Abstract
Depression affects around 10% of the Swiss population. While SSRIs are commonly prescribed, only 30–40% of patients achieve remission. Pharmacogenetic (PGx) factors may explain part of this high rate of SSRI treatment failure. This study examined antidepressant (AD) switching among Swiss patients using escitalopram, focusing on whether they switched to ADs with PGx dosing guidelines (PGx AD) or ADs without PGx dosing guidelines (non-PGx ADs). Data from Swiss health insurance records identified 41 275 patients who used escitalopram between July 2020 and June 2022. While 6.4% (n = 2 638) switched to another antidepressant, only 35.4% of these opted for a PGx AD. Men, younger adults showed higher switching rates, whereas patients on antipsychotic medications switched less. Individuals younger than 20 years old and women were more likely to switch to PGx AD whereas the elderly were less likely to switch to PGx AD.
Similar content being viewed by others
Introduction
Depression is a mental health condition affecting approximately 10% of the Swiss population. The chronic and recurrent nature of depression necessitates effective long-term management [1]. Selective serotonin reuptake inhibitors (SSRIs) represent a fundamental element of the pharmacological management of major depressive disorder (MDD). In many guidelines, SSRIs are considered the first-line AD drug class, although some associations are moving towards patient individualization, such as the Association of Scientific Medical Societies (AWMF) [2,3,4,5]. However, treatment outcomes are often suboptimal, with 30–50% of patients not responding adequately to AD treatment. As a result, many patients experience adverse effects or an insufficient therapeutic response, leading clinicians to consider switching patients to alternative ADs [6,7,8]. A study conducted in the United Kingdom revealed that approximately 9.3% of patients initiating treatment with escitalopram required a switch to another AD [9].
One of the reasons for an inadequate response to escitalopram could be the genetic make-up of the patient. The metabolism of escitalopram and other SSRIs is catalysed by cytochrome P450 enzymes; particularly CYP2C19 and CYP2D6 are of relevance in the metabolism of SSRIs [10]. These enzymes exhibit significant inter-individual variability due to genetic polymorphisms, which can influence drug metabolism and, consequently, the efficacy and tolerability of substrate drugs [10, 11]. In accordance with this assumption, Jukić et al. found that patients categorized as poor metabolizers of CYP2C19 switched from escitalopram 3.3 times more frequently than those with normal metabolic capacity [12].
To the best of our knowledge, AD switching patterns on a population level have not yet been assessed in Switzerland. Not all ADs are affected by PGx and therefore do not have PGx dosing guidelines [10, 13,14,15,16]. The primary objective of this study was to examine the switching pattern of escitalopram to alternative ADs and to compare the rates of switching to PGx AD versus non-PGx ADs in the Swiss population. Rather than aiming to evaluate treatment effects or clinical decision-making at the individual level, the study sought to generate population-level insights into current prescribing practices in the Swiss healthcare context.
Methods
Study design and data source
We conducted a descriptive, retrospective study using claims data from Helsana Group, a Swiss health insurance company that covers approximately 15% of the Swiss population across all age groups. Helsana Group claims data include information on outpatient drug claims, categorized according to the World Health Organization’s Anatomical Therapeutic Chemical (ATC) classification system, as well as demographic information such as canton of residence, year of birth, and sex. However, clinical information, lifestyle factors such as smoking status and weight, use of over-the-counter medications, and treatment indications were not available in our anonymized dataset. The Helsana Group database has already been used in various drug safety and utilization studies [17,18,19,20].
Study population
The study period ranged from July 1, 2020, to June 30, 2022. This study focused on escitalopram, the most frequently claimed AD in 2021. Our study population included all individuals with claims for escitalopram and at least one other medication for AD during the specified period.
