiments). Colony number was analyzed using Image J software, with cell size larger than 20 defined as valid clones. Colony numbers are as follows: KYSE150 (Vector: 18.7 ± 3.2, Flag-LIMK2: 31.3 ± 4.7, shMST4: 10.3 ± 1.5, shMST4-OE-LIMK2: 10.0 ± 1.0) and KYSE450 (Vector: 17.7 ± 3.1, Flag-LIMK2: 33.3 ± 4.5, shMST4: 3.7 ± 1.5, shMST4-OE-LIMK2: 4.0 ± 1.0). J MTT assay was measured for indicated cells (n = 3, independent experiments). All data were shown as means ± SD and were analyzed with the two-tailed Student’s t test.*p < 0.05, **p < 0.01, ***p < 0.001.
MST4 interacts with NPM1 and phosphorylates it at Thr95
Our findings reveal a potent oncogenic role for MST4 in regulating centrosome clustering, but the underlying mechanisms remain unclear. To investigate it, we performed Co-IP experiments using MST4 antibodies followed by LC-MS/MS analysis to identify MST4 binding partners (Fig. 5A). This analysis identified NPM1 as a specific interactor. Both endogenous and exogenous Co-IP experiments confirmed the interaction between MST4 and NPM1 in ESCC cells (Fig. 5B, C).
A Venn diagram showing the overlapping of mass spectrometry results. e.g., yellow region represents proteins specifically pulled down by the MST4 antibody in KYSE150 cells, blue region represents proteins specifically pulled down by the IgG antibody in KYSE150 cells, while the mixed-color region indicates common proteins identified across these two groups. Mass spectrometry analysis revealed that MST4 and NPM1 were specifically detected only in MST4 antibody-treated KYSE150 and KYSE450 cell lines, as indicated by the arrows in the figure. B, C Direct interaction of MST4 and NPM1 was checked by western blot. D Co-IP of Flag-MST4 WT (wild type) or Flag-MST4 mutant T178A with HA-NPM1 in HEK293T cells. E In vitro kinase assay was performed in the presence of [γ-32P] ATP and the phosphorylation signal was visualized by autoradiography. F In vitro kinase assay of the NPM1 by MST4. The phosphorylated protein was detected using p-NPM1 (T95) and p-NPM1 (T199) antibody. G Active MST4 was incubated with the indicated NPM1 mutate protein in kinase reaction buffer. Phosphorylation signals were detected by western blot. The gray value was calculated using Image J software. H MST4 knock down decreased p-NPM1 (T95) expression in indicated cells, but did not affect total NPM1 expression. I In vitro kinase assay of the NPM1 by LIMK2. The phosphorylated protein was detected using p-NPM1 (T95) antibody. J In vitro kinase assay was performed in the presence of [γ-32P] ATP, phosphorylation signal was visualized by autoradiography.
We purified a MST4 mutant (T178A) protein and found that its interaction with NPM1 was significantly weaker compared to the wild-type MST4 in Co-IP assay (Fig. 5D). This finding suggests that the T178 active state of MST4 facilitates its binding to the downstream protein NPM1. An in vitro kinase assay demonstrated that MST4 phosphorylates NPM1 (Fig. 5E). To identify the phosphorylation site, we examined two potential sites, Thr199 and Thr95. GST-tagged NPM1 mutants showed that phosphorylation levels were significantly reduced in the T95A mutant but not in the T199A mutant, indicating that Thr95 as the key site phosphorylated by MST4 (Fig. 5F, G). Further experiments confirmed that knock down of MST4 in ESCC cells decreased NPM1 phosphorylation at Thr95 (Fig. 5H). To clarify whether LIMK2 also regulates NPM1, an in vitro kinase assay showed that LIMK2 does not phosphorylate NPM1 (Fig. 5I, J), ruling out direct regulation by LIMK2. Instead, our findings support a new oncogenic signaling axis in ESCC, with LIMK2 activating MST4, which in turn phosphorylates NPM1 at Thr95. In summary, MST4 is the upstream protein of NPM1, directly phosphorylating Thr95 residue to regulate NPM1 activity, thereby highlighting the LIMK2/MST4/NPM1 axis as a potential oncogenic pathway in ESCC.
