Abstract
Background
Currently, most studies of depression are limited to a single disease endpoint.
Aims
This study aimed to conduct an umbrella review to comprehensively assess the association between depression and health outcomes.
Method
Until December 17, 2024, we conducted a systematic search of systematic reviews and meta-analyses in PubMed, Embase, and Web of Science. We reanalyzed the summary effects and 95% confidence intervals for each study using random models. We assessed the methodological quality and evidence quality of the research with A Measurement Tool to Assess Systematic Reviews 2 and Grade of Recommendations, Assessment, Development and Evaluation, classifying studies into four categories based on evidence classification criteria.
Results
We selected a total of 72 articles from 27,150 resulting in 114 meta-analyses and 109 health outcomes. Depression exposure was associated with 23 mortality, 21 cardiovascular outcomes, 15 offspring outcomes, 9cancer outcomes, 9 neurological outcomes, 5 endocrine outcomes, 5 dental outcomes, 3 digestive outcomes, and 19 other health outcomes. Moderate-quality evidence linked depression to specific mortality in bladder cancer (Class IV), all-cause mortality in myocardial infarction (Class III), mortality within 2 years of initial assessment in coronary artery disease (Class IV), major adverse cardiovascular events after percutaneous coronary intervention (Class III), irritable bowel syndrome (insignificant), fear of falling (Class III), and frailty (Class III).
Conclusions
Depression has a significant impact on health outcomes, primarily mortality and cardiovascular outcomes. However, more definitive conclusions still require randomized controlled trials or prospective studies for validation.
Similar content being viewed by others
Introduction
Depression affects approximately 5% of adults worldwide, as the World Health Organization estimates, and it stands as the fourth most common disease globally [1]. According to the Global Burden of Disease data from 2019, depression was one of the leading causes of disability and mortality [2]. In 2020, there was an addition of 76.2 million cases of Major Depressive Disorder (MDD) worldwide. Majority of countries such as Spain, Mexico, Malaysia, the United States, and Uruguay experienced the most significant increase in depression prevalence [3]. Furthermore, it was estimated that there would be an additional 53.2 million cases of severe depression globally, representing an increase of 27.6% [4].
Recently, increasing number of studies have shown that depression can lead to various health outcomes such as cardiovascular disease, atrial fibrillation, heart failure, coronary artery disease, myocardial infarction, stroke, and hypertension and impacts life quality and longevity [5,6,7,8,9,10]. Depression also increases the risk of mortality, including cardiovascular mortality and all-cause mortality in cancer [11]. As the amount of systematic reviews and meta-analyses accumulates over the past decades and an umbrella review is helpful to provide a broader picture of findings and synthesize the evidence, depression-related umbrella reviews are mostly restricted to mortality, and many relevant meta-analyses have been published subsequently [12,13,14,15,16], and a lack of comprehensive and systematic assessment of the relationship between depression and multiple health outcomes. Additionally, due to the subjectivity or inconsistency of assessment criteria, differing definitions of exposure, as well as limitations in data sources, diversity in study designs, and inconsistencies in statistical methods, the quality of evidence in these reviews varies. An umbrella review, which synthesizes existing systematic reviews and meta-analyses, will provide decision-makers with a comprehensive source of high-quality research on the relationship between depression and various health outcomes.
Therefore, using umbrella review, this study conducted a comprehensive overview to thoroughly outline and assess the association between depression and various health outcomes.
Methods
Umbrella review methods
We comprehensively and systematically searched the existing literature for various systematic reviews and meta-analyses on the relationship between depression and health outcomes. This umbrella review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42023471844) (https://www.crd.york.ac.uk/PROSPERO/).
Literature search
We systematically searched for systematic reviews and meta-analyses of observational studies in PubMed, Embase, and Web of Science databases from the database inception to December 17, 2024 (see Additional File 1: Table S1) written in English. Electronic searches were independently conducted by two authors (XC and PQ). Subsequently, duplicates were removed, titles and abstracts were screened, and full texts were read to identify meta-analyses that met the inclusion criteria. Any discrepancies between the two reviewers during the literature screening process were resolved by a third author. We manually searched the reference lists of all included articles, reviews, and meta-analyses to identify any potentially missed studies.
Eligibility criteria
We included studies that met the following PICOS criteria: (1) population: participants without restrictions based on race, region, or health status; (2) intervention/exposure: with depression; (3) comparison: without depression;(4) outcome: any health outcome, defined as health states or results that affect individuals’ physical, psychological, or social functioning; and (5) study design: systematic reviews and meta-analyses of observational epidemiological studies (cohort, case-control, and cross-sectional studies) or randomized controlled trials.
We excluded articles that met any of the following criteria: (1) did not report summary estimates (e.g., systematic reviews without meta-analyses); (2) reported other mental disorders (such as anxiety) unless separate data on depression, as defined above; (3) were letters, conference abstracts, academic papers, and research protocols; (4) involved animal or cell culture research; and (5) were published not in English. If two or more health outcomes were reported in one article, data for each individual outcome were extracted separately. If studies on depression exposure and the same health outcome were published more than 24 months apart, we included the study with the newest data, usually the one with the largest sample size.
Data extraction
Two researchers (XC and PQ) independently extracted the following data from eligible original articles: the first author’s name; publication date; study population; types of studies included in the meta-analysis (randomized controlled trials, cross-sectional, cohort, or case-control studies); exposure types; outcomes and the number of studies, total number of participants, and the number of cases included in the meta-analysis. Furthermore, the reviewers extracted the summary effect size and 95% confidence interval (CI) of the results, as well as the model for effect (random or fixed), heterogeneity (I2 statistic and Cochran Q test P-value), and publication bias assessment (Egger’s test or P-value of the funnel plot). Any disagreement was determined by a third author.
Quality assessment of methods and evidence
A Measurement Tool to Assess Systematic Reviews 2 (AMSTAR2) was utilized to assess the methodological quality of the included articles [17]. AMSTAR2 is an effective and reliable measurement tool for evaluating the quality of systematic reviews and meta-analyses, categorizing study quality into four levels: “High”, “Moderate”, “Low” and “Critically low”. Simultaneously, Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) was employed to assess the quality of evidence for the association between depression and each health outcome, categorized into four levels: “High”, “Moderate”, “Low”, or “Very low” [18]. Additionally, we classified the evidence into four categories based on evidence classification criteria: Class I (convincing evidence), Class II (highly suggestive evidence), Class III (suggestive evidence), Class IV (weak evidence), and Class NS (not significant) [19].
Data analysis
We reanalyzed the summary effects (risk ratio, odds ratio, weighted mean difference, or standardized mean difference) for each study using a random-effects or fixed-effects model, and also recalculated their 95% confidence intervals. The I2 statistic and P value of Cochran’s Q test for heterogeneity were recalculated. Additionally, we employed Egger’s regression test to calculate estimates of publication bias for any reanalysis involving at least 10 studies, considering a P-value < 0.1 as significant [20]. If reanalysis could not be performed from the meta-analysis, we extracted summary data to evaluate heterogeneity and publication bias. We conducted the reanalysis with Stata MP version 17 and constructed summary forest plots based on the extracted and/or reanalyzed data using R version 4.3.2.
Patient involvement
No patients participated in the planning, design, or implementation of this study.
Results
Characteristics of meta-analyses
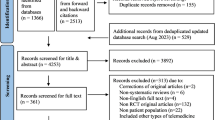
Figure 1 shows the research selection process employed in this study. Following a systematic search, a total of 27,150 articles were retrieved from PubMed, Embase, and Web of Science, and the references of the included studies. After applying inclusion and exclusion criteria, 72 articles were found to meet the conditions, with some articles undergoing multiple meta-analyses, generating a total of 114 meta-analyses and 109 health outcomes. Table 1 summarizes the main characteristics of the conducted meta-analyses. Figure 2 shows the relationship between depression and outcomes related to cancer and mortality. Figure 3 illustrates the relationship between depression and endocrine/metabolic and cardiovascular outcomes. Figure 4 shows the relationship between depression and outcomes related to the digestive system, dental health, and offspring health. Figure 5 displays the relationship between depression and neurological outcomes as well as other types of related health outcomes. The distribution of health outcomes is presented in Fig. 6, with the majority of included meta-analyses focusing on the association between depression and mortality (n = 23, 21.1%). This was followed by cardiovascular outcomes (n = 21,19.8%), other health outcomes (n = 19, 17.4%), offspring health outcomes (n = 15, 13.8%), cancer (n = 9, 8.3%), neurological system health outcomes (n = 9, 8.3%), endocrine/metabolic health outcomes (n = 5, 4.6%), dental health outcomes (n = 5, 4.5%), and digestive system health outcomes (n = 3, 2.8%).
