Abstract
Ghrelin is an orexigenic peptide hormone synthesized in times of stress and hunger and alterations of the ghrelin system following acute stressors could be repeatedly shown in humans. However, little data exists on long-term effects of trauma on the ghrelin system. We aimed to investigate the influence of childhood trauma on total ghrelin serum levels in a large, population-based study. Total serum ghrelin was measured in 1666 participants of a population-based cross-sectional study (‘LIFE study’). The Childhood Trauma Screener (CTS) was used for the assessment of childhood trauma in the final sample (n = 1086; mean age: 57.10 ± 16.23 years; 632 males, 454 females). Multiple linear regression analyses and generalized linear models were chosen to examine the association between childhood trauma and total serum ghrelin concentrations. Childhood sexual abuse went along with significantly higher ghrelin serum levels in the total sample (β = 0.114, t = 3.958; p = 0.00008) and in women (β = 0.142, t = 3.115; p = 0.002), but not in men (β = 0.055; t = 1.388; p = 0.166). Women with severe emotional neglect in the childhood had higher ghrelin levels than those without (odds ratio = 1.204; p = 0.018). For the CTS Sum Score and other CTS sub-scale scores, no significant association with ghrelin serum levels was found. Our study is the first to show associations between childhood sexual trauma and total ghrelin levels in adults in a large, community-based sample. Our results should initiate further research of the role of ghrelin in human stress response in prospective study designs.
Similar content being viewed by others
Introduction
Ghrelin is a 28-amino-acid peptide hormone that is predominantly synthesized in the stomach mucosa [1]. It was first discovered in 1999 as a strong stimulant of Growth-Hormone (GH) release [1] and binds to its almost ubiquitously expressed [2] receptor, the Growth Hormone Secretagogue Receptor (GHRS-1a) after a post-translational acylation by the enzyme Ghrelin O-Acyl Transferase (GOAT) [3, 4]. Ghrelin is secreted in a pulsatile manner [5] and secretion increases in times of stress and hunger [6]. It increases food intake especially of high palatable foods and induces weight gain [7]. Ghrelin has been repeatedly suggested to act as a survival hormone [8, 9], inducing both metabolic and behavioral adaptions to stress and hunger. It decreases energy expenditure [10] while promoting energy intake [11], thus acting towards energy homeostasis. Its involvement in stress response on a behavioral level is complex. While most animals studies show increased levels of serum ghrelin in acute [12,13,14] and chronic [15, 16] stress, ghrelin’s effects on behavior, i.e. if it acts depressiogenic/anxiogenic or antidepressant/anxiolytic seem to depend on a variety of factors, the most important of which seem to be acute vs. chronic exposure (for more information see [17]) and feeding state at the time of the stressor [18]. In humans, data is naturally more scarce, but acute stress exposure was mainly associated with increased serum levels [13, 19,20,21]. Interestingly, in one study in traumatized adolescents, ghrelin levels remained elevated even after the stressor had passed [14, 22]. This suggests that the ghrelin system shows stress-induced alterations considerable time after the stressor occurred. However, to date, it is widely unclear, how long and to what extent these changes persist. One recently published initial study in Anorexia nervosa (AN) patients found a positive association between childhood traumatization and ghrelin levels in their adult AN patients, providing a first indication that increased ghrelin levels can persist into adulthood [23]. Another study, investigating ghrelin’s effect on smoking and relapse in adult smokers, also found a positive association between childhood adverse events and ghrelin serum levels [24]. A very recent publication found a negative association of total ghrelin levels with childhood stress [25]. Childhood adverse events and trauma are known to have long lasting and often severe effects on mental and physical health well into adulthood [26]. Given the high prevalence of childhood trauma in the population [27], better understanding the underlying mechanisms which perpetrate health consequences into adulthood is important for developing new treatment approaches. Also, it has yet to be investigated whether the effects seen by Rossi et al. and Al’Absi et al. occur also in subjects who never developed a psychiatric illness despite experiencing trauma; i.e. whether the ghrelin system is also involved in “healthy” subjects or whether differences can only be seen in disease. Thus, we aimed to investigate the association between traumatic childhood experiences with total ghrelin serum levels in a large, population-based subject sample. Based on the results from previous research, we hypothesized that ghrelin levels would be positively associated with traumatic childhood experiences. Furthermore, we hypothesized that ghrelin levels would be positively associated with the intensity of traumatic childhood experiences. Explorative analyses addressed the question whether there was a significant association between the presence or intensity of specific childhood traumatic experiences and ghrelin serum levels.
Methods
Study design and subjects
Data for the present analysis come from the LIFE-Adult-Study (Leipzig Research Center for Civilization Diseases), a large population-based cohort study comprising 10,000 adults (mainly within the age range 40–79 years), who were randomly recruited in Leipzig, a large city in Germany [28]. All participants gave written informed consent to participate in the study. The study was conducted according to the Declaration of Helsinki and approved by the ethics committee of the University of Leipzig (registration-number: 263-2009-14122009). Blood samples were collected after an overnight fast (at least 8 h of fasting) including abstinence from smoking, between 07:30 and 10:30 h. The blood samples had been immediately processed by the team of the LIFE pre-analytical laboratory which is part of the Leipzig Medical Biobank and sent directly to the Institute of Laboratory Medicine, Clinical Chemistry, and Molecular Diagnostics (ILM) where direct analyses were carried out. Additional samples were frozen within 2 h after blood withdrawal in the vapor phase of liquid nitrogen at temperatures below −150 °C in Askion c-line HS200S (Askion, Gera, Germany) until usage for ghrelin measurements.
