Abstract
The striatum’s role in obsessive-compulsive disorder (OCD) pathology is recognized. However, the specific contributions of individual striatal subregions (SSs) to OCD pathology are underexplored. We recruited 49 drug-naive OCD patients and 53 healthy controls, conducting clinical assessments and resting-state functional magnetic resonance imaging (rs-fMRI) scans pre- and post-4-week paroxetine treatment. Inter-group comparisons were conducted to investigate baseline and treatment-related changes in the patients’ striatum using several fMRI metrics, including amplitude of low-frequency fluctuation, regional homogeneity, and degree centrality (DC). Furthermore, these metrics, along with functional connectivity (FC), and effective connectivity (EC) of SSs, were analyzed. Associations between gene expression patterns and altered information flow patterns in SSs were examined, where information flow was measured using EC, followed by enrichment analysis of relevant genes. While no significant alterations were observed in the patients’ striata in whole-brain statistical analyses, significant changes in DC, FC, and EC were identified in SSs pre- and post-treatment. In particular, the EC analysis unveiled an enhanced top-down control and diminished bottom-up regulation in drug-naive OCD patients. Following treatment, bottom-up EC improved, along with an improvement in clinical symptoms. Additionally, information flow alteration-related genes were enriched in various biological processes and pathways. They substantially overlapped between bidirectional information flows among SSs and the rest of brain and between information flows among homotopical SSs and the rest of brain. This study highlights the diverse contributions of each striatal subregion to OCD pathology. Paroxetine may alleviate OCD symptoms by enhancing bottom-up regulation, specifically the normalization of aberrant connectivity. Furthermore, integrating transcriptomic and rs-fMRI findings offer novel insights into the biological substrates underlying the altered EC of SSs in OCD patients.
Similar content being viewed by others
Introduction
The cortico-striatal-thalamo-cortical (CSTC) circuit is involved in obsessive-compulsive disorder (OCD). This circuit operates as a recurrent loop, with information flowing from the cortex to the striatum, integrating into the thalamus, and then returning to the cortex [1]. The striatum is believed to consist of affective, sensorimotor, and cognitive functional regions, which are based on the cortical and thalamic inputs [2]. Striatal dysfunctions can disrupt thalamic gating functions, leading to cortical hyperactivity and impairing response inhibition, cognitive flexibility, fear extinction, and reward processing [3,4,5]. Thus, the striatum plays a prominent role in the CSTC circuit and the pathology of OCD.
OCD patients characteristically exhibit disturbances in the dual learning system, which consists of the habitual and goal-directed systems [6, 7]. Habitual behaviors, which occur unconsciously and are triggered automatically, are primarily mediated by connections between the putamen and the supplementary motor area (SMA) [6, 8]. In contrast, goal-directed behaviors, which require cognitive effort and intentional control, involve connections between the caudate and the prefrontal cortex (PFC) [6, 8]. Given that both systems are functionally linked to distinct striatal subregions, alterations in these subregions may play a crucial role in OCD pathology. Several studies have reported abnormalities within striatal subregions in OCD patients. For instance, one study identified specific functional connectivity (FC) patterns within the cortico-striatal circuit, particularly involving the ventral rostral putamen and dorsal caudal putamen [9]. Another study found that, compared to healthy controls (HCs), both OCD patients and their unaffected first-degree relatives exhibited decreased effective connectivity (EC) between the left caudate and the lateral PFC, as well as increased EC between the left caudate and the orbitofrontal cortex [10]. These findings suggest that alterations in specific striatal subregions contribute to impaired goal-directed processing in OCD and may serve as a potential biomarker for this disorder [10]. However, while the involvement of the striatum in OCD is well recognized, the precise contributions of its subregions remain underexplored. Further investigation into the distinct roles of striatal subregions is essential for refining our understanding of OCD pathology and identifying potential targets for intervention.
The present study employed a fine-partitioning striatum template and evaluated various neuroimaging measures, including amplitude of low-frequency fluctuation (ALFF), regional homogeneity (ReHo), degree centrality (DC), FC, and EC. These metrics assess the brain’s spontaneous neural activity and connectivity. Specifically, ALFF, ReHo, and DC quantify amplitude-based spontaneous activity, local synchronization, and network centrality, respectively, while FC measures the correlation of brain activity between regions, and EC delineates the directional influence of one region on another [11]. A recent meta-analysis of 41 resting-state functional magnetic resonance imaging (fMRI) studies using ALFF, fractional amplitude of low-frequency fluctuation, or ReHo found that OCD patients exhibited increased spontaneous neural activity in the bilateral medial prefrontal cortex, inferior frontal gyrus, and anterior cingulate cortex, along with decreased spontaneous neural activity in the bilateral paracentral gyrus and cerebellum, the left inferior parietal gyrus and caudate, and the right precuneus [12]. Another meta-analysis of 47 seed-based FC studies in OCD revealed characteristic dysconnectivity patterns in striatum, including caudate hypoconnectivity with the frontoparietal network and hyperconnectivity with the fronto-limbic network, nucleus accumbens hypoconnectivity with the fronto-limbic network, and thalamus-striatum hypoconnectivity [13]. Given these findings, we applied DC, a fundamental graph theory-based metric in resting-state fMRI, to quantify the importance of brain regions within large-scale functional networks. Prior studies have shown that OCD patients exhibit decreased DC in the bilateral cuneus, thalamus, and precuneus, suggesting disrupted functional integration [14]. However, despite extensive research on FC in OCD, the directed connections of striatal subregions remain unclear. To address this gap, we employed Granger causality analysis (GCA), an EC measure based on the principle that past values of a time series can predict future values of another time series, leveraging time-lagged hemodynamic information [15]. This approach enables the identification of whether striatal dysfunction in OCD arises from abnormal top-down or bottom-up information flow. Supporting this rationale, a study by Yoshinari et al. found increased information flow from the orbitofrontal cortex to the ventral striatum in OCD using GCA [16], underscoring its relevance for examining the directional interactions between brain regions in OCD pathology.
Heritability has been observed in EC, including GCA [17,18,19]. Tian et al. found that GCA changes among brain networks were significantly related to trait anxiety and mediated gene–environment effects on trait anxiety [17]. Bianco et al. found that in healthy participants, GCA in the parieto-prefrontal, parieto-prefronto-cerebellar, and posterior cingulate-to-hippocampus connections exhibited the highest and most replicable heritability effects, suggesting a robust role of heritability in shaping GCA within certain cortico-subcortical circuits implicated in cognition [18]. Xu et al. applied structural equation modeling and found that the heritability of EC within the default mode network was 0.54, suggesting that genetic factors play a major role in shaping EC within the default mode network [19]. Furthermore, a genome-wide association study identified genetic variations linked to both OCD and the volumes of the nucleus accumbens and putamen, key regions within the CSTC circuit [20]. However, the associations between gene expression levels and altered GCA in striatal subregions among OCD patients remained unclear. To address this gap, integrating transcriptomic data with neuroimaging findings offers a powerful approach to elucidate the molecular underpinnings of altered EC in OCD. By linking differential gene expression patterns to striatal subregions exhibiting altered GCA, we can identify molecular pathways associated with dysfunctional neural circuits in OCD. This integrative approach bridges the gap between large-scale neural network dysfunction and its underlying genetic determinants, offering deeper mechanistic insights and potential targets for therapeutic intervention.
