Abstract
The potential benefits of music in prenatal and early childhood education are widely acknowledged, yet the neurobiological mechanisms underlying its effects on social behavior, particularly during early development, remain underexplored. This study investigated the impact of musical intervention on social behavior and neurodevelopment in mice, focusing on mechanisms at various developmental stages. Pregnant mice were exposed to music starting at embryonic day 13 (E13) and continuing until postnatal weeks 1, 3, or 5. Behavioral tests, including three-chamber social task and social interaction assays, revealed that musical exposure significantly enhanced social interaction in adult mice. mRNA sequencing revealed notable changes in the expression of genes associated with social behavior in both the medial prefrontal cortex (mPFC) and the amygdala. Additionally, Golgi staining demonstrated increased dendritic complexity, spine density, and dendritic length in these regions following music exposure. Elevated MAP2 expression was observed in the mPFC and amygdala, whereas GFAP expression was reduced in the amygdala. These findings provide valuable insights into the neurobiological mechanisms by which music affects early development and underscore its potential as a noninvasive tool to promote social and emotional well-being. This research has implications for therapeutic interventions in individuals with neurodevelopmental disorders, including autism spectrum disorder (ASD).
Similar content being viewed by others
Introduction
Social interaction is essential for human development and influences cognitive, emotional, and behavioral outcomes across the lifespan [1]. Early social experiences, especially in childhood, are crucial for the formation of neural circuits that support communication, empathy, and social bonding. These early interactions shape not only individual behavior but also long-term emotional health, influencing how individuals engage with others throughout their lives [2, 3]. The development of social behavior is linked to brain regions such as the medial prefrontal cortex (mPFC), amygdala, and superior temporal gyrus, which regulate emotion, social cognition, and reward processing [4, 5]. These regions, especially during critical developmental windows, are highly responsive to sensory and social stimuli, providing a foundation for future social functioning. Disruptions during early neural development can lead to long-lasting deficits in social behavior, as observed in neurodevelopmental disorders such as autism spectrum disorder (ASD) [6,7,8]. Social interactions extend beyond individual development, affecting societal outcomes such as academic achievement, career success, and mental health. Studies have shown that individuals with strong social connections experience better emotional regulation, reduced stress, and enhanced cognitive function, whereas social isolation increases the risk of depression and anxiety [9, 10]. These findings underscore the need for early interventions that foster social development. Among such interventions, music has emerged as a unique tool to enhance social connections and emotional regulation [11, 12].
Music, a universal language that transcends cultural and temporal boundaries, not only serves as a medium for emotional expression but also exerts distinct neurobiological effects that promote social abilities, cognitive development, and emotional regulation [13]. Recent research in music neuroscience has highlighted the profound impact of music interventions, particularly prenatal and infant music exposure, on shaping social behavior in adulthood. Studies have shown that fetuses, starting at 27 weeks of gestation, can perceive pitch, and by 33 weeks, they respond physiologically to complex sounds such as rhythm and melody [14, 15]. Prenatal music exposure can transmit sound vibrations through the placenta, impacting fetal neurodevelopment. For example, rhythmic classical music stabilizes the fetal heart rate, and the mother’s voice, combined with melody, enhances the fetus’s ability to recognize speech patterns [16]. These early sensory stimuli may modulate functional connectivity in brain regions related to social behavior, such as the prefrontal cortex (PFC) and superior temporal gyrus, through synaptic plasticity or epigenetic modifications [17]. Additionally, music therapy in neonatal intensive care units (NICUs) has been shown to improve neurodevelopmental outcomes in preterm infants. Customized music that simulates in utero sounds, such as the mother’s heartbeat with soothing melodies, reduces stress, promotes weight gain, and regulates sleep patterns [18]. These interventions likely increase vagal nerve activity, improving social vigilance and adaptability. music-based interventions (MBI) are increasingly used in therapeutic and educational settings, particularly for individuals with neurodevelopmental disorders such as ASD [19, 20]. However, despite the widespread recognition of music’s role in fostering social interaction, the mechanisms underlying these effects remain poorly understood.