NA not available, RR relative risk, OR odds ratio, HR hazard ratio, AMSTAR2 a measurement tool to assess systematic reviews 2, GRADE grading of recommendations, assessment, development, and evaluation, CVD cardiovascular diseases, HSCT hematopoietic stem cell transplantation, PAD peripheral artery disease, PCI percutaneous coronary intervention, PSD post-stroke depression, HF heart failure, CABG coronary artery bypass grafting, CKD chronic kidney disease, MI myocardial infarction, CHD coronary heart disease.
NA not available, RR relative risk, OR odds ratio, HR hazard ratio, AMSTAR2 a measurement tool to assess systematic reviews 2, GRADE Grading of Recommendations, Assessment, Development, and Evaluation, PAD peripheral artery disease, HF heart failure, PCI percutaneous coronary intervention, CVD cardiovascular diseases, MI myocardial infarction.
Cancer outcomes
A meta-analysis comprising 21 studies indicated that depression increased the incidence of cancer (RR = 1.13). In addition, the same study also found that depression increases the incidence of lung cancer (RR = 1.41), oral cancer (RR = 1.47), prostate cancer (RR = 1.37), and skin cancer (RR = 1.09) [21]. Patients with depression before the treatment of head and neck cancer have a worse overall survival rate compared to those without depression (HR = 1.33) [22]. Similarly, glioma patients with depression have poorer survival outcomes than those without depression (RR = 0.51, 95% CI 0.18–0.83) [23]. An analysis of 11 cohort studies concluded that current epidemiological evidence did not support a connection between depression and breast cancer (RR = 1.13, 95% CI 0.94–1.36) [24]. However, another meta-analysis found a positive correlation between depression and increased risk of breast cancer recurrence (HR = 1.24, 95% CI 1.07–1.43) [25] (Fig. 2).
Mortality
In the community, late-life depression significantly increased all-cause mortality (RR = 1.34) and cardiovascular (CVD) mortality (RR = 1.33) [12] among the elderly. Assessments of depression based on self-reports (HR = 2.51) and clinical interviews (HR = 1.34) show a positive correlation between depression and the risk of all-cause mortality in diabetic patients [26]. Furthermore, depression elevated the risk of death for individuals with coronary artery disease (HR = 1.36) [9] and chronic kidney disease (CKD) (RR = 1.47) [27]. Depression not only increased the all-cause mortality among cancer patients (RR = 1.24) [21] but also heightened the all-cause mortality rates by 34 and 30% in lung cancer and breast cancer patients with depression, respectively. Furthermore, patients with bladder cancer, colorectal cancer, hematological cancers, kidney cancer, and prostate cancer who also have depression experience increases in cancer-specific mortality by 102, 38, 66, 85, and 87%, respectively [21]. Patients with peripheral artery disease (PAD) (HR = 1.06), stroke (HR = 1.59) [16], myocardial infarction (MI) (OR = 2.25) [28], and heart failure (HF) (HR = 1.20) [29] who had depression showed an increased all-cause mortality. Among patients with coronary heart disease (CHD), depression significantly raises mortality risk, with an OR of 2.24 for short-term (within 2 years) mortality and 1.78 for long-term mortality. Compared to patients without depression, those undergoing coronary artery bypass grafting (CABG) [30] or percutaneous coronary intervention (PCI) [15] with depression experienced a 41 and 76% increase in all-cause mortality. Depression had a significant impact on the overall survival of patients undergoing hematopoietic stem cell transplantation (HSCT), with a hazard ratio of 1.06 (HR = 1.06) [31]. However, another meta-analysis of 20 cohort studies found no association between depression and post-transplant mortality (RR = 1.42, 95% CI 0.98–1.86) [32]. (Fig. 2).
Endocrine/metabolic outcomes
A meta-analysis of 9 cohort studies showed individuals with depression had a 34% higher risk of obesity than those without [33]. Furthermore, depression significantly increased the risk of developing diabetes (RR = 1.17) [34], diabetic nephropathy (OR = 1.22) [35], and gestational diabetes (OR = 1.19) [36]. Base on evidence from 31 cross-sectional studies and 18 cohort studies, patients with depression had a higher likelihood of suffering from metabolic syndrome compared to those without depression, with odds ratios of 1.48 and risk ratios of 1.38 [37] (Fig. 3).
Cardiovascular outcomes
Compared to non-depressed individuals, those with depressive symptoms were likelier to face various cardiovascular problems, including atrial fibrillation (RR = 1.13) [6], heart failure (HR = 1.23), coronary heart disease (RR = 1.21) [7], ventricular arrhythmias (HR = 1.33) [38], coronary artery calcification (OR = 1.15) [39], sudden cardiac death (HR = 1.62) [40], ventricular tachycardia/ventricular fibrillation (HR = 1.47) [40], myocardial infarction (HR = 1.31) [9], first stroke (HR = 1.40) [10], hypertension (RR = 1.42) [41], cardiac events following myocardial infarction (OR = 1.59) [28], and major adverse limb events in PAD (RR = 1.18) [14]. In the diabetic population, depressive symptoms significantly increased the risk of non-fatal and fatal cardiovascular events, with risk ratios of 1.35 and 1.47 [42], and diabetes complications involving both large and small blood vessels (HR = 1.39) [43]. PAD patients with depression undergoing percutaneous coronary intervention faced higher risks of adverse outcomes (RR = 1.42) [44], including major adverse cardiovascular events (RR = 1.72) [15]. Furthermore, heart failure patients with depression had increased risks of readmission [45] and stroke recurrence [46] by 45 and 48%. However, some studies indicated no significant link between depression and cardiovascular disease (OR = 1.46, 95% CI 0.99–1.93) [47] or major cardiovascular adverse events in PAD (RR = 0.96, 95% CI 0.53–1.39) [14] (Fig. 3).
Digestive and dental health outcomes
Figure 4 shows the meta-analyses on the association between depression and digestive and dental health outcomes. Depression increased the risk of Crohn’s disease (RR = 1.17) and ulcerative colitis (RR = 1.21) [48], and individuals with depression were twice as likely to suffer from irritable bowel syndrome [49] as those without depression. Moreover, depression seemed to increase the risk of oral diseases in adults and the elderly, especially cavities (OR = 1.27), tooth loss (OR = 1.31) [50], and edentulism (OR = 1.17, 95% CI 1.02–1.34) [50]. However, the association between depression and periodontitis (OR = 0.96, 95% CI 0.84–1.10) [50] as well as gingivitis (OR = 1.00, 95% CI 0.71–1.30) [51] was not significant.
Offspring health outcomes
Paternal depression significantly increases the risk of depression in offspring (OR = 1.42) [52], while maternal perinatal depression has an even greater impact on the risk of depression in offspring (OR = 1.70) [53]. Similarly, paternal perinatal depression was associated with an increase in behavioral and emotional issues in offspring by 21 and 26%, respectively [54]. Maternal depression during pregnancy raised the risk of low Apgar scores in newborns (OR = 1.91) [55] and was linked to asthma (OR = 1.24) [56, 57] as well as social and emotional challenges (OR = 1.56) [58] in children. Offspring of mothers with postpartum depression faced an elevated risk of attention deficit hyperactivity disorder (OR = 1.69) [59], though the impact on anxiety disorders was not significant (OR = 1.73, 95% CI 0.68–2.79) [60]. Additionally, children of mothers with depression or depressive symptoms were more prone to being underweight (OR = 1.50) [61] and experiencing developmental delays (OR = 1.40) [61]. However, studies have shown that prenatal depression was not associated with atopic dermatitis in children [56] or low 1-min [55] and 5-min Apgar scores [55] in newborns. Paternal perinatal depression was related to a statistically non-significant increase in children’s social functioning (OR = 1.30, 95% CI 0.97–1.74) [54] (Fig. 4).
