Abstract
Sex hormones are involved in schizophrenia pathogenesis; however, their direction and genetic overlap remain unknown. By leveraging summary statistics from large-scale genome-wide association studies, we quantified the shared genetic architecture between schizophrenia and four sex hormone traits. Linkage disequilibrium score regression and bivariate causal mixture modeling strategies showed significant positive correlations between sex hormone-binding globulin (SHBG), total testosterone, and schizophrenia, while bioavailable testosterone and schizophrenia were negatively correlated. Estradiol showed a weak positive correlation with schizophrenia, with little polygenic overlap. The conjunctional false discovery rate method identified 303 lead single-nucleotide polymorphisms (SNPs) in jointly shared genomic loci between schizophrenia and SHBG, with 130, 52, and 9 SNPs shared between schizophrenia and total testosterone, bioavailable testosterone, and estradiol, respectively. Functional annotation suggests that mitotic sister chromatid segregation and N-glycan biosynthesis may be involved in common mechanisms underlying sex hormone regulation and schizophrenia onset. In conclusion, this study clarified the inherent relationships between schizophrenia and sex hormone traits, highlighted the roles of mitotic sister chromatid segregation and N-glycan biosynthesis in the pathogenesis of schizophrenia, and delivered potential targets for further validation.
Similar content being viewed by others
Introduction
Schizophrenia is a highly heterogeneous neuropsychiatric syndrome that is likely initiated by early brain developmental abnormalities caused by both genetic and environmental factors [1]. Schizophrenia typically manifests during puberty and is reported to have a significant sex bias, with a male-to-female sex ratio of 1.4:1 [2]. However, the sex risk ratio for schizophrenia incidence is not consistent throughout the lifespan [3]. Males experience a peak of schizophrenia onset in their early twenties and a steady decline thereafter, whereas the trajectory is relatively stable and slow for females [3]. Such biases may be largely driven by dynamic changes in sex hormones, which contribute to the development of normal brain sexual dimorphisms and structural modifications in the central nervous system [4]. Especially in puberty, a critical period during which massive brain reorganization and maturation occur, the disruption of sex hormones may disrupt the normal trajectory of brain development and precipitate neuropsychiatric disease onset [5].
Population-based epidemiological studies have provided evidence linking schizophrenia and sex hormone levels. The most frequently reported sex hormones associated with schizophrenia is estrogen, which has been thought to play a role in the pathogenesis and therapeutics of schizophrenia for decades [6]. The evidence for this mainly manifests in two aspects. First, estrogen deficiency is associated with both increased vulnerability to schizophrenia onset and severity of psychotic symptoms, usually seen in women after menopause [7,8,9]. Second, estrogen can have direct neuroprotective actions by activating two types of estrogen receptors (ERα and ERβ) in the brain [10]. In contrast, the relationship between testosterone levels and schizophrenia remains controversial. A few studies have shown elevated levels of testosterone in schizophrenia [11, 12], while several others have reported lower testosterone levels compared to healthy controls, or no significant difference between groups [13,14,15]. Notably, the levels of sex hormones are disrupted by various factors throughout the life cycle, and few studies have focused on the critical period of puberty during which sex hormones change rapidly and schizophrenia typically begins. Further work should be done to uncover the internal relationship between sex hormones and the onset of schizophrenia.
Progress in genetics has provided alternative options for inferring the internal correlations between phenotypes. Over the past decade, the explosion of genome-wide association studies (GWASs) has led to new analytical methodologies underpinning new discoveries [16]. Approaches, such as linkage disequilibrium score regression (LDSC) and polygenic overlap analysis, provide opportunities to reveal correlations between human diseases from a genetic perspective [17]. By employing such methods, we can quantify the genetic correlations between phenotypes and identify the genetic underpinnings of comorbidity [18,19,20]. In the present study, we aimed to estimate the genetic correlation and polygenic overlap between schizophrenia and four sex hormone traits. We also aimed to identify the shared genetic architecture and explore potential comorbidity mechanisms through functional annotations.
