Abstract
Most of an infant’s day is devoted to sleep – and normal sleep is vital to normal brain development. Sleep disruptions may impair overall health, well-being, and neurodevelopment. Disruptors of sleep and circadian health, such as noise, light, respiratory support, and clinical interventions, are highly prevalent in hospital and nursing care facilities. These factors particularly affect infants who already have an increased risk of sleep disorders and their consequences due to an underlying disease. Preterm infants and infants with disorders such as neonatal abstinence syndrome, craniofacial malformations, congenital heart disease, hypoxic-ischemic encephalopathy, Chiari-malformation/myelomeningocele, congenital musculoskeletal disease, and Down syndrome are all at high risk for impaired development of sleep-wake cycling and for sleep-disordered breathing. Since abnormal sleep is a potentially treatable risk factor for impaired neurodevelopment, there is an urgent need for effective monitoring, timely interventions, and treatment strategies to improve sleep physiology and thereby optimize overall neurodevelopment in these high-risk populations.
Impact
-
Healthy sleep plays a fundamental role in normal infant brain development.
-
Many factors can disrupt sleep during a hospital stay. This is particularly important for infants who have an increased risk of sleep disorders due to neonatal disorders such as prematurity, congenital heart disease, or Chiari malformation.
-
Sleep protective strategies are readily available and need to be systematically implemented into hospital care.
Similar content being viewed by others
Introduction
Sleep is essential for memory consolidation, synaptic plasticity, and the formation of neural connectivity, all of which are important for normal neurodevelopment.1,2,3,4,5 There is much evidence that children who experience inadequate or disrupted sleep show a higher risk of developmental delays, behavioral problems, and difficulties in learning and memory.6,7 For example, higher proportions of total night-time sleep at 18 and 26 months were related to better performance in executive tasks a few months later.8 In neonates at risk for seizures, inefficient sleep, expressed as a higher proportion of quiet sleep and lower electroencephalogram (EEG) delta power during this stage, was associated with worse neurological examination scores and predicted worse 18-month cognitive, language and motor scores.9,10
Beyond sleep-wake cycling (SWC) as a marker of infant brain function, sleep-disordered breathing (SDB) may be a remediable contributor to unfavorable neurodevelopment. Neonates who require Neonatal Intensive Care Unit (NICU) admission commonly have SDB.11 Likewise, newborns with craniofacial anomalies, certain genetic syndromes, neuromuscular diseases, and other congenital anomalies are also at particularly high risk.12 The risk for SDB is also elevated in preterm born infants.13,14,15 Here, repeated, brief, intermittent hypoxemia and resultant sleep disruption caused by SDB could exacerbate the already elevated long-term risk for abnormal cognitive development.
In addition, impaired sleep may have somatic consequences, such as reduced growth.16 Among Singaporean children in the first two years of life, shorter sleep duration was significantly associated with shorter body length and, in some subgroup analyses, with higher body mass index (BMI).17 There is strong evidence from studies in older children and adults that a short sleep duration is associated with an increased risk of obesity, and in addition, adverse sleep characteristics may affect glucose and insulin metabolism and blood pressure, and thus overall cardiovascular risk factors.18
This narrative review aims to give an overview of the current knowledge base of sleep disruptors and how these factors particularly affect infants during their first year of life - a demographic with an increased risk of sleep disorders and their consequences due to an underlying disease.
Role of sleep disruptors
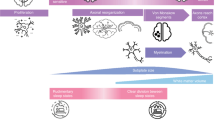
Intensive care units are places with constant activity, alarms, and medical equipment that generates noise and light. Children undergo medically necessary invasive procedures, such as blood draws, or unpleasant interventions, such as physical examinations, ultrasound scans or X-rays and nursing activities. See Fig. 1 for a graphical summary of possible sleep disruptors (Fig. 1). In the following sections, we will provide an overview of selected sleep disruptors.
The infant at risk in its environment of possible sleep disruptors: Light, noise, monitoring and alarms (especially in NICUs/PICUs), diagnostic procedures such as blood takes, physical examinations and ultrasound, nursing activities, the underlying disease itself e.g. myelomeningocele, congenital heart disease, Down syndrome, prematurity, and treatments such as medication e.g. sedatives, surgery, mechanical ventilation.
Noise
Noise measurements in incubators in NICUs showed means of 53–62 dB with maximum levels of up to 113 dB, with no major differences between day and night.19,20,21 These sound levels are frequently above the threshold recommendations of the American Academy of Pediatrics (AAP), which recommends 45 dB as the maximum sound exposure with sound peaks at or below 65 dB22,23,24 and the World Health Organization (WHO), which recommends an even lower maximum of 30 dB for hospital ward rooms during the night.25 Ventilatory support significantly increases the background noise; measurements showed a variable noise level depending on the ventilator, ventilation mode, and precise microphone positioning used, but always between 45 dB and up to 82 dB.26
In preterm infants, noise peaks above 65 dB led to blinking and a startle reflex, facial and body movements in the majority of newborns, as well as changed sleep and wake states in 60%.21 In contrast, others reported no significant correlation between noise levels and wakefulness in preterms.19 One study group performed a period of “quiet time” four times a day for 60 minutes each in a NICU. They found that neonates with a corrected age of 35 weeks’ gestation had longer total sleep time during the 60-minutes “quiet time”, followed by a longer bout of wakefulness.27
There also seems to be a correlation between sound exposure in preterm infants and neurodevelopmental outcomes. A study of 34 preterm infants indicated an association between sound reduction by silicone earplugs and a better mental development index at 18–22 months.28 Here, sleep seems to be the mediator linking noise exposure to poor recovery and adverse neurodevelopmental outcomes. Conversely, there is concern that limiting sound exposure in NICUs could limit necessary exposure to spoken language with long-term consequences for language development. A study comparing auditory exposure in NICU private rooms and in the open ward, found significantly more silent time in the private rooms (1.9 h during a 16h-period)29 and another study described lower language scores in 2-year-old children who were previously hospitalized in NICU private rooms compared to children with previous hospitalization in an open ward.30 Furthermore, exposure to parental talk increased infant vocalization in preterm infants.31 Moreover, live music therapy, especially lullabies, has a positive impact on physiological functions in preterm infants with lower heart and breathing rates, better caloric intake, and improved sleep patterns.32,33,34 Thus, not only noise reduction but also the promotion of positive sounds, such as spoken language and music, seems to be important.