Compared with other sensory-based interventions such as tactile stimulation or language exposure, music provides a temporally structured, multisensory stimulus that simultaneously engages the auditory, emotional, and motor systems. Recent studies have begun to illuminate how music influences brain networks involved in social processing, including the dopaminergic reward pathway, mirror neuron systems, and prefrontal regulatory regions. Music has been shown to activate the reward system, particularly regions such as the ventral striatum and nucleus accumbens, which are key to motivation and social behavior. Listening to pleasant music increases dopamine release in these areas, promoting social engagement [4, 21, 22]. Music exposure also stimulates the mirror neuron system, which is important for empathy and understanding others’ actions, fostering emotional synchronization and strengthening social bonds [23]. Additionally, music enhances PFC activity, which regulates emotional control, social cognition, and decision-making [24]. Despite these advances, significant gaps remain in understanding how early music interventions, particularly during fetal and infant development, influence social behavior into adulthood [25,26,27]. The long-term impact of early music exposure—particularly during prenatal and early postnatal periods—on social behavior and the development of neural circuits remains largely unexplored. In particular, molecular pathways such as synaptic plasticity, neuroimmune signaling, and dopaminergic regulation are still insufficiently studied. Understanding how music modulates these mechanisms during sensitive developmental windows may offer a neurobiological foundation for early interventions aimed at improving social functioning.
MBI have gained increasing attention in clinical settings, especially for autism spectrum disorder (ASD). However, findings remain mixed. Some studies report improvements in social communication, while others show only modest or short-lived benefits—often depending on factors such as patient age, baseline cognitive function, or treatment duration. These inconsistencies underscore the need for well-controlled preclinical models to define optimal exposure timing and duration, as well as to identify the underlying biological mechanisms. To address this gap, we employed a longitudinal mouse model with graded music exposure beginning at embryonic day 13 (E13) and continuing through postnatal weeks 1, 3, and 5. Behavioral assessments and molecular analyses will be conducted in adulthood to evaluate the long-term impact of music on social behavior and its underlying neurobiological mechanisms. By examining the dose‒response relationship between music exposure and social behavior, this study aims to provide valuable insights into the neurobiological effects of music during early development. These findings may provide scientific support for noninvasive, music-based strategies to enhance social functioning and inform the development of music therapy approaches in clinical settings.
Materials and methods
Animal preparation
Pregnant C57BL/6 mice were obtained from SPF (Beijing) Biotechnology Co., Ltd. on gestational day 13 and housed under controlled environmental conditions (23 ± 1 °C, 50 ± 1% humidity, with a 12-h light/dark cycle, lights on from 8 a.m. to 8 p.m.). The mice had ad libitum access to standard rodent chow and water. All groups were maintained in identical housing environments, and aside from the music exposure applied to intervention groups, no additional variables were introduced. All the experimental procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) and were approved by the Animal Care and Use Committee of Minzu University of China.
Experimental procedure
Eight pregnant female C57BL/6 mice (gestational day 13) were randomly assigned to four groups: control, Music-1, Music-2, and Music-3 (n = 2 per group). The music intervention schedule was as follows: Music-1 from gestational day 13 to postnatal day 7; Music-2 from gestational day 13 to postnatal day 21; and Music-3 from gestational day 13 to postnatal day 35. The control group consisted of 7 males and 6 females; the Music-1 group included 6 males and 7 females; the Music-2 group included 7 males and 8 females; and the Music-3 group included 8 males and 6 females. For the experiments, 6 males and 6 females were selected from each group (n = 12 per group). All mice were from the inbred C57BL/6 strain to minimize genetic variability; however, future research may benefit from incorporating genetically diverse or phenotypically characterized mouse populations to explore individual differences in music responsiveness and potential personalized interventions.
Music intervention
The mice in the music groups were exposed to nightly 1.5-h music sessions from 20:30 to 22:00. The music was played in a sequential manner, simulating a CD playback pattern, with the speaker positioned 2 m from the mouse. A curated collection of 25 musical pieces, each averaging 2–3 min in length, was used for auditory stimulation. The music featured high-pitched instruments such as piano, violin, and flute, with rhythmic patterns ranging from cheerful to lyrical. Selections were chosen for their cyclical repetition, dynamic contrast, and rich auditory texture. The specific frequencies of the musical pieces are listed in Supplementary Table 1.
To ensure environmental control, sound levels were measured consistently using a sound level meter and background noise was minimized. While our musical selections focused on high-frequency instrumental music for experimental consistency, we acknowledge the importance of exploring a broader range of auditory stimuli—including low-pitched instruments, vocal music, and mixed-genre compositions—in future studies to better understand differential effects.
Behavioral tests
Three-chamber social interaction test
The three-chamber social interaction test was performed as previously described [28] to assess social behavior in the mice. The experimental apparatus consisted of three rectangular chambers (19 × 45 cm each) connected by passageways. A metal cage, large enough to house a single mouse, was placed in the center of each of the two side chambers. The experiment was conducted in two phases.
In Phase 1, the test mouse was placed in the center chamber and allowed to acclimate for 5 min. A stranger mouse (Mouse-A) was then randomly placed in one of the side chambers, while the other side remained empty. After the partition was removed, the test mouse was allowed to explore all three chambers freely for 10 min. The number of direct contacts and the duration of interaction between the test mouse and Mouse-A or the empty cage were recorded.
