Abstract
Previous studies have found that major depressive disorder (MDD) may accelerate overall structural brain aging. Nevertheless, it still remains unknown whether anhedonia, a critical negative prognostic indicator in MDD, further leads to advanced brain aging in specific regions. A total of 31 MDD with anhedonia (MDD-WA), 41 MDD without anhedonia (MDD-WoA), and 43 healthy controls (HCs) were recruited in this study. The difference between brain structure age (BSA) applied by support vector regression (SVR) and chronological age was calculated to derive the brain structure age gap estimation (BSAGE). Analyses of covariance (ANCOVAs) and intergroup comparisons were performed to obtain brain regions with significant BSAGE differences among three groups. Moreover, a support vector machine (SVM) classification model was used to verify the diagnostic value of altered BSAGE. ANCOVAs revealed significant BSAGE differences among three groups in the bilateral putamen (PU), left cerebellar white matter (CB), left cuneus (CUN), left fusiform gyrus (FuG), left subcallosal area (SCA), left superior occipital gyrus (SOG), left triangular inferior frontal gyrus (IFG-Tri), right lateral ventricle (L-V), right superior frontal gyrus medial segment (SFG-SM), right opercular inferior frontal gyrus (IFG-Oper), right precuneus (pre-CUN), right posterior insula (INS-Post), and right superior temporal gyrus (STG). Compared to HCs, the MDD-WA group showed significant BSAGE increase in all of the aforementioned brain regions, while the MDD-WoA group showed limited BSAGE increase in the CB, FuG, and SCA of left hemisphere only. However, no significant difference was found between MDD-WA and MDD-WoA. The altered BSAGE values showed promising discriminatory performance with an area under the curve (AUC) of 0.944 in classifying MDD-WA and HCs. The current findings emphasize that MDD with anhedonia may exhibit more extensive advanced brain aging, primarily in the frontal-limbic system, temporal lobe, and parietal lobe.
Similar content being viewed by others
Introduction
Anhedonia, a prevalent and cardinal symptom of major depressive disorder (MDD), is defined as a diminished ability to pursue or experience pleasure most of the time [1]. Epidemiological findings indicate that anhedonia is present in about 70% of MDD patients [2]. As the disease progresses, anhedonia may play a role in exacerbating symptoms of depression, increased risk of suicide [3], and resistance to antidepressant treatment [4]. During the remission phase of MDD, anhedonia may persist, affecting the patient’s recovery of social functioning, which may manifest as persistent social withdrawal [5, 6]. In light of this, the symptoms of anhedonia in MDD patients are increasingly being recognized as key indicators of disease progression and treatment response, which emphasize the necessity of exploring their possible biological mechanisms.
Normal brain aging is a complex process accompanied by structural and functional changes [7, 8]. Evidence from longitudinal studies has demonstrated an influential phenomenon that psychiatric disorders may accelerate the process of brain aging [9,10,11,12]. Consequently, the estimation of brain age using brain magnetic resonance imaging (MRI) data has been rapidly adopted to explore the pathophysiology of psychiatric disorders, including MDD, bipolar disorder (BD), and schizophrenia (SZ) [13,14,15]. The majority of previous studies have employed brain structures (including cortical structures and gray matter volumes) to estimate overall brain age [16, 17]. This involves converting the complex pattern of brain structural aging into a single outcome, namely the difference between subject’s chronological age and the estimated age. A large Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) study has shown that the estimated brain age from cortical thickness is 1.1 years higher than chronological age in patients with MDD [17].
However, it is notable that overall brain structural aging is not a pathology exclusive to MDD or other psychiatric disorders, rather the structural changes in specific regions have been demonstrated to be associated with particular symptoms. Accumulating evidence has highlighted structural brain abnormalities in MDD patients with anhedonia mainly in reward-circuit related regions, such as the prefrontal cortex, caudate nucleus, and nucleus accumbens (NAc) [18,19,20,21]. Prior correlational studies have yielded a consistent result, indicating an association between anhedonia and bilateral caudate nucleus volume reductions in MDD patients [18, 22]. Moreover, our previous studies have also reported consistent findings that MDD patients with anhedonia exhibit more severe volumetric atrophy in the left striatum [23], hippocampal subregions [24], and subcortical margins [25] than MDD patients without anhedonia. In this context, the specific regions that may exhibit advanced brain aging associated with anhedonia in MDD should be further elucidated.
In the present study, we extended the concept of overall brain age by estimating the structural age of each brain region after segmentation based on structural MRI and then explored the potential advanced brain aging in MDD patients stratifying groups by anhedonia. For this purpose, we utilized the method for estimating structural ages of the localized brain areas, as proposed by Huy-Dung Nguyen and his colleagues recently [26]. In this method, the brain structure age (BSA) of each region was obtained through the application of an ensemble of deep learning models and a three-dimensional (3D) segmentation mask. Subsequently, the difference between BSA and subject’s chronological age was calculated, resulting in the brain structure age gap estimation (BSAGE). A positive value indicates advanced brain aging, while a negative value indicates the opposite. Based on previous findings, we hypothesized that anhedonia in MDD might be associated with more extensive and severe advanced brain aging mainly in the frontal-limbic areas.