From the LIFE database, data sets were selected according to the following inclusion and exclusion criteria. Inclusion criteria were: valid measurements of total ghrelin; complete data regarding age, gender, alcohol consumption, nicotine consumption, BMI score, cortisol concentration, GAD-7 and CES-D sum scores; written informed consent and sufficient knowledge of the German language. Exclusion criteria (due to possible effects on ghrelin levels) were as follows: current treatment due to an autoimmune disease; treatment in the last year because of a diagnosis of cancer; current diseases of the gastrointestinal system; a history of stroke, multiple sclerosis or epilepsy. (Psycho-)pharmacological treatment was no principal exclusion criterion. Valid measurements of total ghrelin were available for 1666 LIFE participants. 580 individuals had to be excluded due to not meeting in- or exclusion criteria. The resulting final sample consisted of 1086 individuals (632 males and 454 females).
Ghrelin measurements
All ghrelin measurements were done in serum by the use of a radioimmunoassay for total ghrelin (Mediagnost, Reutlingen Germany). Samples were not pre-treated with enzyme inhibitors or acidification. Due to this, only total ghrelin was measured, as it is much more stable than acyl-ghrelin. Sensitivity of the assay was 0.04 ng/mL, mean intra-assay coefficients of variation were 2.7–4.3%; interassay coefficients of variation were between 6.9 and 9.2% for the mean expected range of clinical data around 0.88 and 0.97 ng/mL.
Cortisol measurements
Cortisol was measured in the same serum samples as ghrelin. Measurements were performed by the cobas® e601 fully automated system (Roche Diagnostics, Penzberg). Interassay coefficients of variation of 4.03% and 2.80% with cortisol levels between 65.6–69.2 nmol/L (n = 174) and 762–807 nmol/L (n = 174) were calculated after representative measurement of quality control sera over 6 months.
Childhood Trauma Screener
Childhood trauma was assessed with the German version of the Childhood Trauma Screener (CTS), a 5-item self-rating screening tool [29]. Thus, childhood trauma has not been broadly assessed; this fact was due to the large number of questionnaires which had to be filled in within the LIFE-Adult Study. The questions assess emotional neglect (coded inversely), physical abuse, emotional abuse, sexual abuse, and physical neglect (coded inversely). The possible answers range from 1 (never) to 5 (very often) reflecting the intensity of the corresponding childhood trauma. For each item there is a cut-off that marks the presence or absence of the respective traumatization: For the sub-items physical and emotional abuse, the cut-offs were 3 (“occasionally”), for sexual abuse 2 (“rarely”), for emotional and physical neglect 4 (“frequently”). Furthermore, a sum-score can be calculated by addition of the five item scores. The correlations between the 5 items and the respective subscales of the more detailed Childhood Trauma Questionnaire (CTQ) range between r = 0.55 and r = 0.87 [30]. The CTS has a high internal consistency (Crohnach’s alpha 0.757) [29].
Acquisition of data on tobacco and alcohol consumption
Tobacco consumption was assessed via self-administered questionnaire and interview. Subjects were grouped in three categories: active smoker, former smoker, and never-smoker. Active smokers were considered all those participants who had smoked regularly for at least 6 months consecutively in their lifetime and at least occasionally at the time of examination. Subjects who had smoked continuously for more than 6 months during their lifetime, but were not smoking at the time of assessment, were defined as former smokers [31].
Frequency and amount of consumption of alcoholic beverages (i.e. beer/wine/spirits) during the last 12 months were semiquantitatively assessed using a self-administered food frequency and alcohol questionnaire (FFQ). Possible answers for the frequency of alcohol consumption were “multiple times a day”, “daily”, “multiple times a week”, “once a week”, “two to three times a month”, “once a month or rarer”, or “almost never”. Also, the amount of beverage consumption was assessed by defined categories. From the amount and frequency of alcoholic beverage as well as the average alcohol content of different beverages, the average consumption of pure alcohol (g/day) was calculated [32].
Assessment of BMI
The BMI is defined as the body weight divided by the square of the body height (kg/m2). Body weight was measured with an electronic scale (SECA 701, Seca GmbH & Co KG) with a precision of 0.01 kg, height using a stadiometer (SECA 240) to the nearest 0.1 cm by trained staff according to standardized protocols.
Statistical analysis
In order to answer the question whether the findings of the study were due in part to other factors known to be associated with ghrelin serum concentrations like the BMI score Spearman-Brown correlations between ghrelin serum concentrations and covariates (age, BMI score, alcohol consumption (gram per day), cortisol levels, and GAD-7 and CES-D sum scores) were computed (due to non-normal distribution of the variables as assessed by the Kolmogorov–Smirnov test). In order to assess the association between ghrelin serum levels and binary variables (gender (male/female), smoking status (active smoking: yes versus no)) point-biserial correlation coefficients were chosen.
For primary analyses, generalized linear models with gamma distribution and log-link function were chosen to examine the association between presence of different childhood trauma types according to the CTS items 1–5 (reflecting emotional neglect, physical abuse, emotional abuse, sexual abuse, physical neglect: yes versus no) (as independent variables) and ghrelin serum concentrations (as dependent variable) because of a clearly skewed distribution of the ghrelin serum levels. The models were both unadjusted and adjusted for gender, age (continuous variable in years), BMI score, alcohol consumption (gram per day), smoking status, cortisol levels, GAD-7 and CES-D sum scores. The results were presented as odds ratios (ORs) with corresponding 95% confidence intervals (CI).
For subsequent subgroup analyses, we also applied generalized linear models with gamma distribution and log-link function to examine the gender-specific association between presence of different childhood trauma types and ghrelin serum concentrations (see above) with the effects of age, BMI score, alcohol consumption, smoking status, cortisol levels, GAD-7 and CES-D sum scores being controlled for. Corresponding unadjusted models were computed, too.