Identifying treatment-related neuroimaging alterations in patients can provide novel insights into the pathological mechanisms of OCD and inspire new approaches for developing targeted treatment. However, studies examining the longitudinal effects of treatment on the brains of OCD patients, particularly regarding post-treatment changes in EC, remain limited. The administration of selective serotonin reuptake inhibitors (SSRIs) has been associated with post-treatment functional and structural alterations in the brains of OCD patients [21, 22]. Moreover, a review highlighted that abnormalities in brain structure and function of OCD patients tend to normalize following treatment [23].
Therefore, the study comprehensively investigated baseline aberrations and treatment-related alterations in the precise demarcations of striatal undirected and directed connections in drug-naive OCD patients. We also evaluated the associations between gene expression levels and the altered GCA. Initially, disparities in ALFF, ReHo, and DC of the striatum between OCD patients and HCs, as well as between OCD patients before and after the treatment, were assessed using whole-brain statistical analyses. Subsequently, ALFF, ReHo, and DC signals were extracted from striatal subregions in both OCD patients and HCs at baseline and after treatment to identify baseline characteristics and treatment-related alterations. In the second phase, FC and EC analyses were conducted using the striatal subregions as the regions of interest (ROIs) to reveal striatal undirected and directed connections in OCD patients before and after treatment. Additionally, correlation analysis was conducted to investigate the relationship between clinical symptoms and disrupted functional features. In the third phase, the associations between gene expression levels and the altered GCA were investigated. This study is the first to examine abnormalities in striatal subregions of OCD patients by evaluating multiple neuroimaging parameters, including local activities (ALFF), local connectivity (ReHo), hub regions (DC), undirected connections (FC), and directed connections (EC). Moreover, the influence of treatment on these parameters was examined. Finally, we explored genes associated with altered information flows in the striatal subregions of OCD patients. The study aims to deepen our understanding of the roles of striatal subregions in OCD pathology and their relationship with clinical symptoms and treatment.
Methods
Participants
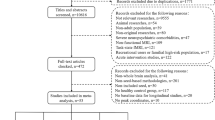
The clinical trial was registered on ClinicalTrials.gov (registration number: NCT03894085) and is accessible at: https://clinicaltrials.gov/ct2/show/NCT03894085. The Ethics Committee of the Second Xiangya Hospital approved the study (registration number: 2018025). All study participants provided written informed consent. To ensure adequate statistical power, our sample size was determined based on prior recommendations for group-level fMRI studies, which suggest a minimum of 30 participants per group to achieve sufficient statistical power [24, 25]. However, considering that increasing the sample size enhances result reproducibility [26], and given the potential for participant attrition at follow-up as well as the possibility of data exclusion due to quality concerns, we aimed for at least 50 participants per group. Between January 2019 and August 2022, we recruited 51 drug-naive OCD patients from the outpatient department of the Department of Psychiatry, Second Xiangya Hospital. Additionally, we recruited 55 HCs from the local community through advertising. Using the Structured Clinical Interview for DSM-5, two psychiatrists confirmed OCD diagnosis [27]. Figure 1A presents a flow chart outlining participant selection criteria and study procedures.
Procedure
Demographic information was collected, and clinical symptoms of OCD patients were evaluated. Participants then underwent an MRI scan. Following the first scan, OCD patients underwent a 4-week course of paroxetine monotherapy. The 4-week treatment duration was selected based on prior studies assessing the early effects of SSRIs in OCD [28, 29]. This timeframe is sufficient to observe symptom changes and is considered an optimal period for detecting early therapeutic effects while minimizing potential dropout rates due to side effects or logistical challenges [28, 29]. Treatment was initiated at a daily dose of 20 mg, which was subsequently increased to 40 mg within one week, following clinical guidelines [30]. The initial dose of 20 mg/day is commonly used to minimize side effects, particularly in patients who are first prescribed SSRIs or those with a heightened sensitivity to medication. The dose was then increased to 40 mg/day within one week to achieve a therapeutic level, as this dosage is generally effective for treating OCD symptoms and well-tolerated by most patients. No additional medication or psychotherapy was administered during the treatment phase. After completing the treatment, participants underwent a second MRI scan and clinical assessments. OCD patients who exhibited more than a 20% reduction in Yale–Brown Obsessive–Compulsive Scale (Y-BOCS) scores were considered positive responders to the 4-week paroxetine treatment [31].
Measures
OCD patients exhibit varying degrees of depressive and anxiety symptoms, along with challenges in social and cognitive functions [32]. They also employ diverse coping mechanisms for navigating daily life events and exhibit distinct personality traits [33]. Noteworthy connections have been observed between OCD severity, anxiety and depressive symptoms, impairments in social and cognitive functions, personality traits, and patterns of brain activity or connectivity in OCD patients [34]. Therefore, we here used the Chinese version of the Y-BOCS (Cronbach’s alpha = 0.90) to assess OCD severity [35]. The Chinese version of the 17-item Hamilton Depression Rating Scale (HAMD, Cronbach’s alpha = 0.71) and Hamilton Anxiety Rating Scale (HAMA, Cronbach’s alpha = 0.86) were used for evaluating depressive and anxiety symptoms [36, 37]. To measure impairments in social and cognitive functions, the Chinese version of the Social Disability Screening Schedule (SDSS, Cronbach’s alpha = 0.85) and the Brief Cognitive Assessment Tool for Schizophrenia (B-CATS, Cronbach’s alpha = 0.73) were applied, respectively [38, 39]. The Simplified Coping Style Questionnaire (CSQ, Cronbach’s alpha = 0.90) was applied to assess diverse ways of coping with daily life events [40]. The Chinese version of the Eysenck Personality Questionnaire (EPQ, Cronbach’s alpha = 0.85) was used to evaluate the personality traits [41]. Y-BOCS comprises two subscales: Obsessions and Compulsions. The B-CATS has four subscales, Digit Symbol Substitution Test (DSST), Trail-Making Test-A (TMT-A), Trail-Making Test-B (TMT-B), and Category Fluency (CF). Lower DSST and CF scores and higher TMT-A and TMT-B scores indicate worse cognitive performance. The CSQ consists of two subscales: Active Coping and Passive Coping. The EPQ has psychoticism, extraversion, neuroticism, and lying as the subscales.
Imaging data acquisition and preprocessing
The blood oxygenation level-dependent (BOLD) and three-dimensional (3D) T1-weighted structural images were acquired using a 3.0 T Philips scanner. Specifically, the BOLD images were acquired through a gradient echo-planar imaging sequence with the following parameters: repetition time/echo time = 2,000/30 ms, number of slices = 33, matrix size = 64 × 64, flip angle = 90°, field of view = 220 × 220 mm2, slice thickness = 4 mm, slice gap = 0 mm, voxel size = 3 × 3 × 3 mm3, and number of volumes = 240. The structural images were acquired using a high-resolution 3D turbo fast-echo T1WI sequence with the following parameters: repetition time/echo time = 8.14/3.72 ms, number of slices = 208, matrix size = 256 × 256, flip angle = 7°, field of view = 256 × 256 mm2, slice thickness = 0.8 mm, slice gap = 0 mm, and voxel size = 1 × 1 × 1 mm3. All participants were scanned on the same scanner under identical acquisition conditions and were instructed to remain still with their eyes closed while remaining awake.