NPM1-T95 phosphorylation promotes centrosome clustering and LIMK2/MST4/NPM1 axis potentiates ESCC growth
To investigate the role of NPM1 in ESCC, we analyzed TCGA data and found elevated NPM1 mRNA levels across multiple cancer types (Fig. S4A). NPM1 knock down significantly inhibited cell proliferation and colony formation, highlighting its potential oncogenic role in ESCC (Fig. 6A–C and S4B). Phosphorylated NPM1 (p-NPM1 at T95) was significantly upregulated in ESCC tissues compared to normal tissues (Fig. S4C). These findings demonstrate that T95 phosphorylation of NPM1 serves as a critical regulatory mechanism governing essential oncogenic signaling pathways in malignant cells. Hirokazu Shiheido et al. discovered that inhibiting NPM1 induces multipolar mitosis by blocking centrosome clustering, leading to cell death [23], which exhibited the underlying function of NPM1 in centrosome clustering for the first time. Our data aligned with this discovery, NPM1 knock down reduced centrosome clustering and increased multipolar mitosis (Fig. 6D). To determine the role of phosphorylation in NPM1’s oncogenic function, KYSE450 cells were transfected with NPM1-WT, phosphorylation-deficient NPM1-T95A, or phosphorylation-mimicking NPM1-T95D plasmids (Fig. 6E). Compared to NPM1-WT, NPM1-T95A reduced proliferation and colony formation, while NPM1-T95D enhanced both, suggesting that T95 phosphorylation promotes NPM1’s function (Fig. 6F, G). While cells expressing NPM1-T95A showed higher rates of multipolar mitosis than those expressing NPM1-WT or NPM1-T95D, confirming that T95 phosphorylation supports centrosome clustering and proliferation (Fig. 6H). To investigate whether NPM1 mediates the oncogenic effects of MST4 in ESCC, rescue experiments were conducted using KYSE150 and KYSE450 cells (Fig. 6I). Overexpression of MST4 significantly promoted cell proliferation and colony formation. However, these effects were notably attenuated when NPM1 was knocked down (Fig. 6J, K). To explore the link between NPM1 and LIMK2/MST4 axis, we overexpressed both LIMK2 and MST4 in NPM1-silenced cells. Both LIMK2 and MST4 overexpression enhanced proliferation and colony formation, but these effects were reversed by NPM1 knock down (Fig. S4D–F). LIMK2 knock down reduced MST4 and NPM1 phosphorylation without affecting their total protein levels across ESCC, STAD, LIHC, and LUSC cell lines (Fig. 6L). In ESCC patient samples, the LIMK2/MST4/NPM1 pathway was frequently activated, with significantly higher expression of LIMK2, MST4, p-MST4 (T178), NPM1, and p-NPM1 (T95) in cancer tissues compared to normal tissues (Fig. S4G). We analyzed the protein expression levels of LIMK2, MST4, and p-NPM1 (T95) in 20 esophageal cancer tissue samples. IHC analysis revealed a significant positive correlation between LIMK2 and the expression levels of MST4 and p-NPM1 (T95). These findings suggest a strong and direct association between LIMK2 and its downstream targets, MST4, and p-NPM1 (T95), in esophageal cancer tissues (Fig. S4H). Correlation analyses from the GEPIA database revealed a strong positive correlation between LIMK2/MST4 and MST4/NPM1 across cancers (database http://gepia.cancer-pku.cn/) (Fig. S4I). These findings establish the LIMK2/MST4/NPM1 axis as a critical oncogenic pathway in ESCC, STAD, LIHC, and LUSC.
