Abstract
Background
Evidence supporting the benefits of delayed cord clamping is increasing; however, there is no clear recommendation on cord management during newborn resuscitation. This study aimed to investigate the effects of resuscitation initiated with an intact umbilical cord, hypothesizing it is a safe stabilization procedure that improves neonatal outcomes.
Methods
Systematic search was conducted in MEDLINE, Embase, CENTRAL, and Web of Science from inception to March 1, 2024. Eligible articles compared neonatal outcomes in newborns receiving initial stabilization steps before and after cord clamping.
Results
Twelve studies met our inclusion criteria, with six RCTs included in the quantitative analysis. No statistically significant differences were found in delivery room parameters, in-hospital mortality, or neonatal outcomes between the examined groups. However, intact cord resuscitation group showed higher SpO2 at 5 min after birth compared to cord clamping prior to resuscitation group (MD 6.67%, 95% CI [−1.16%, 14.50%]). There were no significant differences in early complications of prematurity (NEC ≥ stage 2: RR 2.05, 95% CI [0.34, 12.30], IVH: RR 1.25, 95% CI [0.77, 2.00]).
Conclusion
Intact cord management during resuscitation appears to be a safe intervention; its effect on early complications of prematurity remains unclear. Further high-quality RCTs with larger patient numbers are urgently needed.
Impact
-
Initiating resuscitation with an intact umbilical cord appears to be a safe intervention for newborns.
-
No statistically significant differences were found in delivery room parameters, in-hospital mortality, and neonatal outcomes between the examined groups.
-
The utilization of specialized resuscitation trolleys appears to be promising to reduce the risk of intraventricular hemorrhage in preterm infants.
-
Further high-quality RCTs with larger sample sizes are urgently needed to refine recommendations.
Similar content being viewed by others
Introduction
The first minutes of life and delivery room management of newborns have a fundamental impact on neonatal mortality and morbidity. Establishing interventions and protocols for resuscitation in the delivery room poses a challenge due to the heterogeneous population as term infants differ from preterm infants who were born at the limit of viability. Recommendations and guidelines on stabilization procedures in the delivery room are constantly changing and evolving by integrating new procedures based on recent evidence (e.g., application of sustained inflations and continuous positive airway pressure (CPAP)).1,2,3
Over the past decades, a number of research studies have examined the physiology and outcomes of delayed cord clamping (DCC); thereby, its numerous beneficial effects on infants have been proven, e.g., improved transitional circulation and iron stores, increased hemoglobin level at birth, decreased need for blood transfusion and lower incidence of intraventricular hemorrhage (IVH) and necrotizing enterocolitis (NEC).4,5,6
Therefore, on the basis of the guidelines influenced by the International Liaison Committee on Resuscitation (ILCOR) recommendations, it is suggested to delay cord clamping (CC) by at least 60 s, ideally after ensuring adequate lung aeration.7 However, the timing of CC and the steps of resuscitation are still not synchronized. Unfortunately, non-vigorous and non-breathing infants needing immediate interventions for stabilization are usually clamped immediately and excluded from most studies on different umbilical cord management. In consequence, for preterm and term infants who require resuscitation after birth, we still have insufficient evidence on the optimal time of CC.4,8,9 The purpose of our study was to collect all available data on this most vulnerable newborn population in order to discover the effect of intact cords when initiating resuscitation.
Methods
Our systematic review and meta-analysis is reported based on the recommendations of PRISMA 2020 guideline10 (Supplementary Table 1), and as methodological guidance, the Cochrane Handbook11 was followed. The prestudy protocol was registered in advance on the International Prospective Register of Systematic Reviews with registration number CRD42022370338. A deviation from the protocol occurred as we also conducted subgroup analyses based on the usage of special resuscitation trolleys. Ethical approval was not required due to the inherent design of the systematic review.
Eligibility criteria
To address our research question, we included randomized clinical trials (RCTs) and observational studies comparing the initiation of neonatal resuscitation (airway opening maneuvers, positive pressure ventilation, chest compression, etc. except drying and stimulation only) before (intact cord resuscitation (ICR)) and after CC. Conference abstracts and case reports were excluded. Exclusion criteria for the examined population were monochorionic twins, triplets or higher-order multiple pregnancies, major congenital malformations, fetal hydrops, twin-to-twin transfusion syndrome, placental abruption, and placenta previa.
We defined our primary outcomes in advance as in-hospital mortality; presence of IVH (all grades and severe (≥grade 3)), periventricular leukomalacia, and cerebral palsy. Our secondary outcomes included delivery room parameters, early complications of prematurity and maternal outcomes, etc. (see Supplementary Material, sections 2 and 3 and Supplementary Table 2).