Methods
Participants
A total of 115 subjects were included in the present study, comprising 72 MDD patients and 43 age- and gender-matched healthy controls (HCs). Each MDD patient was subjected to a comprehensive evaluation by two professional psychiatrists. This evaluation was conducted in accordance with the Diagnostic and Statistical Manual of Mental Disorders, IV Edition (DSM-IV), assessed by using Structured Clinical Interview for DSM-IV (SCID). Furthermore, in accordance with Item 2 (loss of interest or pleasure) of the symptom criteria (A) for MDD in DSM-IV and the threshold of transformed score of Snaith- Hamilton Pleasure Scale (SHAPS) [27] for severe anhedonia, MDD patients were divided into MDD with anhedonia (MDD-WA, n = 31) and MDD without anhedonia (MDD-WoA, n = 41). The inclusion criteria for MDD patients were as follows, (1) aged from 18–45; (2) right-handedness, (3) total score of 17-item Hamilton Depression Scale (HAMD-17) [28] ≥ 17; (4) drug-naïve patients or recurrent MDD patients with continued withdrawal ≥ 3 months; (5) keep still during MRI scanning. HCs were recruited through advertisements from community residents, hospital staff, and school students. According to the DSM-IV criteria, all HCs underwent comprehensive interview to confirm the absence of any past or present psychiatric conditions. The general exclusion criteria included, (1) pregnant women; (2) major medical disease, including cardiovascular, respiratory, endocrine, and neurological diseases (e.g., epilepsy, brain trauma, and stroke); (3) present use of any medication that might influence the central nervous system, including drug or alcohol dependence or abuse; (4) history of psychotherapy and physical therapy, such as repetitive Transcranial Magnetic Stimulation (rTMS), transcranial direct current stimulation (tDCS), and electroconvulsive therapy (ECT); (5) contraindications to MRI scanning. The demographic and clinical data were collected from all the participants using a self-designed questionnaire. The severity of depression was evaluated by the HAMD-17. The present study was approved by the local medical ethics committee of The First Affiliated Hospital, Zhejiang University School of Medicine. Prior to the commencement of the study, each participant was required to sign a written informed consent form.
This study is one of our serial investigations focusing on anhedonia in patients with MDD. Details of the recruitment of participants, clinical assessments, and MRI acquisition have been described in our previous studies [23, 25, 29, 30].
Assessment of anhedonia
The Chinese version of SHAPS was used to evaluate anhedonia in the current study [31]. It contains 14 items covering four areas, interests and pastimes, social interactions, sensory experiences, and diet. Each item contains four possible responses, “Strongly agree”, “Agree”, “Disagree”, and “Strongly disagree”. The first two responses and the last two responses were then scored as ‘0’ and ‘1’ respectively to facilitate analysis. The total score after conversion > 5 represents the presence of severe anhedonia. Prior studies have demonstrated that the Chinese version of SHAPS exhibits good internal consistency in non-clinical samples (Cronbach’s α = 0.85) and clinical populations with psychiatric disorders (Cronbach’s α = 0.93), supporting its reliability as a robust tool for assessing anhedonia in Chinese cohorts [31].
MRI acquisition
Imaging data were collected using a 3.0-T scanner (Signa HDxt, GE Healthcare, USA) equipped with a standard birdcage head coil at the Magnetic Resonance Center, The First Affiliated Hospital, Zhejiang University School of Medicine. The detailed MRI scanning parameters were as follows, TR = 7.3 ms, TE = 3.0 ms, TI = 1100 ms, flip angle = 7°, FOV = 256 × 256 mm2, Matrix = 256 × 256, slice thickness = 1 mm, bandwidth = 31.25 KHz, NEX = 1, slices = 192. All subjects were instructed to remain in a supine position on the MRI scanning bed, with their eyes closed and awake.
MRI data preprocessing
Preprocessing is necessary to ensure high quality neuroimaging data suitable for further analysis. Images were first denoised to reduce noise while preserving fine structural details, the denoising step utilized an adaptive non-local means (NLM) filter [32], which dynamically adjusts to spatially heterogeneous noise distributions (both Gaussian and Rician) inherent in MR images. Spatial normalization was then performed using affine transformations to register the images to the MNI152 standard space (181 × 217 × 181 voxels at 1 mm × 1 mm × 1 mm), followed by intensity normalization to reduce intersubject variability in image intensity, and finally intracranial cavities (ICCs) were extracted.