A The expression of NPM1 and p-NPM1 (T95) was evaluated by western blot. B MTT assay were measured for indicated cells. C Resulting cells were subjected to soft agar assay (up panels), and the relative colony numbers were quantified (down panels) (n = 3, independent experiments). D Images of NPM1 shRNA-treated KYSE150 cells stained for the Pericentrin (orange), Centrin 1 (green), α-Tubulin (red), and DNA (DAPI, blue). Scale bar is 5 μm. (n = 3, independent experiments). Each experiment involves more than 80 metaphase cells. E The expression levels of Flag-NPM1 were detected by western blot. GAPDH served as control. F MTT assay were measured for indicated cells (n = 3, independent experiments). G Colony formation assay was performed for the indicated cells and the relative colony numbers were quantified (n = 3, independent experiments). Colony number was analyzed using Image J software, with cell size larger than 20 defined as valid clones. Colony numbers are as follows: KYSE450 (WT: 18.7 ± 1.5, T95A: 7.3 ± 2.3, T95D: 25.7 ± 3.5). H Flag-tagged NPM1-WT, NPM1-T95A, and NPM1-T95D were transfected into KYSE450 cells, images of indicated cells stained for the Pericentrin (orange), Centrin 1 (green), α-Tubulin (red), and DNA (DAPI, blue). Scale bar is 5 μm. (n = 3, independent experiments). Each experiment involves more than 80 metaphase cells. I Cells with NPM1 silencing and Flag-MST4 overexpression were established. NPM1, Flag and NPM1 were determined by western blot. J MTT assay was measured for indicated cells. K Colony formation assay was detected in indicated cells (n = 3, independent experiments). Colony number was analyzed using Image J software, with cell size larger than 20 defined as valid clones. Colony numbers are as follows: KYSE150 (Mock-Flag: 15.3 ± 1.5, Mock-MST4: 25.7 ± 6.0, shNPM1-Flag: 2.3 ± 0.6, shNPM1-MST4: 1.7 ± 0.6). KYSE450 (Mock-Flag: 20.7 ± 6.8, Mock-MST4: 38.3 ± 7.8, shNPM1-Flag: 2.7 ± 2.9, shNPM1-MST4: 2.3 ± 1.5). L LIMK2/MST4/NPM1 signaling pathway related protein expression levels were examined by western blot in LIMK2 shRNA-treated cells. GAPDH served as control. All data were shown as means ± SD and were analyzed with the two-tailed Student’s t test.*p < 0.05, **p < 0.01, ***p < 0.001.
CRT0105950 significantly inhibits LIMK2/MST4/NPM1 signaling pathway and tumor growth in vitro and in vivo
To clarify the effect of chemical inhibition of LIMK2 activity on centrosome clustering and cell growth, we used the LIMKs inhibitor CRT0105950, which inhibit LIMKs activity [29]. Treatment with CRT0105950 significantly reduced colony formation in ESCC, STAD, LIHC and LUSC cells (Fig. S5A). We further examined its effects on centrosome clustering and mitosis in vitro. CRT0105950 treatment caused widespread centrosome de-clustering, leading to multipolar spindle formation, mitotic arrest, and mitotic catastrophe, as indicated by increased p-Histone 3 (S10) levels (Fig. 7A, C). The dispersion of centrosomes leads to cell cycle arrest at the G2/M phase, while the formation of multipolar spindles further induces cell apoptosis. Flow cytometry confirmed that CRT0105950 induced cell apoptosis and cell cycle arrest at G2/M phase, accompanied by increased cleaved PARP expression and decreased CyclinB1 expression (Figs. 7B, C and S5B, C).
A Multipolar spindle formation in CRT0105950-treated cells as shown by the Pericentrin (orange), Centrin 1 (green), α-Tubulin (red), and DNA (DAPI, blue). Scale bar is 5 µm. Quantification of mitotic cells with multipolar spindles from indicated cells. Each experiment involves more than 80 metaphase cells (n = 3, independent experiments). B Flow cytometry analysis Annexin V/PI staining in cells following 48 h treatment with CRT0105950 (n = 3, independent experiments). C Western blot analysis of mitosis marker and apoptosis marker in indicated cells following 48 h treatment with CRT0105950. D LIMK2/MST4/NPM1 signaling pathway related protein expression levels were examined by western blot in cells after CRT0105950 treatment. E Representative images and tumor growth curve of CDX in each treatment group (n = 8/group). All data were shown as means ± SD and were analyzed with the two-tailed Student’s t test. *p < 0.05, **p < 0.01, ***p < 0.001.
