Abstract
Cognitive deficits are prevalent in major depressive disorder (MDD). Given that the dorsolateral prefrontal cortex (DLPFC) is a crucial region within the executive control network, its activity and functional connectivity (FC) may serve as potential indicators of antidepressant response. This prospective cohort study recruited 115 MDD patients and 43 healthy controls. Psychological assessments, electroencephalogram and event-related potential recordings were performed at baseline and 1 week after venlafaxine treatment, with a 12-week follow-up. Independent sample t-tests and Mann-Whitney U tests analyzed group differences, while linear mixed-effects models and logistic regression evaluated associations between DLPFC activity/FC changes and clinical outcomes. The MDD group showed significantly reduced right DLPFC current density during the N2 time window evoked by oddball stimuli (p = 0.028). Higher right DLPFC current density during the N2 time window was correlated with lower HAMD-21 scores one week after treatment (p = 0.041, n = 46). Furthermore, an early increase predicted remission at week 12 (p = 0.005). Decreased beta-band FC between the left DLPFC and both side of posterior cingulate cortex (PCC) (left: p = 0.003; right: p = 0.004) were correlated with lower HAMD-21 scores (n = 71). Moreover, an early reduction in these connectivity measures (left: odds ratio (OR) = 0.534, 95% confidence interval (CI): 0.297–0.972, p = 0.036; right: OR = 0.533, 95% CI: 0.299–0.950, p = 0.033) predicted remission at week 12. Early changes in DLPFC activity and FC may serve as biomarkers for monitoring treatment efficacy and predicting clinical outcomes, informing personalized treatment approaches.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is a widespread and debilitating condition that profoundly affects quality of life [1]. Due to its heterogeneous deficits in emotional, cognitive, and motor functioning, the effectiveness of standard antidepressant treatment is limited, with less than half of MDD patients achieving remission [1,2,3]. Current treatment options, including medications, psychotherapies, and somatic therapies, are predominantly selected based on their tolerability, leading to uncertainty about which patients will benefit from such treatment [4, 5]. Due to the typical 4 to 8-week delay in antidepressant efficacy, many patients must undergo several rounds of treatment trials before identify an effective option often experiencing suboptimal or delayed care until the appropriate therapy is found [6]. These challenges have generated significant interest in identifying biomarkers that could assist in medication selection or facilitate the early termination of ineffective trials.
Recent conceptualizations consider MDD as a systems-level disorder arising from dysregulation among large-scale functional brain networks [7]. Altered emotion regulation and deficits in cognitive control have been reported in individuals with MDD [8]. The dorsolateral prefrontal cortex (DLPFC), an essential region of the executive control network (ECN), plays a vital role in emotion processing and cognitive control through its functional connectivity (FC) with other networks like the default mode network (DMN) and salience network (SN) [9]. Neuroimaging studies have reported disrupted DLPFC connectivity patterns in MDD, particularly altered inter-network communication that may underlie the cognitive and emotional deficits observed in this disorder [10]. Although there is some inconsistency, most functional magnetic resonance imaging (fMRI) findings support antidepressant treatment is associated with change in DLPFC activity in individuals with MDD, mainly characterized by increased activity [11,12,13,14,15]. For instance, one study demonstrated increased DLPFC activity during resting-state fMRI within days of treatment initiation [12], while another task-based fMRI study revealed heightened DLPFC activity in response to unattended fear-related stimuli in patients with MDD at week 8 [13]. However, other studies showed reduced right DLPFC activity during the N-back task after vortioxetine treatment [14], and reduced DLPFC activity during the Go/NoGo task in remitters compared to non-remitters [15]. Additionally, antidepressant treatment has been reported to be associated with changes in DLPFC volumes and DLPFC-seed based FC based on fMRI studies [16].
Previous research has primarily focused on fMRI, which has proven invaluable in identifying structural and functional abnormalities in MDD [17]. However, the high temporal resolution and direct measurement of neuronal activity offered by electroencephalogram (EEG) could provide complementary insights into the rapid neural dynamics and network interactions underlying the cognitive and emotional dysregulation observed in MDD [18, 19]. In addition, most studies predicting the efficacy of depression treatment based on neuroimaging have been conducted at baseline [17, 20]. Nevertheless, treatment efficacy may be more closely associated with dynamic changes in brain function during treatment, which cannot be captured by a single baseline imaging session [16]. EEG signals have been shown to change within days after initiating antidepressant medication. By tracking these changes over time, researchers can investigate the temporal dynamics and trajectories of neural activity and FC patterns related to symptom improvement or treatment response, potentially identifying critical time windows or stages in the recovery process predictive of treatment outcomes [21, 22].
