Abstract
Depression has been linked to cortico-limbic brain regions, and ketamine is known for its rapid antidepressant effects. However, how these brain regions encode depression collaboratively and how ketamine regulates these regions to exert its prompt antidepressant effects through mesoscale brain-wide fluctuations remain elusive. In this study, we used a multidisciplinary approach, including multi-region in vivo recordings in mice, chronic social defeat stress (CSDS), and machine learning, to construct a Mesoscale Brain-Wide Fluctuation Analysis platform (MBFA-platform). This platform analyzes the mesoscale brain-wide fluctuations of multiple brain regions from the perspective of local field potential oscillations and network dynamics. The decoder results demonstrate that our MBFA platform can accurately classify the Control/CSDS and ketamine/saline-treated groups based on neural oscillation and network activities among the eight brain regions. We found that multiple-region LFPs patterns are disrupted in CSDS-induced social avoidance, with the basolateral amygdala playing a key role. Ketamine primarily exerts the compensatory effects through network dynamics, contributing to its rapid antidepressant effect. These findings highlight the MBFA platform as an interdisciplinary tool for revealing mesoscale brain-wide fluctuations underlying complex emotional pathologies, providing insights into the etiology of psychiatry. Furthermore, the platform’s evaluation capabilities present a novel approach for psychiatric therapeutic interventions.
Similar content being viewed by others
Introduction
Depression, a common mental disorder affecting an estimated 3.8% of the global population, is a significant contributor to the overall global burden of disease [1]. Accumulating evidence indicates that chronic social stress is a key risk factor for depression [2]. This kind of stress reduces social function and diminishes social motivation, which is a key component of depression [3].
Chronic social defeat stress (CSDS) has been widely used in study of depression as it effectively mimics the physical and psychological stress that humans suffer from in daily life [4, 5]. The CSDS paradigm exposes mice to severe physical and psychosocial stressors through repeated encounters with aggressive CD1 residents over a 10-day exposure period, which induces depressive-like behavioral typecharacterized by social avoidance, anhedonia, and anxiety-like behaviors. Ketamine, a multifunctional drug with clinical applications, has rapid and sustained antidepressant effects in both humans and rodents with a single administration [6,7,8]. Ample evidence reveals the cellular mechanisms underlying ketamine’s antidepressant effects [9, 10].
Multiple brain regions have been proposed to contribute to depression. Supporting this notion, human neuroimaging has revealed that the neural mechanisms of major depressive disorder (MDD) are not confined to any single brain region, but likely involve aberrant function across brain-wide networks [11]. Research using magnetoencephalography has shown that local neural oscillations are related to depression and highlighted the crucial role of large-scale coupling biomarkers in early antidepressant response prediction [12]. Brain network integration may also reflect antidepressant therapy responses, as evidenced by studies using functional magnetic resonance imaging and transcranial magnetic stimulation [13,14,15]. In rodent studies, manganese-enhanced magnetic resonance imaging and brain-wide c-Fos expression with fluorescence micro-optical sectioning tomography showed a significantly more activation in several brain regions related with depressive (the infralimbic cortex, ventral hippocampus, lateral hypothalamus and so on) after a 10-day social defeat. Additionally, Hultman’s study showed that the prefrontal cortex and its regulated neural networks dysfunction across cortico-limbic brain regions were implicated in stress-related illness [16, 17]. Especially, recent studies investigate that ultraslow PFC-dependent beta networks are associated with behavioral pathology in a chronic stress-based model of MDD and machine learning method contributes to identify these networks for key brain processes from large-scale electrophysiological data [18]. In brief, network science is a powerful tool to explore brain interconnected systems and offers a framework for integrating multi-scale data and complexity [19]. Studying brain-wide fluctuations the perspective of local field potential oscillations and network dynamics serve as a convergence point to our understanding of psychiatric conditions.
Nevertheless, it remains unclear how mesoscale brain-wide network collaborative coding and its dysfunction alter emotion to yield pathology. The quantitative analysis for antidepressant effects of ketamine in mesoscale has also been seldom concerned. To address these gaps, we developed the following platform: First, we implanted custom multi-region electrode arrays in mice with multiple brain regions: prefrontal cortex (PFC), nucleus accumbens (NAc), basolateral amygdala (BLA), ventral tegmental area (VTA), lateral habenula (LHb) and ventral portion of the hippocampus (vHPC) [20,21,22,23,24,25,26]. Next, following 10-day CSDS paradigm, we recorded LFPs during the social interaction test (SIT), which is a well-established translational depression-related assay. We then analyzed the multi-region LFPs from the perspective of neural oscillations and network. The calculated indicators derived from these analyses were selected as the input parameters for our fluctuation analysis, which described the relationship between the behavioral index and mesoscale brain-wide electrophysiology using supervised machine learning. The machine learning methods have been deemed to offer a powerful approach for linking network structure to behavioral performance, enabling effective analysis of brain-wide fluctuations and behavior [27]. Finally, we administered ketamine to alleviate the CSDS-induced dysfunction. Our analytical platform allowed us to investigate the impact on mesoscale brain-wide LFPs’ fluctuations and reveal ketamine’s rapid antidepressant.
In this study, we used an interdisciplinary approach, including multi-region in vivo recordings in mice, chronic social defeat stress, ketamine regulation, neural oscillations and brain network analysis, and machine learning, to construct a mesoscale brain-wide fluctuation analysis platform for exploring collaborative coding of multiple brain regions in depression and assessing quantitatively the effects of ketamine in alleviating depression-like behaviors. This platform will provide essential insights into the etiology of depression and support therapeutic interventions.
Materials and methods
Animals
Male adult (8–12 weeks) C57BL/6 mice and CD-1 male retired breeder mice (4–6 months) were obtained from the Experimental Animal Center of Tianjin Medical University, China. All mice were housed under a 12-h light/dark environment (light on from 7:00 to 19:00), in a suitable humidity and temperature room with ad libitum access to food and water. The sample sizes for each experimental cohort are presented in the Supplementary materials. All experimental procedures were conducted with approved protocols from the Care and Use of Laboratory Animals and the Tianjin Medical University Animal Care and Use Committee (No. TMUaMEC 2021060).
Behavioral assays
Chronic social defeat stress
C57 mice were randomly assigned to control and CSDS groups and underwent the chronic social defeat stress (CSDS) as described previously [27, 28]. All C57 and CD1 mice were singly housed before being subjected to CSDS. A walled cage was divided into two area by transparent plexiglass separators (one CD1 zone and one C57 zone). CD1 mice were housed in the CD1 zone. C57 in CSDS group were randomly introduced to the CD1 zone and were physically defeated by CD1 aggressor mice for 10 min. After each physical interaction, both mice were housed individually in the two zones for constant sensory exposure for 24 h. The above paradigm was repeated daily for 10 consecutive days. C57 mice in control group were housed in identical cages as the CSDS mice but without any stress exposure.
Social interaction test
An open-field test box (40 cm×40 cm x 40 cm) with an empty transparent acrylic cage on one side was prepared for the social interaction test (SIT). A 14 cm × 24 cm rectangular area extending 8 cm around the cage was defined as the social interaction zone. A C57 was allowed to explore simultaneously in the open field with the empty acrylic cage (defined as “No CD1” phase due to no CD1 mouse in the acrylic cage) for 150 s. Subsequently, an unfamiliar male CD1 was placed in the acrylic cage and the C57 was allowed to explore freely in the open field (defined as “With CD1” phase) for another 150 s. The activity of C57 was monitored and recorded by a video camera (C270, Logitech, China) directly above the open-field. The total movement distance, average velocity and social interaction time (defined as the cumulative duration spent in the social interaction zone) were automatically quantified using the behavioral analysis software (Ethovision XT8.5, Noldus, Wageningen, Netherlands). The social index (SI) was calculated as (Interaction time in “With CD1” phase) / (Interaction time in “No CD1” phase). Notably, only susceptible mice that showed social avoidance (SI < 1) were included in subsequent CSDS-related analyses.
Sucrose preference test
Mice were habituated with two bottles of water for 2 days. One bottle of 1% sucrose and one bottle of water were then supplied to mice for a further 2 days. During this period, the liquid consumption was measured twice each day and the position of two bottles was changed every day in case of side preference. The sucrose preference was calculated as (Sucrose consumption) / (Sucrose consumption + Water consumption) × 100%.
Tail suspension test
Mice were suspended 50 cm above the ground with its tail being fixed by tape for 6 min. The immobility time, explained as the time in which mice (contained the body and all limbs) did not move within the last 5 min of the whole test, was recorded.