Mechanistically, CRT0105950 treatment decreased phosphorylation of LIMK1 (Thr508)/LIMK2 (Thr505) within 3 h, suppressed p-MST4 (T178) at 6 h, and inhibited p-NPM1 (T95) at 24 h (Fig. 7D). Additionally, LIMK2 protein levels were significantly reduced after 24 h of treatment, highlighting that CRT0105950 disrupts the LIMK2/MST4/NPM1 pathway. The deleterious effects on regular cell division across different normal tissues have limited the clinical application of small-molecule inhibitors targeting vital mitotic kinases and kinesins [30, 31]. To address this concern, CRT0105950 demonstrated minimal effects on cell growth and division in normal cells across various concentrations (Fig. S5D, E). This suggests CRT0105950 has a favorable toxicity profile compared to myelosuppressive and neurotoxic anti-mitotic agents. To assess its therapeutic potential in vivo, we tested CRT0105950 in CDX model. At a dosage of 60 mg/kg, CRT0105950 significantly reduced tumor growth and tumor weight (Figs. 7E and S5F). Furthermore, IHC staining revealed a significant reduction in PCNA expression in the CRT0105950 treatment group compared to the control group in KYSE450 and MGC803 CDX models (Fig. S5G).
In summary, CRT0105950 effectively disrupts centrosome clustering and reduces proliferation of cancer cells with centrosome amplification by inhibiting the LIMK2/MST4/NPM1 pathway. Its minimal toxicity in normal cells and significant antitumor activity in vivo support its potential as a viable therapeutic strategy for targeting cancer growth.
Discussion
Paclitaxel exert its cytotoxic effect by arresting mitosis through microtubule stabilization, resulting in cellular apoptosis, is a validated and widely applied strategy for treating human cancers [32]. However, the non-specific circulation of paclitaxel often results in systemic toxicity, leading to multi-organ damage, including liver and kidneys [33]. This highlights the need for therapies that specifically target cancer-specific traits. Centrosome amplification and clustering, hallmark features of aggressive cancers, are absent in normal cells, making them attractive therapeutic targets [34]. Thus, targeting centrosome clustering could offer a promising approach in clinical trials, as it selectively eradicates cancer cells with extra centrosomes while largely sparing normal cells.
Centrosome amplification and clustering in human cancers has recently been discussed by others in great detail [2, 6, 35]. Several proteins, including Poly (ADP-ribose) polymerase (PARP), C-terminal kinesin motor protein (KIFC1), Heat shock 70 kDa protein (Hsp70), Cortical actin, anaphase promoting complex/cyclosome (APC/C)-CDH1 complex, kinesin-5 (Eg5) and Polo-like kinase 4 (PLK4) are known to regulate centrosome clustering, and inhibitors targeting these proteins have shown promise in preclinical studies [35]. PLK4 is a serine/threonine protein kinase that regulates centriole duplication [36]. PLK4 inhibitor CFI-400945 has advanced to phase I clinical trials [37, 38]. Our study expands on this field by elucidating the role of LIMK2 in centrosome clustering in ESCC, STAD, LIHC and LUSC. LIMK2 depletion or inhibition leading to centrosome de-clustering, mitotic arrest and cell cycle arrest at G2/M phase, and promoting cell apoptosis.
The primary substrate of LIMK2 is the actin-depolymerizing and severing protein cofilin/ADF. Phosphorylation of cofilin at serine 3 by LIMK2 inactivates cofilin, thereby stabilizing the actin cytoskeleton [39]. It is widely published that disruption of LIM kinases or cofilin has profound effects on the actin cytoskeleton. In terms of centrosome clustering, it is already well established that the actin cytoskeleton plays a key role in centrosome clustering [40]. And, it has already been demonstrated that LIMK inhibition or cofilin activation causes centrosome de-clustering [41, 42]. However, this study reveals that LIMK2 can also regulate centrosome clustering through a novel signaling pathway independent of cofilin. Notably, this pathway has been validated in ESCC, STAD, LIHC, and LUSC, demonstrating the critical role of the LIMK2/MST4/NPM1 signaling pathway in centrosome clustering.