Methods
Study participants and data acquisition
We obtained GWAS summary-level data for schizophrenia from the Psychiatric Genomics Consortium [21]. Briefly, the currently largest GWAS of schizophrenia comprise 76,755 individuals with schizophrenia and 243,649 controls, of whom approximately 80% were of European Ancestry. Our analysis retained only the results for the European population to ensure sample consistency in subsequent analyses. The sex hormone traits data were acquired from the UK Biobank [22]. Four sex hormone traits, namely sex hormone-binding globulin (SHBG), total testosterone, bioavailable testosterone, and estradiol, were measured in up to 425,097 European participants aged 40–69 years old. Genetic association analysis was performed with including genotyping chip, age at baseline and ten genetically derived principal components as covariates. Sex-specific GWAS summary statistics were also extracted for subgroup analysis. Notably, data of estradiol levels in women was not available as the majority of test values were below the limit of detection, therefore the subsequent analysis of estradiol was limited to males only. All original GWASs received ethical approval from the relevant ethics committees, and informed consent was obtained from all participants.
Genetic correlation and polygenic overlap analysis
LDSC was used to investigate the genetic correlations between schizophrenia and sex hormone traits using default settings [23]. Although LDSC is a genome-wide globing measurement and does not capture mixtures of effect directions across shared genetic variants, we applied the bivariate causal mixture model (MiXeR) to characterize polygenic overlap accounting for mixed-effect directions [24]. To achieve this, we first constructed a univariate Gaussian mixture model to estimate the polygenicity and discoverability of each trait using maximum likelihood estimation [25]. Next, we performed a cross-trait analysis by extending the model to bivariate MiXeR to evaluate the polygenicity of shared components between schizophrenia and sex hormone traits [24]. The results were presented as Venn diagrams of unique and shared polygenic components across traits, and conditional quantile-quantile (Q-Q) plots were constructed to provide visual representations of cross-trait polygenic enrichment and overlap. Generally, cross-trait enrichment can be seen as successive leftward deflections from the expected null line in conditional Q-Q plots, with an increasing association significance of the secondary phenotype.
Definition of shared genomic loci
We next used conditional/conjunctional false discovery rate (cond/conjFDR) analysis to identify the shared genomic loci between schizophrenia and sex hormone traits [26]. Cond/conjFDR is a widely used statistical tool that leverages cross-trait polygenic enrichment information to identify genomic loci for a single trait (condFDR) and shared genomic loci for both traits (conjFDR) [27,28,29]. We adopted the recommended threshold of conjFDR < 0.05 to define statistically significant single-nucleotide polymorphisms (SNPs) associated with both traits. We further performed clumping procedure with 500 iterations to define the lead SNP within each linkage disequilibrium (LD) block (r2 < 0.1 within a 250 kb window). Due to strong SNP associations within long-range LD regions, the major histocompatibility complex region was excluded from the analysis to avoid artificially inflated genetic enrichment.
Functional annotation, gene mapping, and gene-set analyses
FUMA v1.5.2 was used to annotate candidate SNPs with biological functions, map candidate SNPs to genes, and characterize their biological functions using gene-based test/gene-set analyses [30]. Functional annotation of SNPs included physical localization of gene sequences using ANNOVAR and annotation of pathogenic potential using the Combined annotation-dependent depletion (CADD) score [31, 32]. Candidate SNPs were mapped to genes through three FUMA-recommended strategies: 1) physical position on the genome, 2) expression quantitative trait loci associations, and 3) 3D chromatin interactions [30]. Next, we performed gene-set analysis based on the mapped genes to determine whether the trait-related genes were enriched in specific biological pathways or gene sets using the MAGMA module embedded in the FUMA platform. A recommended threshold of P < 0.05 was considered statistically significant for enrichment pathways.