Neurological system health outcomes
Compared to healthy individuals, patients with depression experienced a reduction in subsequent cognitive scores, an increased risk of mild cognitive impairment, Alzheimer’s disease, Parkinson’s disease, dementia, and motor cognitive risk syndrome, with respective increases of 33, 52, 79, 78, 63, and154% [62,63,64,65]. Patients with depression had a 91% increased risk of developing postoperative delirium after surgery [66] Additionally, depression linked to atrophy in both right and left hippocampal regions (SMD = −0.43, 95% CI −0.66–−0.21) [67] and (SMD = −0.40, 95% CI −0.66–−0.15) [67] (Fig. 5).
Others health outcomes
Depression had a broad and profound impact on various health outcomes across different age groups and professions. In adolescents, depression significantly increased the risk of internet addiction (OR = 1.25, 95% CI 1.19–1.31) [68]. Among the elderly, those with depression were more likely to experience sleep disturbances (RR = 1.72) and worsening symptoms (RR = 1.73) [69]. Additionally, depressive symptoms were linked to worsening recovery in patients with chronic lumbar disc herniation (RR = 0.92, 95% CI 0.89–0.95), adverse outcomes in tuberculosis treatment (OR = 4.26, 95% CI 2.33–7.79), reduced hip bone mineral density (SMD = −0.35, 95% CI −0.53–−0.17), increased incidence of adult-onset asthma (RR = 1.43, 95% CI 1.28–1.61), and elevated C-reactive protein (CRP) concentrations in patients with PSD (SMD = 0.34, 95% CI 0.12–0.56) [70,71,72,73]. Studies reporting risk ratios and hazard ratios found that the risk of fractures in patients with depression increases by 18 and 30%, respectively [72]. Patients with depression were more likely to be frail (OR = 2.25; OR = 4.07), premature ejaculation (OR = 1.63), and sexual dysfunction (RR = 1.52) [74,75,76]. Doctors who exhibit depressive symptoms are at a higher risk of committing medical errors (RR = 1.97) [77]. Depression was also related to a higher risk of car accidents (OR = 2.00) [78]. A meta-analysis of 13 cohort studies showed that patients with depression symptoms were 59% more likely to engage in suicidal behavior during follow-up than non-depressed individuals [79]. However, symptoms of depression did not correlate with pain intensity in acute lumbar disc herniation patients (OR = 1.05, 95% CI 0.97–1.14) [80]. Elderly patients with depression were not significantly linked to falls (OR = 1.05, 95% CI 0.92–21.17), fear of falling (OR = 2.72, 95% CI 0.99–4.44) [81], or persistent sleep disorders (RR = 1.20, 95% CI 0.94–1.52) [69] (Fig. 5).
Heterogeneity
Reanalysis found that approximately 58 (50.9%) out of the 114 studies that we reanalyzed had significant heterogeneity (I2 > 50% or P value of Cochran’s Q test <0.1). Of these, 42 (36.8%) meta-analyses showed high heterogeneity (I2 > 75%).
Assessment of risk of bias
In our reanalysis, significant publication bias was detected in studies concerning all-cause mortality, cardiovascular mortality, overall survival in hematopoietic stem cell transplantation, all-cause mortality in cancer, heart failure, coronary artery mortality, post-transplant mortality, metabolic syndrome in cross-sectional and cohort studies, coronary heart disease, myocardial infarction, offspring attention deficit hyperactivity disorder, offspring social function, mild cognitive impairment, internet addiction, pain intensity in acute lumbar disc herniation, exacerbation of chronic lumbar disc herniation, falls and fractures (Egger test P value < 0.05).The remaining studies did not exhibit significant publication bias or could not be assessed for publication bias due to an insufficient number of studies.
Amstar, grade, and evidence classification
The AMSTAR 2 analysis revealed that the methodological quality of 7 studies (6.1%) on all-cause mortality in diabetics with depression (self-reported and clinical interviews), all-cause mortality in PAD, coronary heart disease, hypertension periodontitis, and suicidal behavior were classified as “High” quality. Additionally, 4 studies (3.5%) concerning mortality in CKD, major cardiovascular adverse events in PAD, major adverse limb events in PAD, and adult-onset asthma were rated as “Moderate” quality, while 95 studies (83.3%) received a “Low” or “Critically Low” quality (Table S2).
According to GRADE scoring, 7 studies (6.1%) covering bladder cancer mortality, all-cause mortality post-myocardial infarction, mortality risk within two years of coronary heart disease, major adverse cardiovascular events post-percutaneous coronary intervention, irritable bowel syndrome, fear of falling, and frailty were deemed to have “Moderate” epidemiological evidence quality. The epidemiological evidence quality of 107 studies (93.9%) were considered “Low” or “Very Low” (Table S3).
Regarding the classification of evidence, 55 out of 114 outcomes (48.2%) were rated as “III” (suggestive evidence), 40 (35.1%) as “ IV” (weak evidence), and 19 (16.7%) were deemed “NS” (non-significant) (Table S4).
Discussion
Principal findings and possible explanations
Our review encompassed 72 articles, including 114 meta-analyses and 109 health outcomes, and showed depression’s link to adverse health effects. Depression was found to be associated with all-cause mortality and various disease-specific mortality, such as cardiovascular death, coronary artery disease, and lung cancer mortality. Depression was also linked to multiple adverse cardiovascular outcomes, including atrial fibrillation, coronary heart disease, and stroke. Furthermore, depression increased the risk of developing cancers, such as lung cancer, oral cancer, and prostate cancer. Depression also raised the risk of neurological disorders, including Alzheimer’s disease, Parkinson’s disease, and dementia. Our study further indicated that depression was associated with endocrine-metabolic diseases (such as diabetes, obesity, and diabetic nephropathy), digestive system diseases (such as Crohn’s disease and ulcerative colitis), oral diseases (such as dental caries and tooth loss), offspring health outcomes (such as offspring depression and low infant Apgar scores), sleep disorders, adult asthma, traffic accidents, and premature ejaculation, among other adverse health outcomes.
The AMSTAR2 tool assessed articles’ methodological quality on depression’s impact on health outcomes, and the GRADE method analyzed evidence quality. For the methodological quality, only 11 meta-analyses (all-cause mortality in diabetic patients (self-reported and clinical interviews), all-cause mortality in PAD, coronary heart disease, hypertension, mortality in CKD patients, major cardiovascular adverse events in PAD patients, major adverse limb events in PAD patients, periodontitis, suicidal behavior, and adult-onset asthma were rated as “high” or “moderate” in methodological quality. The other health outcomes were found to have “Low” or “Critically low” methodological quality, mainly due to a lack of consideration for publication bias or because the authors did not provide a detailed list of excluded studies with justification for the exclusions. The failure to assess the quality of the original studies also reduced the overall quality of the research [7, 42, 43, 47, 56, 71, 82]. Moreover, the evidence quality for studies on cancer, digestive system, oral health, offspring health, and endocrine and metabolic outcomes was generally not high, with only 7 outcomes (bladder cancer mortality, all-cause mortality after myocardial infarction, mortality risk within two years for coronary heart disease, major adverse cardiovascular events after percutaneous coronary intervention, irritable bowel syndrome, fear of falling, and frailty) having a “moderate” level of evidence quality. The primary reasons for the low quality of evidence were high heterogeneity among studies [6, 8, 9, 12, 14, 16, 21, 22, 24, 26, 27, 29, 37, 39, 42, 43, 54, 59, 61,62,63, 67,68,69,70, 72, 76, 78, 52], lack of precision [6, 14, 21, 23, 24, 31, 38, 39, 48, 50, 55, 63, 69, 70, 76, 80, 82], or wide confidence intervals [21, 28, 45, 49, 63, 74, 81,82,83]. Therefore, future meta-analyses related to depression and health outcomes could reduce publication bias by systematically searching various databases, including unpublished studies, and using comprehensive search strategies to capture all relevant research. It is recommended that authors provide a detailed list of excluded studies with clear reasons for the exclusions to ensure transparency and replicability of the process. Improving study quality also hinges on strictly adhering to research design principles, including, but not limited to, proper randomization methods, allocation concealment, blinding, and appropriate statistical analysis techniques. Researchers should also ensure sufficient sample sizes to achieve statistical significance, thereby enhancing the study’s power.