In this study, we elucidated a novel molecular mechanism by which LIMK2 regulates centrosome clustering in cancer malignancy. Mechanistically, LIMK2 interacted and phosphorylated MST4. MST4 is involved in tumor cell proliferation, migration and invasion, epithelial-mesenchymal transition (EMT), survival, and cancer metastasis [43]. To date, PAK4 is the only reported upstream kinase of MST4 [44], our findings contribute to this field by uncovering that LIMK2 is an additional upstream regulator of MST4 in cancers. Our research findings indicate that LIMK2 phosphorylates MST4 at the T178 site. T178 residue has been reported to be associated with the activation of MST4 kinase [26, 28, 44]. This activation enables MST4 to more effectively interact with downstream protein, enhancing its kinase function. Moreover, NPM1, an identified key downstream regulator of MST4 in our results, is widely expressed in proliferating cells and is involved in a variety of cellular processes [22]. High expression of NPM1 is associated with tumor progression [45]. Masaru Okuda et al. discovered that phosphorylation of NPM1 at Thr199 by CDK2/cyclin E promotes centrosome duplication [24]. However, our kinase assays revealed that MST4 does not phosphorylate NPM1 at T199 site. This finding prompted us to search for other potential phosphorylation sites. Wang et al. found that phosphorylation at T95 can also regulate NPM1’s association with centrosomes [25]. However, the kinase responsible for phosphorylating T95 has not been identified. This perspective clearly indicates the direction of our research. Results from kinase assays and in vitro signaling pathway both indicated that the phosphorylation site of NPM1 by MST4 is T95. Moreover, the activation of the T95 site significantly enhanced cell proliferation and centrosome clustering. When mutated to an inactive form, cells displayed multipolar spindle morphology and inhibited cell proliferation. Rescue result indicated that NPM1 mediates the pro-oncogenic effects of the LIMK2/MST4 axis. Furthermore, LIMK2/MST4/NPM1 signaling pathway has been validated in both LIMK2 knock down cells and cells treated with CRT0105950. Suppression of LIMK2 in cancer (ESCC, STAD, LIHC, and LUSC) cells significantly reduced p-MST4 (T178) and p-NPM1 (T95) protein levels, while the total protein levels of MST4 and NPM1 remained unchanged. These findings suggested that LIMK2/MST4/NPM1 signaling pathway is universally present in ESCC, STAD, LIHC, and LUSC, which plays a crucial role in promoting cancer development and progression.
Our findings present compelling evidence that the targeted depletion of LIMK2 holds promising preclinical efficacy in human cancer models. CRT0105950 is a LIMKs inhibitor, has been reported to disrupt the integrity of the mitotic spindle [29]. Our study identified CRT0105950 not only significantly inhibits the kinase activity of LIMK1 and LIMK2 but also promotes the degradation of LIMK2 protein. CRT0105950 faithfully replicates the mitotic and viability effects observed with LIMK2 knock down across a range of cancer cell lines. Importantly, CRT0105950 selectively affects cancer cells with amplified centrosomes while sparing normal cells. Additionally, inhibition or knock down of LIMK2 reduced phosphorylation of MST4 and NPM1 in cancer cells, providing a strong basis for investigating the effects of LIMK2 on the downstream MST4/NPM1 signaling pathway. At effective concentrations, CRT0105950 shows minimal adverse effects on normal human cells and does not disrupt their mitosis. This distinguishes it from other anti-mitotic agents, which often cause severe adverse events such as neuropathies [46]. In vivo studies further confirmed CRT0105950’s significant anti-cancer activity in CDX models, with good tolerability in mice. This favorable profile suggested that CRT0105950 has the potential for clinical applications.
In conclusion, our study identifies LIMK2 as a key driver of centrosome clustering and cancer progression. Pharmacological inhibition of LIMK2 with CRT0105950 selectively targets cancer cells with amplified centrosomes, inducing mitotic catastrophe and apoptosis while sparing normal cells (Fig. 8). These findings strongly support the clinical testing of CRT0105950 as a promising therapeutic agent for cancers with centrosome amplification.