Light
Light exposure in intensive care units is highly variable. On the one hand, light is necessary to carry out interventions safely and to assess children clinically; on the other hand, children should not be dazzled or disturbed. The AAP and a consensus committee, therefore recommend variably adjustable light sources that provide indirect lighting and ambient light in a range of 10 to 600 lux.23,24 A study in extremely low birth weight infants measured a mean light level of 70.5 lux throughout their hospital stay (excluding phototherapy episodes), and light levels almost always met the AAP recommendations.35 Others reported light levels more than twice the recommended levels at multiple times of the day in the environment of a neonate with chronic heart disease,36 while another study found light levels generally below the recommended values in infants with chronic heart disease.37
Interestingly, high light levels achieved during phototherapy can also alter sleep behavior after the end of the exposure. A large Japanese study found shorter sleep time over 24 hours at one month of age in children who had received phototherapy over 24 hours.38 It is increasingly recommended to adapt the light to day and night time in intensive care units to support the existing or developing circadian rhythm.39,40 Even in preterm and newborn infants, positive effects of light cycling have been shown with shorter length of stay, better weight gain and less crying at 11 weeks’ corrected age.41,42
Overall, optimizing light exposure in the intensive care unit environment seems beneficial for the development of infants and sleep health. However, a recent review emphasized the low level of evidence supporting chronobiology in NICUs.43
Interventions by nurses and physicians
In many intensive care units, an attempt is made to take the infant’s sleep-wake cycle into account and adapt interventions accordingly. However, there is a risk that active sleep may be mistaken for wakefulness due to the presence of frequent body and facial movements, and the infant may thus be disturbed in its most vulnerable sleep state.44,45,46 Active sleep plays a particularly important role in brain development, especially sensorimotor plasticity, which is underscored by the fact that preterm infants, during a period of extremely rapid development, spend more time in active sleep than older children.47,48 Additionally, SDB is typically exacerbated during this sleep stage, and respiratory events are most commonly induced duringthe handling of infants in active sleep.44 A survey of Dutch healthcare providers who were directly involved in neonatal care revealed limited knowledge of sleep physiology, with only 14% correctly answering a question about the difference between wakefulness and active sleep.45 Similarly, in a study of 30 Iranian nurses working in a NICU, only 20% were able to describe the characteristics of active sleep.49
A polysomnography (PSG) study in term and near-term infants at risk of cerebral dysfunction during their NICU stay revealed frequent handling with a total duration of hands-on care of 65.3 ± 33.0 minutes within 4 hours.44 These contacts, including direct contact with the neonate and with objects within the incubator and, in 34% of cases, with the PSG-related technology, occurred in equal proportions during all sleep and wake stages with a mean maximum time interval between contacts of 50.9 ± 26.2 minutes. Only half of the neonates were able to complete a 60-minute sleep-wake cycle without interruption.44 Another study in 12 preterm infants with a corrected age of 35 weeks’ gestation observed a mean of 143 handlings (of which 55 were due to adjustments of polysomnography) for each infant with a total time of 3.9 h in 24 hours with more handlings during the day, but there was no statistically significant association between number and duration of handling episodes and wakefulness.19 Two small postoperative studies in neonates with chronic heart disease also found frequent arousals and only a short duration of sleep episodes with an average of 12.3 minutes37 and sleep durations of less than 30 minutes for 90% of the time.36 In an Irish study of moderate to late preterm infants 23% of overnight sleep cycles were interrupted, almost exclusively for the purposes of feeding.50
Additionally, experiencing procedural pain is common in NICUs or Pediatric Intensive Care Units (PICUs). One study reported a median of 35 skin breaking procedures in the first five days in a group of preterm infants, which showed a significant correlation with maturation on EEG measured with polysomnography at discharge.51 Another study showed that higher levels of stress, as measured by salivary cortisol, prolonged periods of wakefulness.52 Interestingly, this group also found that very and moderately preterm infants were highly resilient to nociceptive stimuli during active sleep and did not awaken, whereas older neonates (34 – 40 PMA) were particularly vulnerable during active sleep and often awakened to the same stimuli.52
In summary, many factors in the hospital environment can disrupt sleep. It is important to optimize the local clinical environment to promote sleep and thus development during a vulnerable period of life. In the following sections, we will take a closer look at some specific patient groups.
Preterm infants
Preterm infants spend up to 90% of their time asleep.53 There is some evidence that preterm infants experience structurally different sleep, e.g., longer sleep cycles, more wakefulness, and higher proportions of quiet sleep at similar post-menstrual ages, demonstrating that being born preterm impacts sleep regulation.54,55,56 This is consistent with the finding that babies born preterm are at significant risk of impaired brain development, which may manifest as altered sleep regulation.57 In addition, significant disruptions in sleep patterns in preterm infants are common and may exacerbate atypical brain development and increase the risk of developing severe cognitive, behavioral, and/or socialization deficits.9,58,59
As outlined earlier, the NICU could be considered a hostile sleep environment for preterm infants. Apart from the external disturbers, inherent pathologies and comorbidities themselves have the potential to hinder normal sleep in this population. In this regard, rapid eye movement sleep (REM) -type sleep plays a particular role. For example, a recent study showed an association between active sleep percentage during 29–32 weeks’ postmenstrual age and white matter volume at term age.60 Additionally, infants with more REM-type (active) sleep proportions also had better neurodevelopmental outcomes as assessed via Bayley II.58
Even after the NICU stay, many former preterm infants still exhibit abnormal sleep features with lower sleep quality and more parental worries about their baby’s sleep.61 Recently published recommendations highlight eight evidence-based practices to protect and promote sleep; as well as noise and light control discussed earlier, emphasis should be placed on sleep team composition, risk factor assessment, sleep assessment tools, positional management, sensory stimulation, and hospital-home transition sleep management.52,62
Neonatal abstinence syndrome
Neonatal abstinence syndrome (NAS), or more specifically neonatal opioid withdrawal syndrome in the case of maternal opioid use, refers to a group of symptoms that occur in newborns who have been exposed to addictive substances in utero. Symptoms can include irritability, tremors, feeding difficulties, vomiting, diarrhea, and specifically sleep disturbances.63 A large proportion of children (39–100%) with NAS showed no SWC during amplitude-integrated electroencephalography (aEEG) monitoring in the first 1–4 postnatal days before medical treatment64,65,66 compared to only 8% of healthy controls.65 Abnormal aEEG patterns and lack of SWC were associated with higher NAS scores and a longer hospital stay.64,66
Compared to healthy infants, those with NAS spend more time in active sleep67,68 and less time in quiet sleep.67,68,69 Additionally, sleep of infants with NAS is more disorganized with a higher percentage of indeterminate sleep68,69 and a higher frequency of change in sleep patterns.67 Overall, infants with symptoms of NAS spent more time awake than controls and, therefore, had poorer sleep efficiency, but when treated with oral morphine, sleep efficiency improved.68,69