Antidepressants
We identified AD claims using the ATC codes N06AA (non-selective monoamine reuptake inhibitors or tricyclic ADs), N06AB (selective serotonin reuptake inhibitors), code), N06AF + N06AG (monoamine oxidase inhibitors (MAOIs)), and N06AX (other ADs) that subsume nonuniform underlying mechanisms of action (atypical ADs), such as serotonin-norepinephrine reuptake inhibitors (SNRIs) and tetracyclic ADs. We classified the respective AD as “pharmacogenetic” if any dosing guidelines were available in the Pharmacogenetic Knowledge Base (PharmGKB) in August 2024 [21]. The presence of a dosing guideline in PharmGKB corresponds to the highest evidence level (Level 1 A), which indicates variant-specific prescribing guidance in a current clinical guideline or FDA-approved drug label, supported by at least one additional peer-reviewed publication [22]. Based on this criterion, we identified dosing recommendations for the following 12 ADs: imipramine, clomipramine, trimipramine, amitriptyline, nortriptyline, doxepin, citalopram, paroxetine, sertraline, fluvoxamine, escitalopram, and venlafaxine. We excluded the lowest strengths for trazodone (50 mg) and mirtazapine (15 mg) because these are mostly prescribed for insomnia. Also, we excluded amitriptyline (10 mg) because it is mostly prescribed for neuropathic pain.
Treatment change
We defined a potential switch in AD therapy as the co-occurrence of an escitalopram claim and a claim for another AD within a 100-day window. To identify switches, we analysed all AD claims for each patient. For each claims date of escitalopram, we checked whether a claim for a different AD was recorded within a 100-day period after the given date. This timeframe reflects the common practice in Switzerland of dispensing drugs for three-month periods, with an allowance for some potential non-adherence. If this co-occurred once during the study period, we checked if the next claim was again the new AD to classify whether it was a real switch or if it was a one-time prescription of another AD. We categorized cases with multiple overlaps during the 100-day window as combination therapies, except for cases in which switching to an AD required a combination of multiple strengths to achieve the correct dosage. We focused only on the first switch if more than one switch occurred during the study period.
Drugs used for other conditions
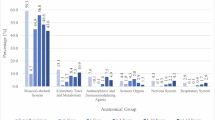
Seven drug categories were identified using ATC codes: drugs used for cardiovascular diseases (B01AA, B01AC, C01, C04A, C02, C07, C08, and C09), diabetes mellitus (A10A, A10B, A10X), cancer (L01), epilepsy (N03), psychoses (N05A), pain (N02A, N02B), and respiratory illnesses (R03). We chose these categories because these drug classes are associated with side effects and interactions of ADs, and the indications for these drugs are associated with depression.
Statistical analyses
We calculated the absolute and relative numbers of patients who switched from escitalopram to alternative ADs during the study period stratified by age, sex and region. Regional variables included the major regions of Switzerland, which are the reference areas defined by the Swiss Federal Office of Statistics (Zurich, Espace Mittelland, Lemanic region, Northwestern Switzerland, Eastern Switzerland, Ticino, and Central Switzerland) of the Swiss Confederation at the cantonal level. We ranked ADs according to the number of patients who switched to these ADs. Additionally, we calculated the number of patients switching each month and the proportion of patients switching to PGx AD or non-PGx AD.
For each drug category used for other conditions, we calculated the prevalence by dividing the number of individuals with at least one medication prescription in one of the defined ATC groups by the total number of patients during the study period. Age at the beginning of the study period was considered in all the calculations. We reported the frequencies and proportions as absolute numbers and percentages of samples.
We employed multivariable logistic regression to examine the factors associated with AD switching. Independent variables included in the regression model were age groups ( ≤ 19, 20–39, 40–64, 65–79, ≥80 years), sex (female or male), residence area (Zurich, Espace Mittelland, Lemanic region, Northwestern Switzerland, Eastern Switzerland, Ticino, Central Switzerland), claiming other drugs (yes/no), and the number of drug categories used for other conditions. We calculated the estimates as odds ratios (ORs) with 95% confidence intervals (CIs). To address the possibility of overfitting or chance findings, we performed a sensitivity analysis in which we re-estimated the logistic regression model including only age group, sex, and the use of antipsychotic medication. We extrapolated our results to the Swiss population in Appendix Tables 1 and 2. The extrapolation factor (ef) is based on a person’s age, gender, and canton of residence. It is provided annually and individually by the joint facility KVG (‘Krankenversicherungsgesetz’). The ef is used for risk balancing among Swiss mandatory basic health insurances and is therefore based on the total number of insured persons of all Swiss insurance companies [23]. All statistical analyses were performed using SAS (version 9.4; SAS Institute Inc., Cary, NC, USA). In accordance with the Swiss Federal Law on Data Protection (Article 22), ethics approval was not required, as the study was retrospective and the data were anonymized [24]. Statistical significance was set at P < 0.05.
Large language models
We used Deepl Write (Deepl SE, Germany) for finale language editing.