LIMK2 promotes centrosome clustering by activating MST4-mediated phosphorylation of NPM1. Targeting LIMK2 with shRNA or the LIMKs inhibitor CRT0105950 can lead to centrosome de-clustering, cell cycle arrest at G2/M phase, mitotic catastrophe and apoptosis, highlighting its potential as a therapeutic target (created using BioRender).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Sauer CM, Hall JA, Couturier DL, Bradley T, Piskorz AM, Griffiths J, et al. Molecular landscape and functional characterization of centrosome amplification in ovarian cancer. Nat Commun. 2023;14:6505.
Mittal K, Kaur J, Jaczko M, Wei G, Toss MS, Rakha EA, et al. Centrosome amplification: a quantifiable cancer cell trait with prognostic value in solid malignancies. Cancer Metastasis Rev. 2021;40:319–39.
Schatten H. The impact of centrosome pathologies on ovarian cancer development and progression with a focus on centrosomes as therapeutic target. Adv Exp Med Biol. 2024;1452:37–64.
Chen JH, Segni M, Payne F, Huang-Doran I, Sleigh A, Adams C, et al. Truncation of POC1A associated with short stature and extreme insulin resistance. J Mol Endocrinol. 2015;55:147–58.
Denu RA, Shabbir M, Nihal M, Singh CK, Longley BJ, Burkard ME, et al. Centriole overduplication is the predominant mechanism leading to centrosome amplification in melanoma. Mol Cancer Res. 2018;16:517–27.
Zhao JZ, Ye Q, Wang L, Lee SC. Centrosome amplification in cancer and cancer-associated human diseases. Biochim Biophys Acta Rev Cancer. 2021;1876:188566.
Hata S, Pastor Peidro A, Panic M, Liu P, Atorino E, Funaya C, et al. The balance between KIFC3 and EG5 tetrameric kinesins controls the onset of mitotic spindle assembly. Nat Cell Biol. 2019;21:1138–51.
Hosea R, Hillary S, Naqvi S, Wu S, Kasim V. The two sides of chromosomal instability: drivers and brakes in cancer. Signal Transduct Target Ther. 2024;9:75.
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46.
Monteiro P, Yeon B, Wallis SS, Godinho SA. Centrosome amplification fine tunes tubulin acetylation to differentially control intracellular organization. EMBO J. 2023;42:e112812.
Kalkan BM, Ozcan SC, Cicek E, Gonen M, Acilan C. Nek2A prevents centrosome clustering and induces cell death in cancer cells via KIF2C interaction. Cell Death Dis. 2024;15:222.
Fan G, Sun L, Meng L, Hu C, Wang X, Shi Z, et al. The ATM and ATR kinases regulate centrosome clustering and tumor recurrence by targeting KIFC1 phosphorylation. Nat Commun. 2021;12:20.
Malvi P, Reddy DS, Kumar R, Chava S, Burela S, Parajuli K, et al. LIMK2 promotes melanoma tumor growth and metastasis through G3BP1-ESM1 pathway-mediated apoptosis inhibition. Oncogene. 2023;42:1478–91.
Sooreshjani MA, Nikhil K, Kamra M, Nguyen DN, Kumar D, Shah K. LIMK2-NKX3.1 engagement promotes castration-resistant prostate cancer. Cancers. 2021; 13:2324.
Nikhil K, Chang L, Viccaro K, Jacobsen M, McGuire C, Satapathy SR, et al. Identification of LIMK2 as a therapeutic target in castration resistant prostate cancer. Cancer Lett. 2019;448:182–96.
Nikhil K, Kamra M, Raza A, Shah K. Negative cross talk between LIMK2 and PTEN promotes castration resistant prostate cancer pathogenesis in cells and in vivo. Cancer Lett. 2021;498:1–18.
Malvi P, Janostiak R, Chava S, Manrai P, Yoon E, Singh K, et al. LIMK2 promotes the metastatic progression of triple-negative breast cancer by activating SRPK1. Oncogenesis. 2020;9:77.