To address the limitations of previous research, this longitudinal cohort study enrolled both patients with MDD and healthy controls (HCs). Psychological assessments, EEG, and event-related potential (ERP) data were collected at baseline and 1 week after initiating antidepressant treatment, with a 12-week follow-up. We hypothesized that: (1) patients with MDD would display abnormal DLPFC activity compared to HCs under the visual oddball paradigm; (2) DLPFC activity might change after treatment, potentially correlating with prognosis; and (3) changes in DLPFC FC with the DMN and SN would correlate with depressive symptoms and treatment prognosis. The posterior cingulate cortex (PCC) and insula cortex (IC) were selected as regions of interest (ROIs) representing the DMN and SN, respectively. The study aimed to determine the stability or recovery signs in DLPFC activity and DLPFC-seed based FC, shedding light on the impact of therapeutic interventions on brain activity and networks. Additionally, it sought to enhance understanding of the cognition-depression relationship and brain functional changes during treatment, providing insights into individual treatment responses.
Materials and methods
Study design
All patients with MDD were treated with venlafaxine for 12 weeks. Depression severity was evaluated utilizing the 21-item Hamilton Depression Rating Scale (HAMD-21) [23] at baseline, week 1 and week 12. Clinical outcomes were evaluated by Visual Analogue Scale (VAS) and HAMD-21 scores. The criteria for clinical outcomes were defined as follows: (1) Remission: VAS score ≥ 5 and HAMD-21 score < 7 at week 12; (2) Response: VAS score ≥ 5 and a ≥ 50% reduction in HAMD-21 scores from baseline to week 12; (3) Non-response: VAS score < 5 or a < 50% reduction in HAMD-21 scores from baseline to week 12 [23]. EEG and ERP recordings were conducted at baseline and week 1.
Participants
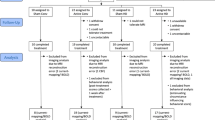
This prospective longitudinal cohort study enrolled 115 untreated patients with MDD at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology from March 2022 to September 2023. The inclusion criteria were as follows: (1) age 18-65 years; (2) meeting the diagnostic criteria for depressive disorder as outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V); (3) HAMD-21 score ≥17; (4) educational level of elementary school or higher; (5) right-handedness; and (6) native Chinese speaker. Exclusion criteria included: (1) history of mental disorders such as schizophrenia, bipolar disorder, severe depressive disorder with suicidal tendencies, alcoholism, or substance abuse; (2) presence of severe or unstable physical disorders; (3) evidence of current or prior head injury, central nervous system disease, or other disorders according to the International Classification of Diseases, Tenth Revision (ICD-10); (4) contraindications to antidepressants; (5) history of antidepressants or long-acting antipsychotic injections within the past month; (6) evidence of aphasia, deafness, blindness, or cognitive impairment; and (7) breastfeeding, pregnancy, or planning pregnancy during the trial. Additionally, 43 HCs matched for age, gender, and education level were recruited. Exclusion criteria for the HCs were the same as mentioned earlier, with the additional of no history of any psychiatric illness. The sample size in this study was determined by the availability of eligible patients within the recruitment period. The participant enrollment flowchart is presented in Fig. 1.
The informed consent was obtained in accordance with the Declaration of Helsinki. This study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ-IRB20220205). The registration number for this trial was ChiCTR2200057365. The URL of the publicly accessible registered website is: http://www.chictr.org.cn/.
EEG recording and preprocessing
Rest-state EEG signals, involving 12-min trials with eyes-open and eyes-closed conditions, were recorded in a dimly illuminated, electrically shielded, and acoustically isolated chamber utilizing a 128-channel EEG system (BrainVision Recorder software, Brain Products GmbH, Germany). Participants maintained stillness, minimizing blinks and eye movements, while fixating on a central cross during eyes-open conditions. Electrodes were positioned according to the standard international 10/5 system at a sampling frequency of 1000 Hz, with the FCz electrode serving as the default reference electrode. Electrode impedance was meticulously maintained below 20 KΩ during recording. EEG data preprocessing was performed using BrainVision Analyzer software (version 2.2, Brain Products GmbH, Germany) (see Supplemental Methods).
