Abstract
Auditory verbal hallucinations (AVH) represent a substantial therapeutic challenge in schizophrenia. Low-frequency repetitive transcranial magnetic stimulation (rTMS) has demonstrated potential in reducing AVH, yet the underlying neurobiological mechanisms remain incompletely understood. This study investigated the genetic and molecular processes associated with functional connectivity density (FCD) changes induced by 1 Hz rTMS in schizophrenia patients with AVH. The results revealed that the active stimulation group exhibited significant improvement in positive symptoms and AVH severity compared to the sham control group. Specifically, rTMS increased FCD within the frontoparietal network while decreasing FCD in the language network. Notably, baseline FCD values in these networks were predictive of the extent of symptom amelioration. Gene enrichment analysis indicated that rTMS-induced FCD changes were linked to molecular pathways critical for cellular homeostasis and neuronal function. Among the identified hub genes, GAL emerged as a key regulator of these alternations. Furthermore, neurotransmitter systems were implicated, with alterations in mu-opioid (MU) receptor density mediating the effects of GAL on FCD modifications. These findings highlight a multifaceted interplay among genetic, molecular, and connectivity-based mechanisms underlying the therapeutic efficacy of rTMS in treating AVH.
Similar content being viewed by others
Introduction
Schizophrenia is a severe psychiatric disorder characterized by hallucinations, delusions, and cognitive impairments [1], with a lifetime prevalence of approximately 0.7% [2] and substantial associated disability [3]. Among its hallmark symptoms, auditory verbal hallucinations (AVH) defined as sensory experiences occurring without external stimulation [4], are particularly prevalent and persistent, affecting up to 70% of individuals with schizophrenia [5]. Although antipsychotic medications remain the cornerstone of treatment for [6], AVH often persist in many patients [7], and these medications can induce significant side effects, inducing weight gain, sedation, dystonia, parkinsonism, and akathisia [8]. These limitations highlight the urgent need for alternative therapeutic approaches.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive brain stimulation technique that has emerged as a promising therapeutic intervention for psychiatric disorders [9, 10]. Low-frequency rTMS targeting the left temporoparietal junction (TPJ) has demonstrated efficacy in alleviating both the frequency and severity of AVH [11,12,13]. In particular, 1 Hz stimulation is believed to exert an inhibitory effect on cortical excitability, thereby mitigating hyperactivity in the auditory cortex, which critically involved in the generation and persistence of AVH [14]. Meta-analyses further corroborate the therapeutic benefits of low-frequency (1 Hz) rTMS stimulation in reducing AV symptoms [15]. The proposed mechanism suggests that rTMS normalizes aberrant cortical excitability and connectivity underlying the pathology of AVH [16,17,18,19]. For instance, studies utilizing 1 Hz rTMS directed at the left TPJ have reported increased connectivity between the stimulation site and the right insula [20], alongside reduced connectivity with the right temporal lobes in patients receiving active stimulation [21]. Specifically, functional connectivity density (FCD) analysis, a data-driven method that quantifies the number of connections between a given voxel and all other voxels in the brain [22], provides highly sensitive to individual variations in connectivity [23] and holds potential as a biomarker for schizophrenia [24]. Previous research has shown that maintenance rTMS targeting the left dorsal prefrontal cortex, when combined with electroconvulsive therapy and olanzapine, enhances FCD in the left prefrontal lobe, parietal lobe, and insular lobe in treatment-resistant schizophrenia [25]. These findings indicate that rTMS can modulate activity within targeted cortical sites and their associated networks, potentially contributing to symptom amelioration. Nevertheless, further investigation is needed to elucidate the neurobiological mechanisms underlying the therapeutic effects of rTMS on AVH in schizophrenia.
Schizophrenia exbibits a strong hereditary component, with genetic factors accounting for approximately 80% of the variability in disease risk [26]. Genetic variations, such as those in ZNF804A, DTNBP1, DAOA, AKT1, NTRK3, and ERBB4, are implicated in schizophrenia by modifying brain structure and function [27], consequently impacting neural connectivity [28]. These genes regulate critical pathways involved in synaptic plasticity, neurotransmitter signalling, and neurodevelopment [29,30,31], which are essential for maintaining brain network integrity [32,33,34]. The public accessible gene transcription data from the Allen Human Brain Atlas (AHBA) [35] serves as a valuable tool for exploring the relationship between transcriptional profiles and neuroimaging phenotypes [36]. Recent studies have demonstrated that abnormal functional connectivity in schizophrenia is closely tied to the expressions of specific genes identified through AHBA analyses [37,38,39]. For instance, reduced connectivity in the superior and middle temporal gyrus and increased connectivity in the thalamus have been associated with differential expression of genes implicated in synaptic signalling and transmission [40, 41]. These findings underscore the genetic contributions of network disruptions in schizophrenia and the value of integrating genomic and neuroimaging data to elucidate its pathophysiology.
The neurochemical foundation of schizophrenia has long been tied to dopamine dysregulation, a cornerstone of both research and clinical management [42]. The dopamine hypothesis posits that hyperactivity in mesolimbic pathways drives positive symptoms, such as hallucinations and delusions, while hypoactivity in the prefrontal cortex contributes to negative symptoms and cognitive deficits [43,44,45]. Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) atlas enable precise mapping of neurotransmitter distributions in the brain [46], offering critical insights into how these neurochemical imbalances disrupt brain network organization and manifest as clinical features of schizophrenia. Studies have shown that reduced connectivity in the medial frontal regions and the occipital cortex in schizophrenia patients, associated with dysfunctions in dopamine, serotonin, and gamma-aminobutyric acid systems [47, 48]. Integrating genetic expression data with neurotransmitter density in FCD analyses offers a comprehensive framework for understanding how genetic and molecular abnormalities influence brain connectivity and contribute to the clinical manifestations of schizophrenia.
Although rTMS has been shown to modulate specific gene expressions and neurotransmitter distributions [49, 50], the precise neurobiological mechanism underlying its therapeutic effects on schizophrenia with AVH remain unclear. The present study aimed to investigate the FCD changes induced by low-frequency rTMS and their associations with genetic and neurotransmitter profiles in schizophrenia patients with AVH. We hypothesize that 1 Hz rTMS modulates functional connectivity by inducing network-specific alternations related to AVH, notably a decrease in FCD within the aberrant speech-processing network while concurrently increasing FCD in prefrontal regions implicated in cognitive regulation. Furthermore, we propose that the neurobiological effects of rTMS may involve specific molecular mechanisms, including the modulation of gene expressions and alternations in neurotransmitter systems. Grounded on existing evidence, our study may provide a theoretical framework for exploring potential biomarkers and therapeutic targets in schizophrenia with AVH.
Methods and Materials
Participants
This study enrolled patients diagnosed with schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V). The inclusion criteria were as follows: (1) daily AVH persisting despite treatment with at least two antipsychotic medications, and (2) a minimum of five AVH episodes per day over the past month. Patients were maintained on stable antipsychotic doses throughout the study. Exclusion criteria included neurological disorders, significant head trauma, current or past substance abuse disorders, and contraindications to MRI.
The sample size was determined using the G*Power program software [51], targeting a statistical power (1-β) of 0.90, an alpha of 0.05, and a medium effect size (Cohen’s d = 0.5). This yielded a required total sample of 44 for detecting group differences in matched pairs. Ultimately, 58 patients were recruited and randomized by a computer-generated random number table by an independent researcher, allocating 32 to the active stimulation group and 26 to the sham control group (Supplementary Figure 1). Randomization was blinded to both the patients and the clinical raters, with only rTMS operators aware of the group assignments, minimizing bias.
The protocol was approved by the Medical Ethics Committee of the Xijing Hospital (approval number:KY20202055-F-1), and all participants provided written informed consent following a detailed study description. This study was registered in Chinese Clinical Trial Registry (https://www.chictr.org.cn; registration number: ChiCTR2100041876).
Clinical assessment
Participants underwent comprehensive clinical assessments to determine the symptom severity and track changes over time. These included the Positive and Negative Syndrome Scale (PANSS) for evaluating schizophrenia symptoms [52] and the Auditory Hallucination Rating Scale (AHRS) for AVH, focusing on frequency, duration, and associated distress [53]. Evaluations were conducted both at baseline and post-treatment.
rTMS protocol
Low-frequency (1 Hz) rTMS was administered targeting the left TPJ, a region implicated in the pathophysiology of AVH, using a Magstim Rapid System (YIRUDE, Wuhan, China) with a 70 mm figure-of-eight coil. The TPJ was located using the 10–20 electrode system (T3-P3). Active stimulation was applied at 110% of each individual’s resting motor threshold (MT), while the sham stimulation using a Magstim sham coil, replicating auditory cues without an effective magnetic field. Each session lasted 15 min, with10-second stimulation periods followed by 5-second interval, repeated daily for 15 consecutive days.