Results
Genetic correlation and polygenic overlap
The LDSC (Table S1) suggested SHBG and total testosterone were positively correlated with schizophrenia (rg = 0.08, P = 4.03 × 10−5 for SHBG and (rg = 0.08, P = 2.98 × 10−5 for total testosterone). Subgroup analysis revealed consistent results in males and females. No significant correlations were observed between bioavailable testosterone, estradiol, and schizophrenia in the LDSC analysis. While the LDSC method usually underestimates the shared genetic underpinnings between phenotypes, we further adopted the bivariate MiXeR approach to provide a more complete quantification of polygenic overlaps between schizophrenia and sex hormone traits. Bivariate MiXeR analysis indicated that schizophrenia had a polygenic overlap with all four sex hormone traits, and sex-specific subgroup analysis showed consistent results (Fig. 1; Table S2). The Venn Diagrams showed consistent results with the LDSC for correlations between SHBG, total testosterone, and schizophrenia, and both shared approximately 0.6k causal variants with schizophrenia. Bioavailable testosterone also showed a negative correlation with schizophrenia (rg = −0.03) and the estimated number of shared variants was about 0.3k. By contrast, the polygenic overlap between estradiol and schizophrenia in males was weak and shared variants were limited (Table S2).
Venn diagrams depicting the unique and shared variants associated with SCZ and SH traits. The value and circle size reflect the extent of polygenicity for each trait. Conditional Q‑Q plots of nominal versus empirical -log10 transformed P values in the primary phenotype as a function of significance of SNP associations with the secondary phenotype at the level of P < 1.00, P < 0.1, P < 0.01, and P < 0.001. The dashed line is the expected Q‑Q plot under the null hypothesis. SHBG sex hormone-binding globulin. Total T total testosterone. Bioavailable T bioavailable testosterone. * The results are only for males as data for females is not available.
Identification of shared genomic loci between schizophrenia and sex hormone traits
Genomic loci jointly associated with the schizophrenia and sex hormone traits were drawn on a circular Manhattan plot (Fig. 2). At conjFDR < 0.05, we identified hundreds of lead SNPs jointly associated with schizophrenia and sex hormone traits, including 303 SNPs with SHBG, 130 SNPs with total testosterone, 52 SNPs with bioavailable testosterone, and 9 SNPs with estradiol (Table S3–S6). We further listed the top SNPs jointly associated with schizophrenia and sex hormone traits at conjFDR < 1 × 10−4 in Table 1. Of the 303 SNPs jointly associated with schizophrenia and SHBG, 184 (60.7%) showed the same direction of effect in schizophrenia and SHBG. In top SNPs with conjFDR < 1 × 10−4, the proportion was even as high as 72.7%. Notably, several genome loci, such as C10orf32-ASMT, are common components that contribute to more than one sex hormone trait and schizophrenia. The sex-specific subgroup results for SHBG, total testosterone, and bioavailable testosterone are shown in Table S3–S6 and Figure S1–S3, whereas data for estradiol were not available. Although the smaller sample size in the subgroup analysis might affect the ability to identify shared genomic loci, the results consistently indicated a much larger number of shared risk loci in males than in females.
Circular Manhattan plot depicting the -log10 conjFDR values for SNPs jointly associated with schizophrenia and sex hormone traits. The red dashed line represents the threshold for significant association set at conjFDR < 0.05. The circles from the outside to the inside successively are SHBG, total testosterone, bioavailable testosterone, and estradiol. * The results are only for males as data for females is not available.
Functional annotation of shared SNPs
Functional annotations of shared candidate SNPs associated with schizophrenia and the four sex hormone traits are shown in Table S3–S6. ANNOVAR results revealed that the majority of the SNPs shared by schizophrenia and sex hormone traits were in intergenic or intronic regions of the genome (71.2%–79.9%), with only a few scattered in exons. CADD annotations revealed 20 SNPs with CADD scores above 12.37 in jointly associated loci of schizophrenia and SHBG. For total testosterone and bioavailable testosterone, the numbers were 9 and 5, respectively, whereas none were above 12.37 for the shared SNPs of schizophrenia and estradiol. We further mapped candidate SNPs showing joint effects on schizophrenia and sex hormone traits to protein-coding genes. Specifically, 362 protein-coding genes in 129 independent genomic loci were mapped to SNPs jointly associated with schizophrenia and SHBG (Table S7). Additionally, dozens to hundreds of protein-coding genes were identified for joint associations between schizophrenia and the other three sex hormone traits (Table S8–S10).