Visual oddball paradigm and event-related potential
The visual oddball paradigm was conducted in a soundproof chamber. It consisted of two visual stimuli: a red car as the oddball stimulus and a blue car as the standard stimulus. The experimental process is illustrated in Fig. 2A and Supplemental Methods. In the study, the ERP analysis focused primarily on the stimulus-associated N1, P2, N2, and P3 components. The selection of time windows and electrodes was guided by a prior study [24], as shown in Supplemental Table S1.
Source localization analyses
Source localization analyses were conducted using the built-in low resolution electromagnetic tomography analysis (LORETA) within the BrainVision Analyzer software. LORETA calculates the current density (i.e., the amount of electrical current flowing through a volume; unit: μA/mm2) of intracranial sources responsible for scalp-recorded EEG signals [25]. The study focused on the current density within the DLPFC during the visual oddball paradigm. The DLPFC ROI was defined by the following coordinates (x, y, z): left DLPFC (−45, 32, 20) and right DLPFC (47, 32, 19).
Region-of-interest selection
To examine the longitudinal changes in FC between the DLPFC and other brain networks (DMN and SN), and their association with alternations in depression symptoms, we identified the PCC and IC as ROIs for DMN and SN, respectively, according to findings by Whitton et al. [22]. The coordinates (x, y, z) for the ROIs were as follows: left PCC (−7, −48, 33), right PCC (7, −47, 33), left IC (−38, −4, 5), and right IC (40, −5, 7). The locations of the DLPFC, PCC and IC ROIs are showed in Fig. 3A. Finally, as described above, the intracortical current density within each ROI was computed using LORETA.
Source-based functional connectivity
Given the practical constraints inherent in measuring FC using scalp electrodes, which are vulnerable to volume conduction effects, this study employed the phase lag index (PLI) to compute FC between sources [26]. As a phase-based connectivity analysis method, PLI relies on the distribution of phase angle differences between two sources [26]. The underlying concept is that oscillatory sequences synchronize in phase when neural clusters are functionally coupled. PLI was calculated for the delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz) and beta (12–30 Hz) frequency bands.
Statistical analysis
The general characteristics of the study population were described as percentages for categorical variables, means ± standard deviations (SDs) for continuous variables with normal distributions, and medians (interquartile ranges, IQRs) for continuous variables without normal distributions. Z-score standardization was performed for DLPFC current density and DLPFC-seed based FC due to their relatively small and scattered nature. Group comparisons were conducted using chi-square tests, independent samples t-tests, and Wilcoxon rank-sum tests.
All statistical analyses were performed using SPSS for Windows, version 26.0, with two- tailed tests set at a significance level of α = 0.05. Pearson correlation analysis was employed to examine the bivariate associations between the changes in DLPFC current density and DLPFC-seed based FC, and the changes in HAMD-21 scores.
To investigate whether baseline measures could predict long-term treatment outcomes, a linear regression model was employed. Variables were entered into the model in the following steps: Step1: Age, gender, and baseline HAMD-21 score included as control variables. Step 2: DLPFC activity and DLPFC-seed based FC, were entered as the independent variables. Step 3: HAMD-21 at week 12 were used as dependent variable.
The changes of these variables were calculated by subtracting the week 1 values from the baseline. To further investigate these associations while accounting for multiple factors, a linear mixed-effects model (LMM) based on maximum likelihood estimation (MLE) was conducted. LME, a statistical model suitable for hierarchical or repeated measures data with fixed and random effects, allows for more accurate characterization of variability without distribution restrictions and is widely utilized across various fields [27]. In the current study, participants were treated as random effects, while age, gender and DLPFC current density or DLPFC-seed based FC were designated as fixed effects, with HAMD-21 score as the dependent variable.
Binary logistic regression analysis was utilized to examine the association between early changes in DLPFC current density and DLPFC-seed based FC, and clinical outcomes at week 12, with corrected odds ratio (OR) and its corresponding 95% confidence interval (CI) serving as the effect size. The variables were entered into the regression model in the following steps: Step1: Age, sex, and baseline HAMD-21 score included as control variables. Step 2: Early change in DLPFC activity and DLPFC-seed based FC, calculated by subtracting the week 1 values from baseline, were entered as the independent variable. Step 3: Clinical outcomes (remission or no-remission) at week 12 were used as dependent variables.