In Phase 2, the empty cage was replaced with a second stranger mouse (Mouse-B). The interaction time and frequency between the test mouse and both Mouse-A and Mouse-B were recorded.
Social interaction test
The experiment was designed to assess the social interaction abilities of the test mice by allowing them to interact with a novel stranger mouse, as previously described [29]. Before the experiment, the test mice were acclimated to the behavioral testing room for at least 30 min. The plants were subsequently placed in a clean cage lined with fresh bedding and allowed to explore the environment freely for 15 min under low light conditions as an adaptation period. A stranger mouse of similar age or slightly younger was subsequently introduced into the cage, and the two mice were allowed to interact freely for 10 min. Interactions were recorded via video, and the direct contact behaviors of the test mouse toward the stranger mouse, including body sniffing, following, and climbing, were observed.
Elevated plus maze
The elevated plus maze (EPM) test was employed to assess exploratory behavior and anxiety levels in mice on the basis of their entry into the open arms and the time spent there [30]. Prior to testing, the mice were allowed to acclimate to the experimental environment for 2 h. At the beginning of the experiment, each mouse was gently placed at the center of the maze, facing the open arms. The number of entries into the open arms and the time spent in these areas were recorded over a 5-min period. An entry was defined as when both forepaws of the mouse crossed into the open arm. After each trial, the maze was cleaned with a 75% ethanol solution to eliminate olfactory cues and allowed to dry before the next trial.
Quantitative real-time polymerase chain reaction (qPCR)
Total RNA was extracted from the mPFC and amygdala TRIzol reagent (Invitrogen, #15596026). qPCR was performed via 2×SYBR Green qPCR Master Mix (Genestar, #A301-10) on a StepOnePlus instrument (Applied Biosystems) [30]. The specific primers used in the qPCR analysis are listed in Supplementary Table 2. Relative mRNA expression levels were quantified via the 2^−ΔΔCt method.
Immunofluorescence
After the completion of the MBI and behavioral testing, the animals were euthanized on day 80. The mice were perfused intracardially with saline, followed by 4% paraformaldehyde. The brains were then dehydrated by immersion in 20% sucrose in PBS for 24 h, followed by immersion in 30% sucrose in PBS for 48 h. Coronal sections (35 μm) were rinsed with PBS and blocked for 10 min with QuickBlock™ Immunostaining Blocking Buffer (Beyotime Biotechnology, P0260). The sections were incubated overnight at 4 °C with the following primary antibodies: rabbit anti-glial fibrillary acidic protein (GFAP, ABclonal, #A0237, 1:200), rabbit anti-ionized calcium-binding adapter molecule 1 (IBA1, Wako, #019-19741, 1:1000), and rabbit anti-microtubule-associated protein 2 (MAP2, Cell Signaling Technology, #14082, 1:400). The following day, the sections were incubated for 2 h with a secondary antibody: goat anti-rabbit IgG Alexa Fluor® 594 (Invitrogen, #A11008, 1:1000). After washing, the sections were counterstained with DAPI and imaged using a Leica TCS SP8 confocal microscope [31].
Golgi-cox staining
Golgi-Cox staining was performed via the FD Rapid GolgiStain™ Kit (FD NeuroTechnologies) to visualize the dendritic spines. Fresh rostral and mid-brain tissues were immersed in a 1:1 mixture of Solution A (potassium dichromate and mercuric chloride) and Solution B (potassium chromate) for 2 weeks, with the solution replaced after 24 h. The tissues were then transferred to Solution C and incubated for 1 week, with another solution change after 24 h. Following incubation, the tissues were frozen in powdered dry ice, sectioned into 100 μm slices, mounted on slides coated with 0.5% gelatin, and then air-dried. The sections were stained for 5 min in a mixture of Solutions D, E, and distilled water, followed by rinsing, dehydration in ethanol, clearing in xylene, and coverslipping.
Six staining sessions were conducted for each condition, with four mice per session. Images of the mPFC and amygdala were captured via a Leica TCS SP8 confocal microscope. ImageJ software was used to analyze 30–36 neurons per condition. Dendritic branching was assessed using Sholl analysis, with individual neurons from the mPFC and amygdala imaged at 20× magnification. Concentric circles were drawn at 10 μm intervals from the cell body, and the number of intersections with dendrites was quantified. Dendritic spine density was measured via a 63× oil immersion lens, and the spines per 10 μm of dendrite were counted. All analyses were conducted in a blinded manner with respect to the treatment conditions.
mRNA sequencing (RNA-seq) and bioinformatics analysis
Total RNA was extracted from the mPFC and amygdala tissues of mice in the control, Music-1, Music-2, and Music-3 groups (n = 6 per group, with an equal distribution of males and females). The RNA samples were then sent to Novogene (Beijing, P. R. China; https://cn.novogene.com/) for mRNA library preparation and sequencing.