Gene-set analyses of the shared loci between schizophrenia and sex hormone traits
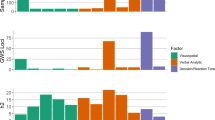
Gene-set analyses of each set of mapped genes uncovered a number of significant pathways involved in the shared biological mechanisms of schizophrenia and sex hormone traits (Table S10–S13). Figure 3 showed the enriched pathways from three important databases, specifically, the Gene Ontology-Biological Process, the Kyoto Encycfigurelopedia of Genes and Genomes, and the Reactome Pathway Database. As shown in Fig. 3, the shared mechanisms of schizophrenia and SHBG mainly involve mitotic sister chromatid segregation, T cell proliferation, and N-glycan biosynthesis, whereas the integration of energy and phospholipid metabolism might participate in the common processes of testosterone regulation and schizophrenia onset.
Bar diagrams showing the enriched gene sets between schizophrenia and (A) SHBG, and (B) Testosterone on three important databases, specifically, the Gene Ontology-Biological Process (GO BP), the Kyoto Encyclopedia of Genes and Genomes (KEGG), and the Reactome Pathway Database (REACTOME). No significant gene sets are enriched on the three databases for shared genes of schizophrenia with bioavailable testosterone and estradiol.
Discussion
In the current study, we investigated the polygenic overlap and shared genetic architecture underlying schizophrenia and four sex hormone traits. Using the MiXeR approach, we found significant positive polygenic correlations between schizophrenia and three sex hormone traits (SHBG, total testosterone, and estradiol), and a negative correlation between schizophrenia and bioavailable testosterone. We reported hundreds of candidate SNPs in jointly associated loci between schizophrenia and sex hormone traits. We also performed a functional annotation of these candidate SNPs to uncover a shared genetic basis and common biological mechanisms between the traits. Our findings verify the extensive associations between schizophrenia and sex hormones, and support the hypothesis that sex hormones play an essential role in the etiology of schizophrenia. Furthermore, our study provides novel insights into the shared genetic architecture between schizophrenia and sex hormone traits and highlights several putative candidate genes and pathways with the potential for drug discovery or etiological exploration for further validation.
Although testosterone has long been recognized as playing a role in the pathophysiology of schizophrenia, whether this effect is positive or negative remains unclear. An essential factor may be that neither total nor free testosterone measured in clinical practice reflects the actual levels of active testosterone. Circulating testosterone typically binds specifically to SHBG (~55%), non-specifically to albumin (~43%), or is unbounded in human plasma (free testosterone, 1%–2%) [33, 34]. Active testosterone refers to the non-SHBG-bound portion (including albumin-bound and free testosterone), also known as bioavailable testosterone. Our present study found that schizophrenia was associated with increased levels of SHBG and total testosterone, but decreased levels of bioavailable testosterone, suggesting that large amounts of testosterone bind specifically to SHBG and thus reduce the level of bioavailable testosterone, which might contribute to the pathogenesis of schizophrenia. The findings were supported by some previous studies. Several studies suggested the testosterone levels were positively correlated with white matter growth whereas SHBG was negatively correlated with the structural changes of cortical GM and whole brain, indicating testosterone played a positive role in cortical maturation [35, 36]. Some other studies found testosterone to be pro-neurogenic and neurotrophic, given its evidence in supporting neurogenesis in the adult male rodent brain [37, 38]. To our knowledge, our study is the first to clarify the internal link between schizophrenia and testosterone levels using genetic strategies, providing novel insights into the understanding of sex biases and differentiated treatment of schizophrenia.