Drug administration
Following the completion of chronic social defeat stress (CSDS) paradigm, C57 mice were randomly assigned to either the ketamine or saline groups. To assess the rapid antidepressant experiment that yielded electrophysiological data reflecting a significant neuronal response, we selected 20 mg/kg ketamine (Hengrui Pharma, Jiangsu, China) to administer via intraperitoneal injection [29, 30]. One hour after drug delivery, the mice were used for the SIT and in vivo electrophysiology studies. For the control group, mice were subjected to physiological saline (0.9% NaCl) same as the ketamine dose.
Multi-region electrode array
A multi-region electrode array was constructed using multi-channel connector and 0.033 mm diameter tungsten wires with formvar insulation (California Fine Wire Co., CA, USA), impendence <1MΩ. The configuration of array was customized based on the region coordinates. The length of each electrode wire was determined based on the depth of the target region. All electrodes were referenced to a common electrode and common ground.
Stereotaxic surgery and neurophysiological recording
Mice were anesthetized with isoflurane and placed in a stereotaxic surgery platform (Reward, Shenzhen, China). The target brain regions were as followed: BLA (AP 1.6, ML −2.75, DV −3.9); Cg1 (AP −1.8, ML −0.3, DV −1.8); IL (AP −1.8, ML −0.3, DV −2.9); LHb (AP 1.72, ML −0.46, DV −2.62); NAc (AP −1.6, ML −1.1, DV −3.5); PrL (AP −1.8, ML −0.3, DV −2.3); vHPC (AP 3.7, ML −3.0, DV −3.5); VTA (AP 3.3, ML −0.5, DV −4.25). All regions included in the final analysis had correct electrode tip positions.
The multi-region electrode arrays were constructed as described above. Once all electrode wires were implanted into the corresponding region, multi-region electrode array and metal ground screws were secured above the cranium with dental cement. All mice were implanted on the right side.
All in vivo electrophysiological recordings were performed using the Cerebus Acquisition System (Blackrock Microsystems Inc., UT, USA), synchronized with behavioral video tracking during SIT. Local field potentials (LFPs) were bandpass filtered (0.5–250 Hz) and sampled at 2 kHz. Notably, we only utilized susceptible mice (SI < 1) for data analysis in CSDS group. All electrophysiological analysis were conducted on the extracted LFPs when mice were in the social interaction zone accompanied by social interacting with cage during “With CD1” and “No CD1” phase.
Data analysis
Spectral analysis of neural oscillations
The short-time Fourier transform was applied to the extracted LFPs to obtain the power spectral density (PSD) with a Hamming window(0.5 s wide, 1 Hz frequency resolution). Building upon established frameworks of CSDS-induced neural oscillatory dysfunction [17, 27], we integrated pivotal evidence from Hultman et al. (2018) demonstrating that baseline states (“No CD1” phase) exhibit stress-encoding signatures through electrophysiological spatiotemporal dynamics [30]. Thus, we calculated the normalized PSD ratio as follows: (PSD of With CD1 - PSD of No CD1) / (PSD of With CD1 + PSD of No CD1). This bounded ratio (−1 to +1) quantifies directional PSD shifts while controlling for baseline neurophysiological heterogeneity across subjects, thereby linking neural oscillatory pattern to behavior. We defined the frequency bands with exact bins as follow: 2–7 Hz for low theta, 7–12 Hz for high theta, 13–30 Hz for beta, 30–60 Hz for low gamma and 60–90 Hz for high gamma. The PSD ratio was used for the subsequent oscillation analysis.
Global efficiency and information flow
Directed transfer function (DTF) method was used for the analysis of causal connectivity within multiple brain regions in the frequency domain [31]. The target regions were defined as the nodes of the causal network and elements of the DTF matrix were defined as the edges of the causal network, which were used to describe the network connectivity of the multi-region LFPs.
According to the multivariate autoregressive (MVAR) model, multi-region LFPs can be represented as a data vector X of N source signals:
Then the MVAR model can be expressed as:
In which p is the model order, calculated by the Bayesian information criterion. \({A}_{n}\) is the coefficient matrix of the MVAR model. E(t) is the vector of multivariate zero mean uncorrelated white noise at time t.
\(f\) refers to a specific frequency, \(H(f)\) is the transfer function matrix, \(A(0)=-I\), \(I\) is an identity matrix.
\({\gamma }_{{ij}}(f)\) represents the ratio between the effect from channel j to channel i and the combined effect from all other nodes to node j, k is the number of nodes, and \(H(f)\) is the transfer matrix of the system. The DTF values among LFPs were calculated and converted into a DTF matrix. \({{DTF}}_{{ij}}\) refers to the connectivity strength from channel \(i\) to channel \(j\).
The global efficiency of the network was calculated as shown followed:
Where \({g}_{i\leftrightarrow j}\) is the shortest path (geodesic) between i and j. \({E}_{i}\) is the efficiency of node i.
Information flow between diverse brain regions [32], \({{IF}}_{{ij}}\), indicates the information transfer from brain region i to region j.
Support vector machines
We used a binary-class support vector machine (SVM) with the Gaussian (RBF, Radial Basis Function) kernel for decoders to discriminate between two pairwise states: control/CSDS mice or ketamine/saline-treated mice. The data for analysis were acquired from control, CSDS, ketamine-treated and saline mice during social interaction test. Behavior (social index) and LFPs characteristics (PSD ratio, global efficiency and information flow in different frequency bands between many brain regions) were selected as SVM feature vectors.
We conducted feature selection by identifying features that exhibited significant differences in both LFPs patterns (Control/CSDS group and ketamine-treated/saline-treated group) and LFPs-behavior correlation (P < 0.05). We randomly divided the final dataset into training set (70%) and testing set (30%). During training, we used tenfold cross validation for parameters optimization to improve generalization ability and reduce overfitting. To control for local dependencies in both the behavioral data and the LFPs characteristics, we repeated the decoding procedure either with the behavioral labels shuffled in relation to the real data (shuffle control, 100 repetitions) or with the individual LFPs characteristic circularly shifted with random shifts relative to the real behavioral description (shift control, 100 repetitions) [33, 34]. Next, we used accuracy, ROC and AUC to evaluate the SVM performance. The accuracy was calculated from the confusion matrices of decoders. The receiver operator characteristic (ROC) curve was constructed by plotting the true positive rate (TPR) against the false positive rate (FPR). The area under ROC (AUC), a popular metric for reporting performance of binary classifiers, was calculated from ROC curve.
We further analyzed the weights of the learned features for binary classification by subtracting the accuracy of the full model (with all regions) to that of a reduced model (by shuffling the individual region LFPs data). This approach allowed us to assess the contribution of each region (or each frequency network) to the model’s overall performance.
A multi-class SVM with the Gaussian RBF kernel and a class-weighted loss function was employed to classify the three states: control, CSDS and ketamine-treated mice using identical feature engineering protocols. Similarly, tenfold cross validation and shuffle control were used. We calculated multiclass accuracy to assess SVM performance.
For the SVM analysis, we used MATLAB 2023a and libsvm 3.32. The detailed sample sizes for the machine learning approaches have been provided in Supplemental Table 3.
Histology
For verifying the multi-region electrode array locus, mice were deeply anesthetized with isoflurane and transcardially perfused with phosphate buffered solution (PBS), followed by a 4% paraformaldehyde (PFA) solution. Brains were post-fixed in 4% PFA overnight then dehydration of gradient sucrose (20% and 30%). Brains were sectioned into 40 μm thick slices using a freezing microtome (CM1950, Leica, Nussloch, Germany). Images were acquired using an optical microscope (VS200, Olympus, Tokyo, Japan).
Statistical analysis
Required sample sizes were estimated on the basis of published studies, although no statistical methods were used to predetermine sample sizes. Mice were randomly assigned to treatment groups. Analyses were performed in a manner blinded to treatment assignments in all behavioral experiments. Based on pre-established criteria, values were excluded from analysis if electrode implant sites were out of the targeted eight regions. All data are presented as mean ± s.e.m., analyzed using MATLAB 2020b and GraphPad Prism version 9. All statistical tests were two-sided, and the significance level was set at 0.05. *P < 0.05, **P < 0.01 and ***P < 0.001. Normal distribution and equal variances between groups were tested for each experiment. Between-group comparisons of behavioral results (SIT, SPT, TST) were conducted using unpaired t-test in Control/CSD groups and Ketamine/Saline-treated groups. Two-way Repeated Measures ANOVA with Šídák’s multiple comparisons test was used for PSD ratio analysis in CSDS/Control and Ketamine/Saline-treated. Ordinary two-way ANOVA with Šídák’s multiple comparisons test was performed for global efficiency analysis in CSDS/Control and Ketamine/Saline-treated. Pearson’s or Spearman correlation was performed for LFPs-behavior correlation analysis when linearity of samples was achieved or not, respectively.