Following sequencing, transcripts with counts greater than 10 per million reads in all samples were selected for further analysis. Differential gene expression between the music and control groups was assessed via edgeR. Genes with a log2(fold change) > = 1 and p < = 0.05 were considered differentially expressed. Heatmaps of gene expression were generated via a bioinformatics platform (http://www.bioinformatics.com.cn/). Pathway enrichment analysis of differentially expressed genes (DEGs) was performed via Metascape (https://Metascape.org/gp/index.html#/main/step1).
Results
The positive impact of music on social behavior in mice
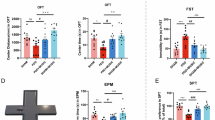
This study examined the effects of prenatal and early childhood music interventions on adult social behavior, modeling three MBI phases corresponding to the neonatal, infant, and preschool stages (Fig. 1A). Social novelty preference was assessed using the three-chamber social interaction test (Fig. 1B), and a social preference index was calculated to compare social behaviors across groups. Both the music and control groups showed a preference for exploring Mouse-A over the empty cage. Furthermore, the music group spent significantly more time exploring Mouse-A the control group. Music-3 group (exposed to music from prenatal week 1 to postnatal week 5) a significantly social preference index (Fig. 1C).
A. Timeline of experimental procedures. B. Three-chamber social test. C. Sociability session. Left: Both music-exposed and control mice spent significantly more time sniffing the cage containing Mouse-A the empty cage. Right: No significant difference was observed in the social preference index (ratio of time spent sniffing Mouse-A vs. the empty cage) between music-exposed and control mice. D. Social novelty session. Left: Both music-exposed and control mice spent significantly more time sniffing the cage containing Mouse-B the cage containing Mouse-A. Right: The preference index (ratio of time spent sniffing Mouse-B vs. Mouse-A) revealed a significantly preference for Mouse-B in the Music-2 and Music-3 groups. E. Social test. F. Music-exposed mice exhibited a significantly stronger preference Stranger mice. G–J. Effect of music exposure on anxiety-like behavior in the EPM. No significant differences were found between the music-exposed and control groups in the time spent in the open arms, distance traveled in the open arms, or total distance. (control, n = 12; Music-1 group, n = 12; Music-2 group, n = 12; Music-3 group, n = 12; one-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; values are the means ± SEM).
In the social novelty test, Mouse-B replaced the empty cage, and all groups preferentially explored Mouse-B over Mouse-A. Notably, the music exposure group spent more time exploring Mouse-B than the control group. Further analysis revealed that the duration of music exposure influenced the social preference index. Specifically, the Music-2 group (exposed to music from prenatal week 1 to postnatal week 3) and the Music-3 group significantly social preference indices the control group (Fig. 1D).
To further assess social behavior, a separate social interaction assay was conducted. Compared with control mice, mice in the music-exposed groups, particularly Music-2 and Music-3, presented a stronger preference for interacting with unfamiliar mice (Fig. 1E, F). To control for potential confounding effects of locomotor activity, behavior in the EPM was also examined. No significant differences were observed between groups in terms of time spent in the open arms, distance traveled in the open arms, or total distance traveled (Fig. 1G–J), indicating that music exposure did not influence general motor activity.
Sex-specific analyses revealed no significant differences between male and female mice in response to musical intervention across all behavioral tests, suggesting a robust and consistent effect of music exposure on neurodevelopment regardless of sex (Figs. S1–S2). Moreover, a positive correlation was observed between the duration of music exposure and social behavior performance. In the three-chamber test, the sociability index (Phase 1) was positively correlated with intervention duration (r = 0.1724, p = 0.0033), as was the social novelty index (Phase 2; r = 0.2678, p = 0.0002). In the social interaction test, a stronger correlation was found (r = 0.3848, p < 0.0001), indicating a dose-dependent relationship between music exposure and enhanced social functioning (Figs. S2E–G). In summary, these findings demonstrate that prenatal and early postnatal music exposure enhances social behaviors—particularly social novelty recognition and interaction—without affecting general locomotor activity. The most pronounced effects were observed in the groups with extended postnatal music exposure (3–5 weeks).