Depression had linked to an increased all-cause and cardiovascular mortality, particularly pronounced among the elderly. This was consistent with findings from two large cohort studies, the China Kadoorie Biobank study and the Dongfeng-Tongji study, which had shown elevated rates of all-cause mortality (HR = 1.21, HR = 1.45) and cardiovascular mortality (HR = 1.33) in depressed patients aged 65 and over [84]. Elderly vulnerability may arise from social isolation, bereavement, health deterioration, and cognitive decline. Early detection and effective management of depression in this demographic were deemed crucial for mitigating its potential adverse effects on health and lifespan. The review also uncovered that in diabetic, depression, whether assessed through self-reported or clinically interviewed, could increase risk of all-cause mortality [26]. The association between self-reported depression and all-cause mortality was notably stronger than that measured through clinical interviews, possibly reflecting the sensitivity of self-reporting in capturing the subjective experience of depressive symptoms. This underscored the importance of considering the method of depression measurement in future studies. It is noteworthy that depression not only increased the mortality rates among specific disease groups, such as patients with coronary artery disease, but also adversely affected the mortality rates of patients undergoing certain surgical treatments, such as those who had undergone coronary artery bypass graft surgery or had received hematopoietic stem cell transplants [30, 31]. A multi-center prospective study had aligned with our findings, showing a positive correlation between depressive symptoms within a year after coronary artery bypass graft surgery and mortality [85]. This could be indirectly attributed to depressed patients struggling to adhere to recommendations for a healthy diet, regular exercise, and medication due to low mood, lack of energy, and reduced motivation. Although our analysis had revealed an association between depression and increased mortality risk, no significant connection was observed in terms of mortality post-organ transplantation and PAD mortality [14, 32]. This suggested a degree of heterogeneity in the impact of depression on different mortality, warranting further investigation in future research. AMSTAR2 and GRADE analyses had indicated that the quality of research for 7 disease outcomes was “High” or “Moderate”. However, the quality of evidence for most studies linking depression to mortality remained low. Therefore, future studies would need to employ more rigorous research designs and methodologies to enhance the quality of evidence.
A retrospective cohort study spanning 5.5 years revealed that over one-tenth of patients with depression developed cardiovascular diseases, with a particularly notable finding that individuals with prolonged durations of depression faced a higher risk of cardiovascular diseases after adjustments were made for multiple variables [86]. This discovery aligned with our comprehensive review, which noted that individuals exhibiting symptoms of depression were more likely to encounter a range of cardiovascular issues, including atrial fibrillation, heart failure, coronary artery disease, myocardial infarction, hypertension [6,7,8,9,10, 41], and significant limb problems due to PAD [14]. Patients with depression often endured sustained psychological stress, leading to chronic stress responses that activated the HPA axis and the autonomic nervous system, causing increases in blood pressure and heart rate, thereby elevating the risk of cardiovascular diseases [87]. The emergence or worsening of cardiovascular diseases could, in turn, exacerbate symptoms of depression, creating a vicious cycle. For instance, cardiovascular diseases could restrict physical activity, further intensifying symptoms of depression [88]. However, a reevaluation of a meta-analysis encompassing seven cohort studies found no significant association between depression and cardiovascular diseases [47], which could be attributed to differences in study designs, methods of assessing depression, sample characteristics, or statistical approaches. Future research should employ standardized tools for assessing depression, control for potential confounding factors, and conduct long-term follow-ups to accurately assess the relationship between depression and cardiovascular diseases.
Our umbrella review revealed that depression, as a risk factor, increased the incidence of cancer, particularly lung cancer, oral cancer, prostate cancer, and skin cancer [21]. Depression was also linked to poorer survival outcomes in patients with head and neck cancers and gliomas [22, 23], suggesting that depression could not only elevate the likelihood of developing cancer but might also adversely affect the treatment responses and quality of life of cancer patients. This could be attributed to factors such as the unhealthy lifestyles of individuals with depression such as smoking and excessive alcohol consumption, diminished immune function due to chronic stress, or reduced adherence to treatment [87, 89, 90]. However, the epidemiological evidence connecting depression with breast cancer outcomes appeared insufficient. Although some meta-analyses identified a positive correlation between depression and the risk of recurrence in breast cancer [25], the high heterogeneity and imprecision of these studies rendered the quality of evidence low, necessitating a cautious interpretation of this conclusion.
Exposure to depression was found to have increased the risk of obesity, diabetes and its complications, as well as metabolic syndrome [33,34,35,36]. Studies indicated that the risk of obesity in patients with depression had risen by 34%, which could be attributed to their reduced physical activity, unhealthy dietary habits, and metabolic changes associated with chronic stress related to depression [87]. Additionally, chronic stress and depression could promote obesity by affecting hormone levels, such as an increase in cortisol. Metabolic syndrome, a key risk factor for cardiovascular diseases and diabetes, was diagnosed in 27.7% of patients with depression in a study from northwestern India [91]. This study also highlighted that individuals with metabolic syndrome engaged in less physical activity and had poorer dietary habits [92, 93] compared to those without metabolic syndrome, suggesting that depression might indirectly heighten the risk of metabolic syndrome by impacting lifestyle factors. Our analysis also revealed limitations in current research, with all included studies rated as “low” in methodological quality, and the quality of evidence for most endocrine/metabolic outcomes being “Low” or “Very low.” This underscores the necessity for further large-scale prospective studies.
As a common mental disorder, depression was found to have complex associations with neurological conditions such as mild cognitive impairment, Alzheimer’s disease, motor cognitive risk syndrome, Parkinson’s disease, dementia, and hippocampal atrophy [62, 64, 67]. For instance, a study conducted on hospitalized adolescents revealed that clinical depression correlated with cognitive features [94], suggesting that depression might have begun impacting the brain early on and potentially accelerated cognitive decline and the progression of neurodegenerative diseases. Moreover, the link between depression and physical changes in the brain was also confirmed to some extent. Research showed that depression could lead to a more rapid decrease in hippocampal volume, which is closely linked to impairments in memory and other cognitive functions, thereby potentially speeding up the development of diseases like Alzheimer’s disease [95]. However, it is crucial to acknowledge the limitations present in studies exploring the relationship between depression and neurological diseases, such as small sample sizes, insufficient control of confounding factors, and heterogeneity in diagnostic criteria [82]. Although evidence indicated a connection between the depression and neurological diseases, the methodological and evidence quality of these studies was generally low due to publication bias and high heterogeneity, limiting our understanding of the depth and mechanisms of these relationships.
Additionally, depression was found to have a positive correlation with various digestive system diseases and oral health issues. Specifically, depression increased the risk of Crohn’s disease, ulcerative colitis, and irritable bowel syndrome [48, 49]. Depression also impacted the oral health of adults and the elderly, such as cavities, tooth loss, and edentulism [50]. A longitudinal study of elderly individuals in the UK further reinforced these findings, showing that participants who exhibited symptoms of depression at baseline were more likely to report poorer self-assessed oral health [96]. This suggests that depression might indirectly affect dental health by impacting individuals’ quality of life, including emotional state, physical vitality, social interactions, and hygiene habits. The use of antidepressant medications could exacerbate this situation [97], as they may cause dry mouth, cariogenic dietary habits, and decreased immune function following oral infections, thereby increasing the risk of cavities or other dental issues. The influence of depression on various digestive system diseases was also supported by a large-scale Mendelian randomization study. This study indicated that depression increased the risk of developing 12 types of digestive system diseases, including irritable bowel syndrome, non-alcoholic fatty liver disease, alcoholic liver disease, and gastroesophageal reflux disease [98]. Additionally, experiments inducing depressive-like behavior in mice found that the depressive state might increase the susceptibility to intestinal inflammation by affecting the function of the vagus nerve [99], further supporting the biological link between depression and digestive system diseases.