For secondary analyses, multivariate linear regression analyses were calculated for both the total sample and two subgroups (males and females). The independent variables were the CTS sum scores and the CTS items 1–5 (reflecting emotional neglect, physical abuse, emotional abuse, sexual abuse and physical neglect) and the dependent variable was the ghrelin concentration. The analyses were adjusted for age, gender, smoking status, alcohol consumption, BMI scores, cortisol levels, and GAD-7 sum scores [33] as well as the CES-D sum scores. Regression coefficients (β) with 95% confidence intervals (CI) were computed. For regression analyses in males and females, the same covariables were selected (except of gender).
Supplementary analyses were as follows:
Gender differences regarding ghrelin serum levels, CTS items and corresponding sum scores were assessed by using Mann–Whitney U tests in view of a non-normal distribution of the dependent variables (Kolmogorov–Smirnov test: p < 0.05).
Differences between individuals with versus without presence of different childhood trauma types according to the CTS items 1–5 (emotional neglect, physical abuse, emotional abuse, sexual abuse, physical neglect: yes versus no) regarding clinically relevant variables (CES-D sum scores, GAD-7 sum scores, BMI scores and alcohol consumption (in gram per day)) in the total sample were tested for statistical significance by using Mann–Whitney U tests. Moreover, two types of multiple regression analyses were chosen for supplementary statistical tests:
First, unadjusted multiple linear regression analyses were calculated for the total sample. The independent variables were the CTS items 1–5 (see above) and the dependent variable was the CES-D sum score. Thus, the association between the intensity of different childhood trauma types and the intensity of depression could be assessed. An analogous approach was selected for the GAD-7 sum score as dependent variable.
Second, unadjusted multiple regression analyses were computed for the total sample with the independent variables being the binary CTS items 1–5 (reflecting emotional neglect, physical abuse, emotional abuse, sexual abuse, and physical neglect: yes versus no; reference category: no) and the dependent variable being the CES-D sum score. Thus, the association between the presence of different childhood trauma types and the intensity of depression could be evaluated. An analogous approach was chosen for the GAD-7 sum score as dependent variable.
The frequency distribution of all 32 combinations of the binary CTS items 1–5 (representing emotional neglect, physical abuse, emotional abuse, sexual abuse, and physical neglect: yes versus no) was presented, too.
Moreover, the Bonferroni–Holm method was used to correct for multiple comparisons.
The SPSS version 26.0 was selected for the statistical analyses and the significance level α = 0.05 was chosen. All statistical tests were two-sided.
Results
Demographic and clinical characteristics of the sample
Demographic characteristics (age and gender distribution) as well as clinical data of the final sample (like severity of anxiety and depression) are summarized in Table 1.
Childhood trauma characteristics of the sample
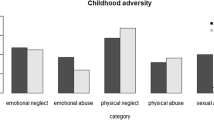
In all, 345 of 1086 reported having experienced childhood trauma (31.77%). Of the total sample, 81 participants (7.46%) had conspicuous CTS scores reflecting emotional neglect. Corresponding numbers were 91 (8.38%) for physical abuse, 77 (7.09%) for emotional abuse, 60 (5.52%) for sexual abuse and 203 (18.69%) for physical neglect.
The frequency distribution of all 32 combinations of the binary CTS items 1–5 representing emotional neglect, physical abuse, emotional abuse, sexual abuse and physical neglect (yes versus no) was summarized in Table S3. The most frequent configuration (besides of absence of any childhood trauma (68.23%)) was physical neglect without further childhood traumas (12.98%), followed by physical abuse without further childhood traumas and sexual abuse without further childhood traumas (3.04% in both cases) The most frequent combination of two traumas was the combination of severe emotional neglect and physical neglect (1.66%), the most frequent triple combination that for severe emotional neglect, physical abuse, and emotional abuse (0.83%) and the most frequent quadruple combination that for severe emotional neglect, physical abuse, emotional abuse, and physical neglect (0.92%). All five childhood traumas were found in only four individuals (0.37%).
Detailed information on the mean intensity of different childhood trauma types and corresponding gender differences were presented in Table 2. Overall, the proportions of individuals with medium/severe emotional abuse and sexual abuse were significantly higher in females than in males (p ≤ 0.019).
Association between ghrelin serum levels and presence of childhood trauma
Ghrelin serum concentrations were found to be significantly associated with the following variables in the total sample: gender (r = 0.269; p < 0.001), age (ρ = −0.110; p < 0.001), the BMI score (ρ = −0.284; p < 0.001) and the GAD-7 sum score (ρ = 0.102; p < 0.001). There was a statistical trend regarding the positive association of ghrelin serum concentrations and the cortisol concentrations (ρ = 0.051; p = 0.09). The associations between ghrelin serum concentrations and active smoking (r = 0.019; p = 0.54), alcohol consumption (ρ = −0.020; p = 0.51) and the CES-D sum score (ρ = 0.004; p = 0.89) failed to be statistically significant in the total sample.
Overall, presence of at least one childhood trauma did not go along with significantly higher total ghrelin serum levels as compared to individuals without such experiences (unadjusted model: OR = 1.014; p = 0.609; adjusted model: OR = 0.999; p = 0.964; see also Table 3).
For severe emotional neglect, a significantly positive association with ghrelin serum levels was found in an unadjusted model (OR = 1.100; p = 0.049); however, this association was not significant in a generalized linear model, corrected for several covariables (age, gender, BMI scores, alcohol consumption, smoking status, cortisol levels, GAD-7 and CES-D sum scores) (OR = 1.070; p = 0.138). Physical abuse, emotional abuse and physical neglect were neither significantly associated with ghrelin serum levels in unadjusted models nor adjusted models (see Table 3). Instead, individuals with sexual abuse had significantly higher total ghrelin serum levels than individuals without sexual abuse in an unadjusted model (OR = 1.217; p = 0.0004); a corresponding statistical tendency was found in the adjusted model (OR = 1.098; p = 0.076).