Results
Baseline characteristics
A total of 69 patients completed two ERP recordings, out of which 46 patients participated in the follow-up stage. Additionally, 93 patients underwent two EEG recordings, with 71 of these patients completing the follow-up stage. The detailed screening process is depicted in Fig. 1.
As shown in Table 1, there were no significant differences between the MDD group and the HC group in terms of age, gender, education level, and marriage status (p > 0.05). After treatment, 58.7% of patients who completed ERP and 64.8% of patients who completed EEG met the remission criteria. No significant differences were observed between remitters and non-remitters concerning age, gender, and baseline HAMD-21 scores (p > 0.05) (Supplemental Table S2).
DLPFC activity difference in the MDD group and the HC group
As showed in Supplemental Table S3, the current density of the right DLPFC during the N2 ((−5.46) × 10^(−5) μA/mm2 vs. 60.28 × 10^(−5) μA/mm2, p = 0.028) and P3 (14.68 × 10^(−5) μA/mm2 vs. 60.58 × 10^(−5) μA/mm2, p = 0.037) time windows under the oddball stimulus were significantly lower in the MDD group compared to the HC group. Liner regression model was used to examine whether baseline DLPFC activity and DLFPC seed-based FC could predict HAMD-21 scores at week 12. However, no significant results were found after controlling for age, gender and baseline HAMD-21 scores, as shown in Tables S4–5.
Comparison of DLPFC activity and DLFPC seed-based FC during the first week of treatment in MDD group
As shown in Supplemental Table S6, the current density of the right DLPFC during the N1 ((−21.54) × 10^(−5) μA/mm2 vs. 21.71 × 10^(−5) μA/mm2, P = 0.015 and P2 ((−8.34) × 10^(−5) μA/mm2 vs. 28.89 × 10^(−5) μA/mm2, p = 0.006) time windows under the oddball stimulus were significantly lower at week 1 compared to the baseline. In addition, theta-band FC between the right DLPFC and the left IC significantly decreased at week 1 compared to baseline (p = 0.028) (Supplemental Table S7). Besides, theta-band FC between the left DLPFC and the right PCC significantly increased (p = 0.044) (Table S8).
DLPFC activity and DLFPC seed-based FC correlated with HAMD-21 scores
Pearson correlation analysis was performed to assess the associations between DLPFC activity, DLPFC seed-based FC, and depression symptoms across 1 week of treatment. Figure 2B shows the unadjusted correlations between the changes in right DLPFC current density during the N1 (r = −0.464, p = 0.001), P2 (r = −0.424, p = 0.003) and N2 (r = −0.348, p = 0.018) time windows were negatively associated with change in HAMD-21scores. Additionally, as shown in Fig. 3B, the changes in theta-band FC between the left DLPFC and both the left IC (r = −0.340, p = 0.004) and the right IC (r = −0.285, p = 0.015) exhibited negative associations with change in HAMD-21 scores, while change in alpha-band FC between the right DLPFC and the right IC showed a positive association with change in HAMD-21 scores (r = 0.382, p = 0.001).
To further assess the relationships, LMM were performed to control for age and gender. Heightened right DLPFC current density during the N1 (β = −1.447, 95% CI: −2.524-(−0.369), p = 0.009), P2 (β = −1.260, 95% CI: −2.416-(−0.103), p = 0.033), and N2 (β = −1.243, 95% CI: −2.433-(−0.054), p = 0.041) time windows under the oddball stimuli were correlated with lower HAMD-21 scores across 1 week of treatment (Table S9). Moreover, as reported in Table S10, elevated theta-band FC between the left DLPFC and both the left PCC (β = 1.001, 95% CI: 0.032–1.970, p = 0.043) and right PCC (β = 1.289, 95% CI: 0.384–2.194, p = 0.006), alpha-band FC between the right DLPFC and right IC (β = 1.152, 95% CI: 0.179–2.125, p = 0.021), and beta-band FC between the left DLPFC and both the left PCC (β = 1.326, 95% CI: 0.446–2.207, p = 0.003) and right PCC (β = 1.415, 95% CI: 0.473–2.357, p = 0.004) were correlated with higher HAMD-21 scores across 1 week of treatment. Conversely, theta-band FC between the left DLPFC and right IC were correlated with lower HAMD-21 scores across 1 week of treatment (β = −1.154, 95% CI: −2.094-(−0.214), p = 0.017).