Parents exposed to depression increased the risk of negative health outcomes for their offspring, including mental health issues such as depression, anxiety, and attention deficit hyperactivity disorder [59, 60], as well as physical health problems like asthma, low Apgar scores, child underweight, and child developmental delays [55, 61]. The stability of the family environment, emotional support, parenting styles, and parents’ interaction methods profoundly influenced children’s mental health and social adaptability. Compared to mothers, fathers’ depression had a lesser impact on children’s social functions, which is related to mothers often being the primary caregivers and emotional supporters in many cultures and family structures [100]. Additionally, pregnancy is a critical period for child development, and the psychological and physical health of the mother can affect fetal development through various mechanisms, such as reduced blood flow to the fetus [101], increased cortisol levels potentially entering the offspring’s growth environment through the placenta, or increased maternal inflammatory cytokines and serotonin [102]. In a study using a mouse model to investigate the effects of prenatal maternal depression on offspring, socially isolated mothers in a depressed state led their offspring to exhibit increased anxiety-like behaviors, cognitive performance changes, and alterations in the amygdala transcriptome in adulthood [103]. Therefore, prevention and intervention for depressed parents and their offspring’s health, such as early screening, psychosocial support, and designing intervention measures, are particularly important.
In our study, we identified significant associations between depression and multiple unique health outcomes, including not only psychological and behavioral issues such as internet addiction and sleep disorders [68, 69] but also severe physical health consequences like suicide, exacerbation of chronic pain, osteoporosis, asthma, and increased risks of fractures and frailty [73, 74, 79]. The link between depression and sleep disorders was especially noteworthy, as sleep problems [69] are not only common symptoms of depression but can also exacerbate the severity of depression, creating a vicious cycle. Moreover, the increase in medical accidents among doctors highlighted the potential impact of depression on professional performance and occupational safety [77], which needs attention in the health management of medical professionals. Depression could affect individuals’ attention and response capabilities, leading to an increased risk of car accidents, potentially exacerbated by the use of antidepressants in depressed patients [78].
Depression not only affected mental health but also extensively impacted individuals’ physical health, daily functioning, and social interactions as well as offspring related health outcomes. Although more high-quality evidence is still needed on the health effects of depression, the findings of the present umbrella review provide more evidence that the health effects of depression are serious, longstanding, and affecting majority of health system as well as the offspring health, which stress the importance of early screening, timely interventions, and regular monitoring of depression in public health and clinical practice.
Strengths and limitations
Our comprehensive review systematically summarizes previous meta-analyses on the relationship between depression and health outcomes, generating 109 health outcomes. These include cardiovascular outcomes, mortality, cancer, offspring health outcomes, neurological outcomes, endocrine/metabolic health outcomes, dental and gastrointestinal health outcomes, and offspring related outcomes. Our umbrella review assessed the impact of depression on various health outcomes, encompassing a wide range of potential health issues, from cardiovascular health to mortality and mental health, allowing for a more comprehensive evaluation of overall patient risk and a more holistic approach to addressing their health concerns, rather than solely focusing on the occurrence of depression. A systematic and comprehensive search strategy was employed from three scientific databases: PubMed, Embase, and Web of Science. Where possible, standardized methods were used to replicate each meta-analysis, including employing random-effects analyses, and generating measures for heterogeneity and publication bias for better comparison of outcomes. Besides providing an extensive overview of the available evidence, we also critically evaluate the methodological quality of the meta-analyses and the quality of evidence for all the reported associations. Three standard methods were used including AMSTAR2, GRADE, and the evidence classification standard. The methodological quality of the included meta-analyses was assessed using the AMSTAR2 tool [17], followed by the evaluation of the evidence quality for each outcome using the GRADE tool [18]. Outcomes were categorized into four classes based on the evidence classification standard [19] to assess our confidence in the estimates and to enhance decision-making quality in clinical practice.
Although Köhler’s umbrella review focuses on depression as a risk factor for various outcomes (such as genetics, environmental exposures) [104], this review is the first to consider depression as the exposure and various health outcomes as the study outcomes. Additionally, existing reviews are mostly limited to singular outcomes like mortality [11], while this paper fills the gap in systematic evidence regarding health outcomes with depression as the exposure, emphasizing the long-term impact of depression on multiple diseases and its public health significance.
The present study has several limitations. First, randomized controlled trials are better suited to identify causal effects compared with cohort studies. However, trials of the long-term effects of depression on the risk of hard end points (such as CVD, cancer, and dementia) are lacking, being unfeasible to conduct because of their high cost and lack of adherence to long-term interventions. No evidence was rated as high-quality for observational studies in this umbrella review, which may have led to some bias in the interpretation of the findings. To increase the quality of evidence, more experimental studies comparing the interventions on depression with health-related outcomes should be conducted and included in systematic reviews. Secondly, 65.8% of the association analyses (n = 75) were conducted with fewer than 10 primary studies; thus, the interpretation of these outcomes might be limited due to the small number of studies. In addition, selection bias may have affected the representativeness of the included studies, as we included only published literature and excluded grey literature and unpublished studies, which may have led to over-estimation or under-estimation of some findings. Recall bias is also a potential limitation of this study, especially in some retrospective studies, where participants’ memories of depression and health outcomes may not be completely accurate, thus affecting the reliability of the data. Finally, to achieve more rigorous and reproducible umbrella review research, it is necessary to adopt standardized methods to reduce the overlap of reviews caused by subjective decisions and different methodologies [105]. We made decisions to select the eligible reviews based on previous umbrella reviews published in leading biomedical journals [19, 106,107,108]. Nonetheless, future umbrella reviews should compare how different methods of selection and analyses influence the results of the umbrella review, thereby improving the quality and consistency of the research.
Conclusions
To sum up, depression was adversely related to a multitude of health outcomes. In our umbrella review, the quality of epidemiological evidence was considered moderate for bladder cancer mortality, all-cause mortality in myocardial infarction, mortality within two years for patients with coronary heart disease, major adverse cardiovascular events after percutaneous coronary intervention, irritable bowel syndrome, fear of falling, and frailty. Evidence for other health outcomes remains limited. Due to the scarcity of high-quality evidence, further large-scale, multicenter, and international randomized controlled trials or prospective studies are needed to validate the impact of depression on various human health outcomes and arrive at more definitive conclusions.
References
Depressive disorder 2023. https://www.who.int/news-room/fact-sheets/detail/depression.
Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–22.
Santomauro DF, Herrera AMM, Shadid J, Zheng P, Ashbaugh C, Pigott DM, et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398:1700–12.
New Global Burden of Disease analyses show depression and anxiety among the top causes of health loss worldwide, and a significant increase due to the COVID-19 pandemic 2021. https://www.healthdata.org/news-events/insights-blog/acting-data/new-global-burden-disease-analyses-show-depression-and.
Jia H, Zack MM, Thompson WW, Crosby AE, Gottesman II. Impact of depression on quality-adjusted life expectancy (QALE) directly as well as indirectly through suicide. Soc Psychiatry Psychiatr Epidemiol. 2015;50:939–49.
Fu Y, Feng S, Xu Y, Yang Y, Chen H, He W, et al. Association of depression, antidepressants with atrial fibrillation risk: a systemic review and meta-analysis. Front Cardiovasc Med. 2022;9:897622. https://doi.org/10.3389/fcvm.2022.897622.
Cao L, Sheng C, Luo G, Ou J. Depression as a risk factor for developing heart failure: a meta-analysis of prospective cohort studies. J Cardiovasc Nurs. 2022;37:112–21.
Cao H, Zhao H, Shen L. Depression increased risk of coronary heart disease: a meta-analysis of prospective cohort studies. Front Cardiovasc Med. 2022;9:913888. https://doi.org/10.3389/fcvm.2022.913888.
Wu Q, Kling JM. Depression and the risk of myocardial infarction and coronary death: a meta-analysis of prospective cohort studies. Medicine. 2016;95:e2815. https://doi.org/10.1097/MD.0000000000002815.
Barlinn K, Kepplinger J, Puetz V, Illigens BM, Bodechtel U, Siepmann T. Exploring the risk-factor association between depression and incident stroke: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2015;11:1–14.