MBI alters mPFC gene expression to facilitate social behavior in mice
The mPFC is essential for emotion regulation, social behavior, and decision-making [32]. To investigate the molecular mechanisms underlying early MBI in the mPFC, we performed RNA-seq on tissue samples from the mPFC of mice exposed to either music or control conditions. Bioinformatics analysis revealed 1,283 DEGs between the music-exposed and control groups (Fig. 2A), suggesting that music exposure significantly mPFC gene expression. Specifically, the Music-1 group (exposed to music from prenatal week 1 to postnatal week 1) presented 276 upregulated and 131 downregulated genes; the Music-2 group (exposed from prenatal week 1 to postnatal week 3) presented 404 upregulated and 371 downregulated genes; and the Music-3 group (exposed from prenatal week 1 to postnatal week 5) presented 239 upregulated and 104 downregulated genes (Fig. 2B). These findings indicate that the duration of music exposure differentially affects mPFC gene expression.
A. Heatmap showing cluster analysis of 1283 mRNAs in the mPFC. B. Volcano plots of DEGs in the three music groups, with labeled genes validated by qPCR. C. Venn diagram of DEGs in the three music groups. D. Enrichment heatmap of the top 20 pathways associated with DEGs in the mPFC. E. Similarity and association analysis within and between clusters of the top 20 enriched pathways in the mPFC. F. Relative mRNA levels of the Galr3, Pomc and Gh genes involved in the Neuroactive ligand-receptor interaction pathway. G. Relative mRNA levels of the Avp, Chrnb4 and Ffar3 genes involved in the regulation of transmission of nerve impulse pathway. H. Relative mRNA 12levels of the Chrna3, Chrm5 and Chrna10 genes in the synaptic signaling pathway. I. Relative mRNA levels of the Slc6a3, Drd4 and Th genes in the synaptic transmission, dopaminergic pathway. (n = 6; one-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; values are the means ± SEM).
Venn diagram analysis revealed 36 overlapping DEGs across all three music-exposed groups (Fig. 2C), suggesting that MBI influences shared molecular pathways, despite variations in exposure duration. Functional enrichment analysis via Metascape revealed several significantly enriched pathways in the mPFC, including neuroactive ligand‒receptor interactions, regulation of nerve impulse transmission, synaptic signaling, and dopaminergic responses (Fig. 2D). These pathways are critical for neural development and synaptic plasticity [4, 33]. Cluster analysis of the top 20 enriched pathways revealed high intracluster similarity, indicating closely associated molecular complexes, with multiple subclusters sharing common signaling mechanisms (Fig. 2E). This finding suggests that while specific molecules modulated by different durations of music exposure may vary, the underlying molecular pathways exhibit significant overlap.
To validate these findings, we selected representative genes from four critical pathways—neuroactive ligand‒receptor interactions, the regulation of nerve impulse transmission, synaptic signaling, and dopaminergic signaling—for further analysis. These pathways were specifically chosen because of their well-established roles in modulating neural circuits that are involved in social behavior, emotional regulation, and cognitive processing. Neuroactive ligand‒receptor interactions, for example, are crucial for neurotransmission and synaptic plasticity, both of which are essential for the brain’s response to social stimuli [34]. The regulation of nerve impulse transmission and synaptic signaling are directly involved in synaptic communication and plasticity, processes that are central to learning and social interactions [35, 36]. Finally, dopaminergic signaling has long been associated with reward-based learning and motivation, both of which are critical for social behavior and the regulation of emotions [37]. qPCR was performed to confirm the expression patterns of 12 selected genes (three genes from each pathway). The results were highly consistent with the RNA-seq findings (Fig. 2F–I). These findings suggest that the upregulation of genes involved in neurotransmitter regulation, synaptic transmission, and dopaminergic signaling in the mPFC may contribute to the enhanced social behavior observed in music-exposed mice through the modulation of these molecular pathways.
Music intervention alters molecular pathways in the amygdala link to social behavior
The amygdala plays a pivotal role in emotion processing and social behavior, particularly in regulating fear responses, emotional memory, and social interactions [38]. Alterations in gene expression within the amygdala, especially DEGs, can reflect changes in how social signals—such as facial expressions and social exclusion—are processed, influencing adult social behaviors such as social anxiety, social acceptance, and emotional resonance [39]. This study aimed to investigate the molecular impact of early MBI on these processes.
Bioinformatics analysis revealed a total of 1542 DEGs in the amygdala between the music-exposed and control groups (Fig. 3A). Specifically, the Music-1 group (exposed from prenatal week 1 to postnatal week 1) presented 644 DEGs, with 457 upregulated and 187 downregulated genes. The Music-2 group (exposed from prenatal week 1 to postnatal week 3) presented 694 DEGs, including 373 upregulated and 321 downregulated genes, and the Music-3 group (exposed from prenatal week 1 to postnatal week 5) presented 507 DEGs, with 225 upregulated and 282 downregulated genes (Fig. 3B). Venn diagram analysis revealed that 47 DEGs were shared across all three music-exposed groups (Fig. 3C), suggesting that MBI modulates core molecular pathways that are conserved across different exposure durations.