Imaging data were preprocessed to calculate fast Fourier transform (FFT)-ALFF, wavelet-ALFF, voxel-wise FC, and voxel-wise and coefficient-based GCA, using the RESTplus toolbox in MATLAB [42]. The detailed preprocessing steps are provided in the Supplementary Material. Moreover, while calculating FC and GCA, the ROIs were 12 striatal subregions (Table S1) based on the Brainnetome atlas [43]. Detailed FFT-ALFF, wavelet-ALFF, FC, and GCA can be found in the papers of Zang et al. [44], Luo et al. [45], Yan et al. [46], and Zang et al. [47], respectively.
The preprocessing steps used for calculating DC, Kendall’s coefficient of concordance (KCC)-ReHo, and coherence (Cohe)-ReHo were identical to those used for calculating FFT-ALFF, wavelet-ALFF, voxel-wise FC, and voxel-wise and coefficient-based GCA. However, spatial smoothing was not performed after the images were normalized to the Montreal Neurological Institute (MNI) space; instead, spatial smoothing was performed after calculating DC, KCC-ReHo, and Cohe-ReHo. In the DC calculation, the correlation coefficient (r) was set at r = 0.25. In the KCC-ReHo and Cohe-ReHo calculations, a cluster size of 27 voxels within the measured cluster was used. Detailed procedures for calculating DC, KCC-ReHo, and Cohe-ReHo can be found in the papers of Zuo et al. [48], Zang et al. [49], and Liu et al. [50], respectively.
Statistical analysis
Gender distribution differences between the OCD patients and HCs were assessed using a chi-square test in SPSS 25.0 software. We conducted two-sample t-tests or Mann–Whitney U tests based on normality test outcomes (Shapiro–Wilk tests) for age and educational level comparisons. Paired t-tests or Wilcoxon signed-rank tests were conducted to compare the clinical symptoms of OCD patients at baseline and after treatment, with the p-value adjusted for multiple comparisons (p < 0.004 for the simultaneous conduct of 12 tests, Bonferroni correction).
The correlations between the fMRI metrics of OCD patients and their clinical symptoms at baseline, as well as the correlations between post-treatment changes in fMRI metrics and changes in clinical symptoms, were examined using Spearman’s correlation for nonparametric data or Pearson’s correlation for normally distributed data. Before performing correlation analyses, we assessed the presence of outliers using boxplots and standardized z-score (\(z=\frac{x-\mu }{\sigma }\)), where \(x\) is the individual data point, \(\mu\) is the mean of the dataset, and \(\sigma\) is the standard deviation of the dataset. Data points with z-scores exceeding ±3 were considered potential outliers. When outliers were detected, we conducted sensitivity analyses by performing correlations both with and without these data points to assess their impact on the results. If an outlier significantly influenced the correlation, we reported the findings with appropriate caution. Additionally, to address multiple tests, p values for baseline correlations (corrected p < 0.003 for the simultaneous conduct of 17 tests), and post-treatment correlations (corrected p < 0.004 for the simultaneous conduct of 12 tests), were adjusted using the Bonferroni correction.
The imaging data were analyzed using the RESTplus toolbox. To pinpoint clusters with abnormal fMRI metrics in the patients at baseline compared with HCs, two-sample t-tests were conducted with a Gaussian random field (GRF) correction (voxel p value < 0.001, cluster p value < 0.05, two-tailed) after incorporating gender, age, education level, and mean framewise displacement as covariates of no interest. Paired t-tests were performed on the pretreatment and posttreatment fMRI metrics of the OCD patients (GRF corrected) to evaluate clusters with changed fMRI metrics after treatment. Additionally, FFT-ALFF, wavelet-ALFF, DC, KCC-ReHo, and Cohe-ReHo in the striatal subregions were extracted for both HCs and OCD patients before and after the treatment. These metrics (extracted values) were compared between the patients and HCs in each striatal subregion using two-sample t-tests (Bonferroni correction, p < 0.01 for the simultaneous conduct of 5 tests). Furthermore, these metrics (extracted values) were compared between OCD patients before and after treatment in each striatal subregion using paired t-tests with Bonferroni correction.
Associations between gene expression patterns and information flow patterns
Figure 1B presents the flowchart for the associations. Regional microarray expression data were obtained from six postmortem brains provided by Allen Human Brain Atlas (https://human.brain-map.org, accessed on January 14, 2024). The data were processed using the abagen toolbox (version 0.1.4) with a 246-region volumetric atlas in the MNI space (Brainnetome atlas). Specifically, the processing steps included probe reannotation, filtering based on expression intensity, selection of the most stable probe per gene, MNI coordinate alignment, and region-wise normalization. Further details of gene expression data processing are available in the Supplementary Material.
Following the processing of gene expression data, a regional expression matrix was generated. This matrix comprised 246 rows and 15,633 columns corresponding to brain regions and the retained genes, respectively. The Spearman correlation analyses were conducted to identify associations between the gene expression patterns and alterations in GCA coefficients. Using the Bonferroni correction, the correlation’s significance level was adjusted for multiple comparisons (p = 0.05/15633).
We then excluded correlations arising from the spatial autocorrelation. Spatial autocorrelation is a tendency of brain feature values in close spatial proximity to exhibit greater similarity than those in spatially distant regions. BrainSMASH was used to generate 1000 surrogate brain maps with spatial autocorrelation matched to that of the target brain map [51]. Subsequently, the Spearman correlation analyses were conducted between surrogate brain maps and gene expression patterns. Correlations attributable to the spatial autocorrelation were considered chance correlations (p = 0.05). For example, if a gene expression pattern correlated with 50 of the 1000 surrogate brain maps, the gene was excluded from those associated with alterations in GCA coefficients.
Following this step, we identified genes associated with alterations in GCA coefficients, excluding the influence of spatial autocorrelation. Subsequently, gene enrichment analyses (Gene Ontology – GO, and Kyoto Encyclopedia of Genes and Genomes - KEGG) were conducted on these genes by using Metascape [52]. Additionally, we tabulated common and distinct genes associated with the alterations in GCA coefficients between the information flow from the ROI to the rest of the brain and vice versa. Similar comparisons were made for information flow from the rest of the brain to homotopical ROI or from homotopical ROI to the rest of the brain. Detailed information is provided in the Supplementary Material.