Machado MO, Veronese N, Sanches M, Stubbs B, Koyanagi A, Thompson T, et al. The association of depression and all-cause and cause-specific mortality: an umbrella review of systematic reviews and meta-analyses. BMC Med. 2018;16:112. https://doi.org/10.1186/s12916-018-1101-z.
Wei J, Hou R, Zhang X, Xu H, Xie L, Chandrasekar EK, et al. The association of late-life depression with all-cause and cardiovascular mortality among community-dwelling older adults: systematic review and meta-analysis. Br J Psychiatry. 2019;215:449–55.
Scierka LE, Mena-Hurtado C, Ahmed ZV, Yousef S, Arham A, Grimshaw AA, et al. The association of depression with mortality and major adverse limb event outcomes in patients with peripheral artery disease: a systematic review and meta-analysis. J Affect Disord. 2023;320:169–77. https://doi.org/10.1016/j.jad.2022.09.098.
Abi-Jaoude JG, Naiem AA, Edwards T, Lukaszewski M-A, Obrand DI, Steinmetz OK, et al. Comorbid depression is associated with increased major adverse limb events in peripheral arterial disease: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2022;64:101–10.
Song X, Song J, Shao M, Gao X, Ji F, Tian H, et al. Depression predicts the risk of adverse events after percutaneous coronary intervention: a meta-analysis. J Affect Disord. 2020;266:158–64. https://doi.org/10.1016/j.jad.2020.01.136.
Cai W, Mueller C, Li YJ, Shen WD, Stewart R. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. 2019;50:102–9. https://doi.org/10.1016/j.arr.2019.01.013.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. https://doi.org/10.1136/bmj.39489.470347.AD.
Huang Y, Chen Z, Chen B, Li J, Yuan X, Li J, et al. Dietary sugar consumption and health: umbrella review. BMJ. 2023;381:e071609. https://doi.org/10.1136/bmj-2022-071609.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. https://doi.org/10.1136/bmj.315.7109.629.
Wang YH, Li JQ, Shi JF, Que JY, Liu JJ, Lappin JM, et al. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry. 2020;25:1487–99. https://doi.org/10.1038/s41380-019-0595-x.
Van der Elst S, Bardash Y, Wotman M, Kraus D, Tham T. The prognostic impact of depression or depressive symptoms on patients with head and neck cancer: a systematic review and meta-analysis. Head Neck. 2021;43:3608–17. https://doi.org/10.1002/hed.26868.
Shi C, Lamba N, Zheng LJ, Cote D, Regestein QR, Liu CM, et al. Depression and survival of glioma patients: a systematic review and meta-analysis. Clin Neurol Neurosurg. 2018;172:8–19. https://doi.org/10.1016/j.clineuro.2018.06.016.
Sun HL, Dong XX, Cong YJ, Gan Y, Deng J, Cao SY, et al. Depression and the risk of breast cancer: a meta-analysis of cohort studies. Asian Pac J Cancer Prev. 2015;16:3233–9. https://doi.org/10.7314/apjcp.2015.16.8.3233.
Wang X, Wang N, Zhong L, Wang S, Zheng Y, Yang B, et al. Prognostic value of depression and anxiety on breast cancer recurrence and mortality: a systematic review and meta-analysis of 282,203 patients. Mol Psychiatry. 2020;25:3186–97. https://doi.org/10.1038/s41380-020-00865-6.
Hofmann M, Köhler B, Leichsenring F, Kruse J. Depression as a risk factor for mortality in individuals with diabetes: a meta-analysis of prospective studies. PLoS ONE. 2013;8:e79809.
Palmer SC, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, et al. Association between depression and death in people with CKD: a meta-analysis of cohort studies. Am J Kidney Dis. 2013;62:493–505.
Meijer A, Conradi HJ, Bos EH, Thombs BD, van Melle JP, de Jonge P. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen Hosp Psychiatry. 2011;33:203–16. https://doi.org/10.1016/j.genhosppsych.2011.02.007.
Gathright EC, Goldstein CM, Josephson RA, Hughes JW. Depression increases the risk of mortality in patients with heart failure: a meta-analysis. J Psychosom Res. 2017;94:82–9. https://doi.org/10.1016/j.jpsychores.2017.01.010.
Stenman M, Holzmann MJ, Sartipy U. Association between preoperative depression and long-term survival following coronary artery bypass surgery — a systematic review and meta-analysis. Int J Cardiol. 2016;222:462–6.
Guillaume M, Endomba FT, Dornier A, Chauvet-Gelinier JC. Association between depression before hematopoietic stem cell transplantation and posttransplant survival: a systematic review and meta-analysis. J Acad Consult-Liaison Psychiatry. 2023;64:166–76.
Dew MA, Rosenberger EM, Myaskovsky L, DiMartini AF, Dabbs AJD, Posluszny DM, et al. Depression and anxiety as risk factors for morbidity and mortality after organ transplantation: a systematic review and meta-analysis. Transplantation. 2016;100:988–1003.
Mannan M, Mamun A, Doi S, Clavarino A. Is there a bi-directional relationship between depression and obesity among adult men and women? Systematic review and bias-adjusted meta analysis. Asian J Psychiatry. 2016;21:51–66.
Graham EA, Deschenes SS, Khalil MN, Danna S, Filion KB, Schmitz N. Measures of depression and risk of type 2 diabetes: a systematic review and meta-analysis. J Affect Disord. 2020;265:224–32. https://doi.org/10.1016/j.jad.2020.01.053.
Fang T, Zhang Q, Wang Z, Liu JP. Bidirectional association between depression and diabetic nephropathy by meta-analysis. PLoS ONE. 2022;17:e0278489.
Zhang C, Jing L, Wang J. Does depression increase the risk of gestational diabetes mellitus? A systematic review and meta-analysis. Pak J Med Sci. 2023;39:285–92.
Moradi Y, Albatineh AN, Mahmoodi H, Gheshlagh RG. The relationship between depression and risk of metabolic syndrome: a meta-analysis of observational studies. Clin Diabetes Endocrinol. 2021;7:4.
Fu Y, Shen X, Huang W. Association between depression and risk of triggering ventricular arrhythmias: a meta-analysis. Int J Clin Pharmacol Ther. 2019;57:306–14.
Lin S, Zhang H, Ma A. The association between depression and coronary artery calcification: a meta-analysis of observational studies. J Affect Disord. 2018;232:276–82.
Shi S, Liu T, Liang J, Hu D, Yang B. Depression and risk of sudden cardiac death and arrhythmias: a meta-analysis. Psychosom Med. 2017;79:153–61. https://doi.org/10.1097/PSY.0000000000000382.
Meng L, Chen D, Yang Y, Zheng Y, Hui R. Depression increases the risk of hypertension incidence: a meta-analysis of prospective cohort studies. J Hypertens. 2012;30:842–51. https://doi.org/10.1097/HJH.0b013e32835080b7.
Inoue K, Beekley J, Goto A, Jeon CY, Ritz BR. Depression and cardiovascular disease events among patients with type 2 diabetes: a systematic review and meta-analysis with bias analysis. J Diabetes Complications. 2020;34:107710.
Nouwen A, Adriaanse MC, van Dam K, Iversen MM, Viechtbauer W, Peyrot M, et al. Longitudinal associations between depression and diabetes complications: a systematic review and meta-analysis. Diabet Med. 2019;36:1562–72.
Zhang WY, Nan N, Song XT, Tian JF, Yang XY. Impact of depression on clinical outcomes following percutaneous coronary intervention: a systematic review and meta-analysis. BMJ Open. 2019;9:e026445. https://doi.org/10.1136/bmjopen-2018-026445.
Kewcharoen J, Tachorueangwiwat C, Kanitsoraphan C, Saowapa S, Nitinai N, Vutthikraivit W, et al. Association between depression and increased risk of readmission in patients with heart failure: a systematic review and meta-analysis. Minerva Cardiol Angiol. 2021;69:389–97.