There have been several attempts to improve sleep in children with NAS. However, sleeping in a rocking bed increased withdrawal symptoms and sleep disruption on day seven compared to sleeping in a normal bed.70 Another study compared sleeping in a prone position with sleeping in the supine position. Neonates in the prone position showed significantly lower mean and peak withdrawal scores and lower daily caloric intake.71 However, sleeping in the prone position has to be considered very critically because of its increased risk of sudden infant death syndrome (SIDS).72 Promoting sleep in infants with NAS is particularly important, as children with NAS are already at increased risk of neurocognitive impairment.73,74
Craniofacial malformations (e.g. Robin Sequence)
Because of their specific anatomy, upper airway obstruction (UAO) is particularly common in neonates, but not always easy to detect at this age. In some infants, UAO only develops some weeks after birth, thus, a normal sleep study result shortly after birth does not preclude the development of potentially severe obstructive sleep apnea (OSA) during the first postnatal months.75 Also, the common belief that sleep-related UAO will lead to frequent oxygen desaturations is not always true.76 In fact, some children have severe OSA on PSG but no clinical symptoms or desaturations, so that pulse oximetry is not sensitive enough to reliably detect OSA in this population.77
Even if the pathophysiology of UAO is evident, as in infants with RS, the expression of mandibular retrognathia or glossoptosis as the main symptoms correlate only poorly with the severity of UAO/OSA,78 which justifies routine screening for OSA in all infants with these conditions. If no PSG is available, cardiorespiratory polygraphy (PG), but not oximetry, may be used as a valid alternative.79 Using a PG will result in similar values for the mixed obstructive apnea hypopnea index but will detect hypopneas less often than with PSG.80 In a study on infants with RS, the mixed-obstructive apnea-hypopnea index was twice as high as the mixed-obstructive apnea index.81 Given that recording a full PSG is more burdensome on these infants, which may already be severely compromised by their respiratory problems, it may be acceptable only to record a PG for infants with RS, even if full PSG is available, but to reduce treatment thresholds. For example, in the authors’ setting, a threshold of 3 instead of 5 events/h is used.75 Assessing the efficacy of treatment also requires a sleep study. Thus, this diagnostic modality should be available in every center where infants with craniofacial malformations are treated.82
Of particular concern in this context is the frequently applied practice of using the prone sleep position as a first-line treatment for infants with craniofacial malformations. This is because of the large body of evidence identifying this position as one of the most important preventable risk factors for SIDS,72 and although a death in an infant with RS would not be classified as SIDS, there is no reason to suggest that a similar pathophysiology as seen in healthy infants would not also apply to infants with RS. In addition, there is accumulating evidence that prone positioning is not effective in most infants with RS.83 Thus, given the combination of limited effectiveness plus an increased risk of sudden death precludes, in our opinion, a recommendation for prone sleep positioning in this patient group.84 This is especially true given that effective non-surgical treatment options exist for these infants, although not yet in every country.82,85,86
Little is yet known about the clinical consequences of recurrent obstructive apneas as seen in infants with craniofacial malformations. While there is little doubt that frequent respiratory events may result in severe failure to thrive (as seen in infants with RS),87 obstructive apneas were also reported as one of the few sleep study results differentiating infants who later succumbed to SIDS from healthy controls.88 An explanation for this finding may lie in the fact that recurrent obstructive apneas, e.g., in RS infants, are associated with an increased arousal threshold, with an impaired arousal also being implied in the pathophysiology of SIDS.89,90 In addition, there is evidence that these patients may have a normal neurocognitive development if their sleep-related upper airway obstruction is identified and treated early,86,91,92 further supporting the relevance of routinely performing sleep studies in this high-risk group.
Congenital heart disease
Sleep physiology is often disturbed in children with congenital heart disease (CHD). In EEG observations, children with CHD showed a lower proportion of active sleep and a shorter sleep cycle duration compared to healthy term neonates.93 Later, at 6–12 months of age, cyanotic CHD infants had increased wake time with decreased sleep efficiency.94 One study reported a calculated functional brain age (FBA) based on the distribution of quiet sleep and non-quiet sleep as an expression of brain maturation.93 They found a lower FBA in children with CHD, and even more in children with dextro-transposition of the great arteries than in healthy neonates. Postoperatively, in this study, sleep organization and FBA improved and no longer differed from healthy controls.93 Several studies report disturbed SWC in up to half of children with CHD before corrective surgery,95,96,97,98 whereas other studies found normal SWC preoperatively in a high proportion of children with CHD (87–97%)99,100,101 Table 1 provides a summary of available literature on sleep wake cycling in infants with congenital heart disease (Table 1). Most of these studies performed aEEG monitoring in the first days of life, with the exception of one study with measurements just before surgery at a mean age of 1.4 months;95 the different findings could not be explained by differences in age.
Postoperatively, many infants with CHD do not return to normal SWC in the first days (normal SWC in 33% within 24 h,99 29% within 48 h,97 and 32% within 48 h 100). Delayed recovery of SWC after surgery was associated with poorer neurocognitive outcome at 2 years102 and lower intelligence quotient at 4 years.100 FBA delay was associated with poorer motor scores at 2 years, but not cognitive and language scores.93
There is some evidence that SDB is also more likely to occur in infants with CHD.94,103,104 In infants with CHD, comorbid SDB was associated with poorer neurocognitive test scores, including 10–12 points lower IQ.105
Hypoxic-ischemic encephalopathy
SWC is not yet developed in many children with neonatal hypoxic-ischemic encephalopathy (HIE) in the first days after birth,106,107,108,109,110,111 and the severity of HIE influenced the time to develop SWC.112 Furthermore, sleep organization was altered in infants with HIE. Asphyxiated infants with mild to moderate encephalopathy showed an increased percentage of quiet sleep (46.5% vs 38.7%) and indeterminate sleep and a decreased percentage of active sleep (18.9% vs 44.7%) compared to healthy neonates.113
Therapeutic hypothermia is the standard treatment for children with moderate to severe HIE,114,115 but hypothermia itself may affect sleep organization. A study of neonates with moderate to severe HIE randomized to either hypothermia or normothermia treatment in clinical trials showed that neonates in hypothermia took longer to normalize aEEG patterns and to develop SWC compared to infants in normothermia.108
The recovery of SWC can be a predictor of neurologic outcome. Asphyxiated infants with pathologic MRI findings needed longer to develop SWC than infants with HIE and normal MRI imaging.107,111 In addition, the time to SWC onset predicted the outcome as measured by Bayley Scales at 18–36 months106,108,116 or by Griffith’s developmental quotient at 12–66 months.110 Failure to develop SWC in the first days of life (48–120 hours) strongly predicted death or disability.108,110,111,117,118,119 Furthermore, seizures were associated with a lack of SWC120 or a longer time interval to onset of SWC110 and a prolonged time to recovery of SWC was a prognostic factor for the later development of epilepsy.121 These findings underline that the development of SWC reflects healthy brain function.