To evaluate the performance of the SVM models, we used standard metrics including accuracy, ROC, and AUC. Additionally, unpaired t test was employed to compare accuracy between Real and Random data from eight brain regions. Ordinary one-way ANOVA followed by post hoc multiple comparisons with Tukey’s test was used to compare the classification effects of the models and the weight analysis of individual brain region or frequency network in Control/CSDS classification model.
Results
Multi-region electrode array records in vivo electrophysiological signals
Eight brain regions that are implicated in social avoidance induced by CSDS were selected for in-vivo recording. The list includes prelimbic cortex (PrL), infralimbic cortex (IL), cingulate area 1 (Cg1), basolateral amygdala (BLA), nucleus accumbent (NAc), lateral habenula (LHb), ventral hippocampus (vHPC) and ventral tegmental area (VTA). Each of these regions has dense connections with other recorded regions. Then we implanted the custom multi-region electrode arrays. Recordings started about one week after the surgery. We simultaneously recorded in vivo electrophysiological activities from eight brain regions during social interaction behavior (Fig. 1A–C). Histology was acquired for all experimental animals. Only correctly targeted brain regions were used for subsequent data analysis (Fig. 1D; n = 30 mice for BLA, Cg1, IL, LHb, NAc and PrL from Control group; n = 28 mice for vHPC and VTA from Control and CSDS group; n = 31 mice for BLA, Cg1, IL, LHb, NAc and PrL from CSDS group; n = 13 mice for BLA, Cg1, IL, LHb, NAc, PrL, vHPC and VTA from Ketamine and Saline-treated group).
Mesoscale brain-wide fluctuation analysis(MBFA) framework
To study the relationship between widespread mesoscale dynamics and depressive pathology, we developed a framework termed the ‘mesoscale brain-wide fluctuation analysis’ (MBFA) platform, based on LFPs from eight brain regions. We collected data on mouse social interaction behavior along with simultaneous neural signals from these widespread brain regions (Fig. 2A). Our framework consists of the following steps: Firstly, the PSD ratio of each brain region in different frequency bands during the social interaction test was calculated (see Fig. 2B for details). Next, we constructed the functional brain network based on the eight brain regions and computed brain network parameters: global efficiency and information flow (Fig. 2C). Then, we introduced oscillation and network indicators with statistical differences as feature selection to the support vector machine (SVM) model (Fig. 2D). Finally, we implemented a hierarchical classification framework comprising: (i) two binary SVMs distinguishing Control vs CSDS and Ketamine vs Saline groups, and (ii) a multiclass SVM discriminating Control/CSDS/Ketamine. The performance of SVM models were visualized via accuracy, ROC, and AUC (Fig. 2E).
A Sample LFP traces recorded concurrently from eight implanted brain regions. Brain activities were obtained during social interaction behavior. B Schematic of the workflow for neural oscillation analysis of multiple brain regions signals. For each mouse, the PSD ratio of eight brain areas during SIT across the designated trial was calculated. C Schematic of the workflow for network analysis of multiple brain regions signals. For each mouse, the network of eight brain areas during SIT across the designated trial was constructed based on the directional transfer function. Global efficiency and information flow were then calculated. D, E Schematic of SVM workflow. There were two separate binary classifications and one multi-class classification. The features used in the SVM included behavioral data (social index) and LFP characteristics (PSD Ratio, global efficiency, and information flow across different frequency bands between brain regions). We selected features that exhibited significant differences in LFPs among the comparison groups (Control vs. CSDS, Ket vs. Sal, Control vs. CSDS vs. Ket) and significant correlation in LFPs characteristics-SI. The dataset was randomly parsed into a training set (70%) and a test set (30%). Accuracy, ROC curves, and AUC metrics were employed for assessing model performance.
Paralleling classic fMRI research that reveals functional connectivity in the human brain, our MBFA platform identified activity correlated across multiple brain regions over seconds. Moreover, our model enabled the analysis of fast oscillatory electrical signals on a millisecond timescale, offering greater temporal resolution than fMRI across brain regions. The application of SVM allowed us to discriminate specific LFP patterns corresponding to different status (normal, depressive and ketamine-treated) from oscillatory and brain network perspective, respectively. Consequently, we could assess the effectiveness of ketamine treatment in alleviating rapidly depressive-like symptoms based on the classification results.
CSDS results in depressive-like behaviors
Male C57 mice were subjected to CSDS and subsequently evaluated depression-like behaviors (Fig. 3A). Following CSDS in C57 mice, we performed a social interaction test (SIT) to assess sociable behavior (Fig. 3B). We found that the CSDS group exhibited less social interaction with the target mouse compared with the control group (Fig. 3C) and had a lower SI ratio (Fig. 3D). These results indicate that CSDS leads to social avoidance in mice. Moreover, CSDS and control group had no significant difference in total movement distance and velocity during SIT (Fig. 3E, F). It shows that CSDS does not impact mice’s ability of locomotion. Next, despair behavior was assessed using the tail suspension test (TST). Mice exposed to CSDS displayed a significantly higher level of desperation compared to stress-naïve mice (Fig. 3G). Lastly, we performed the sucrose preference test (SPT) and found that the CSDS group had a lower sucrose preference than the control group, suggesting CSDS could promote anhedonia (Fig. 3H). Together, these data demonstrate that CSDS drives depressive like behaviors.
A Experimental and 10-day chronic social defeat stress (CSDS) timeline. B Schematic of SIT. Two stages: mouse was placed inside an open field with an empty cage and inside an open field with a cage contained a CD1. Gray area referred to social interaction zone. C Representative traces of control and CSDS mice during SIT (‘with CD1’ phase and ‘No CD1’ phase). D Social index of control and CSDS mice (unpaired t-test; CSDS: 0.46 ± 0.08; Control: 1.30 ± 0.06; t = 8.71, ***P < 0.001). E Total movement distance of control and CSDS mice in SIT (unpaired t-test; CSDS: 2150 cm ± 41.68 cm; Control: 2190 cm ± 57.30 cm; t = 0.57, P = 0.5763). F Velocity of control and CSDS mice in SIT (unpaired t-test; CSDS: 3.04 cm/s ± 0.12 cm/s; Control: 3.06 cm/s ± 0.16 cm/s; t = 0.08, P = 0.9399). G Immobility time of control and CSDS mice in TST (unpaired t-test; CSDS: 180.2 s ± 6.36 s; Control: 135.1 s ± 5.47 s; t = 5.37, ***P < 0.001). H Sucrose preference of control and CSDS mice in SPT (unpaired t-test; CSDS: 60.33% ± 1.48%; Control: 77.54% ± 0.88%; t = 10.04, ***P < 0.001). D–H: All data are shown as mean±s.e.m. For Control group: n = 8 mice; For CSDS group: n = 8 mice.
CSDS impacts neural oscillations in multiple brain regions
Brain electrophysiological activities were recorded in eight brain regions during SIT. To evaluate the neurophysiological responses to CSDS, we analyzed the multiple regions of oscillations in the interaction zone during SIT. Example PSD curves from the Cg1 region of Control (left) and CSDS (right) mice during SIT are shown in Fig. 4A. The PSD ratio curve of CSDS was distinct from the curve of Control and had a higher gradient across all frequencies (Fig. 4B). We further made a statistical comparison of the PSD ratio across diverse frequency ranges in eight brain regions. A two-way Repeated Measures ANOVA (with Šídák’s multiple comparisons test) exhibited that the oscillation in the CSDS group showed significant changes in most frequency ranges and brain regions compared to the control group, except low theta (in BLA, Cg1, IL, LHb, PrL, vHPC and VTA) and high theta in LHb (Fig. 4C; For BLA, a significant effect of groups: F(1,59) = 15.88, P = 0.0002; For Cg1, a significant effect of groups: F(1,59) = 15.89, P = 0.0002; For IL, a significant effect of groups: F(1,59) = 11.96, P = 0.001; For LHb, a significant effect of groups: F(1,59) = 9.788, P = 0.0027; For NAc, a significant effect of groups: F(1,59) = 15.03, P = 0.0003; For PrL, a significant effect of groups: F(1,59) = 15.14, P = 0.0003; For vHPC, a significant effect of groups: F(1,54) = 13.46, P = 0.0006; For VTA, a significant effect of groups: F(1,54) = 11.22, P = 0.0015; The detailed statistic results in frequency bands were shown in Supplemental Table 1). These suggest that CSDS widely impacts oscillation power in multiple regions.