Search strategy and selection process
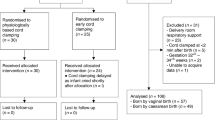
Our systematic search was conducted in four main databases: MEDLINE (via PubMed), Embase, CENTRAL (the Cochrane Central Register of Controlled Trials), and Web of Science on March 1, 2024, using a predefined search key (see in Supplementary Material, section 4). During the search, no filters or language restrictions were applied. Reference and citation lists of the included studies were examined for further eligible articles using the citationchaser.12 After duplicates were removed both automatically and manually, two independent review authors (G.Sz.M. and V.U.) performed the selection process separately via Rayyan (Rayyan Systems, Cambridge, Massachusetts, USA; Qatar Computing Research Institute, Doha, Qatar)13 and Endnote 20 (Clarivate Analytics, Philadelphia, Pennsylvania, USA)14 reference manager programs. Publications were screened according to the eligibility criteria by title and abstract first and then by full text. Disagreements were resolved by involving the corresponding author (Á.G.).
Data were collected from the eligible articles by two authors (G.Sz.M. and V.U.) independently, using a standardized data collection sheet which was created based on the consensus of clinical and methodological experts. The following data were extracted: title, first author, year of publication, countries, number of centers, study period, DOI (digital object identifier), study design, study population, patient demographics, inclusion and exclusion criteria, interventions, and outcomes measured.
Study risk of bias (RoB) assessment
The RoB assessment was performed by two authors (G.Sz.M. and V.U.) separately based on the recommendations of the Cochrane Collaboration, using the Cochrane risk-of-bias tool for randomized trials (RoB2) (Cochrane Bias Methods Group, Cochrane Collaboration).15 The corresponding author (Á.G.) resolved any occurring disagreements.
Synthesis methods
As we assumed considerable between-study heterogeneity in all cases, a random-effects model was used to pool effect sizes.
For binary outcomes, risk ratios (RRs) with a 95% confidence interval (CI) were used for the effect size measure. To calculate the study RRs and the pooled RRs, the total number of patients and those with an event of interest in each group were separately extracted from the studies. We reported the results as the risk of an event of interest in the experimental group versus the risk of an event of interest in the control group. For continuous outcomes, differences between the means (MD) with 95% CI were used for effect size measure. To calculate the study MDs and pooled MDs, the sample size, the mean, and the corresponding standard deviation (SD) were extracted from each study. For the Apgar score, the quartiles were reported in most cases (instead of mean and SD). Therefore, differences between group medians (MedD) were used as effect size measures with 95% CI as recommended by McGrath et al. 16 We reported the results as an experimental group minus control group values.
Results were considered statistically significant if the pooled CI did not contain the null value. We summarized the findings of the meta-analysis in forest plots. As the study number was small, we did not report the prediction intervals (i.e., the expected range of effects of future studies) of results. Between-study heterogeneity was also described by Higgins&Thompson’s I2 statistics.17
Subgroup analysis was performed based on gestational age (GA), usage of special resuscitation trolleys, and the type of intervention in the control group.
Small-study publication bias was assessed by visual inspection of funnel plots and calculating Harbord (modified Egger’s) test p-value18 for RR effect size and classical Egger’s test p-value19 for MD effect size. Unfortunately, the number of studies was too low; therefore, these assessments were meaningless and were not reported.
Potential outlier publications were planned to be explored using different influence measures and plots following the recommendations of Harrer et al. 20. However, the study number was limited; therefore, it was ineffectual to conclude and report.
All statistical analyses were calculated by R software (v4.3.0; R Development Core Team)21 using the meta (v6.5.0)22 package for basic meta-analysis calculations and plots, and dmetar (v0.0.9000)23 package for additional influential analysis calculations and plots.
For additional details see the Supplementary Material, section 5.
Assessing the level of evidence
To evaluate the quality of evidence, we followed the recommendations of the “Grades of Recommendation, Assessment, Development, and Evaluation (GRADE)” workgroup.24
Results
Systematic search and selection
The systematic search yielded 17,141 articles. Following duplicate removal and selection processes, we identified 11 eligible studies and an additional one by reference and citation search (Supplementary Fig. 1). In a subset of the qualified papers,25,26,27,28,29 the study population included infants for whom it was either uncertain or unnecessary to administer any form of resuscitation following birth. After contacting the corresponding authors for more detailed data, we included one more study29 in the analysis. In one article,30 we successfully obtained the necessary information needed to determine the total sample size for in-hospital mortality. In conclusion, a total of six RCTs29,30,31,32,33,34 were included in our quantitative analysis, and ten RCTs25,26,27,28,29,30,31,32,33,34, and two observational studies35,36 in the systematic review. Details and results of the studies included in the systematic review are summarized in Supplementary Table 3.