Infants with moderate HIE showed more SDB as well as sleep initiation and maintenance issues, whereas infants with mild HIE had more circadian rhythm disturbances.122 There are no data on how early family education in HIE may influence later sleep. Identifying and treating sleep problems is particularly important because the occurrence of sleep problems is significantly correlated with lower quality of life and may persist into adulthood.123
Chiari-malformation & myelomeningocele
Myelomeningocele (MMC) is characterized by exposure of the spinal cord through a defect in the spine and is typically accompanied by hindbrain herniation (Chiari II malformation). Infants with myelomeningocele are at high risk of sleep disorders. Abnormal sleep physiology is likely multifactorial in this population, related to MMC level, Chiari-II malformation and resultant brainstem dysfunction, musculoskeletal factors, and pulmonary abnormalities. While fetal surgery reduces the severity of hindbrain herniation, decreases the need for ventriculo-peritoneal shunts, and increases the chances of independent ambulation,124 prenatal MMC closure does not improve cognitive outcomes.125
A large proportion of school-aged children with MMC have sleep apnea.126,127 Sleep apnea and hypoventilation may be highly consequential, though potentially treatable, for this patient population. Importantly, sleep apnea is associated with sudden death in young adults with MMC (relative risk 5.4, 95% CI 2.5–11.8, p = 0.005 for sudden death in patients with vs. without sleep apnea).128 Critically, SDB may be ubiquitous among neonates with MMC. In a matched cohort study of newborns evaluated with gold-standard bedside PSG, the AHI was significantly higher for 19 neonates with MMC (34 ± 22) than for 19 age-matched NICU controls who had no congenital malformations or specific risk factors for SDB (19 ± 11; p = 0.021).129 The AHI was identical among neonates with fetal versus postnatal myelomeningocele repair.
Data from the Children’s Hospital of Philadelphia (CHOP) indicate that “clinically significant apnea” was identified in 58 of 100 neonates with fetal MMC repair (vs. 36% of 78 in the Management of Myelomeningocele Study [MOMS trial]).130 Two additional studies have reported that more than three-quarters of infants <22 months of age had abnormal pneumograms.131,132 Most apneas were central rather than obstructive, perhaps because of compression and dysplasia of the brainstem related to the Chiari II malformation. Based on clinical observations and the above data, the Spina Bifida Association now recommends that “all patients with neural tube defects [the most severe of which is myelomeningocele], whether they are symptomatic or asymptomatic, undergo polysomnography that evaluates for central apneas, hypoventilation, as well as obstructive sleep apneas“.133 An ongoing study (NCT04251806) is evaluating whether SDB in neonates with MMC is associated with cognitive and language development, and persistent sleep disturbances at age 2-years.
Congenital musculoskeletal disorders
SDB, particularly with nocturnal hypercapnic hypoventilation, is highly prevalent (42 – 71%) in children with neuromuscular disorders, depending on disease severity and progression.134,135,136,137,138 Skeletal deformities, such as scoliosis and joint contractures, often accompany neuromuscular disorders or syndromic diseases and may, in turn contribute to SDB.139,140 Furthermore, an association between nocturnal hypercapnic hypoventilation and scoliosis was found in children with neuromuscular disorders,141 and 52% of children with rare skeletal dysplasia had OSA.142 Additionally, sleep quality and quantity may be affected in children with neuromuscular diseases. Children with congenital muscular dystrophies have more frequent nighttime awakenings, lower sleep efficiency, shorter total sleep time, and decreased REM duration compared to healthy controls.135 There is a paucity of studies investigating sleep in this patient group during early infancy. However, based on existing studies in older children, it can be assumed that sleep is also significantly impaired in young infants if the disease manifests early.
Down syndrome
One often ignored comorbidity in Down Syndrome (DS) is OSA. OSA occurs at a significantly higher rate in children with DS, and maybe already be apparent in infancy. The prevalence of OSA in infants with DS ranges from 20 to 30%.143,144 The increased OSA risk in this population can be explained by various predisposing factors, including their unique craniofacial anatomy with a small midface and a protruding tongue, hypotonia, obesity, adenotonsillar hypertrophy, thyroid dysfunction, gastrointestinal problems, and frequent respiratory infections.143,145,146 Untreated OSA in DS has been found to have a negative impact on sleep quality as well as daytime behavior, neuro-development and cardiometabolic health.147 Early diagnosis is needed, especially since children with DS have intellectual disabilities and higher cardiovascular risk, and OSA symptoms may often be overlooked.148 Therefore, guidelines recommend screening sleep studies during early infancy.149 Treatment options in infants with DS include CPAP or high-flow oxygen therapy, myofunctional training and oral appliances.150,151,152 In addition to DS, children with other chromosomal abnormalities, such as Prader-Willi syndrome, are also at high risk for sleep disorders and should be prioritized for evaluation and treatment.153
Conclusion
Certain groups of patients are at particularly high risk of sleep disorders due to the anatomical and physiological characteristics of their underlying disease and the treatment required, which often includes prolonged hospitalization. Measures should be taken to promote sleep, especially in vulnerable infants. These may include, for example, controlling ambient noise and light, assessing risk factors, and training medical and nursing staff in recognizing the infant’s sleep stages and adjusting interventions accordingly. Parents should also be educated about healthy sleep so that they can best support their infant’s sleep and recognize sleep disorders. Additionally, screening protocols for sleep disorders should be applied in at-risk populations to allow for early treatment. More research is needed for optimal evidence to best support sleep health in at-risk infants.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Cao, J., Herman, A. B., West, G. B., Poe, G. & Savage, V. M. Unraveling why we sleep: quantitative analysis reveals abrupt transition from neural reorganization to repair in early development. Sci. Adv. 6, eaba0398 (2020).
Lokhandwala, S. & Spencer, R. M. C. Relations between sleep patterns early in life and brain development: a review. Dev. Cogn. Neurosci. 56, 101130 (2022).
Uchitel, J., Vanhatalo, S. & Austin, T. Early development of sleep and brain functional connectivity in term-born and preterm infants. Pediatr. Res 91, 771–786 (2022).
Wehrle, F. M. et al. Functional networks of working memory abilities in children with complex congenital heart disease: a sleep EEG study. Child Neuropsychol. 29, 1109–1127 (2023).
Picchioni, D., Reith, R. M., Nadel, J. L. & Smith, C. B. Sleep, plasticity and the pathophysiology of neurodevelopmental disorders: the potential roles of protein synthesis and other cellular processes. Brain Sci. 4, 150–201 (2014).
Lenehan, S. M., Fogarty, L., O’Connor, C., Mathieson, S. & Boylan, G. B. The architecture of early childhood sleep over the first two years. Matern Child Health J. 27, 226–250 (2023).
Liu, J. et al. Childhood sleep: physical, cognitive, and behavioral consequences and implications. World J. Pediatr. 2, 1–11 (2022).
Bernier, A., Carlson, S. M., Bordeleau, S. & Carrier, J. Relations between Physiological and Cognitive Regulatory Systems: Infant Sleep Regulation and Subsequent Executive Functioning. Child Dev. 81, 1739–1752 (2010).