A Example PSD curves from the Cg1 of Control (left) and CSDS (right) mice during social interaction test. Red lines indicate With CD1. Black lines indicate No CD1. B Mean PSD ratio curves from the Cg1 of Control and CSDS mice during social interaction. Data are mean ± s.e.m.. Orange lines indicate control group. Blue lines indicate CSDS group. C The comparison diagram of mean PSD ratio within Control and CSDS groups in diverse frequency bands. Two-Way repeated measures ANOVA with Šídák’s multiple comparisons test. The detailed statistic results were shown in Supplemental Table 1. D Pearson correlation analysis between mean PSD ratio and social index in high theta frequency band from Cg1. Pearson correlation analysis. r = −0.5318, *** P < 0.0001. E Pearson correlation analysis between mean PSD ratio and social index for eight brain regions in all frequency bands. The detailed correlation statistic results were shown in Supplemental Table 2. Black dotted line represents the significance level: 0.05. F Binary classification accuracy based on the oscillation features from individual brain region for Real and Shuffle data. Unpaired t-test. BLA: 72.62% ± 0.20%, t = 22.89, P < 0.001; NAc: 71.73% ± 0.18%, t = 21.33, P < 0.001; Cg1: 71.71% ± 0.17%, t = 21.81, P < 0.001; PrL: 70.28% ± 0.17%, t = 18.34, P < 0.001; LHb: 68.75% ± 0.21%, t = 20.28, P < 0.001; vHPC: 68.64% ± 0.19%, t = 22.65, P < 0.001; VTA: 68.59% ± 0.25%, t = 19.05, P < 0.001; IL: 65.59% ± 0.33%, t = 20.06, P < 0.001. G Binary classification accuracy based on the oscillation features from any two brain regions. Colors in bottom left indicate accuracies and some of them are significant different from their random. ***P < 0.001. H Binary classification accuracy based on the oscillation features from multiple brain regions (start from BLA, other brain regions were successively added as participants in order of individual brain area classification accuracy). An ordinary one-way ANOVA with Tukey’s multiple comparisons test. F(7,792) = 48.06, P < 0.001; BLA: 72.62% ± 0.20%, indicators number 2: 74.52% ± 0.35%, BLA vs 2: P = 0.0032; indicators number 3: 76.57% ± 0.36%, BLA vs 3: P < 0.001; indicators number 4: 77.03% ± 0.34%, BLA vs 4: P < 0.001; indicators number 5: 78.57% ± 0.35%, BLA vs 5: P < 0.001; indicators number 6: 78.64% ± 0.35%, BLA vs 6: P < 0.001; indicators number 7: 78.41% ± 0.39%, BLA vs 7: P < 0.001; indicators number 8: 80.18% ± 0.32%, BLA vs 8: P < 0.001. I The weight of individual brain region in Control/CSDS classification model. An ordinary one-way ANOVA with Tukey’s multiple comparisons test. F (7,792) = 12.93, P < 0.001. SBLA: −5.47% ± 0.17%, SVTA: −4.06% ± 0.31%, SBLA vs SLHb: P = 0.0096; SPrL: −3.43% ± 0.27%, SBLA vs SPrL: P < 0.001; SCg1: −3.38% ± 0.30%, SBLA vs SCg1: P < 0.001; SIL: −2.41% ± 0.28%, SBLA vs SIL: P < 0.001. C, E–I: All data were shown as mean±s.e.m. For BLA, Cg1, IL, LHb, NAc, PrL in Control: n = 30 mice; For vHPC, VTA in Control: n = 28 mice; For BLA, Cg1, IL, LHb, NAc, PrL in CSDS: n = 31 mice; For vHPC, VTA in CSDS: n = 28 mice. *P < 0.05, **P < 0.01, ***P < 0.001.
To characterize the relationship of multi-region oscillations to behavioral states, we conducted correlation analysis to investigate the relation between the PSD ratio in different frequency bands in different brain areas and the social index (Fig. 4D, E). We found that the PSD ratio in many frequency bands (high theta, beta, low gamma, and high gamma) was negatively correlated with SI during social interaction, which was consistent with the outcome of the PSD ratio.
Next, we selected the PSD ratios with statistically significant differences to train a support vector machine decoder to classify Control and CSDS mice based on individual brain region. Decoder performance was high when training and testing were performed on the real data (Fig. 4F, Real data), and dropped when testing were performed on shuffle data (Fig. 4F, Shuffle data). It shows all eight brain regions have classification capability based on oscillation and all brain regions might participate in the CSDS-induced social interaction dysfunction. To explore potential coordinated effects between regions, we systematically trained SVM classifiers using all possible two-region feature combinations (28 unique pairs from eight regions). As shown in Fig. 4G, all pairs of region indicators had high accuracies (above 70%), especially in the BLA-NAc, Cg1-LHb, BLA-vHPC, and LHb-NAc brain region pairs (accuracy, 75.6%, 74.8%, 74.7%, and 74.4%, respectively). These results exhibited the classification accuracy was high with individual BLA oscillation features (Fig. 4F) and many brain region pairs involved in BLA also showed high accuracy. Therefore, starting with the BLA, other brain regions were sequentially added as participants based on the order of individual brain area classification accuracy. These oscillatory features were employed to the SVM. The accuracy increased with more brain regions participating and was significantly higher than the accuracy of BLA (Fig. 4H, F (7792) = 48.06, P = 0.001). When all brain areas participated in the decode, the accuracy reached the highest (accuracy, 80.2%). These findings suggest that the oscillations of all eight brain regions contribute to classify Control/CSDS mice.
To further know how much each specific region would contribute to the accuracy of the model, we calculated a reduced model. Our results showed that the model accuracy decreased substantially when the BLA feature was randomized, whereas the accuracy decreased much less when the IL feature was randomized (Fig. 4I). These findings indicate that the BLA likely plays a relatively important role in contributing to CSDS-induced social behavior dysfunction.
CSDS affects the eight-brain-region network and information transmission
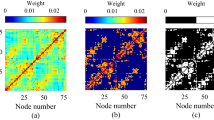
According to the oscillation results, we selected all eight brain regions for the network investigation. We defined the networks using eight regions, from which we extracted the functional connectivity metrics using the graph theory framework. All regions will be defined as nodes in our graph theory analysis. Next, we computed global efficiency as a measure of network functional integration. Our result exhibited a general lower global efficiency in CSDS mice and had a statistically significant reduction in high theta, beta, low gamma, and high gamma compared with the control mice (Fig. 5A). It reflects that CSDS damages brain network function, including the capability of parallel information exchange and integration of processes. Furthermore, global efficiency showed a significantly positive correlation with social interaction ratio in high theta, beta, low gamma, and high gamma (Fig. 5B, C).