Study characteristics
The baseline characteristics of studies included in the meta-analysis can be found in Table 1, and the detailed interventions of the examined groups and the respiratory status at the time of CC are summarized in Table 2. We analyzed six RCTs including 610 preterm29,31,32,33,34 and term30,34 infants. The timing of CC and the rates of different resuscitation procedures were heterogeneous among the studies. In the intervention group, newborns received DCC at various time points (either at 50 s,32 at 60 s,29,31 at least 180 s30,34) or physiological-based cord clamping (PBCC).33 In the control group, immediate/early cord clamping (ICC/ECC) (<60 s)29,30,34 or DCC (>30–60 s)31,32,33 were performed. Therefore, besides the GA, we also performed subgroup analyses based on the type of intervention in the control group and the usage of a special resuscitation trolley (see in the Supplementary Material, Figs. 16–31)
In-hospital mortality
Analysis of in-hospital mortality included five RCTs30,31,32,33,34 and involved 584 patients (Fig. 1). This event occurred in 11 out of 297 patients assigned to the ICR group and 13 out of 287 patients assigned to the CC prior to resuscitation group, which indicates no significant difference between the examined groups (RR 0.89, 95% CI [0.24, 3.36]).
No significant differences were found between the groups either in our subgroup analyses (Supplementary Figs. 2–4), or in any studies included in our systematic review and examined this outcome25,28,35,36 (Supplementary Table 3).
Delivery room parameters (oxygen saturation level, Apgar score) and temperature at NICU admission
The pooled analysis of three RCTs30,32,34 with 392 patients showed higher mean SpO2 at 5 min (MD 6.67%, 95% CI [−1.16%, 14.50%]) and 10 min (MD 2.87%, 95% CI [−5.53%, 11.28%]) after birth in the ICR group; however, the difference was not statistically significant (Fig. 2a, b).
a Forest plot representing the mean difference of SpO2 at 5 min after birth in infants who received ICR or CC prior to resuscitation after birth. b Forest plot representing the mean difference of SpO2 at 10 min after birth in infants who received ICR or CC prior to resuscitation after birth. SpO2 oxygen saturation level by pulse oximetry, min minutes, MD mean difference, SD standard deviation, 95% CI 95% confidence interval, CC cord clamping.
We found no significant difference between the examined groups in terms of Apgar score at 1 min and 5 min after birth (1 min MedD –0.09, 95% CI [−0.55, 0.36]29,31,32,33,34 and 5 min MedD –0.03, 95% CI [−0.36, 0.29]31,32,33,34) (Supplementary Figs. 5 and 6) and temperature at admission to the NICU (MD −0.04 °C, 95% CI [−0.20 °C, 0.12 °C]) (Fig. 3).
In the articles included in our systematic review that examined Apgar scores,26,27,28,35,36 SpO2 shortly after birth27,28 and temperature at NICU admission,25,28,35 no significant difference was found between the intervention and control groups (Supplementary Table 3).
In terms of heart rate (HR), Badurdeen et al. 26 observed that PBCC resulted in a similar mean HR between 60 s to 120 s after birth compared to infants receiving ECC. Andersson et al. 30 reported significantly lower HR values in the ICR group than in the ECC group at 1 min and 5 min after birth. Raina et al. 34 also found significantly lower HRs in the ICR group than in the ECC resuscitation group at 5 and 10 min. In the study of Hoeller et al. 36, the PBCC group had lower HRs during the first 72 h of life than those who underwent standard DCC, reaching significance by 10 h of monitoring (Supplementary Table 3).
Early complications of prematurity (IVH, NEC, ROP, BPD, PDA, and LOS)
For the analysis of all grades of IVH, we had three articles31,32,33 involving 300 patients, and 55 infants with IVH (Fig. 4a). The overall effect size was RR of 1.25, 95% CI [0.77, 2.00]. Regarding severe ( ≥ grade 3) IVH, we had four studies29,31,32,33 covering 326 patients with an event number of 21 patients (Fig. 4b). The rate of severe IVH showed no significant difference between the groups (RR 0.96, 95% CI [0.30, 3.01]), although we found a RR of 0.75, 95% CI [0.06, 10.11] when we examined only those studies that used specialized resuscitation trolleys (Supplementary Fig. 7).
a Forest plot representing the risk ratio of all grades of IVH in infants who received ICR or CC prior to resuscitation after birth. b Forest plot representing the risk ratio of severe IVH (≥grade 3) in infants who received ICR or CC prior to resuscitation after birth. IVH intraventricular hemorrhage, RR risk ratio, 95% CI 95% confidence interval, CC cord clamping.