Shellhaas, R. A. et al. Neonatal sleep-wake analyses predict 18-month neurodevelopmental outcomes. Sleep 40, zsx144 (2017).
Shellhaas, R. A., Burns, J. W., Barks, J. D. & Chervin, R. D. Quantitative sleep stage analyses as a window to neonatal neurologic function. Neurology 82, 390–395 (2014).
Meerkov, M. S. et al. Sleep-disordered breathing is common among term and near term infants in the NICU. Pediatr. Pulmonol. 54, 557–562 (2019).
Mehta, B., Waters, K., Fitzgerald, D. & Badawi, N. Sleep Disordered Breathing (SDB) in neonates and implications for its long-term impact. Paediatr. Respir. Rev. 34, 3–8 (2020).
Emancipator, J. L. et al. Variation of cognition and achievement with sleep-disordered breathing in full-term and preterm children. Arch. Pediatr. Adolesc. Med 160, 203–210 (2006).
Rosen, C. L. et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J. Pediatr. 142, 383–389 (2003).
Durankus, F., Aladag Ciftdemir, N., Vatansever Ozbek, U., Duran, R. & Acunas, B. Comparison of sleep problems between term and preterm born preschool children. Sleep. Med 75, 484–490 (2020).
Tham, E. K., Schneider, N. & Broekman, B. F. Infant sleep and its relation with cognition and growth: a narrative review. Nat. Sci. Sleep. 9, 135–149 (2017).
Zhou, Y. et al. Sleep duration and growth outcomes across the first two years of life in the Gusto study. Sleep. Med 16, 1281–1286 (2015).
Matthews, K. A. & Pantesco, E. J. Sleep characteristics and cardiovascular risk in children and adolescents: an enumerative review. Sleep. Med 18, 36–49 (2016).
Orsi, K. C. et al. Effects of handling and environment on preterm newborns sleeping in incubators. J. Obstet. Gynecol. Neonatal Nurs. 46, 238–247 (2017).
Peng, N. H. et al. To explore relationships between physiological stress signals and stress behaviors in preterm infants during periods of exposure to environmental stress in the hospital. Biol. Res Nurs. 13, 357–363 (2011).
Rodarte, M. D. O. et al. Exposure and reactivity of the preterm infant to noise in the incubator. Codas 31, e20170233 (2019).
Etzel, R., Balk, S. & Bearer, C. Noise: A hazard for the fetus and newborn. American Academy of Pediatrics. Committee on Environmental Health. Pediatrics 100, 724–727 (1997).
Altimier, L. et al. Recommended standards for newborn ICU design. J. Perinatol. 43, 2–16 (2023).
AAP. Guidelines for Perinatal Care (American Academy of Pediatrics, 2017).
Berglund, B., Lindvall, T. & Schwela, D. H. New WHO guidelines for community noise. Noise & Vibration Worldwide 31, 24–29 (2000).
Singh, D. & Fusch, G. Investigating noise exposure to newborn infants from respiratory support: methodological considerations. Cureus 13, e19353 (2021).
Pugliesi, R. R. et al. Correlation of premature infant sleep/wakefulness and noise levels in the presence or absence of “Quiet Time. Adv. Neonatal. Care 18, 393–399 (2018).
Abou Turk, C., Williams, A. L. & Lasky, R. E. A randomized clinical trial evaluating silicone earplugs for very low birth weight newborns in intensive care. J. Perinatol. 29, 358–363 (2009).
Pineda, R. et al. Auditory exposure in the neonatal intensive care unit: room type and other predictors. J. Pediatr. 183, 56–66.e53 (2017).
Pineda, R. G. et al. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. J. Pediatr. 164, 52–60.e52 (2014).
Caskey, M., Stephens, B., Tucker, R. & Vohr, B. Importance of parent talk on the development of preterm infant vocalizations. Pediatrics 128, 910–916 (2011).
Loewy, J., Stewart, K., Dassler, A. M., Telsey, A. & Homel, P. The effects of music therapy on vital signs, feeding, and sleep in premature infants. Pediatrics 131, 902–918 (2013).
Kobus, S. et al. Music Therapy Is Effective During Sleep in Preterm Infants. Int. J. Environ. Res. Public Health 18, 8245 (2021).
Yue, W., Han, X., Luo, J., Zeng, Z. & Yang, M. Effect of music therapy on preterm infants in neonatal intensive care unit: systematic review and meta-analysis of randomized controlled trials. J. Adv. Nurs. 77, 635–652 (2021).
Lasky, R. E. & Williams, A. L. Noise and light exposures for extremely low birth weight newborns during their stay in the neonatal Intensive Care Unit. Pediatrics 123, 540–546 (2009).
Daniels, J. M. & Harrison, T. M. A case study of the environmental experience of a hospitalized newborn infant with complex congenital heart disease. J. Cardiovasc Nurs. 31, 390–398 (2016).
Kalvas, L. B. & Harrison, T. M. Feasibility case series of environment and sleep in infants with congenital heart disease. Nurs. Res. 69, S79–s84 (2020).
Hotta, M. et al. Association between neonatal phototherapy and sleep: the Japan Environment and Children’s Study. J. Sleep. Res 32, e13911 (2023).
Pulak, L. M. & Jensen, L. Sleep in the intensive care unit: a review. J. Intensive Care Med 31, 14–23 (2016).
Rodríguez, R. G. & Pattini, A. E. Neonatal Intensive Care Unit lighting: update and recommendations. Arch. Argent. Pediatr. 114, 361–367 (2016).
Morag, I. & Ohlsson, A. Cycled light in the Intensive Care Unit for preterm and low birth weight infants. Cochrane Database Syst. Rev. 2016, Cd006982 (2016).
Hazelhoff, E. M., Dudink, J., Meijer, J. H. & Kervezee, L. Beginning to see the light: lessons learned from the development of the Circadian system for optimizing light conditions in the Neonatal Intensive Care Unit. Front Neurosci. 15, 634034 (2021).
Lewis, P., Wild, U., Pillow, J. J., Foster, R. G. & Erren, T. C. A systematic review of chronobiology for Neonatal Care Units: What we know and what we should consider. Sleep. Med Rev. 73, 101872 (2024).
Levy, J. et al. Impact of hands-on care on infant sleep in the Neonatal Intensive Care Unit. Pediatr. Pulmonol. 52, 84–90 (2017).
de Groot, E. R. et al. Evaluation of sleep practices and knowledge in Neonatal Healthcare. Adv. Neonatal Care 23, 499–508 (2023).
de Groot, E. R. et al. Creating an optimal observational sleep stage classification system for very and extremely preterm infants. Sleep. Med 90, 167–175 (2022).
Milh, M. et al. Rapid cortical oscillations and early motor activity in premature human neonate. Cereb. Cortex 17, 1582–1594 (2007).
Whitehead, K., Meek, J. & Fabrizi, L. Developmental trajectory of movement-related cortical oscillations during active sleep in a cross-sectional cohort of pre-term and full-term human infants. Sci. Rep. 8, 17516 (2018).