DLPFC activity and DLFPC seed-based FC predicted remission status
Binary logistic regression analysis was conducted to examine the associations between changes in DLPFC activity and DLPFC seed-based FC from baseline to week 1, and remission status at week 12. As demonstrated in Supplemental Table S11, after controlling for age, gender, and baseline HAMD-21 scores, increases in right DLPFC current density during the N1 (OR = 4.295, 95% CI: 1.437–12.833, p = 0.009), P2 (OR = 4.748, 95% CI: 1.527–14.763, p = 0.007), N2 (OR = 5.235, 95% CI: 1.638–16.730, p = 0.005), and P3 (OR = 4.499, 95% CI: 1.457–13.893, p = 0.009) time windows under the oddball stimuli predicted a higher likelihood of achieving remission at week 12. As illustrated in Fig. 4A, changes in right DLPFC current density during these time windows were significantly higher in remitters compared to non-remitters.
A Comparison of changes in right DLPFC current density (μA/mm2) during different time windows under the oddball stimulus between remitters and non-remitters; B Comparison of changes in beta-band FC between the left DLPFC and both the left posterior cingulate cortex (PCC) and right PCC between remitters and non-remitters.
Moreover, as reported in Supplemental Table S12, after controlling for age, gender, and baseline HAMD-21 scores, reductions in beta-band FC between the left DLPFC and both the left PCC (OR = 0.534, 95% CI: 0.297–0.972, p = 0.036) and right PCC (OR = 0.533, 95% CI: 0.299–0.950, p = 0.033) from baseline to week 1 were predictive of a greater probability of achieving remission at week 12. As depicted in Fig. 4B, alterations in beta-band FC between the left DLPFC and both the left PCC and the right PCC were significantly lower in remitters compared to non-remitters.
Discussion
Numerous studies have emphasized the aberrant nature of the DLPFC and its FC with large-scale brain networks in MDD. However, prior research has predominantly focused on MRI methodologies, with EEG and ERP receiving limited attention. Notably, baseline resting-state EEG may serve as a trait-like biomarker for predicting treatment response and identifying depression subtypes, while early EEG changes following antidepressant initiation likely reflect state-dependent alterations in brain activity and connectivity. These state-dependent biomarkers may be more sensitive to the specific neural changes induced by the drug, potentially making them better predictors of treatment response compared to trait-like baseline measures.
The present cohort study aimed to better elucidate the neural mechanisms underlying MDD by leveraging the ability of high-density EEG to directly measure neural activity and to assess the early dynamic changes in brain function associated with treatment response. This approach allows for the identification of state-dependent biomarkers that may be more closely linked to the specific neural changes induced by antidepressant medication, in contrast to the more trait-like baseline measures examined in prior research.
Compared to HCs, patients with MDD exhibited significantly diminished current density in the right DLPFC during the N2 and P3 time windows under the oddball stimulus. These altered activation patterns may reflect impaired attentional modulation and resource allocation when processing rare stimuli, aligning with previous findings from oddball paradigm studies [28, 29], which are widely used to examine attentional and executive control mechanisms. It is consistent with previous findings indicating reduced right hemispheric involvement in emotional processing in MDD, our study extends this concept by demonstrating right-lateralized deficits specifically within task-evoked DLPFC ERP components, providing source-localized evidence under cognitive demands. Importantly, heightened right DLPFC current density during the N1, P2 and N2 time windows were correlated with lower HAMD-21 scores across 1 week of treatment. Furthermore, remitters showed a significantly greater increase in the right DLPFC current density during the N1, P2, N2 and P3 time windows compared to non-remitters. These findings indicate that DLPFC activity patterns are closely linked to depressive symptoms, and increased current density in certain time windows is associated with better treatment outcomes for MDD patients. The N1 and P2 components represent early sensory processes elicited by stimuli, including detection, initial perceptual characterization, and inhibition [30,31,32,33]. In contrast, the N2 and P3 components constitute mid- to late-latency components evoked by oddball stimuli, reflecting automatic attentional mechanisms and cognitive control [34]. The observed changes in these ERP components provide insights into the cognitive and neural processes that are impaired in MDD and those that are involved in the recovery or remission of depressive symptoms.