Brain structure age estimation
To estimate BSA, T1w MRI images were first preprocessed and downscaled to reduce computational complexity and focus on localized brain regions. Overlapping sub-volumes were then extracted, and multiple U-Nets were trained to predict voxel-level brain age, with outputs aggregated to reconstruct a 3D brain age map. To mitigate intensity inhomogeneity artifacts arising from magnetic field variations or radiofrequency coil sensitivity, N4ITK bias correction was applied to the structural MRI data [33]. Subsequently, BSA and BSAGE values were computed using Assembly-Net-based segmentation. The procedures were as follows: (1) Downscaling of T1w MRI, a preprocessed T1w MRI in the MNI space of size 181 × 217 × 181 voxels at 1 mm3 was downscaled with a factor of 2 to the size of 91 × 109 × 91 voxels. We limited the receptive field of each model to a local brain region in order to force it to locally describe the brain age; (2) Sub-Volume Extraction and Distribution, from the downscaled images, k3 (k = 5) overlapping sub-volumes of size 32 × 48 × 32 voxels were extracted, and evenly distributed along the 3 image’s dimensions; (3) Training of U-Net Models, a total of m = k3 (m = 125) U-Nets were trained to predict voxel-level brain age using the extracted sub-volumes. During training, voxels inside the intracranial cavity (ICC) were assigned the subject’s chronological age as ground truth value, while all other voxels were set to zero. The training data was split into training/validation sets with a ratio of 0.8/0.2. During training, we used the mean absolute error (MAE) as loss function and SGD optimizer. The batch size was set to 8 and the training was terminated after 20 epochs without any improvement on validation loss. Finally, we employed different data augmentation techniques to alleviate the overfitting problem; (4) Reconstruction of 3D Brain Age Map, the m = 125 outputs were then used to reconstruct a 3D age map of size 91 × 109 × 91 voxels; (5) Bias Correction and Feature Calculation, each element was corrected for age using the simple method of Smith et al. [34]; (6) Acquisition of BSA features; (7) Computation of BSA and BSAGE Values, the BSA features were used as input metrics for the support vector regression (SVR) model (10-fold cross-validation) and then the BSA values were output with the help of an Assembly Net-based brain segmentation [35]. The BSAGE value was calculated by subtracting chronological age from BSA value. Finally, the MAE, R2, and Pearson’s r were calculated to evaluated the model’s performance, and the results demonstrated that the predicted regional brain ages were closely aligned with chronological ages in HCs, which indicated that the model performed well in predicting brain age. The detailed metrics were provided in Table S1.
Statistical analysis
The demographic and clinical data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 27.0 (SPSS Inc., Chicago, IL, USA). The chi-square test was employed to assess categorical variables, whereas one-way analyses of variance (ANOVAs) were utilized for continuous variables. The differences in BSAGE values among three groups were compared using analyses of covariance (ANCOVAs) and intergroup comparisons. All continuous variables were tested and confirmed to be normally distributed. Finally, partial correlation analyses using R 4.4.1 software were conducted to examine the correlation between altered BSAGE values and clinical features in MDD patients. Gender and education years were included as covariates in ANCOVAs and partial correlation analyses. The Benjamini-Hochberg method (BH) was used for false discovery rate (FDR) correction to adjust the p-values in multi-comparisons.
Classification analysis using support vector machine (SVM)
In this study, the SVM from the LIBSVM machine learning library (www.csie.ntu.edu.tw/~cjlin/libsvm), a frequently used classifier in neuroimaging research [36], was employed to evaluate the discriminative capability of abnormal BSAGE values in brain regions. Specifically, based on the results of ANCOVAs and post-hoc tests, we used the SVM multiple classification model to differentiate MDD patients with anhedonia from HCs. The specific steps were outlined below, (1) Normalization, the data matrix was normalized using the mapminmax function, scaling all feature values to a range between 0 and 1; (2) Train-Test Split, the total dataset was split into a training set and a testing set in a ratio of 0.8/0.2. A random permutation was applied to shuffle the data, ensuring randomness in sample selection; (3) SVM model, a radial basis function (Gaussian kernel) was used; (4) Hyperparameter Tuning, grid search and Cross-validation were performed to identify the optimal values for two key hyperparameters, the penalty parameter C (Box Constraint) and the kernel parameter γ (Gamma) [37]. Finally, the accuracy of the testing set and training set predictions were reported for evaluating the model performance, and the area under the curve (AUC) was calculated for evaluating the model classification ability.
Results
Demographic and clinical characteristics
As shown in Table 1, there was no significant difference in age (F = 2.419, p = 0.094), gender (χ2 = 0.402, p = 0.670), or education years (F = 0.470, p = 0.626) among three groups. No significant difference was observed between the two groups of MDD patients on means of onset age (t = 1.947, p = 0.056), episode times (t = 1.151, p = 0.254), illness duration (t = 0.306, p = 0.761), and HAMD-17 scores (t = −1.949, p = 0.055). As anticipated, the MDD-WA group showed higher scores of SHAPS when compared to the MDD-WoA group (t = 9.499, p < 0.001).
Altered brain structure age estimation among three groups
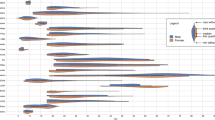
As shown in Table 2 and Fig. 1, the ANCOVAs revealed significant BSAGE differences among three groups in the bilateral putamen (PU) (Left: p = 0.037, Right: p = 0.035), left cerebellum (CB) (p = 0.037), left cuneus (CUN) (p = 0.037), left fusiform gyrus (FuG) (p = 0.02), left subcallosal area (SCA) (p = 0.031), left superior occipital gyrus (SOG) (p = 0.029), left triangular inferior frontal gyrus (IFG-Tri) (p = 0.031), right lateral ventricle (L-V) (p = 0.032), right superior frontal gyrus medial segment (SFG-SM) (p = 0.028), right opercular inferior frontal gyrus (IFG-Oper) (p = 0.032), right precuneus (pre-CUN) (p = 0.029), right posterior insula (INS-Post) (p = 0.032), and right superior temporal gyrus (STG) (p = 0.031). The p-values were then corrected by BH method and all the adjusted p-values were less than 0.05. Further intergroup comparisons showed that compared with HCs, the MDD-WA group showed significantly increased BSAGE values in all of the aforementioned brain regions, with mean BSAGE values ranged from +0.61 to +3.04, while the MDD-WoA group showed limited BSAGE increase in the CB, FuG, and SCA of left hemisphere only, with mean BSAGE values ranged from +2.32 to +5.39. Although the MDD-WA group showed a trend of increased BSAGE values in all of the aforementioned brain regions when compared to the MDD-WoA group, these differences were not statistically significant. As shown in Fig. 2, partial correlation analyses revealed significant negative correlations between onset ages and BSAGE values in the bilateral PU, right L-V, right SFG-SM, right IFG-Oper, left SOG, and left SCA in the MDD-WA group.