Mahmoodi, N., Arbabisarjou, A., Rezaeipoor, M. & Pishkar Mofrad, Z. Nurses’ awareness of preterm neonates’ sleep in the NICU. Glob. J. Health Sci. 8, 226–233 (2015).
Ryan, M. A. et al. Nocturnal sleep architecture of preterm infants in the NICU. Infant 16, 209–214 (2020).
Lavanga, M. et al. The effect of early procedural pain in preterm infants on the maturation of Electroencephalogram and Heart Rate Variability. Pain 162, 1556–1566 (2021).
Georgoulas, A. et al. Sleep-wake regulation in preterm and term infants. Sleep 44, zsaa148 (2021).
Ardura, J., Andres, J., Aldana, J. & Revilla, M. A. Development of sleep-wakefulness rhythm in premature babies. Acta Paediatr. 84, 484–489 (1995).
Scher, M. S., Turnbull, J. P., Loparo, K. A., Johnson, M. W. & Holditch-Davis, D. Physiologic brain dysmaturity and neurodevelopmental outcome. Sleep 27, 104–105 (2004).
Peirano, P., Algarín, C. & Uauy, R. Sleep-wake states and their regulatory mechanisms throughout early human development. J. Pediatr. 143, S70–S79 (2003).
Ryan, M. A. J. et al. Sleep state organisation of moderate to late preterm infants in the Neonatal Unit. Pediatr. Res 93, 595–603 (2023).
Bennet, L., Walker, D. W. & Horne, R. S. C. Waking up too early - the consequences of preterm birth on sleep development. J. Physiol. 596, 5687–5708 (2018).
Arditi-Babchuk, H., Feldman, R. & Eidelman, A. I. Rapid Eye Movement (REM) in premature neonates and developmental outcome at 6 months. Infant Behav. Dev. 32, 27–32 (2009).
Périvier, M. et al. Neonatal EEG and neurodevelopmental outcome in preterm infants born before 32 weeks. Arch. Dis. Child Fetal Neonatal Ed. 101, F253–F259 (2016).
Wang, X. et al. Machine learning-derived active sleep as an early predictor of white matter development in preterm infants. J. Neurosci. 44, e1024232023 (2024).
Trickett, J., Hill, C., Austin, T. & Johnson, S. The impact of preterm birth on sleep through infancy, childhood and adolescence and its implications. Children 9, 626 (2022).
Gu, Y., Tang, Y., Chen, X. & Xie, J. Best evidence summary of sleep protection in premature infants in the neonatal intensive care unit: a narrative review. Transl. Pediatr. 13, 946–962 (2024).
Jansson, L. M. & Patrick, S. W. Neonatal abstinence Syndrome. Pediatr. Clin. North Am. 66, 353–367 (2019).
Lust, C. et al. An amplitude-integrated EEG evaluation of neonatal opioid Withdrawal Syndrome. Am. J. Perinatol. (2022).
Rana, D., Pollard, L., Rowland, J., Dhanireddy, R. & Pourcyrous, M. Amplitude-integrated EEG in infants with neonatal abstinence Syndrome. J. Neonatal Perinat. Med 12, 391–397 (2019).
Limjoco, J., Zawadzki, L., Belden, M., Eickhoff, J. & Ikonomidou, C. Amplitude-integrated EEG use in Neonatal Abstinence Syndrome: A pilot study. J. Matern Fetal Neonatal Med 33, 3565–3570 (2020).
Pinto, F., Torrioli, M. G., Casella, G., Tempesta, E. & Fundarò, C. Sleep in babies born to chronically heroin addicted mothers. A follow up study. Drug Alcohol Depend. 21, 43–47 (1988).
Dinges, D. F., Davis, M. M. & Glass, P. Fetal exposure to narcotics: neonatal sleep as a measure of nervous system disturbance. Science 209, 619–621 (1980).
O’Brien, C. M. & Jeffery, H. E. Sleep deprivation, disorganization and fragmentation during opiate withdrawal in newborns. J. Paediatr. Child Health 38, 66–71 (2002).
D’Apolito, K. Comparison of a rocking bed and standard bed for decreasing withdrawal symptoms in drug-exposed infants. MCN Am. J. Matern Child Nurs. 24, 138–144 (1999).
Maichuk, G. T., Zahorodny, W. & Marshall, R. Use of Positioning to reduce the severity of neonatal narcotic withdrawal Syndrome. J. Perinatol. 19, 510–513 (1999).
Carpenter, R. G. et al. Sudden unexplained infant death in 20 regions in Europe: Case Control Study. Lancet 363, 185–191 (2004).
Monnelly, V. J., Hamilton, R., Chappell, F. M., Mactier, H. & Boardman, J. P. Childhood neurodevelopment after prescription of maintenance Methadone for opioid dependency in pregnancy: a systematic review and meta-analysis. Dev. Med. Child Neurol. 61, 750–760 (2019).
Peterson, B. S. et al. Associations of maternal prenatal drug abuse with measures of newborn brain structure, tissue organization, and metabolite concentrations. JAMA Pediatr. 174, 831–842 (2020).
Evans, K. N. et al. Robin sequence: from diagnosis to development of an effective management plan. Pediatrics 127, 936–948 (2011).
Marcus, C. L. et al. Diagnosis and management of childhood obstructive sleep apnea Syndrome. Pediatrics 130, e714–e755 (2012).
Hizal, M. et al. Obstructive sleep apnea in children with Down syndrome: is it possible to predict severe Apnea? Eur. J. Pediatr. 181, 735–743 (2022).
de Sousa, T. V., Marques, I. L., Carneiro, A. F., Bettiol, H. & Freitas, J. A. Nasopharyngoscopy in Robin sequence: clinical and predictive value. Cleft Palate Craniofac. J. 40, 618–623 (2003).
Brockmann, P. E., Schaefer, C., Poets, A., Poets, C. F. & Urschitz, M. S. Diagnosis of obstructive sleep apnea in children: a systematic review. Sleep. Med. Rev. 17, 331–340 (2013).
Coutier, L. et al. The role of sleep laboratory polygraphy in the evaluation of obstructive sleep apnea syndrome in robin infants. Sleep. Med 72, 59–64 (2020).
Lim, K. et al. Should obstructive hypopneas be included when analyzing sleep studies in infants with Robin sequence? Sleep. Med 98, 9–12 (2022).
Sullivan, N. A. T. et al. Differences in analysis and treatment of upper airway obstruction in Robin sequence across different countries in Europe. Eur. J. Pediatr. 182, 1271–1280 (2023).
Kukkola, H. K., Vuola, P., Seppa-Moilanen, M., Salminen, P. & Kirjavainen, T. Pierre Robin sequence causes position-dependent obstructive sleep apnoea in infants. Arch. Dis. Child 106, 954–960 (2021).