A Global efficiency of Control and CSDS mice in the frequency bands. Ordinary Two-Way ANOVA with Šídák’s multiple comparisons test; a significant effect of group, F (1,270) = 32.11, P < 0.001. For low theta; CSDS: 0.0779 ± 0.0039, Control: 0.0793 ± 0.0030; P = 0.7024. For high theta; CSDS: 0.0644 ± 0.0022, Control: 0.0724 ± 0.0028; P = 0.0267. For beta; CSDS: 0.0734 ± 0.0027, Control: 0.0892 ± 0.0030; P < 0.001. For low gamma; CSDS: 0.0823 ± 0.0030, Control: 0.0950 ± 0.0025; P = 0.0018. For high theta; CSDS: 0.0864 ± 0.0029, Control: 0.0996 ± 0.0023; P = 0.0016. B Pearson correlation analysis between global efficiency and social index in high theta frequency band. Spearman correlation analysis. r = 0.3111, * P = 0.0196. C Pearson correlation analysis between global efficiency and social index for all frequency bands. The detailed correlation statistic results were shown in Supplemental Table 2. Black dotted line represents the significance level: 0.05. D Chord diagrams of information flow with significant change between Control and CSDS mice for individual frequency band. Different colors represent various brain regions. The arrows indicate the direction of information flow (IF), while the width of each arrow corresponds to the significance level of the change in IF between the CSDS and Control groups. E The contribution of individual frequency network in Control/CSDS classification. An ordinary one-way ANOVA with Tukey’s multiple comparisons test. F (4,495) = 21.19, P < 0.001. An ordinary one-way ANOVA with Tukey’s multiple comparisons test. F (4,495) = 21.19, P < 0.001. Slow theta: −5.75% ± 0.45%, Shigh theta: −2.80% ± 0.44%, Slow theta vs Shigh theta: P < 0.001; Slow gamma: −1.05% ± 0.36%, Slow theta vs Slow gamma: P < 0.001; Shigh gamma: −2.47% ± 0.39%, Slow theta vs Shigh gamma: P < 0.001. F Binary classification accuracy based on diverse learned features (oscillation, network and their combination). An ordinary one-way ANOVA with Tukey’s multiple comparisons test. F (2, 297) = 29.20, P < 0.001. Oscillation: 77.19% ± 0.28%, Network: 78.52% ± 0.28%, Both: 80.06% ± 0.24%. Oscillation vs Network: P = 0.0013; Oscillation vs Both: P < 0.001; Network vs Both: P < 0.001. G ROC curves of binary classification model based on different learned features (oscillation, network and their combination). H AUC of binary classification model based on different learned features (oscillation, network and their combination). An ordinary one-way ANOVA with Tukey’s multiple comparisons test. F (2, 297) = 296.5, P < 0.001. Oscillation: 0.7952 ± 0.0024, Network: 0.78581 ± 0.0025, Both: 0.8735 ± 0.0023. Oscillation vs Network: P < 0.001; Oscillation vs Both: P < 0.001; Network vs Both: P < 0.001. All data are shown as mean±s.e.m. Data were from Control group (n = 28 mice) and CSDS group (n = 28 mice). * P < 0.05, ** P < 0.01, *** P < 0.001.
Information flow (IF) of pairs of region was identified based on the functional connectivity metrics. The chord diagrams displayed IF with significant changes between Control and CSDS groups (Fig. 5D). The colors used in the figure represented diverse brain regions The arrows indicate the direction of IF, while the width of each arrow corresponds to the significance level of the change in IF between the CSDS and Control groups. We found that the information output of NAc, PrL, and vHPC and the information inflow of IL varied considerably in all frequency bands. The output of LHb and the input of Cg1 varied greatly in low frequency, while the outflow of Cg1 changed significantly in high frequency. These confirm that CSDS refers to the imbalances of multiple brain regions and damages the brain-wide network.
Oscillation and multi-region brain network could be used for classification
Similarly, we calculated the weight of specific frequency band network to investigate how individual frequency networks contribute to accuracy. The results showed in Fig. 5E. We then used all-region oscillation features, all-frequency network features and both features in Control/CSDS SVM model, respectively. The accuracy with network features was significantly higher than the accuracy with oscillation features and the accuracy with combination of the two (oscillation and network) was significantly higher than their individual (Fig. 5F–H). These data demonstrates multi-region network could better classify CSDS and Control mice compared with oscillatory features. The combination of them (Both features) exhibits the best discrimination performance. These findings indicate that networks across multiple frequency bands likely play a relatively important role in contributing to CSDS-induced social behavior dysfunction.
Ketamine rapidly alleviates social avoidance behavior and improves brain network function
After exposure to 10 days of CSDS, mice were injected with ketamine (intraperitoneal, 20 mg/kg). An hour after ketamine injection, we tested mice for social interaction behavior (Fig. 6A). The social index of the ketamine-treated group was significantly increased without a change in motion ability, but not in the saline group (Fig. 6B–D). It explains that ketamine enhances social interaction capacity and alleviates social avoidance behavior in a rapid response. Similar to the behavioral results, the PSD ratio of eight different brain regions showed significant changes, with notable enhancements observed in the high theta and low gamma frequency bands (Fig. 6E; For BLA, a significant effect of groups: F (1,24) = 5.231, P = 0.0313; For Cg1, a significant effect of groups: F (1,24) = 5.273, P = 0.0307; For IL, no significant effect of groups: F (1,24) = 3.299, P = 0.0818; For LHb, no significant effect of groups: F (1,24) = 3.782, P = 0.0636; For NAc, no significant effect of groups was noted: F (1,24) = 4.120, P = 0.0536; For PrL, no significant effect of groups: F (1,24) = 3.465, P = 0.075; For vHPC, a significant effect of groups: F (1,24) = 8.120, P = 0.0088; For VTA, a significant effect as well: F (1,24) = 6.341, P = 0.0189). The correlation between PSD Ratio and SI displayed a negative relation and had significant correlation in different frequency bands (Fig. 6F, G). The global efficiency of the eight-region brain network in all frequency bands increased and had a significant increase in the low theta and high theta in ketamine-treated animals (Fig. 6H–J). We found that both oscillation and network had prominently variation in high theta frequency band. We thus focused on this specific frequency band and calculated the IF (Fig. 6K). The histograms quantified the inflow and outflow of information for each brain region and exhibited the directionality of information flow between the eight brain regions in high theta (Fig. 6L). The results exhibited that ketamine significantly altered the information outflow from the PrL and vHPC. Additionally, there were significant changes in the information inflow of the VTA and NAc. These indicate ketamine impacts on electrophysiological activities at the brain-wide level and enhances widespread output.
A Experimental design for behavioral tests and in-vivo electrophysiological signals acquisition after intraperitoneal injection of ketamine or saline. B Heatmaps representation of the time spent in SIT in Ketamine- and Saline-treated mice. C Social index of saline and ketamine mice (unpaired t-test, Ketamine-treated: 1.07 ± 0.13, Saline-treated: 0.36 ± 0.02, t = 5.41, P < 0.001). D Velocity of saline and ketamine mice in SIT (unpaired t-test, Ketamine-treated: 2.79 cm/s ± 0.20 cm/s, Saline-treated: 2.93 cm/s ± 0.25 cm/s, t = 0.44, P = 0.6673). E The comparison diagram of mean PSD ratio within Ketamine- and Saline-treated groups in devise frequency bands. Two-Way repeated measures ANOVA with Šídák’s multiple comparisons test. The detailed statistic results were shown in Supplemental Table 1. F Pearson correlation analysis between mean PSD ratio and social index in high theta frequency band from Cg1. Pearson correlation analysis. r = −0.4877, * P = 0.01. G Pearson correlation analysis between mean PSD ratio and social index for eight brain regions in all frequency bands. The detailed correlation statistic results were shown in Supplemental Table 2. Black dotted line represents the significance level: 0.05. H Global efficiency of Ketamine- and Saline-treated mice in all frequency bands. Two-Way ANOVA with Šídák’s multiple comparisons test; a significant effect of group, F (1, 120) = 18.33, P < 0.001. For low theta; Ketamine-treated: 0.0820 ± 0.0035, Saline-treated: 0.0662 ± 0.0057; P = 0.0237. For high theta; Ketamine-treated: 0.0744 ± 0.0039, Saline-treated: 0.0569 ± 0.0025; P = 0.0093. For beta; Ketamine-treated: 0.0837 ± 0.0034, Saline-treated: 0.0704 ± 0.0028; P = 0.0816. For low gamma; Ketamine-treated: 0.0896 ± 0.0038, Saline-treated: 0.0853 ± 0.0034; P = 0.9432. For high theta; Ketamine-treated: 0.0924 ± 0.0045, Saline-treated: 0.0907 ± 0.0043; P = 0.9991. I Pearson correlation analysis between global efficiency and social index in high theta frequency band. Spearman correlation analysis. r = 0.5269, ** P = 0.0057. J Pearson correlation analysis between global efficiency and social index for all frequency bands. The detailed correlation statistic results were shown in Supplemental Table 2. Black dotted line represents the significance level: 0.05. K Chord diagrams of information flow with significant change between Ketamine- and Saline-treated mice in high theta frequency band. Different colors represent various brain regions. The arrows indicate the direction of information flow (IF), while the width of each arrow corresponds to the significance level of the change in IF between the Ketamine- and Saline-treated groups. L Histograms quantify the inflow and outflow of information and exhibit the directionality of information flow between the eight brain regions in high theta. M Binary classification accuracy based on diverse learned features (oscillation, network and their combination). An ordinary one-way ANOVA with Tukey’s multiple comparisons test. F (2, 297) = 393.9, P < 0.001. Oscillation: 79.95% ± 0.30%, Network: 89.37% ± 0.28%, Both: 89.09% ± 0.23%. Oscillation vs Network: P = 0.0013; Oscillation vs Both: P < 0.001; Network vs Both: P = 0.7429. N ROC curves of binary classification model based on different learned features (oscillation, network and their combination). O AUC of binary classification model based on different learned features (oscillation, network and their combination). An ordinary one-way ANOVA with Tukey’s multiple comparisons test. F (2, 297) = 534.8, P < 0.001. Oscillation: 0.8599 ± 0.0032, Network: 0.9532 ± 0.0018, Both: 0.9527 ± 0.0016. Oscillation vs Network: P < 0.001; Oscillation vs Both: P < 0.001; Network vs Both: P = 0.9897. All data are shown as mean±s.e.m. B–D: n = 8 for Ketamine-treated group, n = 8 for Saline-treated group; E–O: n = 13 for Ketamine-treated group, n = 13 for Saline-treated group. * P < 0.05, ** P < 0.01, *** P < 0.001, n.s. P > 0.05.