Su Y, Xu B, Shen Q, Lei Z, Zhang W, Hu T. LIMK2 is a novel prognostic biomarker and correlates with tumor immune cell infiltration in lung squamous cell carcinoma. Front Immunol. 2022;13:788375.
Park JB, Agnihotri S, Golbourn B, Bertrand KC, Luck A, Sabha N, et al. Transcriptional profiling of GBM invasion genes identifies effective inhibitors of the LIM kinase-Cofilin pathway. Oncotarget. 2014;5:9382–95.
Sumi T, Matsumoto K, Takai Y, Nakamura T. Cofilin phosphorylation and actin cytoskeletal dynamics regulated by rho- and Cdc42-activated LIM-kinase 2. J Cell Biol. 1999;147:1519–32.
Lagoutte E, Villeneuve C, Lafanechere L, Wells CM, Jones GE, Chavrier P, et al. LIMK regulates tumor-cell invasion and matrix degradation through Tyrosine phosphorylation of MT1-MMP. Sci Rep. 2016;6:24925.
Grisendi S, Mecucci C, Falini B, Pandolfi PP. Nucleophosmin and cancer. Nat Rev Cancer. 2006;6:493–505.
Shiheido H, Terada F, Tabata N, Hayakawa I, Matsumura N, Takashima H, et al. A phthalimide derivative that inhibits centrosomal clustering is effective on multiple myeloma. PLoS ONE. 2012;7:e38878.
Okuda M, Horn HF, Tarapore P, Tokuyama Y, Smulian AG, Chan PK, et al. Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell. 2000;103:127–40.
Wang W, Budhu A, Forgues M, Wang XW. Temporal and spatial control of nucleophosmin by the Ran-Crm1 complex in centrosome duplication. Nat Cell Biol. 2005;7:823–30.
Huang T, Kim CK, Alvarez AA, Pangeni RP, Wan X, Song X, et al. MST4 phosphorylation of ATG4B regulates autophagic activity, tumorigenicity, and radioresistance in glioblastoma. Cancer Cell. 2017;32:840–855.e848.
Thompson BJ, Sahai E. MST kinases in development and disease. J Cell Biol. 2015;210:871–82.
Zhang H, Lin M, Dong C, Tang Y, An L, Ju J, et al. An MST4-pbeta-catenin(Thr40) signaling axis controls intestinal stem cell and tumorigenesis. Adv Sci. 2021;8:e2004850.
Mardilovich K, Baugh M, Crighton D, Kowalczyk D, Gabrielsen M, Munro J, et al. LIM kinase inhibitors disrupt mitotic microtubule organization and impair tumor cell proliferation. Oncotarget. 2015;6:38469–86.
Komlodi-Pasztor E, Sackett DL, Fojo AT. Inhibitors targeting mitosis: tales of how great drugs against a promising target were brought down by a flawed rationale. Clin Cancer Res. 2012;18:51–63.
Mitchison TJ. The proliferation rate paradox in antimitotic chemotherapy. Mol Biol Cell. 2012;23:1–6.
Zhou L, Tian J, Wang K, Ma Y, Chen X, Luo H, et al. Targeting galectin-1 overcomes paclitaxel resistance in esophageal squamous cell carcinoma. Cancer Res. 2024; 84:3894–908.
Zheng W, Zhu T, Tang L, Li Z, Jiang G, Huang X. Inhalable CAR-T cell-derived exosomes as paclitaxel carriers for treating lung cancer. J Transl Med. 2023;21:383.
Saatci O, Akbulut O, Cetin M, Sikirzhytski V, Uner M, Lengerli D, et al. Targeting TACC3 represents a novel vulnerability in highly aggressive breast cancers with centrosome amplification. Cell Death Differ. 2023;30:1305–19.
Firdous F, Raza HG, Chotana GA, Choudhary MI, Faisal A, Saleem RSZ. Centrosome clustering & chemotherapy. Mini Rev Med Chem. 2023;23:429–51.