Results
Demographic information
Two patients and two controls were excluded from further analysis because they experienced excessive head movement. Subsequently, well-matched 49 patients and 53 healthy controls in age, gender distribution, and educational level were included for the statistical analysis. Table S2 and Figure S1 present the demographic and clinical characteristics of OCD patients and HCs. Furthermore, 34 OCD patients completed the follow-up. No significant side effects were observed in patients taking paroxetine, and no patients dropped out of the study due to the drug. In addition, medication compliance among patients who completed the follow-up was assessed through self-reporting. No significant differences between the baseline demographic and clinical characteristics of patients who completed the follow-up and those of all patients. Following treatment (treatment time: 33.59 ± 5.05 days), the changes in Y-BOCS, Obsessions, Compulsions, HAMD, HAMA, SDSS, Active coping, DSST, and TMT-A scores survived the Bonferroni correction (Figure S2). Moreover, 19 OCD patients exhibited more than 20% reduction in Y-BOCS scores; therefore, these patients were positive responders to 4-week paroxetine treatment [31].
Local activity, local synchronization, and the number of undirected connections in the striatum under whole-brain statistical analyses and these metrics in the striatal subregions under region-specific analyses
Before treatment, no statistically significant differences in FFT-ALFF, Wavelet-ALFF, KCC-ReHo, Cohe-ReHo, and DC were observed in the striatum between OCD patients and HCs based on whole-brain statistical analyses. Furthermore, no significant changes in these metrics were found in the striatum of OCD patients after treatment, as revealed by whole-brain statistical analyses.
In region-specific analyses, before treatment, OCD patients exhibited a decrease in FFT-ALFF and wavelet-ALFF in the right dorsal caudate, left ventral caudate, and right ventromedial putamen, along with a decrease in wavelet-ALFF in the left ventromedial putamen and an increase in KCC-ReHo in the left dorsal and ventral caudate (Figure S3). However, these results were not statistically significant after Bonferroni correction. Furthermore, OCD patients displayed decreased DC in the right globus pallidus (p < 0.01), and the statistical significance of this result persisted in the Bonferroni correction. After treatment, the increase in FFT-ALFF and wavelet-ALFF in the right dorsal caudate, the decrease in FFT-ALFF and wavelet-ALFF in the left ventral caudate, and the decrease in KCC-ReHo in the left dorsal caudate were remarkable compared to pre-treatment values. However, these results were not statistically significant after Bonferroni correction.
FC and EC in striatal subregions
OCD patients had altered FC between brain regions and ROIs, including the right ventral caudate, bilateral globus pallidus, and left dorsal caudate, compared with HCs. After treatment, the patients displayed reduced FC between the right dorsal caudate and the middle and superior temporal gyri, as well as between the right ventral caudate and the triangular part of the inferior frontal gyrus, compared to pre-treatment values (Table S3 and Figure S4).
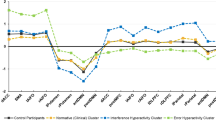
Table 1 and Figs. 2–5 highlight significant differences in EC of the striatal subregions between the patients and HCs before treatment. Before treatment, compared with HCs, OCD patients exhibited increased top-down EC, including the information inflow from the cortex to the striatum and information outflow from the striatum to the cerebellum, as well as decreased bottom-up EC, which includes information outflow from the striatum to the cortex. Compared with that at baseline, an increased bottom-up EC was observed in OCD patients after treatment, including EC from the right globus pallidus to the bilateral medial orbitofrontal cortex (mOFC), medial superior frontal gyrus (mSFG), and left anterior cingulate gyrus (ACC); from the left nucleus accumbens to the bilateral mOFC and ACC; and from the left ventromedial putamen to the right cerebellum VIII and Crus I/VI (Table S4 and Fig. 5).
A Abnormal effective connectivity in the nucleus accumbens of OCD patients at baseline compared to healthy controls, and changes in effective connectivity in the nucleus accumbens of OCD patients after treatment. B Changes in effective connectivity in the right globus pallidus of OCD patients after treatment. C Changes in effective connectivity in the left ventromedial putamen of OCD patients after treatment.
Correlation analysis
Significant associations existed between the imaging metrics in the striatal subregions and clinical assessments (Figures S5–S8 and Table S5). However, only correlations between EC from the right SFG/middle frontal gyrus (MFG) to the left dorsal caudate and passive coping scores (p = 0.001), between EC from the right SFG/MFG to the left dorsal caudate and EPQ psychoticism scores (p = 0.001), and between EC from the right MFG/SFG to the left ventral caudate and EPQ neuroticism scores (p = 0.001) remained significant after Bonferroni correction was applied. Moreover, the change in EC from the right cerebellum VIII and Crus I/VI to the left ventromedial putamen after treatment correlated with the change in SDSS scores (rho = −0.442, p = 0.011) (Figure S9); however, this correlation was not statistically significant after Bonferroni correction.
Associations between gene expression patterns and information flow patterns
Table S6 presents the number of genes associated with alterations in GCA coefficients in the striatal subregions of the patients. Figures S10–S24 display the names of these genes and their correlation coefficients. Additionally, these figures depict the Spearman correlation scatter plots illustrating the correlations with the minimum and maximum p values for each information flow.
Gene enrichment analysis
In the GO enrichment analysis (Figures S10–S20 and S22–24), genes associated with alterations in GCA coefficients were enriched for the biological processes of (1) cellular structure and development, (2) cell signaling and regulation of phosphorylation, (3) regulation of signaling pathways, (4) cellular responses and immune processes, (5) metabolic processes, and (6) gene expression and ribonucleic acid (RNA) processes. Detailed biological processes, annotated with GO terms, are available in the Supplementary Material.
In the KEGG pathway analysis (Figures S17–S20), genes associated with alterations in GCA coefficients were enriched for the pathways that represent a diverse range of cellular processes, including signaling cascades, metabolism, infection responses, and cellular trafficking. Details are available in the Supplementary Material.
Common and distinct GCA coefficient alteration-related genes
Figures S25–S26 present the common and distinct GCA coefficient alteration-related genes between the information flow from the ROI to the rest of brain and from the remaining parts of the brain to the ROI. Table S7 lists the proportion of common genes in each information flow and that among total genes. Figure S27 presents the common and distinct GCA coefficient alteration-related genes between the information flows from the remaining parts of the brain to the homotopical ROIs or from the homotopical ROIs to the remaining parts of the brain. Additionally, the proportion of common genes in each information flow and that in total genes are presented in Table S8. The patterns of common and distinct GCA coefficient alteration-related genes aligned with the structure of the Chinese word ‘Chuan’, which signifies ‘river’ and ‘source’. This alignment aided in elucidating the relationship between gene expression and the altered information flow.