CB cerebellum, CUN cuneus, FuG fusiform gyrus, HCs healthy controls, IFG-Oper opercular inferior frontal gyrus, IFG-Tri triangular inferior frontal gyrus, INS-Post posterior insula, L-V lateral ventricle, L left, MDD-WA major depressive disorder with anhedonia, MDD-WoA major depressive disorder without anhedonia, pre-CUN precuneus, PU putamen, R right, SCA subcallosal area, SOG superior occipital gyrus, SFG-SM superior frontal gyrus medial segment, STG superior temporal gyrus.
CB cerebellum, CUN cuneus, FuG fusiform gyrus, HCs healthy controls, IFG-Oper opercular inferior frontal gyrus, IFG-Tri triangular inferior frontal gyrus, INS-Post posterior insula, L-V lateral ventricle, L left, MDD MDD-WA major depressive disorder with anhedonia, MDD-WoA major depressive disorder without anhedonia, pre-CUN precuneus, PU putamen, R right, SCA subcallosal area, SOG superior occipital gyrus, SFG-SM superior frontal gyrus medial segment, STG superior temporal gyrus.
SVM analysis
Since the major BSAGE differences in this study were found between the MDD-WA group and HCs, the BSAGE values of brain regions with significant group differences were then selected as the feature indicators, which were put into the SVM model to validate their ability in distinguishing MDD-WA from HCs. As shown in Fig. 3, the SVM analysis showed that the optimal parameters of the SVM model were, c = 0.01, γ = 7.20, the test set accuracy was 93.33%, the training set accuracy was 98.31%, and the AUC value was 0.944.
A, B showed the accuracy of distinguishing between HCs and the MDD-WA group in the training and test sets in the SVM model, C showed the SVM model determining the optimal parameter values for c and γ through network searching, D showed the ability of 14 brain regions to discriminate between the MDD-WA group and the HCs group verified by SVM modelling and the AUC calculated. AUC area under the curve, ROC receiver operating characteristic curve, SVM support vector machine.
Discussions
In this study, potential advanced brain aging was explored using an approach to estimate brain age at regional structural level in MDD patients stratifying groups by anhedonia. The BSAGE was calculated for each brain region to measure trends in brain structural aging. Compared to HCs, the MDD-WA group showed significantly increased BSAGE in the bilateral PU, left CB, left CUN, left Fu, left SCA, left SOG, left IFG-Tri, right L-V, right SFG-SM, right IFG-Oper, right pre-CU, right INS-Post, and right STG, and further SVM analysis also verified that BSAGE values in the aforementioned brain regions performed well in distinguishing MDD-WA from HCs. However, the MDD-WoA group showed limited increased BSAGE in the left CB, left FuG, and left SCA only, when comparing to HCs. In addition, partial correlation analyses showed significant negative associations between onset ages and BSAGE values in the bilateral PU, right L-V, right SFG-SM, right IFG-Oper, left SOG, and left SCA. In summary, the present findings demonstrated that the two groups of MDD patients exhibited segregated advanced brain aging pattens, providing empirical evidence for the biological basis of different clinical outcomes between MDD patients with and without anhedonia. Moreover, our findings provided insight into MDD heterogeneity in biological aspect and might represent a promising step to the development of personalized management for MDD.
The current study found significant advanced brain aging in the frontal-limbic areas in MDD patients with anhedonia. Previous evidence has shown that abnormalities in the frontal-limbic system are considered to be a more consistent outcome in MDD patients with anhedonia [25, 30, 38], as well as the fact that the frontal-limbic system is the most sensitive brain network to aging [39]. Based on the results of the current study, the PU is a part of the dorsal striatum that specializes in encoding reward-related feedback [40, 41], as well as linking incentives to action by engaging in the stimulus-response-reward learning process [42,43,44]. A prior study revealed a negative correlation between bilateral PU volume and the severity of anhedonia in adolescents with MDD [20]. Notably, when focusing on the structural impact of age on brain regions, the study reported an accelerated age-related reduction in the PU volume in MDD patients with anhedonia compared to HCs [13]. Moreover, our study found that another brain region closely associated with anhedonia was the right INS-Post, which was involved not only in emotion regulation and cognitive processes, but also in reward decision-making processes especially [45,46,47,48].