Gender-specific subgroup analyses revealed that presence of childhood trauma and ghrelin serum levels were not significantly associated in males (see Table 4). Regarding females, severe emotional neglect went along with significantly higher ghrelin serum levels at the 5% level (unadjusted model: OR = 1.232; p = 0.009; adjusted model: OR = 1.204; p = 0.018). The latter OR did not significantly differ from the corresponding OR in males (0.965) as revealed by the overlapping 95% confidence intervals (males: 0.868–1.072; females: 1.032–1.405).
Regarding the presence of sexual abuse, there was a positive association with ghrelin serum levels in both males and females but failed to be significant (p ≥ 0.08). When comparing medium to severe (CTS item sexual abuse of 3–5) with no or little childhood sexual trauma (CTS item sexual abuse of 1–2), i.e. when the cut-offs were set differently, results were significant at the 5% level (adjusted model: OR = 1.195; p = 0.02).
Physical abuse, emotional abuse and physical neglect in females were neither significantly associated with ghrelin serum levels in unadjusted models nor adjusted models (see Table 4).
Association between ghrelin serum levels and the intensity of childhood abuse
In the total sample (n = 1086), ghrelin serum levels did not show a significant association with the intensity of emotional neglect, physical abuse, emotional abuse and physical neglect as measured by the CTS items 1–3 and 5 when adjusted for age, gender, BMI, alcohol consumption, smoking status, cortisol concentrations, GAD-7 sum scores, and CES-D sum scores (see Table 5, upper part). The same was true for the subgroups of males (see Table 5, middle part) and females (see Table 5, lower part). Whereas there was no significant association between the presence of childhood sexual abuse and ghrelin serum concentrations in adjusted generalized linear models, there was a significantly positive association between the intensity of sexual abuse (as reflected by the CTS item 4) and ghrelin serum concentrations in the total sample (ß = 130.01 (95% CI: 65.55; 194.47), standardized β = 0.114, t = 3.958; p = 0.00008; adjusted for the above mentioned covariables) (see Table 5, upper part) and in the female subgroup (ß = 145.35 (95% CI: 53.64; 237.06), standardized β = 0.142, t = 3.115; p = 0.002; adjusted for the above mentioned covariables without gender; see Table 5, lower part) whereas the corresponding positive association in the male subgroup (ß = 76.08 (95% CI: −31.56; 183.72), standardized β = 0.055; t = 1.388; see Table 5, middle part) failed to be statistically significant (p = 0.166).
We analyzed to what extent distinct trauma types were associated with higher scores in depression (CES-D) and anxiety scores (GAD-7). When associating the presence of childhood abuse with outcomes in the CES-D and GAD-7, we found that for depressive symptomatology, emotional abuse (standardized β = 0.088; t = 2.454; p = 0.014), sexual abuse (standardized β = 0.085; t = 2.796; p = 0.005), and severe physical neglect (standardized β = 0.123; t = 4.036; p = 0.00006) were significantly and positively associated with CES-D sum scores. The two latter findings were also significant after alpha-adjustment. Similarly, in generalized anxiety the following categories were significantly and positively associated with GAD-7 sum scores: emotional abuse (standardized β = 0.077; t = 2.130; p = 0.033), sexual abuse (standardized β = 0.066; t = 2.157; p = 0.031), and severe physical neglect (standardized β = 0.083; t = 2.690; p = 0.007; see Table S2). The latter result was also significant at the alpha-adjusted significance level (α = 0.01).
When associating the intensity of different childhood trauma types with outcomes in the CES-D and GAD-7 it could be shown that emotional neglect (standardized β = 0.110; t = 3.221; p = 0.001), emotional abuse (standardized β = 0.099; t = 2.748; p = 0.006), sexual abuse (standardized β = 0.084; t = 2.765; p = 0.006) and physical neglect (standardized β =β = 0.092; t = 2.971; p = 0.003) were significantly and positively associated with CES-D sum scores. In contrast, only the intensity of emotional abuse (standardized β = 0.126; t = 3.442; p = 0.001) and sexual abuse (standardized β = 0.061; t = 1.994; p = 0.046) were significantly associated with higher GAD-7 sum scores. The latter finding was no longer significant after Bonferroni correction.
Discussion
In this study we showed that childhood sexual abuse was positively associated with ghrelin serum levels in the total sample (standardized β = 0.114, t = 3.958; p = 0.00008) and in women (standardized β = 0.142, t = 3.115; p = 0.002), but not in men (standardized β = 0.055; t = 1.388; p = 0.166). Women who had experienced severe emotional neglect had higher ghrelin serum levels than those who did not (adjusted model: OR = 1.204; p = 0.018). For CTS Sum Score and the other sub-scores, no significant association or differences in serum levels were found.
The difference in total ghrelin serum levels between subjects with and without childhood sexual abuse showed a trend significance and we could show a positive association in a linear regression analysis. Thus, we decided to isolate subjects who had experienced medium to severe sexual abuse and compare them to subjects with no or little sexual abuse. Here, we found a statistically significant difference in the total group (adjusted model: OR = 1.195; p = 0.02), but not in men or women when analyzed separately. This is most likely a statistical effect due to relatively small sample sizes. This, together with the results from the regression analysis, suggests that the intensity of abuse has an influence on ghrelin serum levels.