Discussion
This transcriptome–neuroimaging association study explored OCD-involving neural circuits with fine division within the striatum as well as the associations between gene expression profiles and alterations of information flows. Whole-brain voxel-wise statistical analyses at baseline and after treatment revealed no significant alterations in the striatum of OCD patients. However, when region-specific analyses with a fine division of the striatum were employed, substantial changes in DC, FC, and EC were observed in the striatal subregions of OCD patients at baseline and after treatment. Notably, the increased top-down EC including the information inflow from the cortex to the striatum and the information outflow from the striatum to the cerebellum, as well as the decreased bottom-up EC including the information outflow from the striatum to the cortex, were observed in OCD patients before treatment compared with HCs. These alterations in EC within the CSTC circuit reflected the top-down control was in excess and the bottom-up regulation was attenuated in OCD patients. Moreover, these findings suggest the presence of abnormalities in EC within cortical-striatal-cerebellar circuits in OCD patients. After treatment, the bottom-up information flow from the striatum to the cortex and from the cerebellum to the striatum improved, along with some improvement in the clinical symptoms of OCD patients. Thus, the treatment-induced improvement in bottom-up regulation may affect higher-order brain regions, potentially causing the alleviation of clinical symptoms in OCD patients. Furthermore, the correlation analyses showed that EC in the caudate was linked to coping strategies and personality traits. According to the results of the transcriptome–neuroimaging analysis, EC alterations were associated with specific gene expression profiles. These genes were enriched for various biological processes. Furthermore, these genes were also enriched for the pathways representing various cellular processes. Moreover, GCA coefficient alteration-related genes significantly overlapped between the information flows from the striatal subregions to the remaining parts of the brain and vice versa. Additionally, GCA coefficient alteration-related genes significantly overlapped between information flows from the remaining parts of the brain to the homotopical striatal subregions or from the homotopical striatal subregions to the remaining parts of the brain.
In this study, aberrant information flow was noted both in top-down and bottom-up directions between cortical and striatal regions in the OCD patients. A cortico-striatal drive generally exists to maintain dynamics within a brain-wide network during the resting state [53]. Consequently, excessive flow of information in the top-down direction enhances cortical control, thereby causing impairment in cognitive flexibility, emotion regulation, and decision-making in OCD patients [54,55,56,57]. Attenuation of bottom-up EC from the striatal subregions to the cortex might indicate impairment of feedback regulation within the circuit. This causes substantial information to inflow from the cortex to the striatum. Following the treatment, an improvement in information flow in the bottom-up direction was noted in OCD patients, both from the striatum to the cortex and from the cerebellum to the striatum. This improved feedback regulation facilitates bidirectional information to be continuously transmitted, thereby rendering the up-and-down information flow more susceptible to bottom-up modulation [58]. Consequently, the treatment-induced enhancement of bottom-up regulation influences higher-order brain regions to alleviate clinical symptoms in OCD patients [59]. These findings suggest that therapeutic strategies targeting bottom-up regulation could be explored in future treatment protocols for OCD.
Bidirectional communication between the cortex and striatum is impaired because of the decreased bottom-up and enhanced top-down EC within the cortico-striatal circuit. This reciprocal interaction, particularly involving the cingulate cortex and striatum, along with the impaired bidirectional relationship between them, causes dysfunctions in cognitive control and emotional regulation [60]. Consequently, these concerns significantly influence OCD symptom development [61]. Under normal circumstances, bidirectional communication efficiently stimulates constant information interactions among brain networks [62]. By contrast, under pathological conditions, abnormal bidirectional connections may arise because of rapid interregional adjustments and compensatory changes within functional networks. However, after treatment, EC improved from the striatal subregions to the ACC, mOFC, and mSFG. This confirms that the decrease in bottom-up EC from the striatum to the cortex is a pathological damage induced by OCD. Furthermore, the improvement in EC from the striatal subregions to the ACC, mOFC, and mSFG indicates that drug intervention-related therapeutic effects are not an undifferentiated cortical influence but instead, they preferentially affect specific regions, particularly cognitive function-associated regions [4].
In this study, abnormal FC and EC in OCD patients were observed within the cortical-striatal-cerebellar circuit, a crucial circuit for the formation and maintenance of motor learning. The conscious and purposeful motor execution (i.e., goal-directed behaviors) is the motor learning phase, whereas automatic and unconscious behaviors (i.e., habitual behaviors) are the motor adaptation phase [63]. In the present study, a more complex pattern of connections was identified within the dual-learning system than in previous studies [6, 7]. Specifically, in addition to the established EC between the caudate and PFC in the goal-directed system and that between the putamen and SMA in the habit system, we observed information interaction between the putamen and PFC, and between the caudate and SMA. These findings are consistent with those of Xu et al. who emphasized compulsive behavior arising from abnormal connectivity within the two systems as well as from abnormal interactions in the dual-learning system [8]. Excessive EC from the dorsal caudate to the cerebellum in drug-naive OCD patients may result from enhanced top-level control or compensation for impairments in goal-directed behaviors [64, 65]. Furthermore, we observed enhanced bottom-up information transmission from the cerebellum to the left ventromedial putamen after treatment, and this was linked to improved social function (Figure S9). These findings suggest that paroxetine influences the cortical-striatal-cerebellar circuit, thus alleviating the clinical symptoms in OCD patients.
Similarities and differences exist between the findings of EC and FC in OCD. The FC-related findings highlight the crucial role of the globus pallidus in OCD. The globus pallidus plays a central role in cortico-striatal feedback loops, as each striatal subregion projecting back to the cortex through the pallidum and thalamus [66]. Previous studies have identified the front–pallidal connection as a potential neuro-modulatory target for reducing inflexible habitual behaviors [67], and transcranial magnetic stimulation-induced ventral PFC activity has been shown to enhance front–pallidal connection, correlating with OCD symptom improvement [68]. In the present study, medication-induced changes demonstrated an enhancement in EC from the globus pallidus to the mOFC, mSFG, and ACC. This suggests that the front–pallidal connection plays a crucial role in the OCD pathology and drug-related effects of SSRIs. Furthermore, abnormal EC was observed between the striatum and thalamus in OCD patients at baseline. Although functional changes in the thalamus and disrupted connections within the thalamocortical circuit have been reported in OCD patients [69, 70], this is the first study to observe an increase in EC from the thalamus to the ventral caudate. The thalamus serves as a relay center, connecting with the striatum and cortical areas, and exhibits altered EC, thereby reflecting an impairment in transmission and processing of neuronal information in OCD patients [66]. The observed discrepancies between FC and EC findings may stem from fundamental differences in what these measures capture. The presence of altered FC without corresponding EC changes may indicate synchronized activity that is not necessarily functionally directed, while EC alterations without FC changes could reflect shifts in effective network dynamics that are not detected by correlation-based FC methods. These findings suggest that while FC alterations highlight changes in network co-activation patterns, EC provides additional insights into the underlying pathophysiology by revealing changes in the directed flow of information. The observed EC and FC changes after treatment suggest that SSRIs may influence not just the strength of connectivity between regions but also the directionality of information flow within the cortico-striatal-cerebellar circuit. Future studies integrating FC and EC analyses across different time scales and experimental conditions may help further elucidate the functional implications of these discrepancies in OCD.