No significant differences were found between the intervention and control groups in articles included in our systematic review that examined all grades25,36 and severe25,28 IVH, with the exception of the article by Hocq et al. 35 who reported that the incidence of all grades of IVH decreased during the ICR implementation period (Supplementary Table 3).
The analysis of four studies29,31,32,33 involving 326 patients and 14 outcome events resulted in an RR of 2.05, 95% CI [0.34, 12.30] for NEC ≥ stage 2, although it did not reach significance level (Fig. 5). When we analyzed only studies that compared ICR to DCC prior to resuscitation,31,32,33 the RR for NEC ≥ stage 2 was 2.89, 95% CI [0.51, 16.40]; however, this result was not statistically significant (Supplementary Fig. 8). Analyzing studies29,31,33 where newborn stabilization was performed with a special resuscitation trolley, we found an RR of 1.22, 95% CI [0.12, 12.85] but it did not reach a significance level either (Supplementary Fig. 9).
Hocq et al. 35 reported a lower incidence of NEC ≥ stage 2 during the ICR implementation period. Further studies examining this outcome25,28,36 did not find any significant differences between the examined groups (Supplementary Table 3).
ROP requiring treatment analysis resulted in a RR of 1.60, 95% CI [0.50, 5.13]29,31,32 (Fig. 6), and for BPD, we found a RR of 1.20, 95% CI [0.83, 1.76]29,31,32,33 (Supplementary Fig. 10), these findings did not reach significance level. In PDA requiring treatment (RR 0.88, 95% CI [0.47, 1.66])31,32,33 (Supplementary Fig. 11) and LOS (RR 0.91, 95% CI [0.44, 1.87])29,32,33 (Supplementary Fig. 12), there were no clinically or statistically significant differences between the two groups.
No significant differences were found between the intervention and control groups in articles included in our systematic review that examined ROP,25,28,35,36 BPD,25,35,36 PDA25,35 and LOS28,36 (Supplementary Table 3).
Need for blood transfusion and surfactant therapy in NICU
We found no significant differences between the groups in the need for blood transfusion (RR 0.95, 95% CI [0.73, 1.25])29,31,32,33 (Supplementary Fig. 13) and need for surfactant therapy (RR 0.96, 95% CI [0.75, 1.22])29,31,32,33 (Supplementary Fig. 14).
No significant differences were observed between the groups in any of the articles included in our systematic review that examined the need for blood transfusion25,35 and surfactant therapy35 (Supplementary Table 3).
Safety parameters
In our study, we included the outcomes of the need for phototherapy, hypothermia (<36.0 °C) at NICU admission, and maternal outcomes including maternal blood loss, pp. hemorrhage, and pp. infection as safety parameters.
We found no significant differences between the intervention and control groups in the need for phototherapy (RR 1.10, 95% CI [0.92, 1.30])29,32,33,34 (Supplementary Fig. 15) and studies25,26,35 included in our systematic review that examined this outcome found no differences either (Supplementary Table 3).
For hypothermia25,28,33,35 and maternal outcomes (blood loss,26,32,33 pp. hemorrhage,25,26,28,32,33 pp. infection25,26,28,32) no significant differences were found between the examined groups (Supplementary Table 3).
RoB assessment and quality of evidence
The RoB assessment is summarized in Supplementary Tables 4 and 5. Four trials29,30,31,33 had a high RoB due to deviations from the intended interventions. The level of evidence is presented in Supplementary Table 6, for most of our outcomes the GRADE assessment resulted in low certainty due to serious RoB and imprecision, but certainty had to be downgraded to very low due to serious RoB and very serious imprecision in some cases. The assessment of small study bias was meaningless as we had only a few studies.
Discussion
The present systematic review and meta-analysis examined the optimal umbilical cord management during neonatal resuscitation. According to the resuscitation guidelines influenced by the ILCOR recommendations, delaying CC by a minimum of 60 s is recommended, preferably following adequate lung aeration.7 A recent systematic review and network meta-analysis with individual participant data on preterm infants found that the highest reduction in mortality occurred when CC was deferred for at least 120 s. Furthermore, the study suggests that resuscitation with an intact cord might be beneficial, but more evidence is needed to support this practice.37 The major limitations of the first quantitative analysis examining this question conducted by Avinash et al. 38 were the limited number of studies available for inclusion and their relatively small sample sizes.