We found considerable sex differences in our sample concerning both the regression analysis and the generalized linear models. Despite the large sample size, the percentage of subjects who had experienced sexual abuse and severe emotional neglect was quite low. Also, many more women were subject to abuse than men, so that women were overrepresented in the group with childhood abuse experience. Furthermore, in the regression analysis, there is considerable overlap between the 95%-confidence intervals of men (31.56–183.72) and women (53.64–237.06), which strongly suggests that the statistical difference is not a consequence of sex-specific differences in the ghrelin signal. Thus, we interpret the sex differences most likely as a statistical effect due mainly to the fact that group sizes were larger in women. However, sex-specific effects of ghrelin in stress regulation have been reported in animal studies, suggesting higher ghrelin sensitivity and stronger ghrelin effects in stress response in females [34]. Sex-specific differences in ghrelin signaling in feeding response has been shown in mice [35] and humans [36] and ghrelin-dependent growth hormone secretion was shown to be different in male and female mice [37]. Thus, an innate influence of sex on our results cannot be excluded, even if we found no traces of it in our statistical analysis. Further studies that take sex into account when analyzing stress response in humans are needed to investigate this question.
Our results are largely in line with the little evidence that exists on this subject. In a first study, Yousufzai and colleagues could show that children who had suffered traumatic experiences (n = 49) had higher acyl serum levels than the control group (n = 39), even years after the traumatic event happened [22]. Associated with higher ghrelin levels were increased scores in depressive and anxiety symptomatology and disturbed sleeping behavior [22]. In line with this, in our sample, subjects who suffered emotional abuse, sexual abuse or severe physical neglect had higher scores for depressive (as assessed in the CES-D) and anxiety (as assessed in the GAD-7) symptomatology (see supplementary material), underlining the prolonged negative effect that childhood abuse has on mental health. This finding is supported by recent literature [38, 39]. Comparability of the data published by Yousufzai et al with our sample is, however, reduced due to the fact that subjects were children, predominantly male, only acyl-ghrelin was assessed and blood samples were taken in the afternoon with no standardized meal/fasting period beforehand. From the same group and sample, another analysis showed that subjects with Posttraumatic Stress Disorder (PTSD) had higher acyl ghrelin serum levels than subjects who had experienced trauma but had not developed a PTSD and healthy controls [40]. In our sample, data on the presence of PTSD was only present in 15 subjects, of which only one subject fulfilled criteria of PTSD, so a statistical analysis could not be performed.
In another recent study, authors investigated the association between childhood trauma and total ghrelin serum levels in a sample of female Anorexia Nervosa (AN) patients and healthy controls [23]. Ghrelin was collected, as in our study, after a 12h- overnight fast in the morning. Interestingly, they found that, as in our sample, sexual abuse was (among emotional abuse in their sample) the strongest predictor for higher total ghrelin serum levels. Rossi et al found that physical neglect and total CTQ score were also positively associated with ghrelin serum levels, but effects were markedly lower than for emotional and sexual abuse [23]. This is of note, as it suggests, together with our data, that these types of traumas seem to be associated the strongest with ghrelin serum levels.
Al’Absi and colleagues found that high, but not low or medium, early life adversity [24] was associated with higher total ghrelin serum levels in non-smokers. This finding is relevant, as it backs up our observation that ghrelin serum levels were associated with the intensity of childhood abuse and effects were clearer in subjects with medium to severe childhood abuse [24]. Summing up, the research published so far investigates mainly specific subject samples (traumatized adolescents, AN-patients, smokers), but results are in line with ours from a broad, population-based approach.
Daniels et al investigated the association between early childhood stress and (ELS) and total ghrelin serum levels in young adults [25]. It is the first study to date that found a negative association between ghrelin levels and ELS. However, when the authors corrected for currents psychiatric condition, this association was no longer statistically significant. Results in our study were controlled for depression and anxiety scores as these are known to be associated with both ghrelin levels and childhood trauma [17, 38, 39]. Furthermore, they investigated much younger subjects (mean age 26.6 in control group, 27.8 years in group with ELS) and ghrelin serum levels were much lower than in our sample in general (~250 pg/ml vs ~800 pg/ml in our sample), suggesting differences in the total ghrelin assay that was used [25].
Ghrelin’s role in stress regulation has been subject of intensive animal research and the accumulating evidence paints a picture of a complex, non-linear involvement in stress response regulation, depending on a variety of factors, but mainly on whether ghrelin signaling is acute or chronic and whether animals were stressed or unstressed previous to ghrelin administration [13, 14]. Higher baseline ghrelin levels led to decreased fear memories in unstressed rodents [41] and to reduced aversive behavior following elevated ghrelin signaling, either via overexpression of GHRS-1a in the basolateral complex of the amygdala (BLA) [42] or via ghrelin administration in the amygdala [18]. Similarly, ghrelin administration in acute stress led to anxiolytic and antidepressant effects in animals [16, 43, 44]. However, in chronically stressed animals, ghrelin signaling seems to have the opposite effect [14, 41]. Interestingly, in chronic stress, GHRS-1a receptor expression was reduced in the BLA, the region whose activation led to decreased fear memory in unstressed rodents, suggesting a decreased ghrelin-sensitivity in chronic stress [41]. Other studies point in the same direction [45, 46]. This led Stone et al to hypothesize that ghrelin has a protective role in acute stress and a detrimental role in chronic stress [14].
Applied to our sample, elevated ghrelin levels years after stress events suggest a chronically increased ghrelin signal. In fact, as already discussed above, subjects who experienced severe emotional neglect, sexual abuse or emotional abuse all exhibited higher anxiety and depression scores. Given the cross-sectional design of our study, no causal conclusions can be drawn, but from what we know from animal studies and the human studies to date, an involvement of ghrelin seems at least plausible. Further, longitudinal studies are needed to clarify this.