The MBFA platform could apply to evaluate ketamine’s rapid antidepressant effects
We conducted the rapid antidepressant ketamine experiment to explore its improvement effects on depression through behavioral assessments and LFPs. To further evaluate ketamine’s rapid antidepressant effects, we employed the MBFA platform to achieve binary and three-class classifications.
As mentioned in previous section, we used all-region oscillation features, all-frequency network features and both features in Ketamine/Saline-treated discrimination model, respectively. The binary classification had a greater accuracy in network features compared with oscillation features. The accuracy with combination of the two (oscillation and network) was also significantly higher than the accuracy with oscillation features, while it had no significant different compared with network features (Fig. 6M–O). These results indicate that ketamine exerts influence on both neural oscillations and network features, and its effect on network features is more pronounced.
Moreover, we initially trained a three-class SVM model based on three groups: Control, CSDS, and ketamine-treated group. The results showed that using oscillation features achieved a higher accuracy for classifying the three groups. (Oscillation accuracy: 81.63% ± 0.22%; Network accuracy: 67.35% ± 0.27%; unpaired t test, P < 0.001) The accuracy of three-class SVM model with network features was significantly lower than the accuracy with oscillation features. These results demonstrate that both network features and oscillation features can be utilized to classify the three groups.
In summary, these are consistent with the fact that ketamine acts on NMDA receptors that are widely distributed in the brain, and its modulation of brain network function may play a crucial role in alleviating social avoidance behavior. Our results verify the application of our MBFA platform and might assisted illustrate that ketamine has potent antidepressant effects in a brain-wide range.
Discussion
Numerous animal studies aim to investigate how the coordination of multiple brain regions in time and space affects specific emotional states. They link neural synchrony at the millisecond timescale to emotional and cognitive behavior and promote the causal role of neural synchrony in encoding emotional behavior [16, 30, 35, 36]. The research focuses on the selective manipulation of cellular activity within specific circuit nodes to alter emotional state and behavior [37]. Nonetheless, measures and studies of brain-wide neural synchrony are rarely mentioned. While further studies also reveal that activity within a circuit node has an effect on activity in other brain neural circuits underlying emotional pathology [38]. Given the above, the brain network, as a convergence point for our comprehension of psychiatric and neurological disorders, is the crux for advancing the treatment of neuropsychiatric diseases [39]. We thus constructed a MBFA platform that could quantitatively analyze the synergistic encoding of the brain-wide region network and employed it to explore depressive social interaction dysfunction. The platform is based on a cross-disciplinary approach, including multi-region in vivo recordings in mice, chronic social defeat stress, ketamine regulation, informatics analysis, and machine learning methods. Our analytical platform allowed us to investigate the impact on mesoscale brain-wide LFPs’ fluctuations and reveal ketamine’s rapid antidepressant.
Recent studies provide a comprehensive map of structural connectomics disorders throughout the brain of patients with MDD [40,41,42]. The topological variations of the structural (white matter) brain network indicate an overall decrease in connectivity strength and global efficiency [43]. A literature review of macaques shows that deep brain stimulation induces structural connectivity (white matter) remodeling and functional changes to brain-wide networks [44]. It demonstrates that structural connectivity disturbances entail potential functional consequences. Related research verifies that resting-state functional magnetic resonance imaging (rfMRI) in depressive mice displays a brain-wide abnormality, especially decreased resting-state functional connectivity (rsFC) in mPFC-related pathways and optogenetic activation of mPFC neurons rescues decreased rsFC and depressive-like behaviors [45]. Our prior finding examines differences in PFC oscillation underlying the social behavioral state that accompany the emergence of stress-induced behavioral dysfunction [46]. Strikingly, multiple studies confirm the correctness of our findings: CSDS induces aberrant changes in the intra-regional power of oscillations across multiple frequency bands and a reduced global efficiency of the mesoscale brain-wide network in general frequency bands.
However, we still unclear if LFPs patterns after ketamine is more similar to the Control mice, or whether it is more of a compensatory state. Here, we applied the SVM model, which was initially trained on the Control and CSDS groups, to classify the ketamine-treated group. This binary classification approach aimed to determine whether ketamine treatment resulted in distinct brain activity patterns compared to the Control and CSDS groups. Notably, feature selection for the ketamine-treated group was conducted using the same protocol as for the control and CSDS groups, focusing on neural oscillation and network features that exhibited statistical differences between Control and CSDS groups. When only network features were used, the ketamine-treated mice could be classified as Control group in an accuracy of 63.28%, whereas it was distinguished as CSDS group with 88.28% accuracy (Oscillation features). These results indicated that ketamine-treated mice could more likely to be classified as Control mice when using network features. On the other hand, when applying oscillation features, ketamine-treated mice are more likely to be classified as CSDS group. Combined, the above results indicate that ketamine primarily exerts a compensatory effect through network features (such as network global efficiency and inter-brain-region information flow), which contributes to its rapid antidepressant effect.
Nowadays, pharmacological interventions remain the primary treatment for depression [47, 48]. Ketamine is a non-competitive N-methyl-D-aspartate receptor (NMDAR) antagonist that blocks the ion permeation pore located inside the NMDAR. So far, various hypotheses about ketamine’s mechanism have been proposed. One hypothesis is that of NMDAR disinhibition. The theory postulates that the antidepressant response to ketamine is initiated by selective blockade of NMDARs on gamma-aminobutyric acid (GABA)-ergic inhibitory interneurons. It reduces interneuronal activity and leads to disinhibition of pyramidal neurons, which enhances glutamatergic firing. The above processes could produce α-amino-3-hydroxy- 5-methyl-4-isoxazole propionic acid receptor (AMPAR) activation, resulting in an antidepressant effect [49,50,51,52]. In our study, we focused on developing a mesoscale brain-wide fluctuation analysis platform which is accompanied by additional verification from the rapid antidepressant experiment involving ketamine. Thus, we opted for a rapid antidepressant experiment where we conducted SIT one hour after ketamine (20 mg/k) injection. The acute antidepressant experiment yielded a significant reversal of CDSD-induced social interaction dysfunction and electrophysiological data reflecting a significant neuronal response. Besides, ketamine manifests a sustained antidepressant effect and 10 mg/kg is commonly used as a subanesthetic dose of ketamine without the interference of sedation or other acute effects. Hu’s research reveals it with significant benefits noticeable at 1 h, 24 h, 3 days, and even 7 days post-administration [53]. Evidence also shows the CSDS-induced social avoidance phenotype could persist in a long term (maintain 28 days) [54, 55]. Therefore, assessing the effects of ketamine over a longer period and other dose would provide a more robust evaluation of its potential as an antidepressant for the MBFA platform further development.
Also, evidence shows NMDAR antagonists increase gamma power amplitude in both human and animal models, predominantly for the use of ketamine [56]. In human intracranial recording research, ketamine produces increased gamma oscillation (25–55 Hz) power in the inferior frontal area and a reduction of low-frequency oscillation (8–15 Hz) power in the inferior frontal area [57]. A marked increase in EEG gamma oscillations is a response to ketamine in the Wistar Kyoto rat model of treatment-resistant depression [58]. It is also demonstrated that ketamine-induced power increases in broadband gamma arise from asynchronous activities via genetic alteration of PV interneurons [59]. Our study, in which we exhibit ketamine recovery oscillation power at high theta and low gamma in a widespread brain region, completely supports these findings. Related literature reported that ketamine had an antidepressant effect in animal model of depression and increased brain-wide gamma power [60]. As researches mentioned, ketamine is likely to act on multiple brain regions and multiple molecular partners. LHb and BLA, as primary brain targets of ketamine action, its widespread projections have global effects in many brain regions [61, 62]. And our results of information flow (IF, Fig. 6L) showed most brain regions had significant changes after ketamine in high theta frequency band. These clarified that the different changes of individual oscillatory IF from multiple brain regions might form together a compensating for LHb to achieve an antidepressant effect after ketamine-treated.