Results
Description of the study population
We identified 41 275 individuals who claimed at least one prescription for escitalopram during the study period. Of those 16 581 individuals aged 4 −103 years (mean 56.0 year ± 20.8 years) claimed at least one prescription for escitalopram and another AD during the study period, qualifying them as potential switchers. The study population comprised 66.7% women. The majority of the study population was 40 years or older with the largest age group being 40–64 years (39.9%). More than 95% of the study population had in addition to the prescribed AD at least one prescription for drugs from another drug class, the top 3 being drugs for pain, cardiovascular drugs, and drugs to treat psychoses. Every 7th person (15.9%) in our study population (i.e. people who claimed escitalopram and at least one other medication for AD) switched from escitalopram to another AD during the study period. In relation to all escitalopram users - irrespective of whether or not they also claimed another AD drug - 6.4% switched. Details on the characteristics of the study population stratified by escitalopram switching status are provided in Table 1 and Appendix Table 1.
Switching behaviour
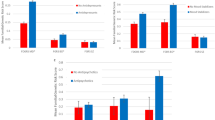
Among those who switched, 15.9% switched to another SSRI, 10.0% to tricyclic AD (TCA), and 74.1% switched to other ADs. Most switches (61.6%) occurred to Mirtazapine, Duloxetine, Venlafaxine, Trimipramine and Amitriptyline. Only 35.4% of patients switched to another PGx AD. In 77.9% of the patients who switched to another SSRI, the patient switched to a PGx SSRI. No switches to PGx TCA were observed. While men were more likely to switch to another AD in general, women were more likely to switch to a PGx AD, yielding an OR of 1.16 (95% CI 1.06–1.26) and of 0.78 (95% CI 0.65, 0.93), respectively. People with a higher number of concomitant medications were more likely to switch to a PGx AD than people without (OR 1.75, 95% CI 0.83–3.82), although results were not statistically significant. Concomitant medication was not associated with switching in general (see Tables 2 and 3 and Appendix Tables 2 for the detailed switching characteristics). The sensitivity analysis showed that the direction and statistical significance of the primary associations remained unchanged, suggesting that the results are robust to model simplification. The number of patients who switched during the year ranged from 167 to 279 per month, with the fewest switches observed in February and the most in June. Figure 1 illustrates the monthly distribution of patient switches throughout the year
Discussion
In this study, we assessed the percentage of people switching from escitalopram to other ADs in the Swiss population. Based on extrapolations from the Helsana Group population in this study, which included 15% of the Swiss population, we estimated that 15.8% of the Swiss population who claimed escitalopram and at least one additional AD switched during 2021. In relation to all escitalopram users, 6.4% switched. Based on our analysis most patients switched from escitalopram to non-PGx ADs. The factors associated with switching were male sex, living in northwest Switzerland, and age between 20 and 39 years. Women were more likely to switch to PGx ADs than men were.
Our findings align with those of previous studies, which reported similar switching behaviors and rates among escitalopram users [9, 25,26,27]. The majority of switches from SSRIs were to other AD classes than SSRIs, a trend also noted in other studies, where venlafaxine and other non-SSRI agents were common alternatives following treatment failure [27,28,29]. Like in other studies, patients in our study most commonly switched from escitalopram to mirtazapine, duloxetine and venlafaxine [9, 28]. In our study, we observed no switch from escitalopram to non-PGx TCAs, possibly because non-PGx TCAs were rarely prescribed. Our study population exhibited demographic characteristics similar to those observed in other studies on antidepressant use, including a higher prevalence among women and older adults, as previously shown in a study using Helsana Group data [30].
Comparing the factors associated with switching in our study to those in other studies, we found that men and younger adults (aged 20–39 years), which partially mirrors findings from similar studies that also reported higher odds for switching among younger adults [9, 27, 29, 31]. We also observed a higher propensity of women to switch to PGx ADs, whereas older adults tend to switch less to PGx ADs, which has both not been assessed in previous studies [9, 27, 29, 31, 32].