Currently, there are well-defined protocols for cases where CC precedes newborn resuscitation. In cases where initial resuscitation interventions and appropriate thermal care can be safely performed with an intact cord without compromising the newborn, CC may be delayed during these interventions.7,8 Therefore, it is essential to establish standardized procedures and equipment for ICR. However, this concept lacks robust evidence; consequently, explicit protocols and equipment for ICR have not yet been defined.
Main findings
In our study, no statistically significant differences were found in terms of in-hospital mortality, delivery room parameters, and early complications of prematurity. Intact cord management during resuscitation appears to be safe and may improve initial oxygenation, although this is in conflict with current standards for delivery room resuscitation.
In some of the examined outcomes, we observed the following findings which might be relevant in patient care.
Delivery room parameters
Although our results did not reach the statistically significant level, they suggest a possible beneficial effect of ICR on oxygenation. This finding was also noted in another study27 that was excluded from our analysis because of the population examined. In addition to CC timing, FiO2 is another critical factor in neonatal stabilization. Current international guidelines recommend starting resuscitation with 21–30% FiO2 to mitigate the potential for hyperoxia-induced tissue damage.39 All included studies reporting on this aspect30,31,32,33 used an initial FiO2 < 40%. Nevertheless, findings from both animal and human studies indicate the potential benefits of starting resuscitation with 100% FiO2.40,41,42 As further larger investigations are necessary for conclusive evidence, the ongoing DOXIE trial was conducted to directly compare the use of 30% FiO2 to 100% FiO2 during ICR.43
Due to a lack of data, we could not perform an HR analysis, which is an important delivery room parameter; however, studies reporting data on this aspect26,30,34,36 found lower HR values after birth in the ICR group which is hypothesized to be a result of the increased blood volume following ICR.36
Early complications in preterm infants
However, the results of early preterm complications did not show statistically significant differences between the groups, interestingly, the risk of NEC ≥ stage 2 and treatment-requiring ROP seemed to be higher in the ICR group. Examining studies that were excluded from the analysis because of the population or the study design, Deng et al. 28 also reported higher rates of NEC ≥ stage 2 and ROP ≥ phase 2 in the DCC + nCPAP group compared to the DCC-only group, but these results did not reach statistical significance either. In contrast, Hocq et al. 35 found a lower incidence of NEC ≥ stage 2 following ICR implementation in their hospital protocol. Free radicals and hyperoxia might play a role in the development of NEC and ROP.44,45,46
Resuscitation trolleys
To provide continuous placenta-newborn connection, while allowing an immediate stabilization of non-vigorous newborns, different resuscitation platforms were developed such as LifeStart Trolley (Inspiration HealthCare Group PLC, Croydon, UK), Concord Birth Trolley (Leiden University Medical Center, Leiden, Netherlands), NOOMA cart (Maternal Life, LLC, Palo Alto, California, USA) and INSPiRe Trolley (Integrated Neonatal Support on Placental Circulation with Resuscitation, Alberta Health Services, Edmonton, Alberta, Canada).47 Although resuscitation with an intact cord can also be achieved with standard equipment, certain challenges such as the warming device to prevent hypothermia and the availability of sufficient respiratory support may persist.48 Among the RCTs we included in our meta-analysis, ICR has performed with29,31,33,34 and without30,32 the use of specialized resuscitation trolleys as well. A subgroup analysis revealed that employing specialized trolleys might improve the impact of ICR on early complications associated with prematurity: although the rate of severe IVH did not significantly differ between the groups in the analysis of all studies included, when examining specifically those using special equipment, there appeared to be a lower risk of severe IVH in the ICR group compared to the CC prior to the resuscitation group. For NEC ≥ stage 2, although the risk was still higher in the ICR group, it was almost halved when a special trolley was used. However, conducting a subgroup analysis resulted in an even smaller sample size and none of these results reached significance.
Common concerns about DCC
For DCC, safety parameters include hypothermia, the necessity for phototherapy, maternal blood loss, and pp. infection. In our analysis, we did not observe significant differences in the need for phototherapy. Although we did not have sufficient data to perform a statistical analysis for the rest of the outcomes, studies25,26,28,32,33 reporting data on these did not find any significant difference between the groups, except Knol et al. 33 and Hocq et al. 35 who found moderate hypothermia on admission to NICU in a higher proportion of very preterms receiving ICR than the control group.
Strengths and limitations
In this systematic review and meta-analysis, we aimed to achieve the highest level of evidence available; therefore, we followed our pre-registered protocol. Studies were included only if they provided explicit information indicating that all or nearly all newborns in both arms had received resuscitation after birth. We conducted a quantitative analysis of the eligible RCTs and included all studies examining this specific question in the systematic review part. As we had a broad population (preterm and term infants) and heterogeneous interventions to examine a wide range of outcomes, we conducted subgroup analyses where feasible to mitigate the effect of these factors.