Prior studies on repetitive transcranial magnetic stimulation (rTMS) or electrical stimulation to activate the DLPFC has shown promise for ameliorating clinical symptoms in patients with MDD, further indicating that increasing DLPFC activity may help alleviate depressive symptoms [35, 36]. However, the discovery of increased DLPFC activity in remitters during the visual oddball paradigm in the present study contradicts findings from a previous task-based fMRI study, which reported decreased DLPFC activity in remitters during the Go/NoGo paradigm [15]. This discrepancy may reflect divergent cognitive and neurobiological processes across different task paradigms. Notably, ERPs can capture distinct stages of cognitive processing, such as early attentional orienting and later cognitive control, whereas fMRI primarily reflects average activity levels across the entire task duration, making it difficult to differentiate activity patterns at different cognitive stages [37, 38]. Additionally, the differences in the temporal measurement time windows of ERPs and fMRI during task performance may also contribute to the observed discrepancies.
Further investigating the influence of DLPFC activity on the DMN and SN, we discovered that higher theta-band FC between the left DLPFC and the right IC, as well as lower alpha-band FC between the right DLPFC and right IC, correlated with lower HAMD-21 scores after one week of treatment. This finding suggests that enhanced communication between the DLPFC, which is associated with cognitive control processes, and the right IC, a key node in the SN responsible for processing salient stimuli and regulating attention, may facilitate more rapid alleviation of depressive symptoms during the initial stages of treatment. This result is partly in line with prior fMRI studies which have documented decreased FC between the ECN and SN in patients with MDD [11]. Additionally, a body of fMRI literature has consistently indicated that MDD primarily manifests as dysfunctions within the prefrontal-limbic circuitry [9, 39, 40]. Notably, the SN has been shown to exhibit increased connectivity with the DMN but reduced connectivity with the ECN individuals with MDD [11]. This pattern may reflect an over-allocation of attention towards internal negative information coupled with a neglect of external stimuli, as well as reduced recruitment of ECN resources for cognitive control processes [11].
Finally, the present study observed that higher FC in the theta-band and beta-band between the left DLPFC and both the left PCC and right PCC correlated with higher HAMD-21 scores over 1 week of treatment. Furthermore, an early decrease in the beta-band FC between the left DLPFC and both the left PCC and right PCC predicted a greater probability of achieving remission at week 12. These findings align with previous fMRI studies indicating increased FC between the ECN and DMN in MDD [11, 41, 42]. Reduced DLPFC activity may impair cognitive control and emotion regulation abilities in MDD patients, the increased FC between the DLPFC and DMN regions in the beta-band may be a compensatory mechanism adopted by the brain to cope with the reduced DLPFC function, aiming to maintain the regulation of DMN activity [43, 44]. However, further research is needed to validate this hypothesized mechanism.
Strengths and limitations
This study offers several key strengths compared to prior research in this area. Firstly, this prospective longitudinal study encompassed both MDD patients and HCs, and collected psychological assessment, EEG and ERP data at baseline and week 1 post-treatment, enabling continuous observation of dynamic alternations in brain network and depressive symptoms, as well as evolving associations between brain networks alterations and symptoms progression. Secondly, by examining changes in brain activity states and FC before and after treatment, this study reduces the impact of individual variances and the inherent diversity of MDD symptomatology. Thirdly, the concurrent examination of DLPFC activity and DLPFC seed-based FC provided further insights into the pathophysiological mechanisms of MDD. Fourthly, unlike previous research predominantly relying on MRI, this study utilized EEG, offering several advantages, including higher temporal resolution, lower cost, greater portability, and insensitivity to motion artifacts. Additionally, the source seed-based FC analysis of the EEG data enhanced interpretability compared to direct calculations between scalp channels, facilitating a more comprehensive understanding of brain areas interactions. Lastly, and importantly, this study revealed that the EEG changes observed soon after initiating antidepressant medication reflect state-dependent modifications in brain function and connectivity. By investigating early treatment response and long-term remission, this study provides crucial insights that have the potential to optimize treatment selection and outcomes for MDD patients, thereby reducing morbidity and economic burden by facilitating timely transitions to more effective interventions when initial antidepressants are predicted to have limited benefit.
However, future research should address several limitations. Firstly, the single-center recruitment and relatively small sample size necessitate further validation. Secondly, the exclusive use of SNRI medications limits generalizability. Additionally, while EEG offers high temporal resolution, cost-effectiveness, and portability compared to MRI, its spatial resolution remains limited. Finally, individual differences in symptoms and treatment response should be carefully considered and controlled for in future research.