As expected, the present study found that MDD patients with anhedonia also exhibited advanced brain aging in the frontal lobe, including the left SCA, left IFG-Tri, right SFG-SM, and right IFG-Oper. To be specific, the IFG belongs to the executive control network involved in reward regulation [49], and the SFG is recognized as a key node in a cognitive emotion regulation network [50, 51]. Interestingly, a previous task-state functional MRI study demonstrated that anhedonia was negatively correlated with connectivity in fronto-temporal regions that process musical structures, including the bilateral IFG-Oper, IFG-Tri, and middle temporal gyrus (MTG)/STG [52]. Moreover, it has also been reported that in the IFG-Oper and SFG, anhedonia is associated with thinner cortical thickness [53, 54]. Recent study has consistently revealed that gray matter volume of the SFG in high-anhedonia group of MDD patients is significantly smaller than that in low-anhedonia group [55]. Notably, the SFG and IFG atrophy have also been recognized as the most replicated findings in brain aging [56, 57]. In addition, significantly increased BSAGE in the SCA, a part of the anterior cingulate gyrus, was found as well in the present study. Indeed, more direct evidence has suggested that the SCA hyperactivation potentially induces a lack of anticipatory and motivational anhedonia [58]. In summary, we found that MDD with anhedonia was associated with advanced brain aging mainly in the frontal-limbic system, which could be a neuropathological alteration specific to anhedonia.
In the temporal lobe, the current study found that compared with HCs, the MDD-WA group showed significantly increased BSAGE in the right STG and left FuG, while the MDD-WoA group also showed increased BSAGE in the left FuG. Morphologically, the STG is connected to the caudate nucleus [59], and volume reduction and abnormal activation of the STG during rewarded learning have been repeatedly reported in MDD [60, 61]. A prior study has reported that cortical thickness in the STG is negatively correlated with scores of anhedonia in MDD patients [53]. Equally important, abundant evidence has been found that remarkable age-associated cortical thinning may be more pronounced in the STG [56, 62, 63]. Besides, the FuG has been commonly recognized as a crucial structure for advanced vision, such as face perception and facial emotion processing [64,65,66]. It is noteworthy that abnormalities of the FuG in psychiatric patients with anhedonia have been widely reported in previous studies [67,68,69].
With regard to the occipital and parietal lobes, this study revealed significantly increased BSAGE in the left SOG, left CUN, and right pre-CUN in the MDD-WA group, while increased BSAGE in the left SOG only in the MDD-WoA group, relative to HCs. In general, the SOG, CUN and pre-CUN are considered to be the vital fields relevant to visual recognition circuits, and importantly, findings in recent years have gradually highlighted their important contributions to the reward circuitry [70,71,72]. Of note, a previous study has reported that the right amygdala-right pre-CUN connectivity is significantly correlated with physical and social trait anhedonia in MDD [73]. In addition, our findings indicated that compared to HCs, the MDD-WA group showed increased BSAGE in the left CB and right L-V, whereas the MDD-WoA group exhibited increased BSAGE in the left CB only. Anatomically, the CB may extend its function from traditionally coordinating movement to engaging in a wide range of cognitive processes and reward processing through connections to the basal ganglia and prefrontal lobes [74,75,76,77]. Moreover, ventricular enlargement, an important pathological alteration of brain aging [78], has been found in MDD patient recently [79, 80]. Importantly, evidence has suggested that lateral ventricle enlargement and reward processes are also closely related [81], which is consistent with our findings.
Notably, in the current exploration, partial correlation analyses revealed that BSAGE values in the bilateral PU, right L-V, right SFG-SM, right IFG-Oper, left SOG, and left SCA were inversely associated with onset ages in the MDD-WA group. The neurobiological mechanisms underlying MDD development may vary according to onset age [82]. It has been found that early-onset MDD patients exhibit more regional homogeneity (ReHo) abnormalities in the prefrontal lobes, especially in the right inferior frontal triangular gyrus, than late-onset MDD patients [83]. Together with our findings, the current evidence may demonstrate that the earlier age of onset corresponds with more severe brain impairments in MDD, and consequently, resulting in enlarged advanced brain aging.
Limitation
The present findings should be interpreted in light of several limitations. Firstly, these findings are cross-sectional and based on a small sample size. When age is included as a covariate, the inter-group differences in BSAGE become non-significant. This may be limited by the small sample size, and future multi-center, large-sample studies are needed to validate the longitudinal effects of anhedonia on accelerated aging. Secondly, the sample size of the current study was small, and the model probably still lacked stability in the SVM analyses, although a network search was used to determine the optimal parameters. Thirdly, the current study predicted structural brain age based mainly on volume changes of brain regions only. Brain age predictions based on structural and functional changes appear to more comprehensively and accurately respond to the effects of MDD or other psychiatric disorders on brain aging. Finally, the study focused solely on consummatory anhedonia as measured by the SHAPS, without distinguishing potential neural differences between consummatory and anticipatory anhedonia subtypes. Future investigations should employ multidimensional tools (e.g., the Temporal Experience of Pleasure Scale, TEPS) to disentangle subtype-specific contributions to advanced brain aging.
Conclusion
In conclusion, the current findings emphasize that MDD with anhedonia may exhibit more extensive advanced brain aging, primarily in the frontal-limbic system, temporal lobe and parietal lobe.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to privacy and ethical restrictions but are available from the corresponding author on reasonable request.
References
American Psychiatric Association D, American Psychiatric Association D. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American psychiatric association; 2013.