Due to the inclusion criteria and the early stage of ICR implementation in clinical practice, a limited number of studies were eligible, posing limitations to our analysis. The generalizability of our findings is challenged by the small sample size and number of events (sometimes zero). In addition, protocols of interventions and definitions of outcomes were heterogeneous or even missing in some cases. Devices used for bedside newborn stabilization can be crucial; however, different resuscitation platforms were used in the studies included. Another limitation was the presence of a moderate and high RoB in most domains. Therefore, caution is needed when interpreting our results.
Implications for clinical practice and future research
The translation of scientific findings into daily practice plays a key role, highlighting that effective implementation significantly improves the quality and cost-efficiency of healthcare. This process is essential to ensure that advancements in medical research directly benefit patient care and public health.49,50
The potential beneficial effect of ICR on oxygenation in the population of term infants suggests that recommending ICR may be justified. Nevertheless, ICR should be applied in preterm infants with caution, and we recommend that this practice be performed only in specialized centers with appropriate expertise, protocols, and equipment, given the potential for complications. Based on our results the use of special resuscitation trolleys appears to be beneficial.
There is still no clear and strong evidence for optimal cord management during neonatal resuscitation. There are ongoing multicenter RCTs51,52,53,54 to examine this question and we encourage researchers to conduct further high-quality RCTs with large sample sizes, homogeneous intervention protocols, and outcome definitions. In addition, it is essential to differentiate outcome data between infants requiring post-birth stabilization measures and those not requiring them. This approach increases the representativeness of the results.
Conclusion
Intact cord management during resuscitation appears to be safe and may improve initial oxygenation, although this is in conflict with current standards for delivery room resuscitation. The early complications of prematurity remain unclear. The use of specialized resuscitation trolleys seems promising to reduce the risk of IVH. There is an urgent need for further high-quality RCTs with larger patient numbers, especially with specialized resuscitation trolleys and physiological-based CC.
Data availability
The datasets used in this study can be found in the full-text articles included in the systematic review and meta-analysis.
References
Richmond, S. & Wyllie, J. European Resuscitation Council Guidelines for Resuscitation 2010: section 7. Resuscitation of babies at birth. Resuscitation 81, 1389–1399 (2010).
Batey, N. et al. The newborn delivery room of tomorrow: emerging and future technologies. Pediatr. Res. https://doi.org/10.1038/s41390-022-01988-y (2022).
Vento, M. & Saugstad, O. D. Resuscitation of the term and preterm infant. Semin. Fetal Neonatal Med. 15, 216–222 (2010).
World Health Organization. Guideline: delayed umbilical cord clamping for improved maternal and infant health and nutrition outcomes. World Health Organization. https://iris.who.int/handle/10665/148793 (2014).
American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Delayed umbilical cord clamping after birth. ACOG Committee Opinion No. 814. Obstet Gynecol. 136, e100–e106 (2020).
Bruckner, M., Katheria, A. C. & Schmölzer, G. M. Delayed cord clamping in healthy term infants: More harm or good? Semin. Fetal Neonatal Med. 26, 101221 (2021).
Madar, J. et al. European Resuscitation Council Guidelines 2021: newborn resuscitation and support of transition of infants at birth. Resuscitation 161, 291–326 (2021).
Aziz, K. et al. Part 5: neonatal resuscitation: 2020 American Heart Association Guidelines for Cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 142, S524–S550 (2020).
Koo, J., Aghai, Z. H. & Katheria, A. Cord management in non-vigorous newborns. Semin. Perinatol. 47, 151742 (2023).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71 (2021).
Higgins, J. P. T. et al. (eds) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.3 (John Wiley and Sons, Ltd., 2022).
Haddaway, N. R., Grainger, M. J. & Gray, C. T. citationchaser: an R package and Shiny app for forward and backward citations chasing in academic searching. Zenodo. https://doi.org/10.5281/zenodo.4543513 (2021).
Ouzzani, M., Hammady, H., Fedorowicz, Z. & Elmagarmid, A. Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 5, 210 https://www.rayyan.ai/ (2021).
The EndNote Team. EndNote https://endnote.com/ (Clarivate, 2013).
Sterne, J. A. C. et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, 4898 (2019).
McGrath, S., Sohn, H., Steele, R. & Benedetti, A. Meta-analysis of the difference of medians. Biom. J. 62, 69–98 (2020).
Higgins, J. P. T. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
Harbord, R. M., Harris, R. J. & Sterne, J. A. C. Updated tests for small-study effects in meta-analyses. Stata J. 9, 197–210 (2009).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Harrer, M., Cuijpers, P., Furukawa, T. & Ebert. D. Doing Meta-Analysis with R: A Hands-On Guide. https://doi.org/10.1201/9781003107347 (Chapman and Hall/CRC, 2021).