The current findings also showed a large proportion of polygenic overlap between schizophrenia and sex hormone traits using the MiXeR method, with nearly 70% (0.58k/0.83k) of the genetic variants underlying SHBG appeared to affect schizophrenia. For bioavailable testosterone the proportion was ~30% (0.27k/0.90k) while for total testosterone the proportion even reached 90% (0.58k/0.64k). These shared polygenic overlaps embrace a massive amount of information underlying the common biological mechanisms for schizophrenia and sex hormone traits. By leveraging pleiotropic enrichment using the conjFDR method, we further localized the genomic loci jointly associated with schizophrenia and sex hormone traits. We noticed that one of the most significant loci, which showed strong associations (conjFDR < 1 × 10−4) with all three testosterone-related traits (SHBG, total testosterone, and bioavailable testosterone), was the C10orf32-ASMT/AS3MT locus on chromosome 10. One difference was that SHBG and total testosterone showed consistently opposite allelic effects on schizophrenia at this locus, whereas the direction was the same for bioavailable testosterone with schizophrenia. Through a literature review, we found that an isoform of AS3MT encoding production, which lacked the first 102 amino acids and lost enzymatic activity compared to AS3MT, was significantly overexpressed in individuals with schizophrenia [39]. Production of AS3MT has also been reported to play a role in male reproductive damage [40]. Interestingly, RNA-seq data suggested that the adrenal gland produced nearly ten times more AS3MT transcripts than other organs [41]. Hence, the mechanism by which AS3MT acts on the adrenal axis, which regulates complex interactions with both the brain and gonads, could be an essential point for future research on this gene. Table 1 also showed three genes with CADD scores > 12.37, which were HSPA9, PRR12, and MSL2 respectively. HSPA9 was reported to control most of the cellular pathways involved in the degeneration of dopaminergic neurons [42], while PRP12 and MSL2 were proposed to have potential roles in neurodevelopment and gene regulation [43, 44], suggesting all these genes might be promising targets for revealing the pathogenesis of schizophrenia.
Gene set enrichment analyses provided comprehensive insights into the common pathophysiological mechanisms underlying schizophrenia and sex hormone traits. Asymmetric division of neural stem cells is the basis for their proliferation and differentiation during brain development [45]. During mitosis, sister neurons arising from asymmetric divisions preferentially develop both vertical and horizontal synaptic connections [46]. Disruption of this process is closely associated with the onset of schizophrenia. DISC1, which participates in the assembly of human mitotic cell spindle bodies, has long been recognized as a high-risk susceptibility gene for schizophrenia [47]. Our study also showed that MAD1L1, a gene encoding a component of the mitotic spindle-assembly checkpoint, was the most significant gene shared by schizophrenia and SHBG. These findings are consistent with another study [48], and support the hypothesis that schizophrenia is a neurodevelopmental disorder related to mitosis during nerve cell proliferation and differentiation.
We also reported that N-glycan biosynthesis is involved in the pathophysiology of schizophrenia. N-glycosylation deficiency is a hallmark of many neuropsychiatric disorders, as it plays an important role in modulating synaptic plasticity, neuronal morphology, neurite outgrowth, and many cognitive processes [49]. Several recent studies have shown that aberrant N-glycosylation of proteins in synaptic vesicles and synaptosomes is common in the brain of people with schizophrenia [50,51,52]. Our study identified ALG12 as a shared significant gene (conjFDR = 2.91 × 10−4) for schizophrenia and SHBG. ALG12 encodes a glycosyltransferase that catalyzes important reactions required for protein glycosylation. Mutations in ALG12 would cause neurodevelopmental delays and male genital hypoplasia in humans [53]. Hence, ALG12 may be a potential target for glycosylation-based interventions and therapeutic discovery in schizophrenia.