A. Heatmap showing cluster analysis of 1542 mRNAs in the amygdala. B. Volcano plots of DEGs in the three music groups, with labeled genes validated by qPCR. C. Venn diagram of DEGs in the three music groups. D. Enrichment heatmap of the top 20 pathways associated with DEGs in the amygdala. E. Similarity and association analysis within and between clusters of the top 20 enriched pathways in the amygdala. F. Relative mRNA levels of the Agtr1b, Mcpt4 and Cga genes involved in the endocrine process pathway. G. Relative mRNA levels of the Pomc, Gh, and Cma1 genes involved in the Peptide hormone metabolism pathway. H. Relative mRNA 12levels of the Drd4, Cysltr2 and Hcar1 genes in the Class A/1 (Rhodopsin-like receptors) pathway. I. Relative mRNA levels of the Col3a1, Gm5127 and F2rl1 genes in the positive regulation of Rho protein signal transduction pathway. (n = 6; one-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; values are the means ± SEM).
Functional enrichment analysis via Metascape revealed several key pathways that were significantly enriched in the amygdala, including Rho protein signaling, A/1 class receptors (rhodopsin-like receptors), endocrine processes, and positive regulation of peptide hormone metabolism (Fig. 3D). These pathways are essential for neural development, synaptic function, and hormonal regulation, which are crucial for shaping social behaviors [40, 41]. Notably, Rho protein signaling, which is involved in neuronal plasticity and synaptic connectivity, and A/1 class receptors, which regulate emotional and sensory processing, are both significantly affected by MBI [42]. Endocrine processes are critical in modulating hormones associated with social behavior, whereas peptide hormone metabolism plays a key role in emotional resonance and social behavior modulation [43,44,45]. Cluster analysis of the top 20 enriched pathways revealed high intracluster similarity, indicating that these pathways are tightly connected within molecular complexes, with several subclusters sharing common signaling mechanisms (Fig. 3E). These findings suggest that MBI induces overlapping molecular effects in the amygdala, irrespective of the exposure duration.
We selected genes from four key pathways—Rho protein signaling, A/1 class receptors, endocrine processes, and peptide hormone metabolism—to further analyze the molecular mechanisms underlying the observed effects. The interactions between these pathways are likely complementary, working together to modulate emotional and social responses [21]. To confirm these findings, we performed qPCR on 12 selected genes, with three genes from each pathway. The qPCR results were in strong agreement with the RNA-seq data, confirming that these pathways are actively modulated by MBI. These findings further support the molecular basis for the observed improvements in social behavior in music-exposed mice (Fig. 3F–I). These findings highlight the integrated role of these molecular pathways in promoting social and emotional responses, underscoring the therapeutic potential of MBI in enhancing neuroplasticity and improving social behavior.
Effects of MBI on synaptic plasticity in the mPFC and amygdala
We assessed the impact of MBI on synaptic plasticity via Golgi staining, with a focus on dendritic structures in the mPFC and amygdala. Dendritic complexity was quantified via Sholl analysis, which measures the number of dendritic intersections at increasing distances from the cell body [46]. A standardized termination radius of 270 μm was applied for the mPFC (Fig. 4A).
A. Representative images of neurons and high-magnification views of dendritic spines in the mPFC region. B. Sholl analysis of dendritic branch points at 10 μm intervals in the mPFC region. C. Quantification of dendritic spine branch numbers in the mPFC region. D. Dendritic spine density in the mPFC region. E. Representative images of neurons and high-magnification views of dendritic spines in the amygdala. F. Sholl analysis of dendritic branch points at 10 μm intervals in the amygdala. G. Quantification of dendritic spine branch numbers in the amygdala. H. Dendritic spine density in the amygdala. (n = 6; one-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; values are the means ± SEM).
Compared with the controls, the music-exposed groups presented significant increases in dendritic intersections, length, branching, and spine density (Fig. 4B–D). These changes were positively correlated with the duration of music exposure, suggesting that longer exposure enhances dendritic growth. Specifically, the Music-2 group (exposed from prenatal week 1 to postnatal week 3) presented significant increases in both dendritic length and branching, whereas the Music-3 group (exposed from prenatal week 1 to postnatal week 5) presented the most pronounced increase in dendritic branching. These findings indicate that prolonged music exposure enhances dendritic growth and synaptic plasticity in the mPFC, which may contribute to improvements in cognitive and social behaviors.