R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. v4.3.0. https://www.R-project.org/ (2023).
Schwarzer, G., Balduzzi S., Rücker G., Schwarzer G. “How to perform a meta-analysis with R: a practical tutorial.” BMJ Mental Health 22, 153–160 (2023).
Cuijpers, P., Furukawa, T. & Ebert, D. D. Dmetar: Companion R Package for the Guide Doing Meta-analysis in R. https://dmetar.protectlab.org (2023).
Schünemann, H., Brożek, J., Guvatt, G., & Oxman, A. GRADE Handbook. https://gdt.gradepro.org/app/handbook/handbook.html (Cochrane Collaboration, 2013).
Duley, L. et al. Randomised trial of cord clamping and initial stabilisation at very preterm birth. Arch. Dis. Child. Fetal Neonatal Ed. 103, F6–F14 (2018).
Badurdeen, S. et al. Physiologically based cord clamping for infants ≥ 32 + 0 weeks gestation: a randomised clinical trial and reference percentiles for heart rate and oxygen saturation for infants ≥35 + 0 weeks gestation. PLoS Med. 19, e1004029 (2022).
Katheria, A. C. et al. Delayed cord clamping in newborns born at term at risk for resuscitation: a feasibility randomized clinical trial. J. Pediatr. 187, 313–317 (2017).
Deng, R. et al. With or without nasal continuous positive airway pressure during delayed cord clamping in premature infants < 32 weeks: a randomized controlled trial using an intention-to-treat analysis. Front. Pediatr. 10, 843372 (2022).
Finn, D. et al. Clamping the umbilical cord in premature deliveries (CUPiD): neuromonitoring in the immediate newborn period in a randomized, controlled trial of preterm infants born at <32 weeks of gestation. J. Pediatr. 208, 121–126.e2 (2019).
Andersson, O. et al. Intact cord resuscitation versus early cord clamping in the treatment of depressed newborn infants during the first 10 minutes of birth (Nepcord III)—a randomized clinical trial. Matern. Health Neonatol. Perinatol. 5, 15 (2019).
Katheria, A. et al. Neonatal resuscitation with an intact cord: a randomized clinical trial. J. Pediatr. 178, 75–80.e3 (2016).
Nevill, E., Mildenhall, L. F. J. & Meyer, M. P. Effect of breathing support in very preterm infants not breathing during deferred cord clamping: a randomized controlled trial (the ABC study). J. Pediatr. 253, 94–100.e1 (2023).
Knol, R. et al. Physiological-based cord clamping in very preterm infants—randomised controlled trial on effectiveness of stabilisation. Resuscitation 147, 26–33 (2020).
Raina, J. S. et al. Resuscitation with intact cord versus clamped cord in late preterm and term neonates: a randomized controlled trial. J. Pediatr. 254, 54–60.e4 (2023).
Hocq, C. et al. Implementing intact cord resuscitation in very preterm infants: feasibility and pitfalls. Eur. J. Pediatr. 182, 1105–1113 (2023).
Hoeller, N. et al. Physiological-based cord clamping stabilised cardiorespiratory parameters in very low birth weight infants. Acta Paediatr. 113, 931–938 (2024).
Seidler, A. L. et al. Short, medium, and long deferral of umbilical cord clamping compared with umbilical cord milking and immediate clamping at preterm birth: a systematic review and network meta-analysis with individual participant data. Lancet. https://doi.org/10.1016/S0140-6736(23)02469-8 (2023).
B, S. A. et al. Outcomes of neonatal resuscitation with and without an intact umbilical cord: a meta-analysis. Cureus 15, e44449 (2023).
Wyckoff, M. H. et al. 2022 International Consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations: summary from the basic life support; advanced life support; pediatric life support; neonatal life support; education, implementation, and teams; and first aid task forces. Pediatrics 151, e2022060463 (2023).
Dekker, J. et al. The effect of initial high vs. low FiO2 on breathing effort in preterm infants at birth: a randomized controlled trial. Front. Pediatr. 7, 504 (2019).
Lakshminrusimha, S., Vali, P., Chandrasekharan, P., Rich, W. & Katheria, A. Differential alveolar and systemic oxygenation during preterm resuscitation with 100% oxygen during delayed cord clamping. Am. J. Perinatol. 40, 630–637 (2023).
Edwards, H., Dorner, R. A. & Katheria, A. C. Optimizing transition: providing oxygen during intact cord resuscitation. Semin. Perinatol. 47, 151787 (2023).