This study had some limitations. First, all GWAS datasets included in our analysis were restricted to European ancestry, and cross-ethnic populations are needed to validate these findings. Second, analysis of estradiol was limited to males as data for females were not available. Third, although our findings clarify the association between schizophrenia and sex hormone traits, further studies are necessary to reveal the biological underpinnings of the interactions between schizophrenia and sex hormones. Fourth, the novel polygenetic targets or biological pathways proposed in our study should be studied further to determine their potential in drug development or etiological exploration of schizophrenia.
In conclusion, the current study clarified the inherent relationships between schizophrenia and sex hormone traits, with a positive association between schizophrenia, SHBG, and total testosterone, and a negative association between schizophrenia and bioavailable testosterone. We also identified abundant shared genomic loci that jointly contributed to both schizophrenia and sex hormone traits. Furthermore, our findings highlight the roles of mitotic sister chromatid segregation and N-glycan biosynthesis in the pathophysiological process of schizophrenia and reveal a series of targets with potential in etiological exploration or drug development.
Data availability
Publicly available datasets are analyzed in this study. Summary statistic GWAS meta-analysis results for schizophrenia was extracted from the Psychiatric Genomics Consortium (https://pgc.unc.edu/). GWAS datasets of testosterone-related traits are available at the GWAS Catalog (https://www.ebi.ac.uk/gwas/).
References
Owen MJ, Sawa A, Mortensen PB. Schizophrenia. The Lancet. 2016;388:86–97.
Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD, et al. Schizophrenia. Nat Rev Dis Primers. 2015;1:15067.
Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. The Lancet. 2022;399:473–86.
Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nat Neurosci. 2015;18:1413–20.
Morrison KE, Rodgers AB, Morgan CP, Bale TL. Epigenetic mechanisms in pubertal brain maturation. Neuroscience. 2014;264:17–24.
Gogos A, Sbisa AM, Sun J, Gibbons A, Udawela M, Dean B. A role for estrogen in schizophrenia: clinical and preclinical findings. Int J Endocrinol. 2015;2015:1–16.
Markham JA. Sex steroids and schizophrenia. Rev Endocr Metab Disord. 2012;13:187–207.
Brzezinski-Sinai NA, Brzezinski A. Schizophrenia and Sex Hormones: What Is the Link? Front Psychiatry. 2020;11:693.
Brand BA, de Boer JN, Sommer IEC. Estrogens in schizophrenia: progress, current challenges and opportunities. Curr Opin Psychiatry. 2021;34:228–37.
Cersosimo MG, Benarroch EE. Estrogen actions in the nervous system: Complexity and clinical implications. Neurology. 2015;85:263–73.
Bergemann N, Parzer P, Mundt C, Auler B. High bone turnover but normal bone mineral density in women suffering from schizophrenia. Psychol Med. 2008;38:1195–201.
Talih F, Fattal O, Malone D Jr. Anabolic steroid abuse: psychiatric and physical costs. Cleve Clin J Med. 2007;74:341–52.
Okita K, Kanahara N, Nishimura M, Yoshida T, Yasui-Furukori N, Niitsu T, et al. Second-generation antipsychotics and bone turnover in schizophrenia. Schizophr Res. 2014;157:137–41.
Li J, Xiao W, Sha W, Xian K, Tang X, Zhang X. Relationship of serum testosterone levels with cognitive function in chronic antipsychotic-treated male patients with schizophrenia. Asia-Pacific Psychiatry. 2015;7:323–9.
Doğan Bulut S, Bulut S, Güriz O. The relationship between sex hormone profiles and symptoms of schizophrenia in men. Compr Psychiatry. 2016;69:186–92.
Visscher PM, Wray NR, Zhang Q, Sklar P, McCarthy MI, Brown MA, et al. 10 years of GWAS discovery: biology, function, and translation. Am J Hum Genet. 2017;101:5–22.
Smeland OB, Frei O, Fan CC, Shadrin A, Dale AM, Andreassen OA. The emerging pattern of shared polygenic architecture of psychiatric disorders, conceptual and methodological challenges. Psychiatr Genet. 2019;29:152–9.