The possible clinical relevance of ghrelin’s involvement in stress and mood regulation has been recently illustrated with ghrelin-based therapeutic approaches to psychiatric conditions. Ghrelin agonists were shown to have antidepressant effects in rodents [47] and for alcohol addiction, drug candidates have been used in humans with promising results [48]. Also, ghrelin measurement might be suitable to differentiate depression subtypes [49] and predict treatment response [50, 51]. Stone et al. [14] hypothesize that in future, characterization of ghrelin activity in humans suffering from PTSD and/or MDD might identify subjects that would profit from a ghrelin-based therapy.
In our analysis, not all categories of traumatic experiences were associated with increased ghrelin serum levels. We could show this for sexual abuse in the regression analysis and for severe emotional neglect in the generalized linear models for women. In multiple cohorts, one in the USA and one in Germany, combat exposure and sexual abuse was associated with significantly higher rates of PTSD than other trauma types [52, 53], suggesting more pronounced detrimental long-term effects. This in line with the finding of an association between ghrelin serum levels and PTSD in war-traumatized adolescents [40]. In a German sample, the chance of developing PTSD was twice as high in subjects experiencing sexual violence when compared to non-sexual violence [53]. A recent study investigating heavily traumatized women also found more pronounced negative effects after sexual violence as compared to non-sexual violence [54]. Thus, of the trauma categories in the screening tool used here, sexual abuse can be seen as the most severe trauma type. This might be one reason why ghrelin serum levels were predominantly associated with sexual abuse. This is line with other, similar studies: sexual abuse was also most strongly associated with ghrelin serum levels in another study and physical abuse was, as in our sample, not associated with ghrelin levels [23]. In summary, sexual abuse is most reliably associated both with negative psychological effects and endocrine changes, suggesting that it might be more destructive than other trauma types and be comparable to war- and combat exposure.
Childhood abuse is a heterogeneous and complex phenomenon and often, subjects did not just experience one type of traumatization in an isolated way. To be able to better interpret our results, we analyzed the frequency distribution of all possible combinations of the CTS items (Table S3). Of the subjects who experienced trauma, 33% experienced two or more types of trauma. This illustrates that the co-occurrence of different types of abuse is common. A slightly higher fraction was observed in the 60 subjects experiencing sexual trauma, of whom 27 also experienced other forms of abuse (45%). Thus, in this cross-sectional design, it cannot be fully excluded that the effects seen for sexual trauma might be due to the fact that subjects who experienced sexual abuse might represent a sub-group which is more heavily traumatized in general.
The rate of childhood abuse in total (31.77%) and of the subcategories are in line with other studies of comparable populations. For example, in another population-based cohort from Germany, in the city of Greifswald [30, 55], 4.3% of subjects reported childhood sexual abuse (vs. 5.52% in our sample). This suggests that our sample was in fact representative and we did not investigate a selected or biased group of subjects.
The main strengths of this investigation were the large sample size and the highly standardized data acquisition. Furthermore, in contrast to other studies, we strictly controlled our statistical analysis for factors known to influence the ghrelin serum levels, most importantly in this context anxiety scores [17], but also alcohol consumption [32] and smoking [31].
The cross-sectional design is the main limitations of this study. As a consequence, all hormones were only assessed at one time point. Also, only total and not acylated ghrelin was measured and blood samples were not pre-treated with protease inhibitors or acidification. Results were not controlled for current medication. Depression and anxiety measures were only assessed by self-rating instruments, childhood traumatic experiences were only assessed by a screening instrument and recent stressors were not assessed in our analysis.
In conclusion, we could show a positive association between childhood traumatic experiences and total serum ghrelin levels in adulthood for the first time in a large, population-based subject sample. Although causative conclusions cannot be drawn, our data suggests that the ghrelin system may be altered years and decades after traumatic experiences, lending further support for the relevance of the ghrelin system in stress response and – processing.
Data availability
The data that support the findings of this study are available from the Leipzig Research Center for Civilisation Diseases (LIFE) but restrictions apply to the availability of these data, which were used under license (project number: PV_0358_Kluge) for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of LIFE.
References
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60.
Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988.
Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–96.
Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105:6320–5.
Natalucci G, Riedl S, Gleiss A, Zidek T, Frisch H. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: maintenance of a meal-related pattern. Eur J Endocrinol. 2005;152:845–50.
Müller TDD, Nogueiras R, Andermann MLL, Andrews ZBB, Anker SDD, Argente J, et al. Ghrelin. Mol Metab. 2015;4:437–60.
Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9.
Mani BK, Zigman JM. Ghrelin as a survival hormone. Trends Endocrinol. Metab. 2017;28:843–54.
Wittekind DA, Kluge M. Ghrelin in psychiatric disorders - a review. Psychoneuroendocrinology. 2015;52:176–94.
Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15:741–5.
Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13.
Kristenssson E, Sundqvist M, Astin M, Kjerling M, Mattsson H, Dornonville de la Cour C, et al. Acute psychological stress raises plasma ghrelin in the rat. Regul Pept. 2006;134:114–7.
Fritz EM, Singewald N, De Bundel D. The good, the bad and the unknown aspects of ghrelin in stress coping and stress-related psychiatric disorders. Front Synaptic Neurosci. 2020;12:1–23.
Stone LA, Harmatz ES, Goosens KA. Ghrelin as a stress hormone: implications for psychiatric illness. Biol. Psychiatry. 2020;88:531–40.
Chuang J-C, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, et al. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest. 2011;121:2684–92.
Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–3.
Wittekind DA, Kratzsch J, Mergl R, Riedel-Heller S, Witte AV, Villringer A, et al. Serum ghrelin is positively associated with physiological anxiety but negatively associated with pathological anxiety in humans: Data from a large community-based study. Psychoneuroendocrinology. 2022;140:105728.