Katheria, A. Delayed cord clamping with oxygen in extremely low gestation infants. J. Pediatr. https://clinicaltrials.gov/study/NCT04413097 (2023).
Li, T.-M. & Liu, D.-Y. Mechanism of neonatal intestinal injury induced by hyperoxia therapy. J. Immunol. Res. 2022, 2316368 (2022).
Chou, H.-C. & Chen, C.-M. Neonatal hyperoxia disrupts the intestinal barrier and impairs intestinal function in rats. Exp. Mol. Pathol. 102, 415–421 (2017).
Saugstad, O. D. Oxygen and retinopathy of prematurity. J. Perinatol. 26, S46–S50 (2006).
Katheria, A., Lee, H., Knol, R., Irvine, L. & Thomas, S. A review of different resuscitation platforms during delayed cord clamping. J. Perinatol. 41, 1540–1548 (2021).
Hutchon, D., Pratesi, S. & Katheria, A. How to provide motherside neonatal resuscitation with intact placental circulation? Children 8, 291 (2021).
Hegyi, P., Erőss, B., Izbéki, F., Párniczky, A. & Szentesi, A. Accelerating the translational medicine cycle: the Academia Europaea pilot. Nat. Med. 27, 1317–1319 (2021).
Hegyi, P. et al. Academia Europaea Position Paper on translational medicine: the cycle model for translating scientific results into community benefits. J. Clin. Med. 9, 1532 (2020).
Knol, R. et al. Physiological-based cord clamping in very preterm infants: the aeration, breathing, clamping 3 (ABC3) trial—study protocol for a multicentre randomised controlled trial. Trials 23, 838 (2022).
Fairchild, K. VentFirst: A Multicenter RCT of Assisted Ventilation During Delayed Cord Clamping for Extremely Preterm Infants. https://clinicaltrials.gov/study/NCT02742454 (University of Virginia, 2023).
Pratesi, S. et al. Placental circulation intact trial (PCI-T)—resuscitation with the placental circulation intact vs. cord milking for very preterm infants: a feasibility study. Front. Pediatr. 6, 364 (2018).
Ekelöf, K. et al. A hybrid type I, multi-center randomized controlled trial to study the implementation of a method for Sustained cord circulation And VEntilation (the SAVE-method) of late preterm and term neonates: a study protocol. BMC Pregnancy Childbirth 22, 593 (2022).
Acknowledgements
The authors express their gratitude to Prof. Ola Andersson, MD, PhD, and Prof. Eugene Dempsey, MD, MSc, MA, FRCPI, for graciously providing additional information from their research. This collaboration enabled the inclusion of their trials in our analysis. None of them have any conflict of interest concerning our systematic review and meta-analysis.
Funding
Funding was provided by the Centre for Translational Medicine, Semmelweis University. Sponsors had no role in the design, data collection, analysis, interpretation, or manuscript preparation. Open access funding provided by Semmelweis University.
Author information
Authors and Affiliations
Contributions
Gréta Szilvia Major: conceptualization, project administration, methodology, formal analysis, visualization, and writing—original draft; Vivien Unger: conceptualization, data curation, and writing—review and editing; Rita Nagy: conceptualization, methodology, visualization, and writing—review and editing; Márk Hernádfői: conceptualization, methodology, visualization, and writing—review and editing; Dániel Sándor Veres: conceptualization, formal analysis, data curation, visualization, and writing—original draft; Ádám Zolcsák: conceptualization, formal analysis, data curation, visualization, and writing—review and editing; Miklós Szabó: conceptualization and writing—review and editing; Miklós Garami: conceptualization and writing—review and editing; Péter Hegyi: conceptualization and writing—review and editing; Péter Varga: conceptualization and writing—review and editing; Ákos Gasparics: conceptualization, supervision, and writing—original draft and final approval of the manuscript submitted. All authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
There are no potential conflicts of interest to declare.
Ethics approval and consent to participate
No ethical approval was required for this systematic review with meta-analysis, as all data were already published in peer-reviewed journals. None of the patients was involved in the design, conduct or interpretation of our study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Consent Statement Patient consent was not required.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Major, G.S., Unger, V., Nagy, R. et al. Umbilical cord management in newborn resuscitation: a systematic review and meta-analysis. Pediatr Res 97, 1481–1491 (2025). https://doi.org/10.1038/s41390-024-03496-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03496-7
This article is cited by
-
Umbilical cord management in newborn resuscitation
Pediatric Research (2025)
-
Feasibility and early outcomes of intact cord resuscitation without special equipment in extremely preterm infants born at 23–25 weeks of gestation: A case series
European Journal of Pediatrics (2025)