Poets, C. F. & Wiechers, C. Reappraising Prone positioning for infants with Robin Sequence: A cautionary tale. Arch. Dis. Child 106, 933–934 (2021).
Choo, H. et al. Disruptive therapy using a nonsurgical orthodontic airway plate for the management of neonatal Robin Sequence: 1-year follow-up. Cleft Palate Craniofac J. 60, 758–767 (2023).
Wiechers, C. et al. Sleep and neurocognitive outcome in primary school children with Robin Sequence. Sleep 46, zsac317 (2023).
Wiechers, C. et al. Retrospective study on growth in infants with isolated Robin sequence treated with the Tuebingen palate plate. Orphanet J. Rare Dis. 16, 338 (2021).
Kahn, A. et al. Polysomnographic studies of infants who subsequently died of sudden infant Death Syndrome. Pediatrics 82, 721–727 (1988).
Kato, I. et al. Incomplete arousal processes in infants who were victims of sudden death. Am. J. Respir. Crit. Care Med. 168, 1298–1303 (2003).
Nino, G. et al. Defining age-related OSA features in Robin sequence using polysomnographic-based analyses of respiratory arousal responses and gas-exchange parameters. Cleft Palate Craniofac. J. 60, 142–150 (2023).
Fauroux, B., Cozzo, M., MacLean, J. & Fitzgerald, D. A. Osa Type-III and neurocognitive function. Paediatr. Respir. Rev. 53, 39–43 (2024).
Naros, A. et al. Neurocognitive development in isolated Robin sequence treated with the Tuebingen palatal plate. Clin. Oral. Investig. 26, 4817–4823 (2022).
Hermans, T. et al. Functional brain maturation and sleep organisation in neonates with congenital heart disease. Eur. J. Paediatr. Neurol. 36, 115–122 (2022).
Ykeda, D. S., Lorenzi-Filho, G., Lopes, A. A. & Alves, R. S. Sleep in infants with congenital heart disease. Clinics 64, 1205–1210 (2009).
Gui, J. et al. Peri- and post-operative amplitude-integrated Electroencephalography in infants with congenital heart disease. Indian Pediatr. 57, 133–137 (2020).
ter Horst, H. J., Mud, M., Roofthooft, M. T. & Bos, A. F. Amplitude integrated Electroencephalographic activity in infants with congenital heart disease before surgery. Early Hum. Dev. 86, 759–764 (2010).
Claessens, N. H. P. et al. Amplitude-integrated Electroencephalography for early recognition of brain injury in neonates with critical congenital heart disease. J. Pediatr. 202, 199–205.e191 (2018).
Mulkey, S. B. et al. Amplitude-integrated EEG in newborns with critical congenital heart disease predicts preoperative brain magnetic resonance imaging findings. Pediatr. Neurol. 52, 599–605 (2015).
Padiyar, S. et al. Continuous Electroencephalography (CEEG) in infants with congenital heart disease (CHD). Pediatr. Res. 94, 715–723 (2023).
Latal, B. et al. Postoperative amplitude-integrated electroencephalography predicts four-year neurodevelopmental outcome in children with complex congenital heart disease. J. Pediatr. 178, 55–60.e51 (2016).
Mebius, M. J. et al. Amplitude-integrated Electroencephalography during the First 72 H after birth in neonates diagnosed prenatally with congenital heart disease. Pediatr. Res 83, 798–803 (2018).
Gunn, J. K., Beca, J., Hunt, R. W., Olischar, M. & Shekerdemian, L. S. Perioperative amplitude-integrated EEG and neurodevelopment in infants with congenital heart disease. Intensive Care Med 38, 1539–1547 (2012).
Hiatt, P. W., Mahony, L. & Tepper, R. S. Oxygen desaturation during sleep in infants and young children with congenital heart disease. J. Pediatr. 121, 226–232 (1992).
Stamm, R. W. et al. Clinically asymptomatic sleep-disordered breathing in infants with single-ventricle physiology. J. Pediatr. 218, 92–97 (2020).
Combs, D. et al. Osa and neurocognitive impairment in children with congenital heart disease. Chest 158, 1208–1217 (2020).
Steiner, M. et al. Outcome prediction in neonatal hypoxic-ischaemic encephalopathy using neurophysiology and neuroimaging. Neonatology 119, 483–493 (2022).
Goeral, K. et al. Prediction of outcome in neonates with hypoxic-ischemic Encephalopathy Ii: Role of amplitude-integrated Electroencephalography and cerebral oxygen saturation measured by near-infrared Spectroscopy. Neonatology 112, 193–202 (2017).
Thoresen, M., Hellström-Westas, L., Liu, X. & de Vries, L. S. Effect of Hypothermia on amplitude-integrated Electroencephalogram in infants with Asphyxia. Pediatrics 126, e131–e139 (2010).
ter Horst, H. J. et al. Prognostic significance of amplitude-integrated EEG during the first 72 h after birth in severely asphyxiated neonates. Pediatr. Res. 55, 1026–1033 (2004).
Osredkar, D. et al. Sleep-wake cycling on amplitude-integrated Electroencephalography in term newborns with Hypoxic-ischemic Encephalopathy. Pediatrics 115, 327–332 (2005).
Takenouchi, T. et al. Delayed onset of sleep-wake cycling with favorable outcome in hypothermic-treated neonates with Encephalopathy. J. Pediatr. 159, 232–237 (2011).
Abramsky, R., Stavsky, M., Novack, V. & Shany, E. Appearance of sleep cycling after birth in term neonates: an electro-physiologic study. Pediatr. Res. 87, 711–715 (2020).
Scher, M. S., Steppe, D. A., Beggarly, M. E., Salerno, D. G. & Banks, D. L. Neonatal EEG-sleep disruption mimicking Hypoxic-ischemic Encephalopathy after Intrapartum Asphyxia. Sleep. Med 3, 411–415 (2002).
Shankaran, S. et al. Whole-body Hypothermia for neonates with hypoxic-ischemic Encephalopathy. N. Engl. J. Med. 353, 1574–1584 (2005).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic Encephalopathy. Cochrane Database Syst. Rev. 2013, Cd003311 (2013).
Meder, U. et al. Longitudinal analysis of amplitude-integrated electroencephalography for outcome prediction in hypoxic-ischemic Encephalopathy. J. Pediatr. 246, 19–25.e15 (2022).
Csekő, A. J. et al. Accuracy of amplitude-integrated Electroencephalography in the prediction of neurodevelopmental outcome in asphyxiated infants receiving hypothermia treatment. Acta Paediatr. 102, 707–711 (2013).
Massaro, A. N. et al. AEEG evolution during therapeutic hypothermia and prediction of nicu outcome in Encephalopathic neonates. Neonatology 102, 197–202 (2012).