MRI data acquisition
MRI data were acquired using a 3.0 Tesla scanner (GE MR750, Milwaukee, WI, United States). Functional MRI used a gradient echo-planar (EPI) sequence for with parameters: repetition time (RT) = 2,000 ms; echo time (ET) = 30 ms; field of view (FOV) = 240 mm × 240 mm; flip angel (FA)= 90o; matrix= 64 × 64; slice thickness = 4.5 mm; voxel size = 3.5 × 3.5 × 3.5 mm, yielding 210 volumes. High-resolution T1-weighted structural images were obtained via a magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence: TR = 8.1 ms, ET = 3.2 ms, FA= 12o, FOV = 256 × 256 mm, matrix=256 × 256, slice thickness = 1 mm, voxel size = 1.0 × 1.0 × 1.0, producing 176 sagittal slices. Participants were instructed to remain still, stay awake, and keep their eyes closed during the scan to reduce motion artifacts.
Image data preprocessing
Image data were preprocessed using the DPABI (http://rfmri.org/DPABI) and SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12) software package. Preprocessing steps included: (1) discarding the first ten volumes for magnetization stabilization; (2) slice timing correction; (3) head motion correction; (4) co-registration of the anatomical image to the mean functional images; (5) segmentation into gray matter, white matter, and cerebrospinal fluid; (6) normalization and resampling to 3 × 3 × 3 mm isotropic voxels in Montreal Neurological Institute (MNI) standard space; (7) band-pass filtering at 0.01 to 0.1 Hz; (8) linear detrending; and (9) regression of nuisance covariates, including motion parameters, global mean signal, cerebrospinal fluid, and white matter signals. Participants with head motion exceeding 3 mm of movement or 3° rotation in any direction were excluded.
FCD calculation
Voxel-wise global FCD was calculated to assess the functional connectivity of each voxel with all other voxels across the brain, identifying hubs with highest connectivity using the Neuroscience Information Toolbox (NIT, http://www.neuro. uestc.edu.cn/NIT.html). Voxel pairs were deemed connected if their Pearson correlation coefficient exceeded a threshold (Tc) of 0.6, based on established guidelines [22]. FCD maps were normalized and smoothed with a 6 mm full-width-at-half-maximum (FWHM) Gaussian kernel to reduce noise and outliers. Group differences in FCD were analysed using two-sample or paired t-tests, with a voxel-level threshold of p < 0.01 and cluster-level Gaussian random field (GRF) correction at p < 0.05.
Association analysis between meta-analytic cognitive terms and FCD changes
The Neurosynth platform [54] (https://neurosynth.org) was used to explore the association between meta-analytic cognitive terms and FCD alterations. Thresholded Z-maps from post-treatment between-group FCD comparisons were split into FCD-positive (active > control) and FCD-negative (active < control) maps. The Neurosynth decoder assessed spatial correlations with cognitive terms, with significance determined via permutation tests correcting for spatial autocorrelation [55].
Prediction of treatment outcome
Support vector regression (SVR) [56](https://www.csie.ntu.edu.tw/~cjlin/libsvm) predicted rTMS treatment responses using baseline FCD values. Model robustness was ensured using 5-fold cross-validation, assessed by mean squared error (MSE) and R². Hyperparameters (C: 0.1–10, ε: 0.01–0.5, kernel: RBF) were optimized via grid search, selecting the lowest MSE configuration. Predictive significance was confirmed with permutation tests (n = 1000).
Transcription-neuroimaging association analysis
Brain-wide gene expression data were obtained from the AHBA (https://human.brain-map.org), and processed following a standardized pipeline [57]: (i) probe re-annotation to genes; (ii) probe signal filtering; (iii) selection of with RNA-seq-validated probes; (iv) sample assignment to the Schaefer1000 atlas [58] within 2-mm Euclidean distance; (v) normalization using a scaled robust sigmoid algorithm; and (vi) gene filtering by differential stability. Due to data limitations, only left hemisphere samples (n = 6) were included, as right hemisphere data (n = 2) were insufficient. A gene expression map averaging 10,027 genes across 500 regions were generated, with 97 regions lacing data. Partial least square (PLS) regression [59] analysed the relationships between FCD changes (t values) in 403 left hemisphere regions and gene expression, selecting the first PSL regression component (PSL1) for maximum variance explanation, with significant genes identified at Z > 3.0 and p < 0.05 with false discovery rate (FDR) correction.
Gene enrichment and PPI analysis
Gene Ontology (GO) biological process analysis of significant genes was conducted using Metascape (https://metascape.org) with a p-value cutoff < 0.05 (FDR-corrected) [60], integrating over 40 knowledge bases for comprehensive annotation. Protein-protein interaction (PPI) analysis used STRING v12.0 (https://cn.string-db.org) [61] with a confidence score ≥ 0.4, visualized in Cytoscape [62] (https://cytoscape.org/). The CytoHubba plugin of the Maximal Clique Centrality (MCC) algorithm [63] identified top 10 hub genes, which were correlated with FCD changes (p < 0.05, Bonferroni-corrected). Schizophrenia-related genes from AHBA ISH data (https://human.brain-map.org/ish/search) were compared to genes linked to six other disorders: major depressive disorder (MDD), Alzheimer’s disease (AD), autism spectrum disorder (ASD), epilepsy (EP), intellectual disability (ID), and Parkinson’s disease (PD), using permutation tests to assess their relative PLS contributions.
Spatial correlation with neurotransmitter density
Neurotransmitter density maps derived from PET /SPCET imaging [64] included receptors and transporters relevant to schizophrenia [65,66,67,68,69]: serotonin receptors (5-HT1a, 5-HT1b, 5-HT2a, and 5-HT4), dopamine receptors (D1/D2), Gamma-aminobutyric acid type A receptor (GABAa), metabotropic glutamate receptor (mGluR5), N-methyl-D-aspartate receptor (NMDA), serotonin transporter (SERT), mu-opioid (MU), and vesicular acetylcholine transporter (VAChT). Maps with the largest sample sizes were selected for reliability. Spearman rank correlations assessed relationships between neurotransmitter densities and FCD changes, with 1000 permutation tests correcting for spatial dependencies (p < 0.05, FDR-corrected).
Mediation effect analysis
Mediation analysis using the PROCESS plugin in SPSS 22.0 (https://www.processmacro.org) explored pathways linking gene expression, neurotransmitter densities, and FCD changes. Hub gene expression served as independent variables, with neurotransmitter densities as mediators, employing Model 4 to delineate direct and indirect effects.
Statistical analyses
Demographic and clinical data are expressed as means and standard deviations. Gender distribution was analysed with chi-square tests, and other variables with t-tests, using SPSS 22.0. Baseline group differences were assessed with independent t-tests, and pre- to post-treatment changes with paired t-tests, with significance set at p < 0.05.
Results
Demographics and clinical characteristics
Due to excessive head movement, two participants from the active stimulation group and one from the sham control group were excluded, resulting in a final analysis of 30 participants in the active stimulation group and 25 in the sham control group. At baseline, no significant differences were observed between the groups in demographic or clinical characteristics (Table 1). Specifically, the two groups were comparable in age (t = 0.644, p = 0.478), gender (χ2 = 0.045, p = 0.832), years of educational (t = 0.458, p = 0.756), and clinical measures, including illness duration (t = 0.525, p = 0.602), medication dosage (t = 0.218, p = 0.828).
Baseline clinical assessments, including Positive and Negative Syndrome Scale (PANSS) and Auditory Hallucination Rating Scale (AHRS) scores, were also similar between groups (Table 1). Post-intervention, the active stimulation group exhibited significant improvements in positive symptom scores (t = 6.197, p < 0.001), general symptom (t = 2.661, p = 0.011), and AHRS scores (t = 6.542, p < 0.001) (Fig. 1a), whereas the sham control group showed no notable changes (Fig. 1b).
a Changes in clinical symptoms from baseline to post-test in the active stimulation group. b Changes in clinical symptoms between baseline and post-test within the sham control group. PANSS_P, positive symptoms of the Positive and Negative Syndrome Scale (PANSS); PANSS_N, negative symptoms of the PANSS; PANSS_G, general symptoms of the PANSS; AHRS, Auditory Hallucination Rating Scale. NS, not significant. *p < 0.05, ***p < 0.001.
Changes in FCD
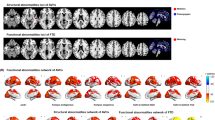
Figure 2 illustrates the distribution of FCD changes from baseline to post-treatment across various regions in both the active stimulation and sham control groups. In the active stimulation group, post-treatment increased FCD in the left supplementary motor area and right superior medial frontal gyrus, while decreasing FCD in the left fusiform gyrus (Table 2; Fig. 3a, b).
a Differences in the FCD between the baseline and post-test in the active stimulation group. b Distribution frequency of FCD scores within the active stimulation group. c FCD changes from baseline to post-test in the sham control group. d Distribution frequency of FCD scores in the sham control group. Cool colours indicate decreased FCD relative to baseline, and warm colours indicate increased FCD relative to baseline.
a Regional variation in global FCD within the active stimulation group. b Distribution of global FCD differences across various networks within the active stimulation group. c Regional change in global FCD within the sham control group. d Distribution of global FCD differences across different networks within the sham control group. e Regional change in global FCD between the active stimulation group and the sham control group posttreatment. f Distribution of global FCD differences across the networks between the active stimulation and sham control groups. g Cognitive terms associated with increased FCD. h Cognitive terms linked to decreased FCD. Warm colours represent increased FCD, whereas cool colours represent decreased FCD in the post-test comparisons or the active stimulation group. SFGmedial, superior medial frontal gyrus; FFG, fusiform gyrus; SMA, supplementary motor area; IFGtriang, interior triangular frontal gyrus; ITG, inferior temporal gyrus; SPG, superior parietal gyrus; SMG, supramarginal gyrus; MTG, middle temporal gyrus; MOG, middle occipital gyrus; IFGorb, inferior orbital frontal gyrus; DMN, default model network; FPN, frontoparietal network; LIB, limbic network; SAN, salient network; DAN, dorsal attention network; SOM, somatomotor network; VIS, visual network.