The transcriptome–neuroimaging analysis revealed EC alterations associated with specific gene expression profiles. These genes were enriched in various biological processes. The cellular structure and development including neuron projection development, regulation of axonogenesis and dendritic spine development, ensheathment of neurons, myelination, maintenance of the blood−brain barrier, etc., are crucial steps in constructing the central nervous system, and these biological processes are implicated in OCD [71, 72]. Moreover, a study reported that an OCD-associated mutation altered cGMP-dependent protein kinase-mediated serotonin transporter phosphorylation [73]. Cell signaling and signaling pathway regulation are the bases of information flow. The involved cellular responses and immune processes support the hypothesized immune-mediated pathophysiology in OCD [74]. Metabolic abnormalities have been consistently reported in OCD patients [75]. Abnormal gene expression and RNA process disruptions have been reported in OCD patients [76]. Furthermore, the results of the KEGG-based enrichment analysis of GCA coefficient alteration-related genes were consistent with those of the GO analysis, which reinforces the robustness and validity of the findings. These findings may have important implications for treatment by identifying potential molecular targets for OCD. Specifically, targeting disrupted neurodevelopmental processes, such as axonogenesis and dendritic spine development, may help restore neural circuitry integrity. Additionally, interventions aimed at modulating immune-related pathways could mitigate the hypothesized immune-mediated contributions to OCD pathophysiology. Furthermore, therapies addressing metabolic dysregulation and abnormal RNA processing may provide novel avenues for treatment. By integrating transcriptome–neuroimaging insights, these molecular targets may contribute to the foundation for developing more targeted treatment approaches for OCD. Importantly, the combination of neuroimaging findings with transcriptomic data allows for the identification of key molecular mechanisms underlying altered connectivity patterns in OCD, offering a more holistic perspective on the disorder’s pathophysiology.
A greater number of changed information flows corresponded to a higher count of GCA coefficient alteration-related genes, evident in more genes being associated with the changed information flows from the remaining parts of the brain to the ROI than with the changed information flows from the ROI to the remaining parts of the brain. These quantitative relationships, to some extent, support the reliability of the results. Furthermore, a substantial overlap of GCA coefficient alteration-related genes was observed between information flows from the striatal subregions to the remaining parts of the brain and vice versa. This bidirectional overlap suggests that the genetic underpinnings of altered effective connectivity in OCD may influence both top-down and bottom-up information flows within the CSTC circuit. Given that the striatum plays a crucial role in integrating cortical input and modulating motor, cognitive, and affective processes, shared genetic factors may contribute to dysregulated connectivity patterns that underlie OCD symptomatology. Additionally, the GCA coefficient alteration-related genes significantly overlapped between information flows from the remaining parts of the brain to the homotopical striatal subregions or from the homotopical striatal subregions to the remaining parts of the brain. This finding indicates that homotopical striatal regions may be governed by similar molecular mechanisms. The homotopic organization of the striatum has been implicated in balancing motor and cognitive functions [77], and disruptions in these circuits have been linked to compulsive behaviors and cognitive rigidity observed in OCD. Together, these findings suggest that reciprocal information flows in the striatal subregions of OCD patients share a common genetic basis, as do information flows in the homotopical striatal subregions of OCD patients. The significant genetic overlap highlights the potential role of specific molecular pathways in shaping large-scale network dysfunctions, providing further evidence that genetic factors contribute to the disrupted neural dynamics observed in OCD. Future studies integrating transcriptomic, epigenetic, and connectivity analyses could further clarify the mechanisms by which these shared genes influence striatal connectivity and OCD pathophysiology.
Some study limitations should be acknowledged. First, the relatively medium sample size at follow-up and the reliance on a single data source may lead to bias. Second, we observed improvements in the bottom-up information flows from the striatum to the cortex and from the cerebellum to the striatum, as well as improvements in the clinical symptoms of OCD patients. Furthermore, the enhanced bottom-up information flow from the cerebellum to the left ventromedial putamen after treatment was linked to improved social function. While we speculated that the treatment-induced improvement in bottom-up regulation may affect higher-order brain regions, potentially causing the alleviation of clinical symptoms in OCD patients, the causal relationship between connectivity changes and symptom improvement cannot been determined from this study. Third, the gene expression and neuroimaging data were derived from distinct sets of participants. However, existing studies suggest substantial consistency in gene expression patterns across diverse human populations [78]. Fourth, while associations between gene expression patterns and information flow patterns were observed, the lack of causal inference due to the cross-sectional nature of the gene expression data is a limitation.
Conclusion
In summary, the current study underscores the significance of delving into OCD-associated neural circuits by finely examining striatal subregions. Through a detailed division of the striatum, substantial changes in DC, FC, and EC were observed in the striatal subregions of the OCD patients both before and after treatment. EC application revealed an aberrant flow of information within CSTC circuits and cortical-striatal-cerebellar circuits, which indicated that top-down control was excessive and bottom-up regulation was attenuated in the drug-naive OCD patients. After treatment, bottom-up information flow improved along with a reduction in the clinical symptoms of OCD patients. This suggests that treatment-induced enhancement of bottom-up regulation influences higher-order brain regions, causing the alleviation of OCD symptoms. Moreover, GCA coefficient alteration-related genes were enriched in the biological processes and pathways related to (1) cellular structure and development, (2) cell signaling and regulation of phosphorylation, (3) regulation of signaling pathways, (4) cellular responses and immune processes, (5) metabolic processes, and (6) gene expression and RNA processes. Interestingly, the information flow alteration-related genes substantially overlapped between bidirectional information flows among striatal subregions and the rest of brain, as well as between information flows among homotopical striatal subregions and the rest of brain. These findings offer new insights into biological substrates underlying the changed EC of the striatum in OCD patients. Furthermore, this study extensively decodes the role of the striatum in OCD.
Data availability
The data of the patients and healthy controls are available from the corresponding author, WG, upon reasonable request. Regional microarray expression data were obtained from six post-mortem brains provided by the Allen Human Brain Atlas (https://human.brain-map.org).
References
Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81.
Di Martino A, Scheres A, Margulies DS, Kelly A, Uddin LQ, Shehzad Z, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–47.
Del Casale A, Kotzalidis G, Rapinesi C, Serata D, Ambrosi E, Simonetti A, et al. Functional neuroimaging in obsessive-compulsive disorder. Neuropsychobiology. 2011;64:61–85.
de Joode NT, Thorsen AL, Vriend C, Pouwels PJ, van Balkom AJ, Hagen K, et al. Linking resting state metabolite concentrations in the dorsal anterior cingulate cortex to response inhibition in OCD, a combined fMRI-MRS study. J Affect Disord Rep. 2023;14:100641.
Grassi G, Pallanti S, Righi L, Figee M, Mantione M, Denys D, et al. Think twice: impulsivity and decision making in obsessive–compulsive disorder. J Behav Addict. 2015;4:263–72.
Gillan CM, Papmeyer M, Morein-Zamir S, Sahakian BJ, Fineberg NA, Robbins TW, et al. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry. 2011;168:718–26.
Banca P, Voon V, Vestergaard MD, Philipiak G, Almeida IS, Pocinho F, et al. Imbalance in habitual versus goal directed neural systems during symptom provocation in obsessive-compulsive disorder. Brain. 2015;138:798–811.
Xu C, Hou G, He T, Ruan Z, Chen J, Wei Z, et al. Imbalance in functional and structural connectivity underlying goal-directed and habitual learning systems in obsessive-compulsive disorder. Cereb Cortex. 2022;32:3690–705.