Shankman SA, Katz AC, DeLizza AA, Sarapas C, Gorka SM, Campbell ML, et al. The different facets of anhedonia and their associations with different psychopathologies. In: Anhedonia: A Comprehensive Handbook, Vol Ⅰ. 2014:3–22.
Lynch CJ, Gunning FM, Liston C. Causes and consequences of diagnostic heterogeneity in depression: paths to discovering novel biological depression subtypes. Biol Psychiatry. 2020;88:83–94.
Gong L, Yin Y, He C, Ye Q, Bai F, Yuan Y, et al. Disrupted reward circuits is associated with cognitive deficits and depression severity in major depressive disorder. J Psychiatr Res. 2017;84:9–17.
Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, Mors O, et al. Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med. 2012;42:967–80.
Buckner JD, Joiner TE, Pettit JW, Lewinsohn PM, Schmidt NB. Implications of the DSM’s emphasis on sadness and anhedonia in major depressive disorder. Psychiatry Res. 2008;159:25–30.
Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR Am J Neuroradiol. 2002;23:1327–33.
Peters R. Ageing and the brain. Postgrad Med J. 2006;82:84–8.
Wolkowitz OM, Reus VI, Mellon SH. Of sound mind and body: depression, disease, and accelerated aging. Dialogues Clin Neurosci. 2011;13:25–39.
Frodl TS, Koutsouleris N, Bottlender R, Born C, Jäger M, Scupin I, et al. Depression-related variation in brain morphology over 3 years: effects of stress? Arch Gen Psychiatry. 2008;65:1156–65.
Soriano-Mas C, Hernández-Ribas R, Pujol J, Urretavizcaya M, Deus J, Harrison BJ, et al. Cross-sectional and longitudinal assessment of structural brain alterations in melancholic depression. Biol Psychiatry. 2011;69:318–25.
Dunlop K, Victoria LW, Downar J, Gunning FM, Liston C. Accelerated brain aging predicts impulsivity and symptom severity in depression. Neuropsychopharmacology. 2021;46:911–9.
Sacchet MD, Camacho MC, Livermore EE, Thomas EAC, Gotlib IH. Accelerated aging of the putamen in patients with major depressive disorder. J Psychiatry Neurosci. 2017;42:164–71.
Yatham LN, Kapczinski F, Andreazza AC, Trevor Young L, Lam RW, Kauer-Sant’anna M. Accelerated age-related decrease in brain-derived neurotrophic factor levels in bipolar disorder. Int J Neuropsychopharmacol. 2009;12:137–9.
Brambilla P, Harenski K, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, et al. Differential effects of age on brain gray matter in bipolar patients and healthy individuals. Neuropsychobiology. 2001;43:242–7.
Liu C, Jing J, Jiang J, Wen W, Zhu W, Li Z, et al. Relationships between brain structure-function coupling in normal aging and cognition: a cross-ethnicity population-based study. Neuroimage. 2024;299:120847.
Han LKM, Dinga R, Hahn T, Ching CRK, Eyler LT, Aftanas L, et al. Brain aging in major depressive disorder: results from the ENIGMA major depressive disorder working group. Mol Psychiatry. 2021;26:5124–39.
Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–10.
Harvey PO, Pruessner J, Czechowska Y, Lepage M. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry. 2007;12:703.
Auerbach RP, Pisoni A, Bondy E, Kumar P, Stewart JG, Yendiki A, et al. Neuroanatomical prediction of anhedonia in adolescents. Neuropsychopharmacology. 2017;42:2087–95.
Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46:327–37.
Enneking V, Krüssel P, Zaremba D, Dohm K, Grotegerd D, Förster K, et al. Social anhedonia in major depressive disorder: a symptom-specific neuroimaging approach. Neuropsychopharmacology. 2019;44:883–9.
Lu S, Wu C, Jia L, Fang Z, Lu J, Mou T, et al. Increased plasma levels of IL-6 are associated with striatal structural atrophy in major depressive disorder patients with anhedonia. Front Psychiatry. 2022;13:1016735.
Wu C, Jia L, Mu Q, Fang Z, Hamoudi HJAS, Huang M, et al. Altered hippocampal subfield volumes in major depressive disorder with and without anhedonia. BMC Psychiatry. 2023;23:540.
Mu Q, Cui D, Zhang K, Ru Y, Wu C, Fang Z, et al. Volume changes of the subcortical limbic structures in major depressive disorder patients with and without anhedonia. Psychiatry Res Neuroimaging. 2023;336:111747.
Nguyen H-D, Clément M, Mansencal B, Coupé P. Brain structure ages-A new biomarker for multi-disease classification. Hum Brain Mapp. 2024;45:e26558.
Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure scale. Br J Psychiatry. 1995;167:99–103.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Lu S, Shao J, Feng Q, Wu C, Fang Z, Jia L, et al. Aberrant interhemispheric functional connectivity in major depressive disorder with and without anhedonia. BMC Psychiatry. 2022;22:688.
Fang Z, Mu Q, Wu C, Jia L, Wang Z, Hu S, et al. The impacts of anhedonia on brain functional alterations in patients with major depressive disorder: a resting-state functional magnetic resonance imaging study of regional homogeneity. J Psychiatr Res. 2022;156:84–90.
Liu WH, Wang LZ, Zhu YH, Li MH, Chan RCK. Clinical utility of the Snaith-Hamilton-Pleasure scale in the Chinese settings. BMC Psychiatry. 2012;12:184.