In the sham control group, post-treatment FCD increased in the left inferior temporal gyrus and right superior parietal gyrus, with a decrease observed in the left inferior triangular frontal gyrus (Table 3; Fig. 3c, d).
Comparative analysis between the groups revealed significant post-treatment differences: the active stimulation group exhibited reduced FCD in the left middle temporal gyrus and right middle occipital gyrus, alongside increased FCD in the left supramarginal gyrus and right inferior orbital frontal gyrus (Table 4; Fig. 3e, f).
Meta-analytic cognitive functions related to FCD alterations
Regions with increased FCD were associated with meta-analytic cognitive terms linked to executive control, memory, and inference functions (Fig. 3g), crucial for higher-order cognition and decision-making. Conversely, regions with decreased FCD correlated with cognitive terms such as object encoding, recognition, and language processing (Fig. 3h), essential for the sensory perception and interpretation.
Clinical responses related to FCD
Baseline FCD values in the active stimulation group significantly predicted post-rTMS changes in positive symptom (r = −0.311, p = 0.047, one-tailed permutation test; Fig. 4a). The most predictive features were within the default mode network (21.04% of total feature weights in SVR feature weights), somatomotor network (22.35%), and frontoparietal network (20.02%) (Fig. 4b). However, baseline FCD did not predict changes in AHRS scores or other clinical measures.
a Scatter plot of the correlation between the observed positive symptom change following active stimulation treatment and the predicted positive symptom change derived from the support vector regression (SVR) analysis (permutation tests; p < 0.05). b Mapping of weights from 5-fold cross-validation onto the brain surface. Regions with higher and lower predictive power are coloured warm and cool, respectively. The radar map provides an overview of the distribution of predictive power across different networks. DMN, default model network; FPN, frontoparietal network; LIB, limbic network; SAN, salient network; DAN, dorsal attention network; SOM, somatomotor network; VIS, visual network.
Gene expression profiles
Comparative analysis of FCD changes between the active stimulation and the sham control groups identified significant gene enrichment in biological processes, including regulation of cell projection organization, cellular secretion, cytosolic calcium ion concentration, transmembrane transport, cell-cell adhesion, and cellular component organization (Fig. 5a). These processes are closely tied to neuroplasticity, synaptic function, and brain connectivity.
a Enrichment of associated genes within various biological processes. Each node represented by a circle varies in size according to the number of input genes categorized under that term. Nodes and their corresponding labels share colours to indicate clustering within the same biological process. b Sankey plot of KEGG (Kyoto Encyclopedia of Genes and Genomes) enrichment analysis, where genes are mapped to specific pathways on the y-axis against their respective enrichment factors on the x-axis.
KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway analysis highlighted key signalling pathways, such as calcium signalling, cAMP signalling, and neurotrophins signalling (Fig. 5b), which are vital for cell growth, neurotransmission, immune response, and hormone signalling.
A PPI network further elucidated genes associated with FCD changes (Fig. 6a). The MCC algorithm identified the top 10 hub genes, including pivotal genes including CALM3, CASP3, and GAL (Fig. 6b).
Schizophrenia-related genes from the ISH dataset demonstrated stronger PLS weights compared to genes associated with other disorders (Fig. 7a), with 48.65% (18 out of 37) of these genes correlating with FCD changes, a higher proportion than in other disorder categories (Fig. 7b).
a Partial least square (PLS) regression weights assigned to genes linked with different disorders with FCD changes, with asterisks denoting significant differences between patients with schizophrenia and those with other disorders (*p < 0.05; **p < 0.01). b Proportion of disorder-related genes whose expression profiles and FCD changes are significantly correlated. MDD, major depression disorder; AD, Alzheimer’s disease; ASD, autism; EP, epilepsy; ID, intellectual disability; PD, Parkinson’s disease.
Relationship between FCD changes and gene expression and neurotransmitter distribution
Pearson correlation analysis revealed significant associations between FCD changes and several hub genes, including GAL (r = 0.276, p < 0.001), OPRM1(r = 0.157, p = 0.001), PRKCD (r = 0.138, p = 0.005), and JAK2 (r = −0.158, p = 0.001) (Fig. 8a–d). These correlations remained their significance following Bonferroni correction. Additionally, significant correlations were observed between FCD changes and specific neurotransmitters, particularly MU (r = 0.156, p = 0.004) and GABAa (r = −0.155, p = 0.011) (Fig. 8e, f). These associations remained significance after adjusting for multiple comparisons using the FDR correction.
a Correlation with GAL (Galanin and GMAP Prepropeptide) expression; b correlation with OPRM1 (Opioid Receptor Mu 1) expression; c correlation with PRKCD (Protein Kinase C Delta) expression; d correlation with JAK2 (Janus Kinase 2) expression; e correlation with MU (mu-opioid receptor) density; f correlation with GABAa (GABA type A receptor) density.
Mediation effect
Mediation analysis provided deeper insight into the pathways linking gene expression and neurotransmitter systems to FCD changes. GAL expression positively predicted FCD changes (Beta = 0.126, t = 2.477, p = 0.014). The neurotransmitter MU partially mediated this relationship, with an indirect effect of 0.149, accounting for 54.30% of the total effect (Fig. 9).
Discussion
The present study investigated the effects of low-frequency rTMS on FCD in schizophrenia with AVH, emphasizing its associations with gene expression and neurotransmitter density. The main findings include: (1) significant clinical improvement in positive symptoms and AVH following 1 Hz rTMS treatment; (2) rTMS induced FCD changes in brain regions linked to langue network (e.g., left middle temporal gyrus) and default model network (e.g., left supramarginal gyrus and right inferior orbital frontal gyrus); (3) gene expression related to FCD changes was enriched in biological process involved in neuroplasticity, synaptic function, and brain connectivity;(4) correlations between FCD changes and neurotransmitter receptor densities, notably MU and GABAa; and (5) MU receptor density mediating the relationship between hub gene GAL expression and FCD changes. These results align with prior studies [70,71,72,73] and our previous work [74,75,76,77], reinforcing the efficacy of low-frequency rTMS in ameliorating AVH in schizophrenia.
FCD changes following rTMS treatment
FCD changes observed following low-frequency rTMS underscore its potential to modulate specific brain networks implicated in schizophrenia with AVH. In the active stimulation group, post-treatment FCD alterations were distinct from those in the sham control group, underscoring network-specific effects. Notably, increased FCD in the left supramarginal gyrus and right inferior orbital frontal gyrus, key hubs of the frontoparietal network, points the capacity of rTMS to enhance cognitive control and executive functions [78, 79]. This network is essential for regulating attention, working memory, and goal-directed behaviours [80,81,82], facilitated by dynamic modulation of connectivity across brain regions [83]. Dysfunctions in the frontoparietal network are consistently associated with cognitive deficits in schizophrenia [84], such as disorganized thinking, which contributes to AVH [85]. The increased FCD in these regions suggest that rTMS may strengthen higher-order cognitive control, potentially improving reasoning and working memory that regulate the internally generated thoughts driving AVH [86, 87]. While these findings align with meta-analytic evidence connecting these areas to executive control and memory functions, this relationship with cognitive improvement remains hypothetical and requires validation through standardized cognitive assessments and symptom change metrics.
Conversely, decreased FCD in the left middle temporal gyrus, a core region for language processing [88], and right middle occipital gyrus, involved in visual processing [89], indicates that rTMS may suppress sensory network hyperactivity linked to AVH. This aligns with meta-analysis evidence of reduced FCD in regions tied to object perception and language processing. Previous studies have showed that low-frequency rTMS improves verbal and visual learning in patients with AVH [77], consistent with our findings. These reductions likely reflect normalization of hyperconnectivity and overactivation, which underpin sensory misperceptions characteristic of AVH [90]. However, the assumption that decreased FCD equates to reduced hyperactivity remains a hypothesis needing further empirical support.