Similar trends were observed in the amygdala. Golgi staining revealed that MBI promotes synaptic plasticity in this region as well. Sholl analysis with a 200 μm termination radius revealed a significant increase in the number of dendritic intersections in the music-exposed groups, although no significant differences were found in the dendritic spine length (Fig. 4E‒F). Notably, dendritic branching and spine density were significantly greater in the music-exposed groups, particularly in the Music-2 and Music-3 groups (Fig. 4G–H).
In summary, these results demonstrate that MBI enhances dendritic branching and spine density in both the mPFC and amygdala, suggesting that music exposure promotes synaptic plasticity in key brain regions involved in social and cognitive functions. The observed cumulative effects, with increased dendritic complexity associated with longer exposure, highlight the potential long-term benefits of sustained music intervention on brain function. These findings underscore the promise of music as a noninvasive intervention to increase neuroplasticity, with potential therapeutic applications in neuroprotection and mental health.
Music intervention enhances the number of mature neurons in the mPFC and amygdala
Neurons play a pivotal role in regulating social and emotional behaviors by integrating sensory input and modulating neural circuits, particularly in brain regions such as the mPFC and amygdala [47]. MAP2 is a marker of mature neurons and is crucial for stabilizing dendritic structures and supporting synaptic plasticity [48].
To investigate the effects of MBI on mature neurons, we performed MAP2 immunofluorescence staining in the mPFC and amygdala. The MAP2 levels in the Music-1 group (exposed to music from prenatal week 1 to postnatal week 1) and the Music-2 group (exposed to music from prenatal week 1 to postnatal week 3) were significantly greater than those in the control group (Fig. 5A, B), with p values of 0.0096 and 0.0019, respectively. Similarly, MAP2 expression in the amygdala was significantly greater in both MBI groups than in the control group (Fig. 5C, D), with p values of 0.0045 for the Music-1 group, 0.0267 for the Music-2 group, and 0.0189 for the Music-3 group. These results suggest that MBI enhances the expression of mature neurons in the mPFC and amygdala.
A. Representative images of mature neurons in the mPFC. B. Quantification of mature neurons in the mPFC. C. Representative images of mature neurons in the amygdala. D. Quantification of mature neurons in the amygdala. (n = 6; one-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; values are the means ± SEM).
Interestingly, the duration of music intervention did not exhibit a dose-dependent relationship with the increase in mature neurons. These findings suggest that short-term interventions (e.g., Music-1) may strongly stimulate early neural development, whereas longer interventions (e.g., Music-2 and Music-3) may induce more complex long-term adaptive responses. These changes may not be directly reflected in the number of MAP2-labeled mature neurons but could influence neuronal function or synaptic plasticity through other mechanisms. This interpretation is consistent with our Golgi staining data, which revealed that MBI enhanced dendritic branching and spine density in the mPFC and amygdala.
In conclusion, the increase in mature neurons, particularly in brain regions critical for social behavior, such as the mPFC and amygdala, may increase the number of neural circuits involved in emotional regulation, social interaction, and cognitive processing. These findings suggest that MBI could promote the maturation of neural networks that support social behavior, providing new insights into the neurobiological mechanisms underlying social cognition.
Impact of MBI on glial cell activation
Glial cells, including astrocytes and microglia, are essential nonneuronal cells distributed throughout the brain. These cells are involved in neuronal development, synapse formation, and the regulation of brain function [49, 50]. They participate in processes such as neuronal migration, differentiation, and positioning during neurodevelopment, as well as in the regulation of synaptic activity [49]. Additionally, glial cells produce neurotrophic factors and signaling molecules that modulate neuronal growth and connectivity [51,52,53]. In addition to supporting neuronal functions, glial cells influence metabolism, immune responses, and behavior by regulating the neuroendocrine system and the neuroendocrine–immune axis [54].
To assess the impact of MBI on neuroimmune function, we investigated the activation of microglia and astrocytes, two key glial cell types that regulate neuronal activity and neuroimmune interactions. Microglia, as resident immune cells of the central nervous system, play crucial roles in synaptic plasticity through cytokine release and the clearance of neurotransmitters from synaptic spaces, thereby influencing neuronal communication [55, 56]. Following music intervention, we performed immunofluorescence staining for IBA1, a marker of mature microglia, to examine the distribution in the mPFC and amygdala. Compared the control group, IBA1 expression was in both regions of the music-exposed group (Fig. 6A–D). This reduction in IBA1 expression suggests that music intervention may modulate microglial activity, potentially reducing neuroinflammation or promoting alterations in the neuroenvironment. These changes could underlie the positive effects of MBI on emotional and cognitive functions.
A. Representative images of IBA1 immunofluorescence in the mPFC. B. Quantification of microglial activation in the mPFC. C. Representative images of IBA1 immunofluorescence in the amygdala. D. Quantification of microglial activation in the amygdala. E. Representative images of GFAP immunofluorescence in the amygdala. F. Quantification of astrocyte activation in the amygdala (n = 6; one-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; values are the means ± SEM).