Regarding pharmacogenetics, CYP2C19 slow and ultrarapid metabolizers switch ADs more frequently than do normal metabolizers [12, 32, 33]. In Switzerland, the prevalence of poor and (ultra)rapid metabolizers is 3% and 30%, respectively [34]. We did not have information on the genotype of the registered persons and were unable to assess whether ultrarapid or slow metabolizers were more prevalent among switchers. Whether the genetic predisposition of CYP2C19 affects AD switching remains unclear, as a meta-analysis showed that some studies found no impact of metabolic phenotypes on escitalopram efficacy [35]. While some of the observed switches may align with pharmacogenetically informed decisions, it is equally likely that they reflect prescribers’ adherence to clinical guidelines, patient tolerability, or institutional prescribing habits. These inconsistencies, due to methodological differences, highlight the need for further research on PGx-guided AD prescription and its real-world effectiveness.
A key strength of our study is the use of a large health insurance claims database that captures data from approximately 15% of the total Swiss population, thereby providing a substantial level of representativeness. Another strength is the inclusion of various sources for PGx recommendations to allow a comprehensive analysis, while restricting the analysis to drugs with PGx guidelines to ensure that they are relevant for current clinical decision-making. Our analysis relies on claims data and is subject to the limitations inherent in this type of data. The main limitation of this study was that the claims data did not provide genetic information or information about drug efficacy or tolerance. As a result, the reason for switching ADs remains unknown. Additionally, we had no information regarding OTC use or drug administration in hospitals. Therefore, the results for drugs that are also available as OTC drugs, as well as drugs prescribed in primary care but typically used in hospitals, will be underestimated. Regarding ADs, this should not be a major limitation because most depressed individuals are treated as outpatients and only hypericum herba is available OTC [36]. In Switzerland, there is a certain (small) number of out-of-pocket payments on an annual basis before health care insurance reimburses the bills. As out-of-pocket payments affect acute medication more than chronic medication, we may have missed a small number of drug claims, leading to a slight underestimation of acute medication. Additionally, the Helsana Group database does not provide information about the indication of the claimed ADs; therefore, we cannot ascertain whether ADs were indeed used for depression or other conditions.
Conclusions
To the best of our knowledge, this is the first study to assess the patterns of AD switching including PGx factors using Swiss health care claims data at a population level. We found a switching rate of 6.4%, mostly from escitalopram to a non-PGx AD. To gain more insight into the influence of PGx on AD switching, further studies including genetic information are needed.
Data availability
The datasets analysed and/or generated during the current study are not available for the public due to confidentiality requirements issued by Helsana Group. Datasets and analysis codes can be made available by the corresponding author (s.allemann@unibas.ch) upon reasonable request and with permission of Helsana Group.
References
Bundesamt für Statistik. Schweizerische Gesundheitbefragung 2022 - Übersicht. 2023;1–28.
Kapur N, Chew-Graham C, Clark J, Ekers D, Gangadharan SK, Gupta T, et al. Depression in adults: treatment and management NICE guideline. 2022 [cited 2024 Sep 23]; Available from: www.nice.org.uk/guidance/ng222
Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), Arbeitsgemeinschaft der Wissenschaftli- chen Medizinischen Fachgesellschaften (AWMF). Nationale VersorgungsLeitlinie Unipolare Depression – Langfassung, Version 3.2. 2022 [cited: 2024 Oct 30]. https://doi.org/10.6101/AZQ/000505. www.leitlinien.de/depression.