Significant between-group differences affirm the therapeutic specificity of active stimulation in normalizing aberrant network activity in schizophrenia [91,92,93], thereby offering a mechanistic basis for its efficacy in reducing AVH. Additional subgroup findings further contextualize these effects. In the active stimulation group, increased FCD in the left supplementary motor area and right superior medial frontal gyrus, involved in motor planning and decision-making [94, 95], while decreased FCD in the left fusiform gyrus, linked to object recognition [96], align with the modulation of rTMS on motor and perceptual networks. In the sham control group, however, FCD increased in the left inferior temporal gyrus and right superior parietal gyrus, linked to a series of perceptive processing [97, 98], alongside decreased FCD in the left inferior triangular frontal gyrus, tied to semantic processing [99], suggesting non-specific placebo effects adjustments unrelated to the therapeutic intent of rTMS. These changes may stem from patient expectations of the sham procedure, which are known to elicit connectivity shifts in placebo settings [100]. Alternatively, these FCD variations might reflect spontaneous fluctuations in network activity to the experimental context, though they lack the directional specificity seen in active rTMS. These findings underscore the importance of distinguishing placebo-driven changes from targeted effects of rTMS in interpreting therapeutic outcome.
FCD prediction on clinical responses
SVR analysis revealed that baseline FCD values in the default mode, sensorimotor, and frontoparietal networks strongly predicted improvements in positive symptoms post- rTMS. The default mode network, implicated in self-referential and introspective thought processes, is frequently dysregulated in schizophrenia, contributing to hallucinations and delusions [101]. Modulation of default mode network activity through rTMS may therefore play a crucial role in alleviating psychotic symptoms by normalizing the hyperactivity and dysconnectivity that are characteristic of schizophrenia.
The sensorimotor network, which is involved in sensory and motor integration, underlies perceptual abnormalities in schizophrenia patients [102]. The disruption of the sensorimotor network could lead to inefficient integration of bottom-up sensory information with high-order cognitive processes [103, 104], and is directly linked to sensory processing deficits [105]. FCD changes within the sensorimotor network may help mitigate sensory processing deficits associated with AVH. The frontoparietal network, vital for cognitive control and executive functions, is often compromised in schizophrenia [106], driving disorganized thinking and cognitive deficits [107]. Enhanced FCD in this network suggests improved regulation of internally generated thoughts, highlighting the potential of FCD as a predictive marker for rTMS outcomes.
Biological processes and pathways of enriched gene
Gene enrichment analysis identified biological processes tied to FCD changes, including cellular component organization, transmembrane transport, and cytosolic calcium ion concentration, all critical for neuronal function and homeostasis. Cellular organization maintains neuronal structure, such as the cytoskeleton, organelles, and membrane systems [108], and its dysregulation in schizophrenia may underlie connectivity deficits [109]. rTMS may exert its therapeutic effects by normalizing these organizational processes, thereby restoring neuronal function and improving connectivity within key brain networks. Transmembrane transport involves the movement of ions, nutrients, and other molecules across cell membranes, which is essential for neuronal excitability, neurotransmitter release, and synaptic plasticity [110, 111]. Disruptions in transmembrane transport can lead to neurotransmitter imbalances, contributing to the symptoms of schizophrenia [112]. By modulating transmembrane transport, rTMS could restore these imbalances [113], reducing the hyperexcitability or hypoactivity in specific brain regions associated with AVH. Calcium signalling, foundational to long-term potentiation (LTP) and long-term depression (LTD) [114], which underlie long-lasting synaptic plasticity [115]. Dysfunctions in calcium signalling have been implicated in schizophrenia [116], where they may contribute to the sensory perception, cognition, and consciousness disturbances characteristic of the disorder [117]. rTMS may induce calcium-dependent synaptic plasticity by increasing intracellular calcium ion concentrations and activating downstream signalling cascades that promote LTP or LTD [118]. These calcium-dependent mechanisms are likely the basis for rTMS-induced neuroplasticity in brain regions associated with cognitive control, sensory processing, and auditory hallucinations [119].
The identified KEGG pathways, including calcium, cAMP, and neurotrophins signalling, further underscore the molecular effects of rTMS. The calcium signalling pathway plays a central role in regulating neuronal activities such as neurotransmitter release and synaptic plasticity [120, 121], which are critical for cognitive functions [122, 123]. Dysregulation of this pathway is linked to cognitive deficits in schizophrenia [124], suggesting that rTMS may contribute to restore normal brain function by modulating calcium signalling [125]. Similarly, the cAMP signalling pathway is involved in neuronal excitability and synaptic connections [126], processes that are often disrupted in schizophrenia[127]. By enhancing AMP signalling [128], rTMS could shape neural connectivity and contribute to symptom relief [129]. The neurotrophins signalling pathway, which supports neuronal growth and survival [130], is a key driver of neural plasticity [131]. Its involvement in schizophrenia indicates that rTMS could enhance the recovery of disrupted neural circuits by promoting neurogenesis and activating neurotrophins signalling [132, 133], thus facilitating the plasticity of neuronal circuits [134]. Notably, studies have revealed that rTMS can increase the expression of brain-derived neurotrophic factor (BDNF) in psychiatric disorders [135], a pivotal neurotrophins involved in synaptic strengthening and neuronal survival. These effects of rTMS may be instrumental in reshaping dysfunctional neural networks and alleviating AVH symptoms in schizophrenia [136]. These enriched biological processes and pathways suggest that rTMS may exert its therapeutic effects by influencing critical molecular mechanisms that underlie neuroplasticity, synaptic function, and brain connectivity.
Hub genes from PPI analysis
PPI analysis identified GAL, OPRM1, PRKCD, and JAK2 as hub genes central to the molecular mechanisms underlying rTMS induced FCD changes. GAL, an endogenous neuroprotective factor [137], involved in neuroplasticity and synaptic transmission [138, 139]. Its positive correlation with FCD changes suggests that rTMS may enhance neuroplasticity by GAL mediated pathways. OPRM1, encodes the mu-opioid receptor, regulates mood regulation and reward processing[140, 141], indicating a potential role for opioid signalling in the therapeutic effects of rTMS [142]. Similarly, PRKCD, a member of the protein kinase C family, influences cell growth, apoptosis, and inflammation [143, 144]. Its association with FCD alterations implies that rTMS may modulate these processes, potentially reducing neuroinflammation while enhancing synaptic function.
JAK2, a key component of the JAK-STAT signalling pathway [145], governs immune responses and cell growth [146, 147]. The negative correlation between JAK2 expression and FCD changes suggests that rTMS may normalize aberrant JAK-STAT signalling, thereby improving neuroplasticity and modulating immune activity. These findings highlight the involvement of these hub genes in pathways critical to neuroplasticity, synaptic function, and neuroinflammation, likely underpinning the therapeutic benefits of rTMS on schizophrenia.
Associated with neurotransmitters profiles
The associations with neurotransmitter receptor densities, particularly MU and GABAa, provides deeper insights into modulation of rTMS on brain connectivity. The positive correlation between MU receptor density and FCD changes indicates that regions with higher MU receptor densities may exhibit enhanced responsiveness to rTMS, potentially increasing FCD. The MU-opioid system, implicated in the cognitive control and the reward system [148, 149], is a core element in the pathophysiology of schizophrenia [150]. Elevated MU receptor density in specific regions may amplify the efficacy of rTMS by facilitating neuroplasticity and strengthening connectivity within key networks, such as the frontoparietal and limbic networks [151, 152], thereby improving cognitive control and reward processing to reduce AVH and other psychotic symptoms [153, 154].
In contrast, the negative correlation between GABAa receptor density and FCD changes suggests that regions with higher GABAa densities may show reduced responsiveness to rTMS. GABAa receptors, which mediate inhibitory neurotransmission, are crucial for maintaining the balance between excitation and inhibition in the brain [155,156,157]. Beyond regulating neuronal excitability, they stabilize neural networks by limiting excessive connectivity, a feature implicated in schizophrenia. In the context of rTMS treatment, this inhibitory effect of GABAa receptor could serve as both a regulator of excitability in neuronal networks [158] and a gating mechanism counteracting maladaptive hyperconnectivity [159, 160]. Thus, rTMS-induced FCD modulation appears contingent upon the regional distribution and functionality of GABAa receptors, influencing the balance of excitatory and inhibitory interactions. Given the critical role of excitatory-inhibitory imbalance in AVH, targeting GABAa receptor activity could enhance rTMS efficacy by stabilizing networks, and may inform personalized treatment protocols based on receptor density.
Meditation effects on FCD changes
Mediation analysis revealed that MU receptor density partially mediates the relationship between GAL gene expression and FCD changes, highlighting a complex interplay between genetic and neurotransmitter systems in rTMS responses. As mentioned above, GAL, a neuropeptide, drives neurogenesis, synaptic plasticity, and neurotransmitter modulation [161, 162] via G-protein-coupled receptor interactions [163], influencing signalling pathways that regulate neuronal excitability and plasticity. Its expression is tied to mood regulation [164] and to neural circuits supporting learning and memory [165], positioning GAL as a key modulator of synaptic strength and connectivity in AVH-related networks.