Conclusions
In summary, this study indicates that the activity of the DLPFC and its FC with the DMN and SN are associated with overall depression severity, as measured by total HAMD-21 scores. Specifically, for patients with MDD receiving treatment with venlafaxine, early alterations in DLPFC activity and its beta-band FC with the DMN could potentially act as predictive biomarkers of improvement in depressive symptoms. Utilizing these early changes detected via EEG during the treatment course may reflect individual variations in neural response to this specific antidepressant medication, enabling more tailored predictions of treatment outcomes based on each patient’s unique neural pattern changes.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Weitz ES, Hollon SD, Twisk J, van Straten A, Huibers MJ, David D, et al. Baseline depression severity as moderator of depression outcomes between cognitive behavioral therapy vs pharmacotherapy: an individual patient data meta-analysis. JAMA Psychiatry. 2015;72:1102–9.
Dunlop BW, Kelley ME, Aponte-Rivera V, Mletzko-Crowe T, Kinkead B, Ritchie JC, et al. Effects of patient preferences on outcomes in the Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT) Study. Am J Psychiatry. 2017;174:546–56.
Lee J, Gierc M, Vila-Rodriguez F, Puterman E, Faulkner G. Efficacy of exercise combined with standard treatment for depression compared to standard treatment alone: a systematic review and meta-analysis of randomized controlled trials. J Affect Disord. 2021;295:1494–511.
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40.
Stimpson N, Agrawal N, Lewis G. Randomised controlled trials investigating pharmacological and psychological interventions for treatment-refractory depression. Systematic review. Br J Psychiatry. 2002;181:284–94.
Oliva V, Possidente C, De Prisco M, Fico G, Anmella G, Hidalgo-Mazzei D, et al. Pharmacological treatments for psychotic depression: a systematic review and network meta-analysis. Lancet Psychiatry. 2024;11:210–20.
Yang Z, Jian L, Qiu H, Zhang C, Cheng S, Ji J, et al. Understanding complex functional wiring patterns in major depressive disorder through brain functional connectome. Transl Psychiatry. 2021;11:526.
Heller AS, Johnstone T, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Increased prefrontal cortex activity during negative emotion regulation as a predictor of depression symptom severity trajectory over 6 months. JAMA Psychiatry. 2013;70:1181–9.
Gong Q, He Y. Depression, neuroimaging and connectomics: a selective overview. Biol Psychiatry. 2015;77:223–35.
Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38.
Yu M, Linn KA, Shinohara RT, Oathes DJ, Cook PA, Duprat R, et al. Childhood trauma history is linked to abnormal brain connectivity in major depression. Proc Natl Acad Sci USA. 2019;116:8582–90.
Meyer BM, Rabl U, Huemer J, Bartova L, Kalcher K, Provenzano J, et al. Prefrontal networks dynamically related to recovery from major depressive disorder: a longitudinal pharmacological fMRI study. Transl Psychiatry. 2019;9:64.
Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ, et al. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord. 2009;112:206–11.
Smith J, Browning M, Conen S, Smallman R, Buchbjerg J, Larsen KG, et al. Vortioxetine reduces BOLD signal during performance of the N-back working memory task: a randomised neuroimaging trial in remitted depressed patients and healthy controls. Mol Psychiatry. 2018;23:1127–33.
Gyurak A, Patenaude B, Korgaonkar MS, Grieve SM, Williams LM, Etkin A. Frontoparietal activation during response inhibition predicts remission to antidepressants in patients with major depression. Biol Psychiatry. 2016;79:274–81.
Lee KH, Shin J, Lee J, Yoo JH, Kim JW, Brent DA. Measures of connectivity and dorsolateral prefrontal cortex volumes and depressive symptoms following treatment with selective serotonin reuptake inhibitors in adolescents. JAMA Netw Open. 2023;6:e2327331.
Kang SG, Cho SE. Neuroimaging biomarkers for predicting treatment response and recurrence of major depressive disorder. Int J Mol Sci. 2020;21:2148.
Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, et al. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. Am J Psychiatry. 2018;175:1111–20.
Fingelkurts AA, Fingelkurts AA. Altered structure of dynamic electroencephalogram oscillatory pattern in major depression. Biol Psychiatry. 2015;77:1050–60.