Biologically relevant oscillations are considered reliable markers of brain operation. Thereinto, LFPs exhibit multiple spectral and temporal gradients and widespread task dependence. Neural signals in beta power could predict the rapid antidepressant effects of deep brain stimulation. The research on depression aims to reveal pathogenesis comprehensively and potentially derive mechanisms of therapeutic pharmacological effects; both activity and connectivity have to be estimated for many brain regions [63,64,65]. Prior work presents an optimized, interpretable machine-learning approach to extracting and quantifying neural changes caused by anti-dopaminergic drugs [66]. These investigations provide powerful support for our findings. Another report shows an optimal balance between model complexity and performance with models using three LFP features [67]. These are in accordance with the predicted results from our mesoscale brain-wide network analysis platform, which show that accuracy reaches nearly 90% when both oscillation and network features are included in the SVM classifier.
Overall, our mesoscale brain-wide network analysis platform provides direct insight into the application of LFP-related biomarkers to discriminate between depression-related brain states and reveal ketamine’s rapid antidepressant. However, it is important to consider the limitations of the platform. While this platform fails to introduce cross-frequency features into the model prediction, Existing literature shows that cross-frequency connectivity for delta-alpha and delta-gamma bands is associated with ketamine’s antidepressant effects in MDD participants [68]. We will add the relevant analytical methods for a thorough depression investigation. Like other machine learning approaches, the results of the platform identified for a brain state will change depending on the model used to conduct the analysis. After networks are identified in a variety of depressive-like behavioral dysfunctions with a generalizable mesoscale brain-wide network, variations can be analyzed on an individual basis based on the differences. Long term, we can learn a complete platform of many different depressive-like behavior-related networks for a better mechanistic interpretation of these relevant neurophysiological events.
Code availability
The code that generates the findings of this study are available from the corresponding author, upon reasonable request.
References
World Health O. Comprehensive mental health action plan 2013–2030. Geneva: World Health Organization; 2021. p. 2021.
Tseng Y-T, Zhao B, Ding H, Liang L, Schaefke B, Wang L. Systematic evaluation of a predator stress model of depression in mice using a hierarchical 3D-motion learning framework. Transl Psychiatry. 2023;13:178.
Bordes J, Miranda L, Reinhardt M, Narayan S, Hartmann J, Newman EL, et al. Automatically annotated motion tracking identifies a distinct social behavioral profile following chronic social defeat stress. Nat Commun. 2023;14:4319.
Fan L, Yang L, Li X, Teng T, Xiang Y, Liu X, et al. Proteomic and metabolomic characterization of amygdala in chronic social defeat stress rats. Behav Brain Res. 2021;412:113407.
Tian X, Russo SJ, Li L. Behavioral animal models and neural-circuit framework of depressive disorder. Neurosci Bull. 2025;41:272–88.
Yavi M, Lee H, Henter ID, Park LT, Zarate CA. Ketamine treatment for depression: a review. Discover Mental Health. 2022;2:9.
Murphy N, Tamman AJF, Lijffijt M, Amarneh D, Iqbal S, Swann A, et al. Neural complexity EEG biomarkers of rapid and post-rapid ketamine effects in late-life treatment-resistant depression: a randomized control trial. Neuropsychopharmacology. 2023;48:1586–93.
Dong Y, Li Y, Xiang X, Xiao Z-C, Hu J, Li Y, et al. Stress relief as a natural resilience mechanism against depression-like behaviors. Neuron. 2023;111:3789–3801.e3786.
Hack LM, Zhang X, Heifets BD, Suppes T, van Roessel PJ, Yesavage JA, et al. Ketamine’s acute effects on negative brain states are mediated through distinct altered states of consciousness in humans. Nat Commun. 2023;14:6631.
Daws RE, Timmermann C, Giribaldi B, Sexton JD, Wall MB, Erritzoe D, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28:844–51.
Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38.
Carboni E, Carta AR. BDNF alterations in brain areas and the neurocircuitry involved in the antidepressant effects of ketamine in animal models, suggest the existence of a primary circuit of depression. JIN. 2022;21:144.
Tura A, Goya-Maldonado R. Brain connectivity in major depressive disorder: a precision component of treatment modalities? Transl Psychiatry. 2023;13:196.
Du L, Liu H, Du W, Chao F, Zhang L, Wang K, et al. Stimulated left DLPFC-nucleus accumbens functional connectivity predicts the anti-depression and anti-anxiety effects of rTMS for depression. Transl Psychiatry. 2018;7:3.
Mague SD, Talbot A, Blount C, Walder-Christensen KK, Duffney LJ, Adamson E, et al. Brain-wide electrical dynamics encode individual appetitive social behavior. Neuron. 2022;110:1728–1741.e1727.
Laine MA, Sokolowska E, Dudek M, Callan S-A, Hyytiä P, Hovatta I. Brain activation induced by chronic psychosocial stress in mice. Sci Rep. 2017;7:15061.
Hultman R, Mague SD, Li Q, Katz BM, Michel N, Lin L, et al. Dysregulation of prefrontal cortex-mediated slow-evolving limbic dynamics drives stress-induced emotional pathology. Neuron. 2016;91:439–52.
Barabási DL, Bianconi G, Bullmore E, Burgess M, Chung S, Eliassi-Rad T, et al. Neuroscience needs network science. J Neurosci. 2023;43:5989.
Kondev V, Najeed M, Yasmin F, Morgan A, Loomba N, Johnson K, et al. Endocannabinoid release at ventral hippocampal-amygdala synapses regulates stress-induced behavioral adaptation. Cell Rep. 2023;42:113027.
Fetcho RN, Parekh PK, Chou J, Kenwood M, Chalençon L, Estrin DJ, et al. A stress-sensitive frontostriatal circuit supporting effortful reward-seeking behavior. Neuron. 2024;112:473–487.e474.
Zheng Z, Guo C, Li M, Yang L, Liu P, Zhang X, et al. Hypothalamus-habenula potentiation encodes chronic stress experience and drives depression onset. Neuron. 2022;110:1400–1415.e1406.
Shen C-J, Zheng D, Li K-X, Yang J-M, Pan H-Q, Yu X-D, et al. Cannabinoid CB1 receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat Med. 2019;25:337–49.
Clemm von Hohenberg C, Weber-Fahr W, Lebhardt P, Ravi N, Braun U, Gass N, et al. Lateral habenula perturbation reduces default-mode network connectivity in a rat model of depression. Transl Psychiatry. 2018;8:68.
Liu D, Zheng X, Hui Y, Xu Y, Du J, Du Z, et al. Lateral hypothalamus orexinergic projection to the medial prefrontal cortex modulates chronic stress-induced anhedonia but not anxiety and despair. Transl Psychiatry. 2024;14:149.
Li M, Yang X-K, Yang J, Li T-X, Cui C, Peng X, et al. Ketamine ameliorates post-traumatic social avoidance by erasing the traumatic memory encoded in VTA-innervated BLA engram cells. Neuron. 2024;112:3192–3210.e6.
Veličković P. Everything is connected: Graph neural networks. Curr Opin Struct Biol. 2023;79:102538.
Liu T, Qi C, Bai W, Tian X, Zheng X. Behavioral state-dependent oscillatory activity in prefrontal cortex induced by chronic social defeat stress. Front Neurosci. 2022;16:885432.
Golden SA, Covington HE 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–91.
Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA Jr. Effects of striatal DeltaFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. 2014;76:550–8.
Hultman R, Ulrich K, Sachs BD, Blount C, Carlson DE, Ndubuizu N, et al. Brain-wide electrical spatiotemporal dynamics encode depression vulnerability. Cell. 2018;173:166–180.e114.
Xia M, Liu T, Bai W, Zheng X, Tian X. Information transmission in HPC-PFC network for spatial working memory in rat. Behav Brain Res. 2019;356:170–8.
Kumar S, Black SJ, Hultman R, Szabo ST, DeMaio KD, Du J, et al. Cortical control of affective networks. J Neurosci. 2013;33:1116.
Fustinana MS, Eichlisberger T, Bouwmeester T, Bitterman Y, Luthi A. State-dependent encoding of exploratory behaviour in the amygdala. Nature. 2021;592:267–71.
Courtin J, Bitterman Y, Muller S, Hinz J, Hagihara KM, Muller C, et al. A neuronal mechanism for motivational control of behavior. Science. 2022;375:eabg7277.