Alvarez-Crespo M, Skibicka KP, Farkas I, Molnár CS, Egecioglu E, Hrabovszky E, et al. The amygdala as a neurobiological target for ghrelin in rats: neuroanatomical, electrophysiological and behavioral evidence. PLoS ONE. 2012;7:e46321.
Raspopow K, Abizaid A, Matheson K, Anisman H. Psychosocial stressor effects on cortisol and ghrelin in emotional and non-emotional eaters: influence of anger and shame. Horm Behav. 2010;58:677–84.
Monteleone P, Tortorella A, Scognamiglio P, Serino I, Monteleone AM, Maj M. The acute salivary ghrelin response to a psychosocial stress is enhanced in symptomatic patients with bulimia nervosa: a pilot study. Neuropsychobiology. 2012;66:230–6.
Rouach V, Bloch M, Rosenberg N, Gilad S, Limor R, Stern N et al. The acute ghrelin response to a psychological stress challenge does not predict the post-stress urge to eat. Psychoneuroendocrinology. 2007;32:693–702.
Yousufzai MIUA, Harmatz ES, Shah M, Malik MO, Goosens KA Ghrelin is a persistent biomarker for chronic stress exposure in adolescent rats and humans. Transl Psychiatry. 2018; 8. https://doi.org/10.1038/s41398-018-0135-5.
Rossi E, Cassioli E, Gironi V, Idrizaj E, Garella R, Squecco R, et al. Ghrelin as a possible biomarker and maintaining factor in patients with eating disorders reporting childhood traumatic experiences. Eur Eat Disord Rev. 2021;29:588–99.
al’Absi M, DeAngelis B, Nakajima M, Hatsukami D, Allen S. Early life adversity and appetite hormones: the effects of smoking status, nicotine withdrawal, and relapse on ghrelin and peptide YY during smoking cessation. Addict Behav. 2021;118. https://doi.org/10.1016/j.addbeh.2021.106866.
Daniels TE, Mathis KJ, Gobin AP, Lewis-de los Angeles WW, Smith EM, Chanthrakumar P, et al. Associations of early life stress with leptin and ghrelin in healthy young adults. Psychoneuroendocrinology. 2023;149:106007.
Nelson CA, Scott RD, Bhutta ZA, Harris NB, Danese A, Samara M. Adversity in childhood is linked to mental and physical health throughout life. BMJ. 2020;371. https://doi.org/10.1136/bmj.m3048.
Stoltenborgh M, Bakermans-Kranenburg MJ, Alink LRA, van IJzendoorn MH. The prevalence of child maltreatment across the globe: review of a series of meta-analyses. Child Abuse Rev. 2015;24:37–50.
Engel C, Wirkner K, Zeynalova S, Baber R, Binder H, Ceglarek U et al. Cohort profile: the LIFE-adult-study. Int J Epidemiol. 2022. https://doi.org/10.1093/IJE/DYAC114.
Grabe H, Schulz A, Schmidt C, Appel K, Driessen M, Wingenfeld K, et al. [A brief instrument for the assessment of childhood abuse and neglect: the childhood trauma screener (CTS)]. Psychiatr Prax. 2012;39:109–15.
Glaesmer H, Schulz A, Häuser W, Freyberger H, Brähler E, Grabe HJ. Der childhood trauma screener (CTS) - entwicklung und validierung von schwellenwerten zur klassifikation. Psychiatr Prax. 2013;40:220–6.
Wittekind DA, Kratzsch J, Mergl R, Enzenbach C, Witte V, Villringer A et al. Higher fasting ghrelin serum levels in active smokers than in former and never-smokers. World J Biol Psychiatry. 2020;21. https://doi.org/10.1080/15622975.2019.1671610.
Wittekind DA, Kratzsch J, Mergl R, Enzenbach C, Witte AVV, Villringer A, et al. Alcohol consumption is positively associated with fasting serum ghrelin in non-dependent adults: Results from the population-based LIFE-Adult-Study. Psychoneuroendocrinology. 2018;97:143–8.
Wittekind DA, Kratzsch J, Biemann R, Mergl R, Riedel-Heller S, Witte V, et al. Association between self-rating depression scores and total ghrelin and adipokine serum levels in a large population-based sample. Front Psychiatry. 2022;13:895.
Börchers S, Krieger JP, Maric I, Carl J, Abraham M, Longo F et al. From an empty stomach to anxiolysis: molecular and behavioral assessment of sex differences in the ghrelin axis of rats. Front Endocrinol (Lausanne). 2022;13. https://doi.org/10.3389/FENDO.2022.901669.
Prins K, Huisman M, McLuskey A, Mies R, Karels B, Delhanty PJD, et al. Ghrelin deficiency sex-dependently affects food intake, locomotor activity, and adipose and hepatic gene expression in a binge-eating mouse model. Am J Physiol Endocrinol Metab. 2022;322:E494–E507.
Leone A, De Amicis R, Pellizzari M, Bertoli S, Ravella S, Battezzati A. Appetite ratings and ghrelin concentrations in young adults after administration of a balanced meal. Does sex matter? Biol Sex Differ. 2022;13:25.
Hassouna R, Fernandez G, Lebrun N, Fiquet O, Roelfsema F, Labarthe A et al. Ghrelin gene deletion alters pulsatile growth hormone secretion in adult female mice. Front Endocrinol (Lausanne). 2021;12. https://doi.org/10.3389/FENDO.2021.754522.
Hailes HP, Yu R, Danese A, Fazel S. Long-term outcomes of childhood sexual abuse: an umbrella review. The Lancet Psychiatry. 2019;6:830–9.