MediX. Depression [Internet]. [cited 2024 Oct 30]. Available from: https://www.medix.ch/wissen/guidelines/depression/
Mednet Bern. Guideline Depression. [cited 2024 Oct 30]; Available from: http://www.dgppn.de/fileadmin/user_upload/_medien/download/pdf/kurzversion-leitlinien/s3-nvl-unipolare-depression-lf.pdf
Walter HJ, Abright AR, Bukstein OG, Diamond J, Keable H, Ripperger-Suhler J, et al. Clinical practice guideline for the assessment and treatment of children and adolescents with major and persistent depressive disorders. J Am Acad Child Adolesc Psychiatry. 2023;62:479–502. Available from: https://pubmed.ncbi.nlm.nih.gov/36273673/
Middleton H, Shaw I, Hull S, Feder G. NICE guidelines for the management of depression. BMJ. 2005;330:267–8. Available from: https://pubmed.ncbi.nlm.nih.gov/15695252/
Tsermpini EE, Serretti A, Dolžan V. Precision medicine in antidepressants treatment. Handb Exp Pharmacol. 2023;280:131–86. Available from: https://pubmed.ncbi.nlm.nih.gov/37195310/
Mars B, Heron J, Gunnell D, Martin RM, Thomas KH, Kessler D. Prevalence and patterns of antidepressant switching amongst primary care patients in the UK. J Psychopharmacol. 2017;31:553–60. Available from: https://pubmed.ncbi.nlm.nih.gov/28460603/
Bousman CA, Stevenson JM, Ramsey LB, Sangkuhl K, Hicks JK, Strawn JR, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A Genotypes and Serotonin Reuptake Inhibitor Antidepressants. Clin Pharmacol Ther. 2023;114:51–68. Available from: https://pubmed.ncbi.nlm.nih.gov/37032427/
Milosavljević F, Bukvić N, Pavlović Z, Miljević Č, Pešić V, Molden E, et al. Association of CYP2C19 and CYP2D6 poor and intermediate metabolizer status with antidepressant and antipsychotic exposure: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78:270–80. Available from: https://pubmed.ncbi.nlm.nih.gov/33237321/
Jukić MM, Haslemo T, Molden E, Ingelman-Sundberg M. Impact of CYP2C19 genotype on escitalopram exposure and therapeutic failure: a retrospective study based on 2087 patients. Am J Psychiatry. 2018;175:463–70. Available from: www.pharmvar.org
Brouwer JMJL, Nijenhuis M, Soree B, Guchelaar HJ, Swen JJ, van Schaik RHN, et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2C19 and CYP2D6 and SSRIs. Eur J Hum Genet. 2022;30:1114–20.
Beunk L, Nijenhuis M, Soree B, de Boer-Veger NJ, Buunk A-M, Guchelaar H-J, et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2D6, CYP2C19 and non-SSRI/non-TCA antidepressants. [cited 2024 Jul 4];15:17. Available from: https://doi.org/10.1038/s41431-024-01648-1
Hicks JK, Bishop JR, Sangkuhl K, Muller DJ, Ji Y, Leckband SG, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98:127–34. Available from: https://pubmed.ncbi.nlm.nih.gov/25974703/
Hicks JK, Sangkuhl K, Swen JJ, Ellingrod VL, Müller DJ, Shimoda K, et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharmacol Ther. 2017;102:37–44. Available from: https://pubmed.ncbi.nlm.nih.gov/27997040/
Wittwer NL, Meier CR, Huber CA, Schwabedissen HEMZ, Allemann S, Schneider C. Utilization of drugs with pharmacogenetic dosing recommendations in Switzerland: a descriptive study using the helsana database. Pharmgenomics Pers Med. 2022;15:967–76. Available from: https://pubmed.ncbi.nlm.nih.gov/36447837/
Spoendlin J, Blozik E, Graber SM, Rauch M, Marxer CA, Rüegg S, et al. Use of valproate in pregnancy and in women of childbearing age between 2014 and 2018 in Switzerland: a retrospective analysis of Swiss healthcare claims data. Swiss Med Wkly. 2021;151:w20386. Available from: https://smw.ch/index.php/smw/article/view/2936
Daphne R, Nadine S, Sibylle T, Eva B, Mathias F, Andri S, et al. Utilisation patterns and costs of lipid-lowering drugs in Switzerland 2013–2019. Swiss Med Wkly. 2021;151:1–8.
Schneider R, Reinau D, Schur N, Blozik E, Früh M, Signorell A, et al. Coverage rates and timeliness of nationally recommended vaccinations in Swiss preschool children: a descriptive analysis using claims data. Vaccine. 2020;38:1551–8. Available from: https://doi.org/10.1016/j.vaccine.2019.11.057.
Whirl-Carrillo M, Huddart R, Gong L, Sangkuhl K, Thorn C, Whaley R. An evidence‐based framework for evaluating pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2021;110:563–72.
Whirl-Carrillo M, Huddart R, Gong L, Sangkuhl K, Thorn CF, Whaley R, et al. An evidence-based framework for evaluating pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2021;110:563–72.