The MU receptor system, linked to dopaminergic modulation [166], underpins reward processing and cognitive control, functions frequently disrupted in schizophrenia [167]. Regions with higher MU receptor density may the modulatory effects of GAL on FCD, enhancing neuroplasticity and cognitive control within relevant circuits [168, 169]. This mediation suggests MU acts as an intermediary, translating GAL expression into connectivity changes [170]. rTMS treatment may amplify MU-mediated neuroplasticity, thereby augmenting the impacts of GAL on network dynamics. The interplay points to potential therapeutic targets, such as GAL receptor agonists to boost rTMS-induced plasticity, or MU receptor modulators to optimize dopaminergic regulation, which could further alleviating AVH.
This study has several limitations. First, the modest sample size may constrain the generalizability of findings, necessitating larger, more diverse cohorts in future research. Second, the associations among gene expression, neurotransmitter density, and FCD changes were based on correlational analyses, which do not establish causality. Experimental approaches such as targeted molecular interventions are required to elucidate underlying mechanisms. Finally, gene expression data from the AHBA were restricted to the left hemisphere, potentially introducing bias by excluding right-hemisphere contributions to AVH networks. Future studies should incorporate bilateral analyses for a comprehensive understanding of gene expression patterns.
Conclusion
This study investigated FCD changes induced by low-frequency rTMS and their associations with genetic and neurotransmitter profiels in schizophrenia patients with AVH. The results demonstrated significant clinical symptom improvement, accompanied by notable FCD alterations across key brain networks. Gene enrichment analysis emphasized biological processes related to neuroplasticity, synaptic function, and neural connectivity, with GAL and MU emerging as central modulators of rTMS-induced connectivity changes. These findings provide valuable insights into the neurobiological mechanisms through which rTMS exerts its therapeutic effects in schizophrenia with AVH.
Data availability
The data will be made available upon reasonable request from the corresponding authors.
References
McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia—an overview. JAMA Psychiatry. 2020;77:201–10.
McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67–76.
Rössler W, Joachim Salize H, van Os J, Riecher-Rössler A. Size of burden of schizophrenia and psychotic disorders. Eur Neuropsychopharmacol. 2005;15:399–409.
Beck AT, Rector NA. A cognitive model of hallucinations. Cognit Ther Res. 2003;27:19–52.
Upthegrove R, Broome MR, Caldwell K, Ives J, Oyebode F, Wood SJ. Understanding auditory verbal hallucinations: a systematic review of current evidence. Acta Psychiatr Scand. 2016;133:352–67.
Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Salanti G, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379:2063–71.
Shergill SS, Murray RM, McGuire PK. Auditory hallucinations: a review of psychological treatments. Schizophrenia Res. 1998;32:137–50.
Sommer IEC, Slotema CW, Daskalakis ZJ, Derks EM, Blom JD, van der Gaag M. The treatment of hallucinations in schizophrenia spectrum disorders. Schizophrenia Bull. 2012;38:704–14.
Slotema CW, Blom JD, Hoek HW, Sommer IEC, Sommer IEC. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71:873–84.
Guo Q, Li C, Wang J. Updated review on the clinical use of repetitive transcranial magnetic stimulation in psychiatric disorders. Neurosci Bull. 2017;33:747–56.
Ray P, Sinha VK, Tikka SK. Adjuvant low-frequency rTMS in treating auditory hallucinations in recent-onset schizophrenia: A randomized controlled study investigating the effect of high-frequency priming stimulation. Ann Gen Psychiatry. 2015;14:8.
Poulet E, Brunelin J, Bediou B, Bation R, Forgeard L, Dalery J, et al. Slow transcranial magnetic stimulation can rapidly reduce resistant auditory hallucinations in schizophrenia. Biol Psychiatry. 2005;57:188–91.
Vercammen A, Knegtering H, Bruggeman R, Westenbroek HM, Jenner JA, Slooff CJ, et al. Effects of bilateral repetitive transcranial magnetic stimulation on treatment resistant auditory–verbal hallucinations in schizophrenia: A randomized controlled trial. Schizophrenia Res. 2009;114:172–9.
Hallmayer J. Repetitive transcranial magnetic stimulation over temporoparietal cortices in the treatment of refractory auditory hallucinations in patients with schizophrenia. Curr Psychiatry Rep. 2005;7:160–1.
Li J, Cao X, Liu S, Li X, Xu Y. Efficacy of repetitive transcranial magnetic stimulation on auditory hallucinations in schizophrenia: a meta-analysis. Psychiatry Res. 2020;290:113141.
Homan P, Kindler J, Hauf M, Hubl D, Dierks T. Cerebral blood flow identifies responders to transcranial magnetic stimulation in auditory verbal hallucinations. Transl Psychiatry. 2012;2:e189.
Xie Y, Guan M, Wang Z, Ma Z, Fang P, Wang H. Cerebral blood flow changes in schizophrenia patients with auditory verbal hallucinations during low-frequency rTMS treatment. Eur Arch Psychiatry Clin Neurosci. 2023;273:1851–61.
Thomas F, Moulier V, Valéro-Cabré A, Januel D. Brain connectivity and auditory hallucinations: in search of novel noninvasive brain stimulation therapeutic approaches for schizophrenia. Rev Neurologique. 2016;172:653–79.
Xie Y, He Y, Guan M, Wang Z, Zhou G, Ma Z, et al. Low-frequency rTMS treatment alters the topographical organization of functional brain networks in schizophrenia patients with auditory verbal hallucination. Psychiatry Res. 2022;309:114393.
Vercammen A, Knegtering H, Liemburg EJ, den Boer JA, Aleman A. Functional connectivity of the temporo-parietal region in schizophrenia: effects of rTMS treatment of auditory hallucinations. J Psychiatr Res. 2010;44:725–31.
Xie Y, Guan M, He Y, Wang Z, Ma Z, Fang P, et al. The static and dynamic functional connectivity characteristics of the left temporoparietal junction region in schizophrenia patients with auditory verbal hallucinations during low-frequency rTMS treatment. Front Psychiatry. 2023;14:1071769.
Tomasi D, Volkow ND. Functional connectivity density mapping. Proc Natl Acad Sci. 2010;107:9885–90.
Tomasi D, Volkow ND. Functional connectivity hubs in the human brain. Neuroimage. 2011;57:908–17.
Gutiérrez-Gómez L, Vohryzek J, Chiêm B, Baumann PS, Conus P, Cuenod KD, et al. Stable biomarker identification for predicting schizophrenia in the human connectome. Neuroimage Clin. 2020;27:102316.
Xu D, Li G, Zhang Y, Jiang D, Tian H, Wang W, et al. Maintenance repetitive transcranial magnetic stimulation (rTMS) weakly improved treatment effect in patients with treatment-resistant schizophrenia who responded to maintenance ECT and adjunct olanzapine treatment - A pilot study. Psychiatry Clin Psychopharmacol. 2020;30:254–63.
Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: Evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–92.
Lin Z, Long Y, Wu Z, Xiang Z, Ju Y, Liu Z. Associations between brain abnormalities and common genetic variants for schizophrenia: a narrative review of structural and functional neuroimaging findings. Ann Palliat Med. 2021;10:10031–52.
Zhang Y, Yan H, Liao J, Yu H, Jiang S, Liu Q, et al. ZNF804A variation may affect hippocampal-prefrontal resting-state functional connectivity in schizophrenic and healthy individuals. Neurosci Bull. 2018;34:507–16.
Xiao X, Chang H, Li M. Molecular mechanisms underlying noncoding risk variations in psychiatric genetic studies. Mol Psychiatry. 2017;22:497–511.
Orozco IJ, Koppensteiner P, Ninan I, Arancio O. The schizophrenia susceptibility gene DTNBP1 modulates AMPAR synaptic transmission and plasticity in the hippocampus of juvenile DBA/2J mice. Mol Cell Neurosci. 2014;58:76–84.
John JP, Thirunavukkarasu P, Halahalli HN, Purushottam M, Jain S. A systematic review of the effect of genes mediating neurodevelopment and neurotransmission on brain morphology: Focus on schizophrenia. Neurol Psychiatry Brain Res. 2015;21:1–26.
Ikuta T, Peters BD, Guha S, John M, Karlsgodt KH, Lencz T, et al. A schizophrenia risk gene, ZNF804A, is associated with brain white matter microstructure. Schizophrenia Res. 2014;155:15–20.
Su W, Zhu T, Xu L, Wei Y, Zeng B, Zhang T, et al. Effect of DAOA genetic variation on white matter alteration in corpus callosum in patients with first-episode schizophrenia. Brain Imaging Behav. 2021;15:1748–59.
Powell F, LoCastro E, Acosta D, Ahmed M, O’Donoghue S, Forde N, et al. Age-related changes in topological degradation of white matter networks and gene expression in chronic schizophrenia. Brain Connect. 2017;7:574–89.
Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–9.
Shen EH, Overly CC, Jones AR. The Allen Human Brain Atlas: Comprehensive gene expression mapping of the human brain. Trends Neurosci. 2012;35:711–4.
Cai M, Ji Y, Zhao Q, Xue H, Sun Z, Wang H, et al. Homotopic functional connectivity disruptions in schizophrenia and their associated gene expression. Neuroimage. 2024;289:120551.