Hindley G, Frei O, Shadrin AA, Cheng W, O’Connell KS, Icick R, et al. Charting the landscape of genetic overlap between mental disorders and related traits beyond genetic correlation. Am J Psychiatry. 2022;179:833–43.
Bahrami S, Hindley G, Winsvold BS, O’Connell KS, Frei O, Shadrin A, et al. Dissecting the shared genetic basis of migraine and mental disorders using novel statistical tools. Brain. 2022;145:142–53.
Ahangari M, Everest E, Nguyen TH, Verrelli BC, Webb BT, Bacanu SA, et al. Genome-wide analysis of schizophrenia and multiple sclerosis identifies shared genomic loci with mixed direction of effects. Brain Behav Immun. 2022;104:183–90.
Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–8.
Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26:252–8.
Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Patterson N, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–5.
Frei O, Holland D, Smeland OB, Shadrin AA, Fan CC, Maeland S, et al. Bivariate causal mixture model quantifies polygenic overlap between complex traits beyond genetic correlation. Nat Commun. 2019;10:2417.
Holland D, Frei O, Desikan R, Fan CC, Shadrin AA, Smeland OB, et al. Beyond SNP heritability: polygenicity and discoverability of phenotypes estimated with a univariate Gaussian mixture model. PLoS Genet. 2020;16:e1008612.
Smeland OB, Frei O, Shadrin A, O’Connell K, Fan CC, Bahrami S, et al. Discovery of shared genomic loci using the conditional false discovery rate approach. Hum Genet. 2020;139:85–94.
Hindley G, Shadrin AA, van der Meer D, Parker N, Cheng W, O’Connell KS, et al. Multivariate genetic analysis of personality and cognitive traits reveals abundant pleiotropy. Nat Hum Behav. 2023;7:1584–1600.
Smeland OB, Bahrami S, Frei O, Shadrin A, O’Connell K, Savage J, et al. Genome-wide analysis reveals extensive genetic overlap between schizophrenia, bipolar disorder, and intelligence. Mol Psychiatry. 2020;25:844–53.
Hope S, Shadrin AA, Lin A, Bahrami S, Rødevand L, Frei O, et al. Bidirectional genetic overlap between autism spectrum disorder and cognitive traits. Transl Psychiatry. 2023;13:295.
Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8:1826.
Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164.
Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–5.
Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72.
Chung MC, Gombar S, Shi RZ. Implementation of automated calculation of free and bioavailable testosterone in epic beaker laboratory information system. J Pathol Inform. 2017;8:28.
Perrin JS, Herve PY, Leonard G, Perron M, Pike GB, Pitiot A, et al. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28:9519–24.
Lian X, Bai Y, Du P, Jing Z, Gao J, Liu F, et al. Causal influences of testosterone on brain structure change rate: a sex-stratified Mendelian randomization study. J Steroid Biochem Mol Biol. 2025;245:106629.
Wainwright SR, Lieblich SE, Galea LA. Hypogonadism predisposes males to the development of behavioural and neuroplastic depressive phenotypes. Psychoneuroendocrinology. 2011;36:1327–41.
Owens SJ, Murphy CE, Purves-Tyson TD, Weickert TW, Shannon Weickert C. Considering the role of adolescent sex steroids in schizophrenia. J Neuroendocrinol. 2018;30:e12538.
Li M, Jaffe AE, Straub RE, Tao R, Shin JH, Wang Y, et al. A human-specific AS3MT isoform and BORCS7 are molecular risk factors in the 10q24.32 schizophrenia-associated locus. Nat Med. 2016;22:649–56.
Wu L, Li H, Ye F, Wei Y, Li W, Xu Y, et al. As3MT-mediated SAM consumption, which inhibits the methylation of histones and LINE1, is involved in arsenic-induced male reproductive damage. Environmental Pollution. 2022;313:120090.
Yoshinaga-Sakurai K, Rossman TG, Rosen BP. Regulation of arsenic methylation: identification of the transcriptional region of the human AS3MT gene. Cell Biol Toxicol. 2021;38:765–80.