Hu Y-T, Tan Z-L, Hirjak D, Northoff G. Brain-wide changes in excitation-inhibition balance of major depressive disorder: a systematic review of topographic patterns of GABA- and glutamatergic alterations. Mol Psychiatry. 2023;28:3257–66.
Cruces-Solis H, Babaev O, Ali H, Piletti Chatain C, Mykytiuk V, Balekoglu N, et al. Altered theta and beta oscillatory synchrony in a genetic mouse model of pathological anxiety. FASEB J. 2021;35:e21585.
Han S, Li X-X, Wei S, Zhao D, Ding J, Xu Y, et al. Orbitofrontal cortex-hippocampus potentiation mediates relief for depression: A randomized double-blind trial and TMS-EEG study. Cell Rep Med. 2023;4:101060.
Cai G, Lu Y, Chen J, Yang D, Yan R, Ren M, et al. Brain-wide mapping of c-Fos expression with fluorescence micro-optical sectioning tomography in a chronic sleep deprivation mouse model. Neurobiol Stress. 2022;20:100478.
Tian S, Wang Q, Zhang S, Chen Z, Dai Z, Zhang W, et al. Local and large-scale resting-state oscillatory dysfunctions for early antidepressant response prediction in major depressive disorder. J Affect Disord. 2023;340:751–7.
Cardenas VA, Bhat JV, Horwege AM, Ehrlich TJ, Lavacot J, Mathalon DH, et al. Anatomical and fMRI-network comparison of multiple DLPFC targeting strategies for repetitive transcranial magnetic stimulation treatment of depression. Brain Stimul. 2022;15:63–72.
Li J, Seidlitz J, Suckling J, Fan F, Ji G-J, Meng Y, et al. Cortical structural differences in major depressive disorder correlate with cell type-specific transcriptional signatures. Nat Commun. 2021;12:1647.
Taraku B, Woods RP, Boucher M, Espinoza R, Jog M, Al-Sharif N, et al. Changes in white matter microstructure following serial ketamine infusions in treatment resistant depression. Hum Brain Mapp. 2023;44:2395–406.
Fujimoto SH, Fujimoto A, Elorette C, Seltzer A, Andraka E, Verma G, et al. Deep brain stimulation induces white matter remodeling and functional changes to brain-wide networks. bioRxiv. [Preprint] 2024, https://doi.org/10.1101/2024.06.13.598710:2024.2006.2013.598710.
Mitra A, Raichle ME, Geoly AD, Kratter IH, Williams NR. Targeted neurostimulation reverses a spatiotemporal biomarker of treatment-resistant depression. PNAS. 2023;120:e2218958120.
Liu J, Mo J-W, Wang X, An Z, Zhang S, Zhang C-Y, et al. Astrocyte dysfunction drives abnormal resting-state functional connectivity in depression. Sci Adv. 2022;8:eabo2098.
Krystal JH, Kavalali ET, Monteggia LM. Ketamine and rapid antidepressant action: new treatments and novel synaptic signaling mechanisms. Neuropsychopharmacology. 2024;49:41–50.
Bottemanne H, Berkovitch L, Gauld C, Balcerac A, Schmidt L, Mouchabac S, et al. Storm on predictive brain: a neurocomputational account of ketamine antidepressant effect. Neurosci Biobehav Rev. 2023;154:105410.
Kim J-W, Suzuki K, Kavalali ET, Monteggia LM. Bridging rapid and sustained antidepressant effects of ketamine. Trends Mol Med. 2023;29:364–75.
Yang C, Yang J, Luo A, Hashimoto K. Molecular and cellular mechanisms underlying the antidepressant effects of ketamine enantiomers and its metabolites. Transl Psychiatry. 2019;9:280.
Pham TH, Gardier AM. Fast-acting antidepressant activity of ketamine: highlights on brain serotonin, glutamate, and GABA neurotransmission in preclinical studies. Pharmacol Ther. 2019;199:58–90.
Fagerholm ED, Leech R, Williams S, Zarate CA, Moran RJ, Gilbert JR. Fine-tuning neural excitation/inhibition for tailored ketamine use in treatment-resistant depression. Transl Psychiatry. 2021;11:335.
Susin E, Destexhe A. A network model of the modulation of γ oscillations by NMDA receptors in cerebral cortex. eNeuro. 2023;10:ENEURO.0157-0123.2023.
Ma S, Chen M, Jiang Y, Xiang X, Wang S, Wu Z, et al. Sustained antidepressant effect of ketamine through NMDAR trapping in the LHb. Nature. 2023;622:802–9.
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8.
Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404.
Tian F, Lewis LD, Zhou DW, Balanza GA, Paulk AC, Zelmann R, et al. Characterizing brain dynamics during ketamine-induced dissociation and subsequent interactions with propofol using human intracranial neurophysiology. Nat Commun. 2023;14:1748.
Kantor S, Lanigan M, Giggins L, Lione L, Magomedova L, de Lannoy I, et al. Ketamine supresses REM sleep and markedly increases EEG gamma oscillations in the Wistar Kyoto rat model of treatment-resistant depression. Behav Brain Res. 2023;449:114473.
Guyon N, Zacharias LR, Fermino de Oliveira E, Kim H, Leite JP, Lopes-Aguiar C, et al. Network asynchrony underlying increased broadband gamma power. J Neurosci. 2021;41:2944.
Kapanaiah SKT, Rosenbrock H, Hengerer B, Kätzel D. Neural effects of dopaminergic compounds revealed by multi-site electrophysiology and interpretable machine-learning. Front Pharmacol. 2024;15:1412725.
Li Q, Takeuchi Y, Wang J, Gellert L, Barcsai L, Pedraza LK, et al. Reinstating olfactory bulb-derived limbic gamma oscillations alleviates depression-like behavioral deficits in rodents. Neuron. 2023;111:2065–2075 e2065.
Chen M, Ma S, Liu H, Dong Y, Tang J, Ni Z, et al. Brain region–specific action of ketamine as a rapid antidepressant. Science. 2024;385:eado7010.
Xia F, Fascianelli V, Vishwakarma N, Ghinger FG, Kwon A, Gergues MM, et al. Understanding the neural code of stress to control anhedonia. Nature. 2025;637:654–62.
DePass M, Falaki A, Quessy S, Dancause N, Cos I. A machine learning approach to characterize sequential movement-related states in premotor and motor cortices. J Neurophysiol. 2022;127:1348–62.
Golshan HM, Hebb AO, Mahoor MH. LFP-Net: A deep learning framework to recognize human behavioral activities using brain STN-LFP signals. J Neurosci Methods. 2020;335:108621.
Sendi MSE, Waters AC, Tiruvadi V, Riva-Posse P, Crowell A, Isbaine F, et al. Intraoperative neural signals predict rapid antidepressant effects of deep brain stimulation. Transl Psychiatry. 2021;11:551.
Jang K-I, Kim S, Chae J-H, Lee C. Machine learning-based classification using electroencephalographic multi-paradigms between drug-naïve patients with depression and healthy controls. J Affect Disord. 2023;338:270–7.
Nugent AC, Ballard ED, Gilbert JR, Tewarie PK, Brookes MJ, Zarate CA. The effect of ketamine on electrophysiological connectivity in major depressive disorder. Front Psychiatry. 2020;11:519.
Richards BA, Lillicrap TP, Beaudoin P, Bengio Y, Bogacz R, Christensen A, et al. A deep learning framework for neuroscience. Nat Neurosci. 2019;22:1761–70.
Acknowledgements
We thank Huiyun Yang for helpful comments and Yinglong Liu for technical support.
Funding
This work was supported by the National Natural Science Foundation of China (61501329 to TL, 61976157 to XZ, and 61971311 to WB) and the Major Project of Guangzhou National Laboratory (GZNL2023A03001 to XX).
Author information
Authors and Affiliations
Contributions
TL and XZ designed the study; TL, QC, XX, XW, FH, WB, DG, and YL collected the data and performed analysis. TL,QC, XX, XZ and BG wrote the manuscript and all authors participated in revising it and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study involved only animal research. All experimental procedures were conducted with approved protocols from the Care and Use of Laboratory Animals and the Tianjin Medical University Animal Care and Use Committee (No. TMUaMEC 2021060).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cao, Q., Xu, X., Wang, X. et al. Mesoscale brain-wide fluctuation analysis: revealing ketamine’s rapid antidepressant across multiple brain regions. Transl Psychiatry 15, 155 (2025). https://doi.org/10.1038/s41398-025-03375-7
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03375-7