Cao H, Wei X, Zhang W, Xiao Y, Zeng J, Sweeney JA, et al. Cerebellar functional dysconnectivity in drug-naïve patients with first-episode schizophrenia. Schizophrenia Bull. 2023;49:417–27.
Romme IAC, de Reus MA, Ophoff RA, Kahn RS, van den Heuvel MP. Connectome disconnectivity and cortical gene expression in patients with schizophrenia. Biol Psychiatry. 2017;81:495–502.
Ji Y, Cai M, Zhou Y, Ma J, Zhang Y, Zhang Z, et al. Exploring functional dysconnectivity in schizophrenia: Alterations in eigenvector centrality mapping and insights into related genes from transcriptional profiles. Schizophrenia. 2024;10:37.
Fan Y-S, Xu Y, Bayrak Ş, Shine JM, Wan B, Li H, et al. Macroscale thalamic functional organization disturbances and underlying core cytoarchitecture in early-onset schizophrenia. Schizophrenia Bull. 2023;49:1375–86.
Lau C-I, Wang H-C, Hsu J-L, Liu M-E. Does the dopamine hypothesis explain schizophrenia? Rev Neurosci. 2013;24:389–400.
Kapur S, Agid O, Mizrahi R, Li M. How antipsychotics work—from receptors to reality. NeuroRx. 2006;3:10–21.
Walter H, Kammerer H, Frasch K, Spitzer M, Abler B. Altered reward functions in patients on atypical antipsychotic medication in line with the revised dopamine hypothesis of schizophrenia. Psychopharmacology (Berl). 2009;206:121–32.
Juckel G, Schlagenhauf F, Koslowski M, Wüstenberg T, Villringer A, Knutson B, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29:409–16.
Dukart J, Holiga S, Rullmann M, Lanzenberger R, Hawkins PCT, Mehta MA, et al. JuSpace: A tool for spatial correlation analyses of magnetic resonance imaging data with nuclear imaging derived neurotransmitter maps. Hum Brain Mapp. 2021;42:555–66.
Hou C, Jiang S, Liu M, Li H, Zhang L, Duan M, et al. Spatiotemporal dynamics of functional connectivity and association with molecular architecture in schizophrenia. Cereb Cortex. 2023;33:9095–104.
He H, Long J, Song X, Li Q, Niu L, Peng L, et al. A connectome-wide association study of altered functional connectivity in schizophrenia based on resting-state fMRI. Schizophrenia Res. 2024;270:202–11.
Ikeda T, Kurosawa M, Morimoto C, Kitayama S, Nukina N. Multiple effects of repetitive transcranial magnetic stimulation on neuropsychiatric disorders. Biochemical Biophysical Res Commun. 2013;436:121–7.
Soundara Rajan T, Ghilardi MFM, Wang H-Y, Mazzon E, Bramanti P, Restivo D, et al. Mechanism of action for rTMS: a working hypothesis based on animal studies. Front Physiol. 2017;8:457.
Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–60.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bull. 1987;13:261–76.
Hoffman RE, Hawkins KA, Gueorguieva R, Boutros NN, Rachid F, Carroll K, et al. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry. 2003;60:49–56.
Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–70.
Burt JB, Helmer M, Shinn M, Anticevic A, Murray JD. Generative modeling of brain maps with spatial autocorrelation. Neuroimage. 2020;220:117038.
Chang C-C, Lin C-J. LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol. 2007;2:27.
Arnatkevic̆iūtė A, Fulcher BD, Fornito A. A practical guide to linking brain-wide gene expression and neuroimaging data. Neuroimage. 2019;189:353–67.
Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo X-N, Holmes AJ, et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 2018;28:3095–114.
Krishnan A, Williams LJ, McIntosh AR, Abdi H. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 2011;56:455–75.
Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1523.
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:607–13.
Otasek D, Morris JH, Bouças J, Pico AR, Demchak B. Cytoscape automation: empowering workflow-based network analysis. Genome Biol. 2019;20:185 https://doi.org/10.1186/s13059-019-1758-4.
Chin C-H, Chen S-H, Wu H-H, Ho C-W, Ko M-T, Lin C-Y. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8:S11.
Hansen JY, Shafiei G, Markello RD, Smart K, Cox SML, Nørgaard M, et al. Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. Nat Neurosci. 2022;25:1569–81.
Selvaraj S, Arnone D, Cappai A, Howes O. Alterations in the serotonin system in schizophrenia: a systematic review and meta-analysis of postmortem and molecular imaging studies. Neurosci Biobehav Rev. 2014;45:233–45.
Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment: meta-analysis of imaging Studies. Arch Gen Psychiatry. 2012;69:776–86.
Egerton A, Modinos G, Ferrera D, McGuire P. Neuroimaging studies of GABA in schizophrenia: a systematic review with meta-analysis. Transl Psychiatry. 2017;7:e1147.
Kruse AO, Bustillo JR. Glutamatergic dysfunction in Schizophrenia. Transl Psychiatry. 2022;12:500.
Jiang Y, Palaniyappan L, Luo C, Chang X, Zhang J, Tang Y, et al. Neuroimaging epicenters as potential sites of onset of the neuroanatomical pathology in schizophrenia. Sci Adv. 2024;10:eadk6063.
Slotema CW, Blom JD, van Lutterveld R, Hoek HW, Sommer IEC. Review of the efficacy of transcranial magnetic stimulation for auditory verbal hallucinations. Biol Psychiatry. 2014;76:101–10.
Hoffman RE, Gueorguieva R, Hawkins KA, Varanko M, Boutros NN, Wu Y-t, et al. Temporoparietal transcranial magnetic stimulation for auditory hallucinations: safety, efficacy and moderators in a fifty patient sample. Biol Psychiatry. 2005;58:97–104.
Mehta DD, Siddiqui S, Ward HB, Steele VR, Pearlson GD, George TP. Functional and structural effects of repetitive transcranial magnetic stimulation (rTMS) for the treatment of auditory verbal hallucinations in schizophrenia: a systematic review. Schizophrenia Res. 2024;267:86–98.
Bais L, Vercammen A, Stewart R, van Es F, Visser B, Aleman A, et al. Short and long term ffects of left and bilateral repetitive transcranial magnetic stimulation in schizophrenia patients with auditory verbal hallucinations: a randomized controlled trial. PLoS ONE. 2014;9:e108828.
Xie Y, Li C, Guan M, Zhang T, Ma C, Wang Z, et al. Low-frequency rTMS induces modifications in cortical structural connectivity - functional connectivity coupling in schizophrenia patients with auditory verbal hallucinations. Hum Brain Mapp. 2024;45:e26614.
Guan M, Xie Y, Li C, Zhang T, Ma C, Wang Z, et al. Rich-club reorganization of white matter structural network in schizophrenia patients with auditory verbal hallucinations following 1 Hz rTMS treatment. Neuroimage Clin. 2023;40:103546.
Xie Y, Guan M, Wang Z, Ma Z, Wang H, Fang P, et al. rTMS induces brain functional and structural alternations in schizophrenia patient with auditory verbal hallucination. Front Neurosci. 2021;15:722894.
Xie Y, He Y, Guan M, Zhou G, Wang Z, Ma Z, et al. Impact of low-frequency rTMS on functional connectivity of the dentate nucleus subdomains in schizophrenia patients with auditory verbal hallucination. J Psychiatr Res. 2022;149:87–96.
Bahlmann J, Beckmann I, Kuhlemann I, Schweikard A, Münte TF. Transcranial magnetic stimulation reveals complex cognitive control representations in the rostral frontal cortex. Neuroscience. 2015;300:425–31.
Patel R, Silla F, Pierce S, Theule J, Girard TA. Cognitive functioning before and after repetitive transcranial magnetic stimulation (rTMS): a quantitative meta-analysis in healthy adults. Neuropsychologia. 2020;141:107395.
Waskom ML, Kumaran D, Gordon AM, Rissman J, Wagner AD. Frontoparietal representations of task context support the flexible control of goal-directed cognition. J Neurosci. 2014;34:10743–55.
Wallis G, Stokes M, Cousijn H, Woolrich M, Nobre AC. Frontoparietal and cingulo-opercular networks play dissociable roles in control of working memory. J Cognit Neurosci. 2015;27:2019–34.
Scolari M, Seidl-Rathkopf KN, Kastner S. Functions of the human frontoparietal attention network: evidence from neuroimaging. Curr Opin Behav Sci. 2015;1:32–9.
Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–17.
Sakurai T, Gamo NJ, Hikida T, Kim S-H, Murai T, Tomoda T, et al. Converging models of schizophrenia – Network alterations of prefrontal cortex underlying cognitive impairments. Prog Neurobiol. 2015;134:178–201.
Mitropoulos GB. Auditory verbal hallucinations in psychosis: abnormal perceptions or symptoms of disordered thought? J Nerv Ment Dis. 2020;208:81–4.
Axelrod V, Rees G, Bar M. The default network and the combination of cognitive processes that mediate self-generated thought. Nat Hum Behav. 2017;1:896–910.