Texier B, Prime M, Atamena D, Belenguer P, Szelechowski M. Mortalin/Hspa9 involvement and therapeutic perspective in Parkinson’s disease. Neural Regen Res. 2023;18:293–8.
Chowdhury F, Wang L, Al-Raqad M, Amor DJ, Baxova A, Bendova S, et al. Haploinsufficiency of PRR12 causes a spectrum of neurodevelopmental, eye, and multisystem abnormalities. Genet Med. 2021;23:1234–45.
Karayol R, Borroto MC, Haghshenas S, Namasivayam A, Reilly J, Levy MA, et al. MSL2 variants lead to a neurodevelopmental syndrome with lack of coordination, epilepsy, specific dysmorphisms, and a distinct episignature. Am J Hum Genet. 2024;111:1330–51.
Florio M, Huttner WB. Neural progenitors, neurogenesis and the evolution of the neocortex. Development. 2014;141:2182–94.
Lin Y, Zhang X-J, Yang J, Li S, Li L, Lv X, et al. Developmental neuronal origin regulates neocortical map formation. Cell Reports. 2023;42:112170.
Ishizuka K, Kamiya A, Oh EC, Kanki H, Seshadri S, Robinson JF, et al. DISC1-dependent switch from progenitor proliferation to migration in the developing cortex. Nature. 2011;473:92–6.
McKinney BC, McClain LL, Hensler CM, Wei Y, Klei L, Lewis DA, et al. Schizophrenia-associated differential DNA methylation in brain is distributed across the genome and annotated to MAD1L1, a locus at which DNA methylation and transcription phenotypes share genetic variation with schizophrenia risk. Transl Psychiatry. 2022;12:340.
Sun RC, Young LEA, Bruntz RC, Markussen KH, Zhou Z, Conroy LR, et al. Brain glycogen serves as a critical glucosamine cache required for protein glycosylation. Cell Metab. 2021;33:1404–17.e9.
Mealer RG, Williams SE, Daly MJ, Scolnick EM, Cummings RD, Smoller JW. Glycobiology and schizophrenia: a biological hypothesis emerging from genomic research. Mol Psychiatry. 2020;25:3129–39.
Mealer RG, Williams SE, Noel M, Yang B, D’Souza AK, Nakata T, et al. The schizophrenia-associated variant in SLC39A8 alters protein glycosylation in the mouse brain. Mol Psychiatry. 2022;27:1405–15.
Pradeep P, Kang H, Lee B. Glycosylation and behavioral symptoms in neurological disorders. Transl Psychiatry. 2023;13:154.
Murali C, Lu JT, Jain M, Liu DS, Lachman R, Gibbs RA, et al. Diagnosis of ALG12-CDG by exome sequencing in a case of severe skeletal dysplasia. Mol Genet Metab Rep. 2014;1:213–9.
Acknowledgements
We thank the authors and participants of all data sources from which we extracted GWAS summary statistics. We also thank the High-Performance Computing Cluster of the First Affiliated Hospital of Xi’an Jiaotong University for data processing.
Funding
This study was funded by grant YXJLRH2022027 from Fundamental-clinical Research Program of the First Affiliated Hospital of Xi’an Jiaotong University. The funding sources had no role in the design of this study, execution, analyses, interpretation of the data, or decision to submit results.
Author information
Authors and Affiliations
Contributions
JY and XM conceptualized and designed the study. XH and QM carried out the initial analyses, and drafted the manuscript. JL and PL contributed to data extraction. HP and WL developed methodology. YL and XZ helped with the interpretation of results. BY was responsible for data management. All authors critically revised and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
GWAS summary statistics for all traits were extracted from the public domain. All original GWASs received ethical approval from the relevant ethics committees, and informed consent was obtained from all participants. All methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, X., Ma, Q., Liu, J. et al. Investigating the shared genetic architecture between schizophrenia and sex hormone traits. Transl Psychiatry 15, 83 (2025). https://doi.org/10.1038/s41398-025-03305-7
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03305-7