Wu QE, Zhou AM, Han YP, Liu YM, Yang Y, Wang XM, et al. Poststroke depression and risk of recurrent stroke: a meta-analysis of prospective studies. Medicine. 2019;98:e17235. https://doi.org/10.1097/MD.0000000000017235.
Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22:613–26. https://doi.org/10.1002/gps.1723.
Piovani D, Armuzzi A, Bonovas S. Association of depression with incident inflammatory bowel diseases: a systematic review and meta-analysis. Inflamm Bowel Dis. 2023;30:573–84. https://doi.org/10.1093/ibd/izad109.
Sibelli A, Chalder T, Everitt H, Workman P, Windgassen S, Moss-Morris R. A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol Med. 2016;46:3065–80. https://doi.org/10.1017/S0033291716001987.
Cademartori MG, Gastal MT, Nascimento GG, Demarco FF, Corrêa MB. Is depression associated with oral health outcomes in adults and elders? A systematic review and meta-analysis. Clin Oral investig. 2018;22:2685–702.
Araujo MM, Martins CC, Machado Costa LC, Miranda Cota LO, Araujo Melo Faria RL, Cunha FA, et al. Association between depression and periodontitis: a systematic review and meta-analysis. J Clin Periodontol. 2016;43:216–28.
Dachew B, Ayano G, Duko B, Lawrence B, Betts K, Alati R. Paternal depression and risk of depression among offspring: a systematic review and meta-analysis. JAMA Netw Open. 2023;6:e2329159. https://doi.org/10.1001/jamanetworkopen.2023.29159.
Tirumalaraju V, Suchting R, Evans J, Goetzl L, Refuerzo J, Neumann A, et al. Risk of depression in the adolescent and adult offspring of mothers with perinatal depression. JAMA Netw Open. 2020;3:e208783. https://doi.org/10.1001/jamanetworkopen.2020.8783.
Cui C, Li M, Yang Y, Liu C, Cao P, Wang L. The effects of paternal perinatal depression on socioemotional and behavioral development of children: a meta-analysis of prospective studies. Psychiatry Res. 2020;284:112775. https://doi.org/10.1016/j.psychres.2020.112775.
Sun YF, Chang Q, Wu QJ, Gao SY, Zang ST, Liu YS, et al. Association between maternal antenatal depression and neonatal Apgar score: a systematic review and meta-analysis of prospective cohort studies. J Affect Disord. 2021;278:264–75.
Chen S, Chen S. Are prenatal anxiety or depression symptoms associated with asthma or atopic diseases throughout the offspring’s childhood? An updated systematic review and meta-analysis. BMC Pregnancy Childbirth. 2021;21:435. https://doi.org/10.1186/s12884-021-03909-z.
Jia X, Lu L, Lou S, Han S, Deng L, Liu S. Perinatal maternal depression and the risk of childhood asthma in offspring: A meta-analysis. PLoS ONE 2024;19:e0310647.
Madigan S, Oatley H, Racine N, Fearon RMP, Schumacher L, Akbari E, et al. A meta-analysis of maternal prenatal depression and anxiety on child socioemotional development. J Am Acad Child Adolesc Psychiatry. 2018;57:645–57 e8. https://doi.org/10.1016/j.jaac.2018.06.012.
Christaki V, Ismirnioglou I, Katrali A, Panagouli E, Tzila E, Thomaidis L, et al. Postpartum depression and ADHD in the offspring: systematic review and meta-analysis. J Affect Disord. 2022;318:314–30. https://doi.org/10.1016/j.jad.2022.08.055.
Chithiramohan T, Eslick GD. Association between maternal postnatal depression and offspring anxiety and depression in adolescence and young adulthood: a meta-analysis. J Dev Behav Pediatr. 2023;44:e231–e8.
Surkan PJ, Kennedy CE, Hurley KM, Black MM. Maternal depression and early childhood growth in developing countries: systematic review and meta-analysis. Bull World Health Organ. 2011;89:608–15. https://doi.org/10.2471/BLT.11.088187.
Mehta K, Thandavan SP, Mohebbi M, Pasco JA, Williams LJ, Walder K, et al. Depression and bone loss as risk factors for cognitive decline: a systematic review and meta-analysis. Ageing Res Rev. 2022;76:101575.
Zhou S, Ye N, Liu X, Li Y, Ai Y, Wang X, et al. Association of motoric cognitive risk syndrome with depression in older adults: a meta-analysis and systematic review of cross-sectional and cohort studies. BMC Geriatr. 2024;24:973. https://doi.org/10.1186/s12877-024-05507-y.
Bareeqa SB, Samar SS, Kamal S, Masood Y, Allahyar, Ahmed SI, et al. Prodromal depression and subsequent risk of developing Parkinson’s disease: a systematic review with meta-analysis. Neurodegener Dis Manag. 2022;12:155–64. https://doi.org/10.2217/nmt-2022-0001.
Santabárbara J, Villagrasa B, Gracia-García P. Does depression increase the risk of dementia? Updated meta-analysis of prospective studies. Actas Esp Psiquiatr. 2020;48:169–80.
Diep C, Patel K, Petricca J, Daza JF, Lee S, Xue Y, et al. Incidence and relative risk of delirium after major surgery for patients with pre-operative depression: a systematic review and meta-analysis. Anaesthesia. 2024;79:1237–49. https://doi.org/10.1111/anae.16398
Santos MAO, Bezerra LS, Carvalho A, Brainer-Lima AM. Global hippocampal atrophy in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Trends Psychiatry Psychother. 2018;40:369–78. https://doi.org/10.1590/2237-6089-2017-0130.
Ye XL, Zhang W, Zhao FF. Depression and internet addiction among adolescents: a meta-analysis. Psychiatry Res. 2023;326:115311.
Bao YP, Han Y, Ma J, Wang RJ, Shi L, Wang TY, et al. Cooccurrence and bidirectional prediction of sleep disturbances and depression in older adults: meta-analysis and systematic review. Neurosci Biobehav Rev. 2017;75:257–73. https://doi.org/10.1016/j.neubiorev.2017.01.032.
Chen W, Wang X, Xia S. Increased C-reactive protein in patients with post-stroke depression: a meta-analysis of cohort study. Alpha Psychiatry. 2024;25:124–31. https://doi.org/10.5152/alphapsychiatry.2024.231338.
Ruiz-Grosso P, Cachay R, De La Flor A, Schwalb A, Ugarte-Gil C. Association between tuberculosis and depression on negative outcomes of tuberculosis treatment: a systematic review and meta-analysis. PLoS ONE. 2020;15:e0227472.
Wu Q, Liu B, Tonmoy S. Depression and risk of fracture and bone loss: an updated meta-analysis of prospective studies. Osteoporos Int. 2018;29:1303–12. https://doi.org/10.1007/s00198-018-4420-1.
Gao Y-h, Zhao H-s, Zhang F-r, Gao Y, Shen P, Chen R-c, et al. The relationship between depression and asthma: a meta-analysis of prospective studies. PLoS ONE. 2015;10:e0132424.
Soysal P, Veronese N, Thompson T, Kahl KG, Fernandes BS, Prina AM, et al. Relationship between depression and frailty in older adults: a systematic review and meta-analysis. Ageing Res Rev. 2017;36:78–87.
Xia Y, Li J, Shan G, Qian H, Wang T, Wu W, et al. Relationship between premature ejaculation and depression a PRISMA-compliant systematic review and meta-analysis. Medicine. 2016;95:e4620.
Atlantis E, Sullivan T. Bidirectional association between depression and sexual dysfunction: a systematic review and meta-analysis. J Sex Med. 2012;9:1497–507.
Pereira-Lima K, Mata DA, Loureiro SR, Crippa JA, Bolsoni LM, Sen S. Association between physician depressive symptoms and medical errors: a systematic review and meta-analysis. JAMA Netw Open. 2019;2:e1916097.
Hill LL, Lauzon VL, Winbrock EL, Li G, Chihuri S, Lee KC. Depression, antidepressants and driving safety. Inj Epidemiol. 2017;4:10. https://doi.org/10.1186/s40621-017-0107-x.
McGinty J, Sayeed Haque M, Upthegrove R. Depression during first episode psychosis and subsequent suicide risk: a systematic review and meta-analysis of longitudinal studies. Schizophr Res. 2018;195:58–66. https://doi.org/10.1016/j.schres.2017.09.040.