Cohen SE, Zantvoord JB, Wezenberg BN, Bockting CLH, van Wingen GA. Magnetic resonance imaging for individual prediction of treatment response in major depressive disorder: a systematic review and meta-analysis. Transl Psychiatry. 2021;11:168.
Pizzagalli DA, Webb CA, Dillon DG, Tenke CE, Kayser J, Goer F, et al. Pretreatment rostral anterior cingulate cortex theta activity in relation to symptom improvement in depression: a randomized clinical trial. JAMA Psychiatry. 2018;75:547–54.
Whitton AE, Webb CA, Dillon DG, Kayser J, Rutherford A, Goer F, et al. Pretreatment rostral anterior cingulate cortex connectivity with salience network predicts depression recovery: findings from the EMBARC randomized clinical trial. Biol Psychiatry. 2019;85:872–80.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Shao X, Yan D, Kong W, Sun S, Liao M, Ou W, et al. Brain function changes reveal rapid antidepressant effects of nitrous oxide for treatment-resistant depression:Evidence from task-state EEG. Psychiatry Res. 2023;322:115072.
Pascual-Marqui RD, Lehmann D, Koenig T, Kochi K, Merlo MC, Hell D, et al. Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Res. 1999;90:169–79.
Stam CJ, Nolte G, Daffertshofer A. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum Brain Mapp. 2007;28:1178–93.
Detry MA, Ma Y. Analyzing repeated measurements using mixed models. JAMA. 2016;315:407–8.
Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–48.
Linden DE. The p300: where in the brain is it produced and what does it tell us? Neuroscientist. 2005;11:563–76.
Vogel EK, Luck SJ. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37:190–203.
Bidet-Caulet A, Mikyska C, Knight RT. Load effects in auditory selective attention: evidence for distinct facilitation and inhibition mechanisms. Neuroimage. 2010;50:277–84.
Chait M, de Cheveigne A, Poeppel D, Simon JZ. Neural dynamics of attending and ignoring in human auditory cortex. Neuropsychologia. 2010;48:3262–71.
Tong Y, Melara RD, Rao A. P2 enhancement from auditory discrimination training is associated with improved reaction times. Brain Res. 2009;1297:80–88.
Kayser J, Bruder GE, Tenke CE, Stewart JE, Quitkin FM. Event-related potentials (ERPs) to hemifield presentations of emotional stimuli: differences between depressed patients and healthy adults in P3 amplitude and asymmetry. Int J Psychophysiol. 2000;36:211–36.
O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–16.
Boggio PS, Rigonatti SP, Ribeiro RB, Myczkowski ML, Nitsche MA, Pascual-Leone A, et al. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychopharmacol. 2008;11:249–54.
Woodman GF. A brief introduction to the use of event-related potentials in studies of perception and attention. Atten Percept Psychophys. 2010;72:2031–46.
Poldrack RA. The future of fMRI in cognitive neuroscience. Neuroimage. 2012;62:1216–20.
Lui S, Wu Q, Qiu L, Yang X, Kuang W, Chan RC, et al. Resting-state functional connectivity in treatment-resistant depression. Am J Psychiatry. 2011;168:642–8.
Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage. 2012;61:677–85.
Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: a review. Neurosci Biobehav Rev. 2015;56:330–44.
Dutta A, McKie S, Deakin JF. Resting state networks in major depressive disorder. Psychiatry Res. 2014;224:139–51.
Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA. 2009;106:1942–7.
Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206.
Funding
The study was supported by the National Natural Science Foundation of China (82090034).
Author information
Authors and Affiliations
Contributions
YY, HZ, and KW were responsible for the conceptualization and design of this research. CL, KS, YX, YHS, JF, and ZWW collaborated in data collection. CL performed the data analysis and wrote the initial draft of the manuscript. HZ and YY provided comprehensive revisions to the manuscript. All authors participated in the manuscript revisions and approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
The authors report there are no conflict of interest to declare.
Consent for publication
All participants signed informed content for publication.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ-IRB20220205). The registration number for this trial was ChiCTR2200057365. All methods were performed in accordance with the Declaration of Helsinki. All participants signed informed consent to participate.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, H., Li, C., Shi, K. et al. Early treatment-related changes in dorsolateral prefrontal cortex activity and functional connectivity as potential biomarkers for antidepressant response in major depressive disorder. Transl Psychiatry 15, 350 (2025). https://doi.org/10.1038/s41398-025-03576-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-025-03576-0