Park J, Kim T, Kim M, Lee TY, Kwon JS. Functional connectivity of the striatum as a neural correlate of symptom severity in patient with obsessive-compulsive disorder. Psychiatry Investig. 2020;17:87.
Dong C, Yang Q, Liang J, Seger CA, Han H, Ning Y, et al. Impairment in the goal-directed corticostriatal learning system as a biomarker for obsessive–compulsive disorder. Psychol Med. 2020;50:1490–500.
Seth AK, Barrett AB, Barnett L. Granger causality analysis in neuroscience and neuroimaging. J Neurosci. 2015;35:3293–97.
Yang Z, Xiao S, Su T, Gong J, Qi Z, Chen G, et al. A multimodal meta-analysis of regional functional and structural brain abnormalities in obsessive–compulsive disorder. Eur Arch Psychiatry Clin Neurosci. 2024;274:165–80.
Liu J, Cao L, Li H, Gao Y, Bu X, Liang K, et al. Abnormal resting-state functional connectivity in patients with obsessive-compulsive disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2022;135:104574.
Teng C, Zhang W, Zhang D, Shi X, Wu X, Qiao H, et al. Association between clinical features and decreased degree centrality and variability in dynamic functional connectivity in the obsessive–compulsive disorder. NeuroImage: Clin. 2024;44:103665.
Schippers MB, Renken R, Keysers C. The effect of intra-and inter-subject variability of hemodynamic responses on group level Granger causality analyses. Neuroimage. 2011;57:22–36.
Abe Y, Sakai Y, Nishida S, Nakamae T, Yamada K, Fukui K, et al. Hyper-influence of the orbitofrontal cortex over the ventral striatum in obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2015;25:1898–905.
Tian T, Liu D, Zhang G, Wang J, Wan C, Fang J, et al. Dynamic effective connectivity among large-scale brain networks mediates risk of anxiety. Hum Brain Mapp. 2023.
Bianco MG, Duggento A, Nigro S, Conti A, Toschi N, Passamonti L. Heritability of human “directed” functional connectome. Brain Behav. 2023;13:e2839.
Xu J, Yin X, Ge H, Han Y, Pang Z, Liu B, et al. Heritability of the effective connectivity in the resting-state default mode network. Cereb Cortex. 2017;27:5626–34.
Hibar DP, Cheung JW, Medland SE, Mufford MS, Jahanshad N, Dalvie S, et al. Significant concordance of genetic variation that increases both the risk for obsessive–compulsive disorder and the volumes of the nucleus accumbens and putamen. Br J Psychiatry. 2018;213:430–36.
Godlewska B, Norbury R, Selvaraj S, Cowen P, Harmer C. Short-term SSRI treatment normalises amygdala hyperactivity in depressed patients. Psychol Med. 2012;42:2609–17.
Wu P, Zhang A, Sun N, Lei L, Liu P, Wang Y, et al. Cortical thickness predicts response following 2 weeks of SSRI regimen in first-episode, drug-naive major depressive disorder: an MRI study. Front Psychiatry. 2022;12:751756.
Bracco L, Dusi N, Moltrasio C, Brambilla P, Delvecchio G. Structural and functional brain imaging after treatment with selective-serotonin reuptake-inhibitors in obsessive-compulsive disorder: a mini review. J Affect Disord. 2023.
Murphy K, Garavan H. An empirical investigation into the number of subjects required for an event-related fMRI study. Neuroimage. 2004;22:879–85.
Pajula J, Tohka J. How many is enough? Effect of sample size in inter-subject correlation analysis of fMRI. Comput Intel Neurosci. 2016;2016:2–2.
Churchill NW, Yourganov G, Strother SC. Comparing within-subject classification and regularization methods in fMRI for large and small sample sizes. Hum Brain Mapp. 2014;35:4499–517.
Association AP. Diagnostic and statistical manual of mental disorders. Am J Psychiatry; 2013.
Issari Y, Jakubovski E, Bartley CA, Pittenger C, Bloch MH. Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a meta-analysis. J Clin Psychiatry. 2016;77:21133.
Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016;55:851–59.e2.
Bandelow B, Sher L, Bunevicius R, Hollander E, Kasper S, Zohar J, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive–compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77–84.
Brar J, Sidana A, Chauhan N, Bajaj MK. Early improvement as a predictor of treatment response in patients with obsessive-compulsive disorder: a 12-week randomized trial of sertraline and fluvoxamine. Prim Care Companion CNS Disord. 2022;24:40361.
Goodwin GM. The overlap between anxiety, depression, and obsessive-compulsive disorder. Dialogues Clin Neurosci. 2022.
Asselmann E, Wittchen H-U, Lieb R, Beesdo-Baum K. A 10-year prospective-longitudinal study of daily hassles and incident psychopathology among adolescents and young adults: interactions with gender, perceived coping efficacy, and negative life events. Soc Psychiatry Psychiatr Epidemiol. 2017;52:1353–62.
Hazari N, Narayanaswamy JC, Venkatasubramanian G. Neuroimaging findings in obsessive–compulsive disorder: a narrative review to elucidate neurobiological underpinnings. Indian J psychiatry. 2019;61(Suppl 1):S9.
Zhang CC, Gong H, Zhang Y, Jin H, Yang Y, Li B, et al. Development and psychometric evaluation of the Mandarin Chinese version of the yale-brown obsessive-compulsive scale–second edition. Braz J Psychiatry. 2019;41:494–98.
Clark DB, Donovan JE. Reliability and validity of the hamilton anxiety rating scale in an adolescent sample. J Am Acad Child Adolesc Psychiatry. 1994;33:354–60.
Zheng Y, Zhao J, Phillips M, Liu J, Cai M, Sun S, et al. Validity and reliability of the Chinese Hamilton depression rating scale. Br J Psychiatry. 1988;152:660–64.
Hurford IM, Ventura J, Marder SR, Reise SP, Bilder RM. A 10-min measure of global cognition: validation of the Brief Cognitive Assessment Tool for Schizophrenia (B-CATS). Schizophr Res. 2018;195:327–33.
Xiang Y, Weng Y, Li W, Gao L, Chen G, Xie L, et al. Training patients with schizophrenia with the community re-entry module: a controlled study. Soc Psychiatry Psychiatr Epidemiol. 2006;41:464–69.
Xie Y. Reliability and validity of the simplified coping style questionnaire. Chin J Clin Psychol. 1998.
Gong Y. Eysenck personality questionnaire revised in china. Inf Psychol Sci. 1984;4:11–18.
Jia X-Z, Wang J, Sun H-Y, Zhang H, Liao W, Wang Z, et al. RESTplus: an improved toolkit for resting-state functional magnetic resonance imaging data processing. Sci Bull. 2019;64:953–54.
Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016;26:3508–26.
Yu-Feng Z, Yong H, Chao-Zhe Z, Qing-Jiu C, Man-Qiu S, Meng L, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91.
Luo F-F, Wang J-B, Yuan L-X, Zhou Z-W, Xu H, Ma S-H, et al. Higher sensitivity and reproducibility of wavelet-based amplitude of resting-state fMRI. Front Neurosci. 2020;14:224.