Hogg B, Gardoki-Souto I, Valiente-Gómez A, Rosa AR, Fortea L, Radua J et al. Psychological trauma as a transdiagnostic risk factor for mental disorder: an umbrella meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2022. https://doi.org/10.1007/S00406-022-01495-5.
Malik MO, Shah M, Irfan Ul, Akbar Yousufzai M, Ullah N, Burgess JCA et al. Association of acyl-ghrelin with posttraumatic stress disorder in adolescents who experienced severe trauma. JAMA Netw Open. 2020;3. https://doi.org/10.1001/jamanetworkopen.2020.13946.
Harmatz ES, Stone L, Lim SH, Lee G, McGrath A, Gisabella B, et al. Central ghrelin resistance permits the overconsolidation of fear memory. Biol Psychiatry. 2016;81:1003–13.
Jensen M, Ratner C, Rudenko O, Christiansen SH, Skov LJ, Hundahl C, et al. Anxiolytic-like effects of increased ghrelin receptor signaling in the amygdala. Int J Neuropsychopharmacol. 2016;19:1–12.
Spencer SJ, Xu L, Clarke MA, Lemus M, Reichenbach A, Geenen B, et al. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol Psychiatry. 2012;72:457–65.
Stark R, Santos VV, Geenen B, Cabral A, Dinan T, Bayliss JA, et al. Des-acyl ghrelin and ghrelin O-acyltransferase regulate hypothalamic-pituitary-adrenal axis activation and anxiety in response to acute stress. Endocrinology. 2016;157:3946–57.
Hansson C, Haage D, Taube M, Egecioglu E, Salomé N, Dickson SL. Central administration of ghrelin alters emotional responses in rats: Behavioural, electrophysiological and molecular evidence. Neuroscience. 2011;180:201–11.
Meyer RM, Burgos-Robles A, Liu E, Correia SS, Goosens KA. A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol Psychiatry. 2013;19:1–11.
Walker AK, Rivera PD, Wang Q, Chuang J-C, Tran S, Osborne-Lawrence S, et al. The P7C3 class of neuroprotective compounds exerts antidepressant efficacy in mice by increasing hippocampal neurogenesis. Mol Psychiatry. 2014;20:500–8.
Lee MR, Tapocik JD, Ghareeb M, Schwandt ML, Dias AA, Le AN et al. The novel ghrelin receptor inverse agonist PF-5190457 administered with alcohol: preclinical safety experiments and a phase 1b human laboratory study. Mol Psychiatry. 2018;25. https://doi.org/10.1038/s41380-018-0064-y.
Simmons WK, Burrows K, Avery JA, Kerr KL, Taylor A, Bodurka J et al. Appetite changes reveal depression subgroups with distinct endocrine, metabolic, and immune states. Mol Psychiatry. 2018. https://doi.org/10.1038/s41380-018-0093-6.
Ricken R, Bopp S, Schlattmann P, Himmerich H, Bschor T, Richter C, et al. Ghrelin serum concentrations are associated with treatment response during lithium augmentation of antidepressants. Int J Neuropsychopharmacol. 2017;20:692–7.
Ishitobi Y, Kohno K, Kanehisa M, Inoue A, Imanaga J, Maruyama Y, et al. Serum ghrelin levels and the effects of antidepressants in major depressive disorder and panic disorder. Neuropsychobiology. 2012;66:185–92.
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52:1048–60.
Maercker A, Forstmeier S, Wagner B, Glaesmer H, Brähler E. Posttraumatische belastungsstörungen in Deutschland. Nervenarzt. 2008;79:577–86.
Rowland GE, Mekawi Y, Michopoulos V, Powers A, Fani N, Bradley B, et al. Distinctive impacts of sexual trauma versus non-sexual trauma on PTSD profiles in highly trauma-exposed, Black women. J Affect Disord. 2022;317:329–38.
Glaesmer H. Assessing childhood maltreatment on the population level in Germany: findings and methodological challenges. Child Adolesc Psychiatry Ment Health. 2016;10. https://doi.org/10.1186/s13034-016-0104-9.
Hautzinger M, Bailer M. Allgemeine Depressions skala (ADS). Die deutsche Version des CES-D. Weinheim: Beltz Test; 1993.
Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401.
Löwe B, Decker O, Müller S, Brähler E, Schellberg D, Herzog W, et al. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med Care. 2008;46:266–74.
Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–7.
Acknowledgements
This work was supported by LIFE − Leipzig Research Center for Civilization Diseases, University of Leipzig. LIFE is funded by means of the European Union, by means of the European Social Fund (ESF), by the European Regional Development Fund (ERDF), and by means of the Free State of Saxony within the framework of the excellence initiative. Part of the work was funded within the framework of the Collaborative Research Center “Adiposity Mechanisms“ 209933838/Deutsche Forschungsgemeinschaft (DFG, German Research Foundation). The author(s) acknowledge support from the German Research Foundation (DFG) and Universität Leipzig within the program of Open Access Publishing.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
DAW: conceptualization and design, validation, project administration, and writing-original draft. JK: investigation, writing – review and editing. RM: validation, statistical analysis, writing – review and editing. CS: investigation, resources, funding acquisition, writing – review and editing. VW: funding acquisition, resources, writing – review and editing. AV: funding acquisition and project administration. MK: conceptualization and design, validation, project administration, writing-original draft, and supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The procedures were conducted according to the Declaration of Helsinki and approved by the ethics committee of the University of Leipzig (registration-number: 263-2009-14122009).
Informed consent
All participants gave written informed consent to take part in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wittekind, D.A., Kratzsch, J., Mergl, R. et al. Childhood sexual abuse is associated with higher total ghrelin serum levels in adulthood: results from a large, population-based study. Transl Psychiatry 13, 219 (2023). https://doi.org/10.1038/s41398-023-02517-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-023-02517-z