Circulars and statistics | Gemeinsame Einrichtung KVG [Internet]. [cited 2024 Nov 20]. Available from: https://www.kvg.org/en/insurer/risk-compensation/circulars-statistics/
Fedlex [Internet]. Fed. Act Data Prot. 2019. Available from: https://fedlex.admin.ch/eli/cc/1993/1945_1945_1945/en
Andersson Sundell K, Petzold MG, Wallerstedt SM. Factors associated with switching and combination use of antidepressants in young Swedish adults. Int J Clin Pract. 2013;67:1302. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC4282276/
Ball S, Classi P, Dennehy EB. What happens next?: a claims database study of second-line pharmacotherapy in patients with major depressive disorder (MDD) who initiate selective serotonin reuptake inhibitor (SSRI) treatment. Ann Gen Psychiatry. 2014;13:8 https://pubmed.ncbi.nlm.nih.gov/24645830/
Milea D, Guelfucci F, Bent-Ennakhil N, Toumi M, Auray JP. Antidepressant monotherapy: a claims database analysis of treatment changes and treatment duration. Clin Ther. 2010;32:2057–72. https://pubmed.ncbi.nlm.nih.gov/21118742/
Connolly KR, Thase ME. If at first you don’t succeed: a review of the evidence for antidepressant augmentation, combination and switching strategies. Drugs. 2011;71:43–64. https://pubmed.ncbi.nlm.nih.gov/21175239/
Saragoussi D, Chollet J, Bineau S, Chalem Y, Milea D. Antidepressant switching patterns in the treatment of major depressive disorder: a General Practice Research Database (GPRD) Study. Int J Clin Pract. 2012;66:1079–87. https://pubmed.ncbi.nlm.nih.gov/23067031/
Haller E, Watzke B, Blozik E, Rosemann T, Reich O, Huber CA, et al. Antidepressant prescription practice and related factors in Switzerland: a cross-sectional analysis of health claims data. BMC Psychiatry. 2019;19:196. https://doi.org/10.1186/s12888-019-2178-4
Marcus SC, Hassan M, Olfson M. Antidepressant switching among adherent patients treated for depression. Psychiatr Serv. 2009;60:617–23. https://pubmed.ncbi.nlm.nih.gov/19411348/
Wong WLE, Fabbri C, Laplace B, Li D, van Westrhenen R, Lewis CM, et al. The Effects of CYP2C19 Genotype on Proxies of SSRI Antidepressant Response in the UK Biobank. Pharmaceuticals (Basel). 2023;16:1277. https://pubmed.ncbi.nlm.nih.gov/37765085/
Mahajna M, Abu Fanne R, Berkovitch M, Tannous E, Vinker S, Green I, et al. Effect of CYP2C19 pharmacogenetic testing on predicting citalopram and escitalopram tolerability and efficacy: a retrospective, longitudinal cohort study. Biomedicines. 2023;11:3245. Available from: https://pubmed.ncbi.nlm.nih.gov/38137466/
Hodel F, De Min MB, Thorball CW, Redin C, Vollenweider P, Girardin F, et al. Prevalence of actionable pharmacogenetic variants and high-risk drug prescriptions: a Swiss hospital-based cohort study. Clin Transl Sci. 2024;17:70009. www.cts-journal.com
Li D, Pain O, Fabbri C. Metabolic activity of CYP2C19 and CYP2D6 on antidepressant response from 13 clinical studies using genotype imputation: a meta-analysis. Transl Psychiatry. 2024;14:296.
Barkil-Oteo A. Collaborative care for depression in primary care: how psychiatry could “Troubleshoot” current treatments and practices. Yale J Biol Med. 2013;86:139. https://pmc.ncbi.nlm.nih.gov/articles/PMC3670434/
Acknowledgements
This study was not sponsored.
Funding
Open access funding provided by University of Basel.
Author information
Authors and Affiliations
Contributions
Conceptualization, MR, CM, CH, HM, SA, CS; methodology, MR, CS, CM; software, MR; validation MR, CM, CH, HM, SA, CS; formal analysis, MR; investigation, MR; resources, CM, CH; data curation, MR; writing—original draft preparation, MR; writing—review and editing CM, CH, HM, SA, CS; visualization, MR; supervision, CM, HM, SA, CS; project administration MR; funding acquisition, n.a. no external funding. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
According to article 22 of the Swiss Federal Law on data protection ethics approval was not necessary, as the study was retrospective and used anonymized data [24].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roth, M.M., Meier, C.R., Huber, C.A. et al. Antidepressant drug switching in the Swiss population with a focus on Escitalopram and drugs with pharmacogenetic dosing guidelines: a drug utilization study using claims data. Pharmacogenomics J 25, 24 (2025). https://doi.org/10.1038/s41397-025-00382-1
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41397-025-00382-1