Yan H, Han Y, Shan X, Li H, Liu F, Xie G, et al. Altered resting-state cerebellar-cerebral functional connectivity in patients with panic disorder before and after treatment. Neuropharmacology. 2023;240:109692.
Zang Z-X, Yan C-G, Dong Z-Y, Huang J, Zang Y-F. Granger causality analysis implementation on MATLAB: a graphic user interface toolkit for fMRI data processing. J Neurosci Methods. 2012;203:418–26.
Zuo X-N, Ehmke R, Mennes M, Imperati D, Castellanos FX, Sporns O, et al. Network centrality in the human functional connectome. Cereb Cortex. 2012;22:1862–75.
Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400.
Liu D, Yan C, Ren J, Yao L, Kiviniemi VJ, Zang Y. Using coherence to measure regional homogeneity of resting-state FMRI signal. Front Syst Neurosci. 2010;4:1366.
Burt JB, Helmer M, Shinn M, Anticevic A, Murray JD. Generative modeling of brain maps with spatial autocorrelation. NeuroImage. 2020;220:117038.
Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1523.
Nakhnikian A, Rebec GV, Grasse LM, Dwiel LL, Shimono M, Beggs JM. Behavior modulates effective connectivity between cortex and striatum. PLoS One. 2014;9:e89443.
Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22.
Norman LJ, Carlisi CO, Christakou A, Murphy CM, Chantiluke K, Giampietro V, et al. Frontostriatal dysfunction during decision making in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:694–703.
Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cognit Sci. 2012;16:43–51.
Ahmari SE, Rauch SL. The prefrontal cortex and OCD. Neuropsychopharmacology. 2022;47:211–24.
Carhart-Harris RL, Friston KJ. REBUS and the anarchic brain: toward a unified model of the brain action of psychedelics. Pharmacol Rev. 2019;71:316–44.
Vollenweider FX, Preller KH. Psychedelic drugs: neurobiology and potential for treatment of psychiatric disorders. Nat Rev Neurosci. 2020;21:611–24.
Long J, Luo L, Guo Y, You W, Li Q, Li B, et al. Altered spontaneous activity and effective connectivity of the anterior cingulate cortex in obsessive–compulsive disorder. J Comp Neurol. 2021;529:296–310.
Thorsen AL, Hagland P, Radua J, Mataix-Cols D, Kvale G, Hansen B, et al. Emotional processing in obsessive-compulsive disorder: a systematic review and meta-analysis of 25 functional neuroimaging studies. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:563–71.
Riedl V, Utz L, Castrillón G, Grimmer T, Rauschecker JP, Ploner M, et al. Metabolic connectivity mapping reveals effective connectivity in the resting human brain. Proc Natl Acad Sci. 2016;113:428–33.
Tzvi E, Stoldt A, Witt K, Krämer UM. Striatal–cerebellar networks mediate consolidation in a motor sequence learning task: An fMRI study using dynamic causal modelling. Neuroimage. 2015;122:52–64.
Xu Y, Han S, Wei Y, Zheng R, Cheng J, Zhang Y. Abnormal resting-state effective connectivity in large-scale networks among obsessive-compulsive disorder. Psychol Med. 2023;54:350–8.
Liu J, Bu X, Hu X, Li H, Cao L, Gao Y, et al. Temporal variability of regional intrinsic neural activity in drug-naïve patients with obsessive–compulsive disorder. Hum Brain Mapp. 2021;42:3792–803.
Sherman SM. Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci. 2016;19:533–41.
Kim T, Kim M, Jung WH, Kwak YB, Moon S-Y, Kyungjin Lho S, et al. Unbalanced fronto-pallidal neurocircuit underlying set shifting in obsessive-compulsive disorder. Brain. 2022;145:979–90.
Xu B, Sandrini M, Wang WT, Smith JF, Sarlls JE, Awosika O, et al. PreSMA stimulation changes task-free functional connectivity in the fronto-basal-ganglia that correlates with response inhibition efficiency. Hum Brain Mapp. 2016;37:3236–49.
Dogan B, Ertekin E, Turkdogan FT, Memis CO, Sevincok L. Cortico-thalamo-striatal circuit components’ volumes and their correlations differ significantly among patients with obsessive–compulsive disorder: a case–control MRI study. Psychiatry Clin Psychopharmacol. 2019;29:162–70.
Weeland CJ, Kasprzak S, de Joode NT, Abe Y, Alonso P, Ameis SH, et al. The thalamus and its subnuclei—a gateway to obsessive-compulsive disorder. Transl Psychiatry. 2022;12:70.
Yang Z, Wu H, Lee PH, Tsetsos F, Davis LK, Yu D, et al. Investigating shared genetic basis across tourette syndrome and comorbid neurodevelopmental disorders along the impulsivity-compulsivity spectrum. Biol Psychiatry. 2021;90:317–27.
Guehl D, Benazzouz A, Aouizerate B, Cuny E, Rotgé J-Y, Rougier A, et al. Neuronal correlates of obsessions in the caudate nucleus. Biol Psychiatry. 2008;63:557–62.
Zhang Y-W, Gesmonde J, Ramamoorthy S, Rudnick G. Serotonin transporter phosphorylation by cGMP-dependent protein kinase is altered by a mutation associated with obsessive–compulsive disorder. J Neurosci. 2007;27:10878–86.
da Rocha FF, Correa H, Teixeira AL. Obsessive–compulsive disorder and immunology: a review. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:1139–46.
Park S-E, Choi N-G, Jeong G-W. Metabolic abnormality in the right dorsolateral prefrontal cortex in patients with obsessive–compulsive disorder: proton magnetic resonance spectroscopy. Acta Neuropsychiatr. 2017;29:164–69.
Rodríguez N, Lázaro L, Ortiz AE, Morer A, Martínez-Pinteño A, Segura AG, et al. Gene expression study in monocytes: evidence of inflammatory dysregulation in early-onset obsessive-compulsive disorder. Transl Psychiatry. 2022;12:134.
Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26.
Stranger BE, Nica AC, Forrest MS, Dimas A, Bird CP, Beazley C, et al. Population genomics of human gene expression. Nat Genet. 2007;39:1217–24.
Acknowledgements
We thank all the research participants. This study was supported by grants from the National Natural Science Foundation of China (Grant No. 82171508) and Natural Science Foundation of Tianjin (Grant No. 18JCQNJC10900).
Author information
Authors and Affiliations
Contributions
YH and HY: Data curation, Formal analysis, Writing – original draft. XS and HL: Data curation, Conceptualization. FL: Funding acquisition, Conceptualization. PL and DL: Formal analysis, Methodology. JZ: Supervision, Conceptualization. WG: Data curation, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Ethics Committee of the Second Xiangya Hospital approved the study (registration number: 2018025). All study participants provided written informed consent. All methods were performed in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, Y., Yan, H., Shan, X. et al. Decoding the striatum of drug-naive patients with obsessive-compulsive disorder: a transcriptome and longitudinal functional magnetic resonance imaging study. Transl Psychiatry 15, 258 (2025). https://doi.org/10.1038/s41398-025-03475-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-025-03475-4