Manjón JV, Coupé P, Martí-Bonmatí L, Collins DL, Robles M. Adaptive non-local means denoising of MR images with spatially varying noise levels. J Magn Reson Imaging. 2010;31:192–203.
Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310–20.
Smith SM, Vidaurre D, Alfaro-Almagro F, Nichols TE, Miller KL. Estimation of brain age delta from brain imaging. Neuroimage. 2019;200:528–39.
Coupé P, Mansencal B, Clément M, Giraud R, Denis de Senneville B, Ta V-T, et al. AssemblyNet: a large ensemble of CNNs for 3D whole brain MRI segmentation. Neuroimage. 2020;219:117026.
Chang C, Lin CJ. LIBSVM-a library for support vector machines. ACM Trans Intell Syst Technol. 2011;2:1–27.
Kohavi R. A study of cross-validation and bootstrap for accuracy estimation and model selection. In: IJCAI international joint conference on artificial intelligence, USA: IEEE; 1995, p. 1137–43.
Wang X, Xia Y, Yan R, Wang H, Sun H, Huang Y, et al. The relationship between disrupted anhedonia-related circuitry and suicidal ideation in major depressive disorder: a network-based analysis. Neuroimage Clin. 2023;40:103512.
Briceño EM, Rapport LJ, Kassel MT, Bieliauskas LA, Zubieta J-K, Weisenbach SL, et al. Age and gender modulate the neural circuitry supporting facial emotion processing in adults with major depressive disorder. Am J Geriatr Psychiatry. 2015;23:304–13.
Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159.
Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35:1219–36.
Delgado MR. Reward-related responses in the human striatum. Ann N Y Acad Sci. 2007;1104:70–88.
O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–4.
Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26.
Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, Boucher O. Structure and function of the human insula. J Clin Neurophysiol. 2017;34:300–6.
Kirk U, Brown KW, Downar J. Adaptive neural reward processing during anticipation and receipt of monetary rewards in mindfulness meditators. Soc Cogn Affect Neurosci. 2015;10:752–9.
Von Siebenthal Z, Boucher O, Lazzouni L, Taylor V, Martinu K, Roy M, et al. Expected value and sensitivity to punishment modulate insular cortex activity during risky decision making. Sci Rep. 2020;10:11920.
Geugies H, Groenewold NA, Meurs M, Doornbos B, de Klerk-Sluis JM, van Eijndhoven P, et al. Decreased reward circuit connectivity during reward anticipation in major depression. Neuroimage Clin. 2022;36:103226.
Zhang Y, Dai C, Shao Y, Peng J, Yang Y, Hou Y. Decreased functional connectivity in the reward network and its relationship with negative emotional experience after total sleep deprivation. Front Neurol. 2021;12:641810.
Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, et al. Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci Biobehav Rev. 2014;45:202–11.
Morawetz C, Bode S, Derntl B, Heekeren HR. The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: a meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2017;72:111–28.
Young CB, Chen T, Nusslock R, Keller J, Schatzberg AF, Menon V. Anhedonia and general distress show dissociable ventromedial prefrontal cortex connectivity in major depressive disorder. Transl Psychiatry. 2016;6:e810.
Zhu X, Ward J, Cullen B, Lyall DM, Strawbridge RJ, Lyall LM, et al. Phenotypic and genetic associations between anhedonia and brain structure in UK Biobank. Transl Psychiatry. 2021;11:395.
Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–93.
Wang W, Zhou E, Nie Z, Deng Z, Gong Q, Ma S, et al. Exploring mechanisms of anhedonia in depression through neuroimaging and data-driven approaches. J Affect Disord. 2024;363:409–19.
Lemaitre H, Goldman AL, Sambataro F, Verchinski BA, Meyer-Lindenberg A, Weinberger DR, et al. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging. 2012;33:617.e611–19.
Ziegler G, Dahnke R, Jäncke L, Yotter RA, May A, Gaser C. Brain structural trajectories over the adult lifespan. Hum Brain Mapp. 2012;33:2377–89.
Alexander L, Gaskin PLR, Sawiak SJ, Fryer TD, Hong YT, Cockcroft GJ, et al. Fractionating blunted reward processing characteristic of anhedonia by over-activating primate subgenual anterior cingulate cortex. Neuron. 2019;101:307–20.e6.
Yeterian EH, Pandya DN. Corticostriatal connections of the superior temporal region in rhesus monkeys. J Comp Neurol. 1998;399:384–402.
Xia J, Fan J, Du H, Liu W, Li S, Zhu J, et al. Abnormal spontaneous neural activity in the medial prefrontal cortex and right superior temporal gyrus correlates with anhedonia severity in obsessive-compulsive disorder. J Affect Disord. 2019;259:47–55.
Yang X-h, Huang J, Lan Y, Zhu C-y, Liu X-q, Wang Y-f, et al. Diminished caudate and superior temporal gyrus responses to effort-based decision making in patients with first-episode major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:52–9.
Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–30.
Crivello F, Tzourio-Mazoyer N, Tzourio C, Mazoyer B. Longitudinal assessment of global and regional rate of grey matter atrophy in 1172 healthy older adults: modulation by sex and age. PLoS ONE. 2014;9:e114478.