Murray, D. M., Boylan, G. B., Ryan, C. A. & Connolly, S. Early EEG findings in hypoxic-ischemic Encephalopathy predict outcomes at 2 years. Pediatrics 124, e459–e467 (2009).
Pavel, A. M. et al. Machine learning for the early prediction of infants with electrographic seizures in neonatal hypoxic-ischemic Encephalopathy. Epilepsia 64, 456–468 (2023).
Nyman, J. et al. Poor AEEG background recovery after perinatal hypoxic ischemic encephalopathy predicts postneonatal epilepsy by age 4 years. Clin. Neurophysiol. 143, 116–123 (2022).
Ding, X. et al. Distinctive sleep problems in children with perinatal moderate or mild hypoxic-ischemia. Neurosci. Lett. 614, 60–64 (2016).
Zareen, Z. et al. An observational study of sleep in childhood post-neonatal Encephalopathy. Acta Paediatr. 110, 2352–2356 (2021).
Adzick, N. S. et al. A randomized trial of prenatal versus postnatal repair of Myelomeningocele. N. Engl. J. Med. 364, 993–1004 (2011).
Farmer, D. L. et al. The management of Myelomeningocele study: full cohort 30-month pediatric outcomes. Am. J. Obstet. Gynecol. 218, e1–256 (2017).
Waters, K. A. et al. Sleep-disordered breathing in children with Myelomeningocele. J. Pediatrics 132, 672–681 (1998).
Alsaadi, M. M., Iqbal, S. M., Elgamal, E. A. & Gozal, D. Sleep-disordered breathing in children with Chiari malformation Type Ii and Myelmeningocele. Pediatr. Int. 54, 623–626 (2012).
Jernigan, S. C. et al. Risk factors of sudden death in young adult patients with Myelomeningocele. J. Neurosurg. Pediatr. 9, 149–155 (2012).
Shellhaas, R. A. et al. Sleep-disordered breathing among newborns with Myelomeningocele. J. Pediatr. 194, 244–247 (2018).
Moldenhauer, J. S. & Adzick, N. S. Fetal surgery for Myelomeningocele: After the management of Myelomeningocele Study (Moms). Semin Fetal Neonatal Med 22, 360–366 (2017).
Davidson Ward, S. L., Jacobs, R. A., Gates, E. P., Hart, L. D. & Keens, T. G. Abnormal ventilatory patterns during sleep in infants with Myelomeningocele. J. Pediatrics 109, 631–634 (1986).
Bendel-Stenzel, E., Linabery, A., Jorgenson, A., Ferrara, T. B. & Spaulding, A. B. Sleep-disordered breathing: an under-recognized problem in infants with 1 Myelomeningocele defects regardless of timing of repair. J. Perinatol. (2019).
Walker W. O. Jr & Perez I. A. Sleep Related Breathing Disorders. https://www.spinabifidaassociation.org/resource/sleep-related-disorders/.
Mellies, U. et al. Daytime predictors of sleep disordered breathing in children and adolescents with neuromuscular disorders. Neuromuscul. Disord. 13, 123–128 (2003).
Pinard, J. M. et al. Sleep-disordered breathing in children with congenital muscular dystrophies. Eur. J. Paediatr. Neurol. 16, 619–624 (2012).
Zambon, A. A. et al. Respiratory function and sleep disordered breathing in pediatric duchenne muscular dystrophy. Neurology 99, e1216–e1226 (2022).
Senel, G. B. et al. Obstructive sleep apnea syndrome and autonomic dysfunction in duchenne muscular dystrophy. Sleep. Breath. 25, 941–946 (2021).
Cheminelle, M. et al. Respiratory function and sleep in children with myotonic dystrophy Type 1. Neuromuscul. Disord. 33, 263–269 (2023).
MacKintosh, E. W. et al. Referral indications and prevalence of sleep abnormalities in children with early onset Scoliosis. Spine Deform 8, 523–530 (2020).
Jon, C., Mosquera, R. A., Mitchell, S. & Mazur, L. J. Obstructive sleep apnoea and arthrogryposis. BMJ Case Rep. 2014, bcr2013201638 (2014).
Frohlich, M. et al. Daytime predictors of nocturnal hypercapnic hypoventilation in children with neuromuscular disorders. Pediatr. Pulmonol. 57, 1497–1504 (2022).
Nguyen, D. B. et al. Sleep-disordered breathing and its management in children with rare skeletal Dysplasias. Am. J. Med. Genet. A 185, 2108–2118 (2021).
Goffinski, A. et al. Obstructive sleep apnea in young infants with Down Syndrome evaluated in a Down Syndrome Specialty Clinic. Am. J. Med Genet A 167a, 324–330 (2015).
Stebbens, V. A., Dennis, J., Samuels, M. P., Croft, C. B. & Southall, D. P. Sleep related upper airway obstruction in a cohort with Down’s Syndrome. Arch. Dis. Child 66, 1333–1338 (1991).
Chamseddin, B. H., Johnson, R. F. & Mitchell, R. B. Obstructive sleep apnea in children with Down Syndrome: Demographic clinical, and polysomnographic features. Otolaryngol. Head. Neck Surg. 160, 150–157 (2019).
Lal, C., White, D. R., Joseph, J. E., van Bakergem, K. & LaRosa, A. Sleep-disordered breathing in Down Syndrome. Chest 147, 570–579 (2015).
Chawla, J. K., Bernard, A., Heussler, H. & Burgess, S. Sleep, function, behaviour and cognition in a cohort of children with Down Syndrome. Brain Sci. 11, 1317 (2021).
Friedman, N. R., Ruiz, A. G., Gao, D. & Ingram, D. G. Accuracy of parental perception of nighttime breathing in children with Down Syndrome. Otolaryngol. Head. Neck Surg. 158, 364–367 (2018).
Bull, M. J. et al. Health supervision for children and adolescents with Down Syndrome. Pediatrics 149, e2022057010 (2022).
Linz, A. et al. Treatment of obstructive sleep apnea in infants with Trisomy 21 using oral appliances. Cleft Palate Craniofac J. 50, 648–654 (2013).
von Lukowicz, M. et al. Effect of a 1-Week Intense myofunctional training on obstructive sleep apnoea in children with Down Syndrome. Arch. Dis. Child 104, 275–279 (2019).
Gastelum, E. et al. Treatment considerations for obstructive sleep apnea in pediatric Down Syndrome. Children 8, 1074 (2021).
Duis, J. et al. Diagnosis and management of sleep disorders in Prader-Willi Syndrome. J. Clin. Sleep. Med. 18, 1687–1696 (2022).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Consortia
Contributions
A.C.N. drafted the initial manuscript together with C.F.P., M.Q. and R.A.S. M.Q. and R.A.S. guided the drafting of the initial article and reviewed it critically for important intellectual content. All authors approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Neukamm, AC., Quante, M., Poets, C.F. et al. The impact of sleep in high-risk infants. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04049-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-025-04049-2