Langland-Hassan P. Hearing a voice as one’s own: two views of inner speech self-monitoring deficits in schizophrenia. Rev Philosophy Psychol. 2016;7:675–99.
Papeo L, Agostini B, Lingnau A. The large-scale organization of gestures and words in the middle temporal gyrus. J Neurosci. 2019;39:5966–74.
Wei L, Li X, Huang L, Liu Y, Hu L, Shen W, et al. An fMRI study of visual geometric shapes processing. Front Neurosci. 2023;17:1087488.
Telles-Correia D, Moreira AL, Gonçalves JS. Hallucinations and related concepts—their conceptual background. Front Psychol. 2015;6:991.
Bais L, Liemburg E, Vercammen A, Bruggeman R, Knegtering H, Aleman A. Effects of low frequency rTMS treatment on brain networks for inner speech in patients with schizophrenia and auditory verbal hallucinations. Prog Neuro-psychopharmacol Biol Psychiatry. 2017;78:105–13.
Gornerova N, Brunovsky M, Klirova M, Novak T, Zaytseva Y, Koprivova J, et al. The effect of low-frequency rTMS on auditory hallucinations, EEG source localization and functional connectivity in schizophrenia. Neurosci Lett. 2023;794:136977.
Briend F, Leroux E, Delcroix N, Razafimandimby A, Etard O, Dollfus S. Impact of rTMS on functional connectivity within the language network in schizophrenia patients with auditory hallucinations. Schizophrenia Res. 2017;189:142–5.
Makoshi Z, Kroliczak G, van Donkelaar P. Human supplementary motor area contribution to predictive motor planning. J Mot Behav. 2011;43:303–9.
Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cognit Sci. 2004;8:410–7.
Weiner KS, Zilles K. The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia. 2016;83:48–62.
Onitsuka T, Shenton ME, Salisbury DF, Dickey CC, Kasai K, Toner SK, et al. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: An MRI study. Am J Psychiatry. 2004;161:1603–11.
Sulpizio V, Fattori P, Pitzalis S, Galletti C. Functional organization of the caudal part of the human superior parietal lobule. Neurosci Biobehav Rev. 2023;153:105357.
Zhu Y, Xu M, Lu J, Hu J, Kwok VPY, Zhou Y, et al. Distinct spatiotemporal patterns of syntactic and semantic processing in human inferior frontal gyrus. Nat Hum Behav. 2022;6:1104–11.
Wu G-R, Wang X, Baeken C. Baseline functional connectivity may predict placebo responses to accelerated rTMS treatment in major depression. Hum Brain Mapp. 2020;41:632–9.
Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76.
Javitt DC, Freedman R. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am J Psychiatry. 2014;172:17–31.
Dong D, Yao D, Wang Y, Hong S-J, Genon S, Xin F, et al. Compressed sensorimotor-to-transmodal hierarchical organization in schizophrenia. Psychological Med. 2023;53:771–84.
Kaufmann T, Skåtun KC, Alnæs D, Doan NT, Duff EP, Tønnesen S, et al. Disintegration of sensorimotor brain networks in schizophrenia. Schizophrenia Bull. 2015;41:1326–35.
Leitman DI, Sehatpour P, Higgins BA, Foxe JJ, Silipo G, Javitt DC. Sensory deficits and distributed hierarchical dysfunction in schizophrenia. Am J Psychiatry. 2010;167:818–27.
Briend F, Armstrong WP, Kraguljac NV, Keilhloz SD, Lahti AC. Aberrant static and dynamic functional patterns of frontoparietal control network in antipsychotic-naïve first-episode psychosis subjects. Hum Brain Mapp. 2020;41:2999–3008.
Chari S, Minzenberg MJ, Solomon M, Ragland JD, Nguyen Q, Carter CS, et al. Impaired prefrontal functional connectivity associated with working memory task performance and disorganization despite intact activations in schizophrenia. Psychiatry Research: Neuroimaging. 2019;287:10–8.
Uldry A-C, Maciel-Dominguez A, Jornod M, Buchs N, Braga-Lagache S, Brodard J, et al. Effect of sample transportation on the proteome of human circulating blood extracellular vesicles. Int J Mol Sci. 2022;23:4515.
Cannon TD. Abnormalities of brain structure and function in schizophrenia: Implications for aetiology and pathophysiology. Ann Med. 1996;28:533–9.
Schwarz E, Izmailov R, Liò P, Meyer-Lindenberg A. Protein interaction networks link schizophrenia risk loci to synaptic function. Schizophrenia Bull. 2016;42:1334–42.
Upadhyay RK. Transendothelial transport and its role in therapeutics. Int Sch Res Not. 2014;2014:309404.
Bansal V, Chatterjee I. Role of neurotransmitters in schizophrenia: a comprehensive study. Kuwait J Sci. 2021;48:1–27.
Hong J, Chen J, Li C, An D, Tang Z, Wen H. High-Frequency rTMS improves cognitive function by regulating synaptic plasticity in cerebral ischemic rats. Neurochem Res. 2021;46:276–86. https://doi.org/10.1007/s11064-020-03161-5.
Connor JA, Petrozzino J, Pozzo-Miller LD, Otani S. Calcium signals in long-term potentiation and long-term depression. Can J Physiol Pharmacol. 1999;77:722–34.
Maren S, Baudry M. Properties and mechanisms of long-term synaptic plasticity in the mammalian brain: relationships to learning and memory. Neurobiol Learn Mem. 1995;63:1–18.
Lidow MS. Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain Res Rev. 2003;43:70–84.
Berridge MJ. Calcium signalling and psychiatric disease: bipolar disorder and schizophrenia. Cell Tissue Res. 2014;357:477–92.
King ES, Tang AD. Intrinsic plasticity mechanisms of repetitive transcranial magnetic stimulation. Neuroscientist. 2022;30:260–74.
Dufor T, Lohof AM, Sherrard RM. Magnetic stimulation as a therapeutic approach for brain modulation and repair: Underlying molecular and cellular mechanisms. Int J Mol Sci. 2023;24:16456.
Nanou E, Catterall WA. Calcium channels, synaptic plasticity, and neuropsychiatric disease. Neuron. 2018;98:466–81.
Monday HR, Younts TJ, Castillo PE. Long-term plasticity of neurotransmitter release: Emerging mechanisms and contributions to brain function and disease. Annu Rev Neurosci. 2018;41:299–322.
Akhondzadeh S. Hippocampal synaptic plasticity and cognition. J Clin Pharm Therapeutics. 1999;24:241–8.
Sarter M, Bruno JP, Parikh V. Abnormal neurotransmitter release underlying behavioral and cognitive disorders: Toward concepts of dynamic and function-specific dysregulation. Neuropsychopharmacology. 2007;32:1452–61.
Kirchner SK, Ozkan S, Musil R, Spellmann I, Kannayian N, Falkai P, et al. Polygenic analysis suggests the involvement of calcium signaling in executive function in schizophrenia patients. Eur Arch Psychiatry Clin Neurosci. 2020;270:425–31.
Clarke D, Beros J, Bates KA, Harvey AR, Tang AD, Rodger J. Low intensity repetitive magnetic stimulation reduces expression of genes related to inflammation and calcium signalling in cultured mouse cortical astrocytes. Brain Stimulation. 2021;14:183–91.
Lee D. Global and local missions of cAMP signaling in neural plasticity, learning, and memory. Front Pharmacol. 2015;6:161.
Wu X-L, Yan Q-J, Zhu F. Abnormal synaptic plasticity and impaired cognition in schizophrenia. World J Psychiatry. 2022;12:541–57.
Hellmann J, Jüttner R, Roth C, Bajbouj M, Kirste I, Heuser I, et al. Repetitive magnetic stimulation of human-derived neuron-like cells activates cAMP-CREB pathway. Eur Arch Psychiatry Clin Neurosci. 2012;262:87–91.
Nicol X, Gaspar P. Routes to cAMP: shaping neuronal connectivity with distinct adenylate cyclases. Eur J Neurosci. 2014;39:1742–51.
Arévalo JC, Wu SH. Neurotrophin signaling: Many exciting surprises! Cell Mol Life Sci CMLS. 2006;63:1523–37.
Cowansage KK, LeDoux JE, Monfils M-H. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol. 2010;3:12–29.
Lee JY, Park HJ, Kim JH, Cho BP, Cho S-R, Kim SH. Effects of low- and high-frequency repetitive magnetic stimulation on neuronal cell proliferation and growth factor expression: A preliminary report. Neurosci Lett. 2015;604:167–72.
Luo J, Zheng H, Zhang L, Zhang Q, Li L, Pei Z, et al. High-frequency repetitive transcranial magnetic stimulation (rTMS) improves functional recovery by enhancing neurogenesis and activating BDNF/TrkB signaling in ischemic rats. Int J Mol Sci. 2017;18:455.