Wong JJ, Tricco AC, Côté P, Liang CY, Lewis JA, Bouck Z, et al. Association between depressive symptoms or depression and health outcomes for low back pain: a systematic review and meta-analysis. J Gen Intern Med. 2022;37:1233–46.
Gambaro E, Gramaglia C, Azzolina D, Campani D, Molin AD, Zeppegno P. The complex associations between late life depression, fear of falling and risk of falls. A systematic review and meta-analysis. Ageing Res Rev. 2022;73:101532.
Gustafsson H, Nordstrom A, Nordstrom P. Depression and subsequent risk of Parkinson disease: a nationwide cohort study. Neurology. 2015;84:2422–9. https://doi.org/10.1212/WNL.0000000000001684.
Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802–13.
Meng R, Yu C, Liu N, He M, Lv J, Guo Y, et al. Association between depression and all-cause and cardiovascular mortality in Chinese adults. JAMA Netw Open. 2020;3:e1921043.
Geulayov G, Novikov I, Dankner D, Dankner R. Symptoms of depression and anxiety and 11-year all-cause mortality in men and women undergoing coronary artery bypass graft (CABG) surgery. J Psychosom Res. 2018;105:106–14. https://doi.org/10.1016/j.jpsychores.2017.11.017.
Zhang Y, Li X, Chan VKY, Luo H, Chan SSM, Wong GHY, et al. Depression duration and risk of incident cardiovascular disease: a population-based six-year cohort study. J Affect Disord. 2022;305:188–95. https://doi.org/10.1016/j.jad.2022.03.005.
Eliwa H, Brizard B, Le Guisquet AM, Hen R, Belzung C, Surget A. Adult neurogenesis augmentation attenuates anhedonia and HPA axis dysregulation in a mouse model of chronic stress and depression. Psychoneuroendocrinology. 2021;124:105097. https://doi.org/10.1016/j.psyneuen.2020.105097.
Kim H, Yoo J, Han K, Jeon HJ. Physical activity and cardiovascular health in depression: Links between changes in physical activity and cardiovascular risk. Gen Hosp Psychiatry. 2022;78:35–41.
Kariis HM, Kasela S, Jurgenson T, Saar A, Lass J, Krebs K, et al. The role of depression and antidepressant treatment in antihypertensive medication adherence and persistence: utilising electronic health record data. J Psychiatr Res. 2023;168:269–78. https://doi.org/10.1016/j.jpsychires.2023.10.018.
Zhao Y, Yang L, Sahakian BJ, Langley C, Zhang W, Kuo K, et al. The brain structure, immunometabolic and genetic mechanisms underlying the association between lifestyle and depression. Nat Ment Health. 2023;1:736–50.
Nebhinani N, Sharma P, Suthar N, Pareek V, Kunwar D, Purohit P, et al. Correlates of metabolic syndrome in patients with depression: A study from north-western India. Diabetes Metab Syndr. 2020;14:1997–2002. https://doi.org/10.1016/j.dsx.2020.10.013.
Jacka FN, Kremer PJ, Leslie ER, Berk M, Patton GC, Toumbourou JW, et al. Associations between diet quality and depressed mood in adolescents: results from the Australian Healthy Neighbourhoods Study. Aust N Z J Psychiatry. 2010;44:435–42.
Jia H, Zack MM, Gottesman II, Thompson WW. Associations of smoking, physical inactivity, heavy drinking, and obesity with quality-adjusted life expectancy among US Adults with depression. Value Health. 2018;21:364–71.
Mihailescu I, Efrim-Budisteanu M, Andrei LE, Buică AM, Moise M, Nicolau IG, et al. Cognitive coping strategies among inpatient adolescents with depression and psychiatric comorbidity. Children. 2023;10:1870.
Heijer TD, Tiemeier H, Luijendijk HJ, Lijn FVD, Koudstaal PJ, Hofman A, et al. A study of the bidirectional association between hippocampal volume on magnetic resonance imaging and depression in the elderly. Biol Psychiatry. 2011;70:191–7.
Zwick L, Schmitz N, Shojaa M. Oral health-related quality of life and depressive symptoms in adults: longitudinal associations of the English Longitudinal Study of Ageing (ELSA). BMC Oral Health. 2023;23:1029. https://doi.org/10.1186/s12903-023-03722-4.
Reese LR, MSC U. Depression and dental health. Clinical Update. 2003;25.
Ruan X, Chen J, Sun Y, Zhang Y, Zhao J, Wang X, et al. Depression and 24 gastrointestinal diseases: a Mendelian randomization study. Transl Psychiatry. 2023;13:146. https://doi.org/10.1038/s41398-023-02459-6.
Ghia JE, Blennerhassett P, Collins SM. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J Clin Invest. 2008;118:2209–18. https://doi.org/10.1172/JCI32849.
Williamson T, Wagstaff DL, Goodwin J, Smith N. Mothering ideology: a qualitative exploration of Mothers’ perceptions of navigating motherhood pressures and partner relationships. Sex Roles. 2023;88:101–17. https://doi.org/10.1007/s11199-022-01345-7.
Teixeira JM, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. BMJ. 1999;318:153–7.
Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–89. https://doi.org/10.1016/j.yhbeh.2010.06.007.
Scarborough J, Mueller FS, Weber-Stadlbauer U, Mattei D, Opitz L, Cattaneo A, et al. A novel murine model to study the impact of maternal depression and antidepressant treatment on biobehavioral functions in the offspring. Mol Psychiatry. 2021;26:6756–72. https://doi.org/10.1038/s41380-021-01145-7.
Kohler CA, Evangelou E, Stubbs B, Solmi M, Veronese N, Belbasis L, et al. Mapping risk factors for depression across the lifespan: an umbrella review of evidence from meta-analyses and Mendelian randomization studies. J Psychiatr Res. 2018;103:189–207. https://doi.org/10.1016/j.jpsychires.2018.05.020.
Shi X, Wallach JD. Umbrella reviews: a useful study design in need of standardisation. BMJ. 2022;378:o1740.
Liuqiao S, Xiaoping L, Yaoyao W, Sui Z, Qian O, Hang X, et al. Fruit consumption and multiple health outcomes: an umbrella review. Trends Food Sci Technol. 2021;118:505–18.
Okoth K, Chandan JS, Marshall T, Thangaratinam S, Thomas GN, Nirantharakumar K, et al. Association between the reproductive health of young women and cardiovascular disease in later life: umbrella review. Bmj. 2020;371:m3502.
Neuenschwander M, Ballon A, Weber KS, Norat T, Aune D, Schwingshackl L, et al. Role of diet in type 2 diabetes incidence: umbrella review of meta-analyses of prospective observational studies. BMJ. 2019;366:l2368. https://doi.org/10.1136/bmj.l2368.
Acknowledgements
Thanks to all the authors of the included studies for giving us the availability to use the data to perform the present systematic review and meta-analysis. Thanks for Mengna Liu giving advice in revising the manuscript.
Funding
This study was supported by the Shenzhen Nanshan District Science and Technology Program Key Project (grant number NS2022009), the Shenzhen Nanshan District Health System Science and Technology Major Project (grant number NSAD2023047), and the Shenzhen Nanshan District Health System Science and Technology Major Project-Clinical Research Talent Program (grant number NSZD2024040). The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
PQ and XC conceived the study, designed the methodology, developed screening strategies, and wrote the manuscript. XNL and FL performed literature searches, data extraction, and quality assessments. HTH and XYL verified data extraction and conducted evidence grading. TQ performed statistical analyses and interpreted results. BJ and JM supervised the review process and ensured methodological rigor. YC and HLH interpreted evidence and reviewed the manuscript. YW and YS created visualizations and forest plots. XW, LL, and JW contributed to methodology design and supplementary analyses. FH optimized study protocols and quality control.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, X., Liu, X., Li, F. et al. Depression and health outcomes: An umbrella review of systematic reviews and meta-analyses of observational studies. Transl Psychiatry 15, 298 (2025). https://doi.org/10.1038/s41398-025-03463-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03463-8