Singh CK, Denu RA, Nihal M, Shabbir M, Garvey DR, Huang W, et al. PLK4 is upregulated in prostate cancer and its inhibition reduces centrosome amplification and causes senescence. Prostate. 2022;82:957–69.
Murphy T, Mason JM, Leber B, Bray MR, Chan SM, Gupta V, et al. Preclinical characterization and clinical trial of CFI-400945, a polo-like kinase 4 inhibitor, in patients with relapsed/refractory acute myeloid leukemia and higher-risk myelodysplastic neoplasms. Leukemia. 2024;38:502–12.
Chan CY, Yuen VW, Chiu DK, Goh CC, Thu KL, Cescon DW, et al. Polo-like kinase 4 inhibitor CFI-400945 suppresses liver cancer through cell cycle perturbation and eliciting antitumor immunity. Hepatology. 2023;77:729–44.
Petrilli A, Copik A, Posadas M, Chang LS, Welling DB, Giovannini M, et al. LIM domain kinases as potential therapeutic targets for neurofibromatosis type 2. Oncogene. 2014;33:3571–82.
Kwon M, Godinho SA, Chandhok NS, Ganem NJ, Azioune A, Thery M, et al. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 2008;22:2189–203.
Oku Y, Tareyanagi C, Takaya S, Osaka S, Ujiie H, Yoshida K, et al. Multimodal effects of small molecule ROCK and LIMK inhibitors on mitosis, and their implication as anti-leukemia agents. PLoS ONE. 2014;9:e92402.
Konotop G, Bausch E, Nagai T, Turchinovich A, Becker N, Benner A, et al. Pharmacological inhibition of centrosome clustering by slingshot-mediated cofilin activation and actin cortex destabilization. Cancer Res. 2016;76:6690–6700.
Getu AA, Zhou M, Cheng SY, Tan M. The mammalian sterile 20-like kinase 4 (MST4) signaling in tumor progression: implications for therapy. Cancer Lett. 2023;563:216183.
Jiang H, Wang W, Zhang Y, Yao WW, Jiang J, Qin B, et al. Cell polarity kinase MST4 cooperates with cAMP-dependent Kinase to orchestrate histamine-stimulated acid secretion in gastric parietal cells. J Biol Chem. 2015;290:28272–85.
Lakshmi Ch NP, Sivagnanam A, Raja S, Mahalingam S. Molecular basis for RASSF10/NPM/RNF2 feedback cascade-mediated regulation of gastric cancer cell proliferation. J Biol Chem. 2021;297:100935.
Penna LS, Henriques JAP, Bonatto D. Anti-mitotic agents: are they emerging molecules for cancer treatment? Pharm Ther. 2017;173:67–82.
Acknowledgements
We thank the members of Dong’s lab for their excellent technical assistance and useful discussions. This work was supported by the National Natural Science Foundation of China (Nos. 8207112153, 82073075); the Henan Provincial Science and Technology Research Project (No. 242102311218); the Postdoctoral Science Foundation of China (No. 2023M733222); the Project of Zhongyuan Scholar Talent (No. 234000510008) and the Major Science and Technology Projects in Henan Province (No. 221100310100).
Author information
Authors and Affiliations
Contributions
JT designed and performed the most of experiments and the data analysis; MMG helped in animal experimentation; JT and MQS prepared and revised the manuscript; HJJ, WNN, RY, and KDL provided technique supports; SHL and YQZ helped perform in vitro and in vivo assays. MQS and ZGD designed and supervised the overall experimental design.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal experiments and clinical sample collections were conducted in strict accordance with ethical guidelines and were approved by the Ethics Committee of the China-US (Henan) Hormel Cancer Institute (CUHCI2020038). Clinical tissues were acquired from the Affiliated Cancer Hospital of Zhengzhou University. Written informed consent was obtained from all participating patients for the use of the tissue samples.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tian, J., Liu, S., Zhang, Y. et al. LIMK2 promotes centrosome clustering and cancer progression by activating MST4-mediated phosphorylation of NPM1. Oncogene 44, 3625–3639 (2025). https://doi.org/10.1038/s41388-025-03518-6
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03518-6