Ma J, Zhang Z, Su Y, Kang L, Geng D, Wang Y, et al. Magnetic stimulation modulates structural synaptic plasticity and regulates BDNF–TrkB signal pathway in cultured hippocampal neurons. Neurochemistry Int. 2013;62:84–91.
Valiuliene G, Valiulis V, Dapsys K, Vitkeviciene A, Gerulskis G, Navakauskiene R, et al. Brain stimulation effects on serum BDNF, VEGF, and TNFα in treatment-resistant psychiatric disorders. Eur J Neurosci. 2021;53:3791–802.
Gao X, Ni Y, Hu W, Wang G, He X. Comparative study about the therapeutic effect of cTBS and rTMS in the treatment of auditory verbal hallucinations in schizophrenia. Postgrad Med J. 2025;101:203–11.
Elliott-Hunt CR, Marsh B, Bacon A, Pope R, Vanderplank P, Wynick D. Galanin acts as a neuroprotective factor to the hippocampus. Proc Natl Acad Sci. 2004;101:5105–10.
Coumis U, Davies CH. The effects of galanin on long-term synaptic plasticity in the CA1 area of rodent hippocampus. Neuroscience. 2002;112:173–82.
Burazin TCD, Larm JA, Ryan MC, Gundlach AL. Galanin-R1 and -R2 receptor mRNA expression during the development of rat brain suggests differential subtype involvement in synaptic transmission and plasticity. Eur J Neurosci. 2000;12:2901–17.
Daniel AM, Rushing BG, Tapia Menchaca KY. Variation of the human mu-opioid receptor (OPRM1) gene predicts vulnerability to frustration. Sci Rep. 2020;10:21840.
Varastehmoradi B, Wegener G, Sanchez C, Smith KL. Opioid system modulation of cognitive affective bias: implications for the treatment of mood disorders. Behav Pharmacol. 2020;31:122–35.
DosSantos MF, Oliveira AT, Ferreira NR, Carvalho ACP, Rosado de Castro PH. The contribution of endogenous modulatory systems to TMS- and tDCS-induced analgesia: Evidence from PET Studies. Pain Res Manag. 2018;2018:2368386.
Zhan J, Fegg FN, Kaddatz H, Rühling S, Frenz J, Denecke B, et al. Focal white matter lesions induce long-lasting axonal degeneration, neuroinflammation and behavioral deficits. Neurobiol Dis. 2021;155:105371.
Speidel JT, Affandi T, Jones DNM, Ferrara SE, Reyland ME. Functional proteomic analysis reveals roles for PKCδ in regulation of cell survival and cell death: Implications for cancer pathogenesis and therapy. Adv Biol Regul. 2020;78:100757.
Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–3.
Zhong J, Yang P, Muta K, Dong R, Marrero M, Gong F, et al. Loss of Jak2 selectively suppresses DC-Mediated innate immune response and protects mice from lethal dose of LPS-induced septic shock. PLoS ONE. 2010;5:e9593.
Zouein FA, Duhé RJ, Booz GW. JAKs go nuclear: Emerging role of nuclear JAK1 and JAK2 in gene expression and cell growth. Growth Factors. 2011;29:245–52.
Nummenmaa L, Saanijoki T, Tuominen L, Hirvonen J, Tuulari JJ, Nuutila P, et al. μ-opioid receptor system mediates reward processing in humans. Nat Commun. 2018;9:1500.
van Steenbergen H, Eikemo M, Leknes S. The role of the opioid system in decision making and cognitive control: a review. Cognitive, Affective, Behav Neurosci. 2019;19:435–58.
Simon JJ, Cordeiro SA, Weber M-A, Friederich H-C, Wolf RC, Weisbrod M, et al. Reward system dysfunction as a neural substrate of symptom expression across the general population and patients with schizophrenia. Schizophrenia Bull. 2015;41:1370–8.
Ryan J, Pouliot JJ, Hajcak G, Nee DE. Manipulating reward sensitivity using reward circuit–targeted transcranial magnetic stimulation. Biol Psychiatry: Cognit Neurosci Neuroimaging. 2022;7:833–40.
Lantrip C, Gunning FM, Flashman L, Roth RM, Holtzheimer PE. Effects of transcranial magnetic stimulation on the cognitive control of emotion: potential antidepressant mechanisms. J ECT. 2017;33:73–80.
Chen J, Wei Y, Xue K, Han S, Li W, Zhou B, et al. Abnormal effective connectivity of reward network in first-episode schizophrenia with auditory verbal hallucinations. J Psychiatr Res. 2024;171:207–14.
Swyer A, Powers AR. Voluntary control of auditory hallucinations: phenomenology to therapeutic implications. NPJ Schizophr. 2020;6:19 https://doi.org/10.1038/s41537-020-0106-8.
Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABAa receptors. Trends Neurosci. 2004;27:569–75.
Sears SMS, Hewett SJ. Influence of glutamate and GABA transport on brain excitatory/inhibitory balance. Exp Biol Med. 2021;246:1069–83.
Wu C, Sun D. GABA receptors in brain development, function, and injury. Metab Brain Dis. 2015;30:367–79. https://doi.org/10.1007/s11011-014-9560-1.
Roth FC, Draguhn A. GABA metabolism and transport: effects on synaptic efficacy. Neural Plast. 2012;2012:805830.
Stagg CJ, Bachtiar V, Amadi U, Gudberg CA, Ilie AS, Sampaio-Baptista C, et al. Local GABA concentration is related to network-level resting functional connectivity. eLife. 2014;3:e01465.
Lee V, Maguire J. The impact of tonic GABAa receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front Neural Circuits. 2014;8:3.
Zaben MJ, Gray WP. Neuropeptides and hippocampal neurogenesis. Neuropeptides. 2013;47:431–8.
Shen P-J, Larm JA, Gundlach AL. Expression and plasticity of galanin systems in cortical neurons, oligodendrocyte progenitors and proliferative zones in normal brain and after spreading depression. Eur J Neurosci. 2003;18:1362–76.
Duan J, Shen D-D, Zhao T, Guo S, He X, Yin W, et al. Molecular basis for allosteric agonism and G protein subtype selectivity of galanin receptors. Nat Commun. 2022;13:1364 https://doi.org/10.1038/s41467-022-29072-3.
Barde S, Rüegg J, Prud’homme J, Ekström TJ, Palkovits M, Turecki G, et al. Alterations in the neuropeptide galanin system in major depressive disorder involve levels of transcripts, methylation, and peptide. Proc Natl Acad Sci. 2016;113:8472–81.
Rustay NR, Wrenn CC, Kinney JW, Holmes A, Bailey KR, Sullivan TL, et al. Galanin impairs performance on learning and memory tasks: Findings from galanin transgenic and GAL-R1 knockout mice. Neuropeptides. 2005;39:239–43.
Campos-Jurado Y, Martí-Prats L, Zornoza T, Polache A, Granero L, Cano-Cebrián MJ. Regional differences in mu-opioid receptor-dependent modulation of basal dopamine transmission in rat striatum. Neurosci Lett. 2017;638:102–8.
Clark SD, Abi-Dargham A. The role of dynorphin and the kappa opioid receptor in the symptomatology of schizophrenia: A review of the evidence. Biol Psychiatry. 2019;86:502–11.
Befort K, Filliol D, Ghate A, Darcq E, Matifas A, Muller J, et al. Mu-opioid receptor activation induces transcriptional plasticity in the central extended amygdala. Eur J Neurosci. 2008;27:2973–84.
Light SN, Bieliauskas LA, Zubieta J-K. “Top-down” mu-opioid system function in humans: mu-opioid receptors in ventrolateral prefrontal cortex mediate the relationship between hedonic tone and executive function in major depressive disorder. J Neuropsychiatry Clin Neurosci. 2017;29:357–64.
Mechling AE, Arefin T, Lee H-L, Bienert T, Reisert M, Ben Hamida S, et al. Deletion of the mu opioid receptor gene in mice reshapes the reward–aversion connectome. Proc Natl Acad Sci. 2016;113:11603–8.
Funding
This study was funded by the Natural Science Basic Research Program of Shaanxi Province (No.2024JC-YBQN-0209), National Natural Science Foundation of China (No. 32471081, 61806210, and 82330043), Key Research and Development Program of Shaanxi Province (No. 2023-ZDLSF-07).
Author information
Authors and Affiliations
Contributions
XYJ and GMZ conceived and design the project, data curation and writing original draft; ZT, MCZ, LCX and WLL analysed and interpreted the data; LXX, MZJ, LYJ and WZH collected and organized the data; WHN and FP provided supervision, resources, and funding. All authors reviewed the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study received approval from the Medical Ethics Committee of Xijing Hospital (KY20202055-F-1) and carried out in accordance with the principles of the Declaration of Helsinki. All procedures adhered to relevant guidelines and regulations. Informed consent was signed by all participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xie, Y., Guan, M., Zhang, T. et al. Unravelling the genetic and molecular basis of low-frequency rTMS induced changes in functional connectivity density in schizophrenia patients with auditory verbal hallucinations. Transl Psychiatry 15, 237 (2025). https://doi.org/10.1038/s41398-025-03459-4
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03459-4