Abramovitch A, Pizzagalli DA, Reuman L, Wilhelm S. Anhedonia in obsessive-compulsive disorder: beyond comorbid depression. Psychiatry Res. 2014;216:223–9.
Weiner KS, Zilles K. The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia. 2016;83:48–62.
Kawasaki H, Tsuchiya N, Kovach CK, Nourski KV, Oya H, Howard MA, et al. Processing of facial emotion in the human fusiform gyrus. J Cogn Neurosci. 2012;24:1358–70.
Yan C, Yang T, Yu Q-J, Jin Z, Cheung EFC, Liu X, et al. Rostral medial prefrontal dysfunctions and consummatory pleasure in schizophrenia: a meta-analysis of functional imaging studies. Psychiatry Res. 2015;231:187–96.
Du H, Xia J, Fan J, Gao F, Wang X, Han Y, et al. Spontaneous neural activity in the right fusiform gyrus and putamen is associated with consummatory anhedonia in obsessive compulsive disorder. Brain Imaging Behav. 2022;16:1708–20.
Uldall SW, Madsen KH, Siebner HR, Lanius R, Frewen P, Fischer E, et al. Processing of positive visual stimuli before and after symptoms provocation in posttraumatic stress disorder - a functional magnetic resonance imaging study of trauma-affected male refugees. Chronic Stress. 2020;4:2470547020917623.
Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, Dudas RB, et al. Abnormal frontostriatal activity during unexpected reward receipt in depression and schizophrenia: relationship to anhedonia. Neuropsychopharmacology. 2016;41:2001–10.
Wang X, Xia Y, Yan R, Sun H, Huang Y, Xia Q, et al. Sex differences in anhedonia in bipolar depression: a resting-state fMRI study. Eur Arch Psychiatry Clin Neurosci. 2024;275:1087–97.
Loeffler LAK, Radke S, Habel U, Ciric R, Satterthwaite TD, Schneider F, et al. The regulation of positive and negative emotions through instructed causal attributions in lifetime depression - a functional magnetic resonance imaging study. Neuroimage Clin. 2018;20:1233–45.
Fan J, Gao F, Wang X, Liu Q, Xia J, Han Y, et al. Right amygdala-right precuneus connectivity is associated with childhood trauma in major depression patients and healthy controls. Soc Cogn Affect Neurosci. 2023;18:nsac064.
Carta I, Chen CH, Schott AL, Dorizan S, Khodakhah K. Cerebellar modulation of the reward circuitry and social behavior. Science. 2019;363:eaav0581.
Raymond JL. Research on the cerebellum yields rewards. Nature. 2020;579:202–3.
Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013;17:241–54.
Bostan AC, Strick PL. The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci. 2018;19:338–50.
McNair BD, Yusifov A, Thornburg JP, Hoopes CR, Satyanarayana SB, Roy T, et al. Molecular and physiological mechanisms of aging are distinct in the cardiac right and left ventricles. Aging Cell. 2024;24:e14339.
Bradley KAL, Mao X, Case JAC, Kang G, Shungu DC, Gabbay V. Increased ventricular cerebrospinal fluid lactate in depressed adolescents. Eur Psychiatry. 2016;32:1–8.
Bravi B, Paolini M, Maccario M, Milano C, Raffaelli L, Melloni EMT et al. Abnormal choroid plexus, hippocampus, and lateral ventricles volumes as markers of treatment-resistant major depressive disorder. Psychiatry Clin Neurosci. 2024. https://doi.org/10.1111/pcn.13764.
Robles CF, Johnson AW. Disruptions in effort-based decision-making and consummatory behavior following antagonism of the dopamine D2 receptor. Behav Brain Res. 2017;320:431–9.
Chen JD, Liu F, Xun GL, Chen HF, Hu MR, Guo XF, et al. Early and late onset, first-episode, treatment-naive depression: same clinical symptoms, different regional neural activities. J Affect Disord. 2012;143:56–63.
Zhang Z, Chen Y, Wei W, Yang X, Meng Y, Yu H, et al. Changes in regional homogeneity of medication-free major depressive disorder patients with different onset ages. Front Psychiatry. 2021;12:713614.
Acknowledgements
All authors would sincerely thank all participants who took part in this study, and the experts at the Magnetic Resonance Center of The First Affiliated Hospital, Zhejiang University School of Medicine for providing scan time and technical assistant.
Funding
This study was supported by the STI2030-Major Projects (2021ZD0200600 to Shaojia Lu) and the National Natural Science Foundation of China (82071521 to Shaojia Lu).
Author information
Authors and Affiliations
Contributions
QLM wrote the first draft of the manuscript and conducted the statistical analyses. SHH and MLH recruited the sample. YC and YWX finished the clinical assessments. DC conducted the statistical analyses. KJZ revised the manuscript (English editing). SJL and PZ also designed the study and had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to and have approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the local medical ethics committee of The First Affiliated Hospital, Zhejiang University School of Medicine. All subjects provided written informed consent prior to participation. All methods were carried out in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mu, Q., Zhang, K., Chen, Y. et al. Altered brain structure age gap estimation in major depressive disorder patients with and without anhedonia: a machine learning-based study. Transl Psychiatry 15, 309 (2025). https://doi.org/10.1038/s41398-025-03555-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-025-03555-5