Astrocytes, another key glial cell type, are vital for supporting and maintaining neuronal function [57, 58]. Their activation is typically observed following central nervous system injury and is characterized by increased expression of GFAP, a reliable marker for astrocyte activation [59, 60]. To assess astrocyte activation in the amygdala after MBI, we performed GFAP immunofluorescence staining on brain slices (Fig. 6E). Quantitative analysis of GFAP expression revealed a reduction in the music-exposed groups, with the Music-3 group (exposed from prenatal week 1 to postnatal week 5) showing a marked decrease in GFAP activation compared with the control group (Fig. 6F). In summary, the reduction in GFAP expression observed in adult mice following early music exposure may reflect astrocyte deactivation or functional recovery. This shift could indicate a more homeostatic state and a healthier neuroenvironment. These changes in glial cell activity, alongside the modulation of microglial function, suggest that early music exposure promotes neuroplasticity and reduces neuroinflammation. This, in turn, may enhance synaptic activity and contribute to the improved cognitive and social behaviors observed in the music-exposed groups.
Discussion
The present study provides compelling evidence that prenatal and early postnatal music exposure enhances social behavior in mice, with concomitant molecular, synaptic, and cellular changes in brain regions critical for social cognition. Our findings that MBI induces neuroplasticity in the mPFC and amygdala, modulates neuroimmune responses, and promotes synaptic and neuronal maturation, collectively supporting improved social novelty recognition and interaction. These results align with translational psychiatry frameworks by bridging behavioral outcomes with neurobiological mechanisms, offering insights into noninvasive interventions for neurodevelopmental and psychiatric disorders, such as ASD and schizophrenia [4].
The behavioral improvements observed in music-exposed mice—particularly enhanced social novelty preference and interaction—mirror findings in human studies where early musical training correlates with improved social communication and emotional regulation [61]. Notably, the duration-dependent effects of MBI (with the Music-2 and Music-3 groups showing the most pronounced benefits) suggest a critical window for neurodevelopmental plasticity, similar to the sensitive periods identified in human neurodevelopment [33]. The lack of locomotor differences in the EPM confirms that these behavioral enhancements are specific to social cognition rather than generalized activity changes, ruling out potential confounding variables. These findings parallel clinical observations in children with ASD, where music therapy improves social engagement and communication, further underscoring the translational relevance of our model [62, 63].
At the molecular level, our RNA-seq data revealed that MBI induces widespread transcriptional changes in the mPFC and amygdala, two regions integral to social decision-making and emotional processing [64]. The upregulation of pathways such as neuroactive ligand‒receptor interactions, synaptic signaling, and dopaminergic responses in the mPFC aligns with their established roles in social reward processing and cognitive flexibility. Dopaminergic signaling, in particular, modulates motivation and reward-seeking behaviors, which may drive the increased social exploration observed in music-exposed mice [65, 66]. Similarly, Rho protein signaling and endocrine pathways enriched in the amygdala are known to regulate synaptic connectivity and stress responses, mechanisms implicated in social anxiety and emotional resonance [4]. The overlap of 36 DEGs in the mPFC and 47 DEGs in the amygdala across all MBI groups suggests conserved molecular targets of music exposure, irrespective of duration, pointing to core pathways that may be leveraged for therapeutic interventions.
The Golgi staining results indicate that MBI enhances dendritic complexity and spine density in the mPFC and amygdala, which are structural correlates of synaptic plasticity. Furthermore, the duration-dependent increases in dendritic branching and spine density further support the hypothesis that sustained auditory enrichment strengthens the neural circuits underlying social cognition. Notably, the absence of a linear dose‒response relationship in MAP2 expression suggests that early exposure to MBI may prime neuronal maturation, whereas prolonged exposure may refine synaptic networks through activity-dependent mechanisms. This form of neuroplasticity is likely to enhance the integration of sensory and emotional inputs, thereby facilitating adaptive social behaviors. This concept aligns with findings from other models of neuroplasticity and enriched environments, which have demonstrated similar enhancements in brain structure and function [46].
In mice exposed to MBI, the reduced expression of microglial and astrocytic markers suggests a neuroprotective effect, potentially mediated by attenuated neuroinflammation. Microglial deactivation has been linked to enhanced synaptic pruning and circuit refinement during critical developmental periods, whereas astrocyte homeostasis supports neurotrophic signaling and balance [67]. These glial changes may create a permissive environment for synaptic plasticity, complementing the observed upregulation of neurodevelopmental genes. This finding aligns with emerging evidence suggesting that neuroimmune modulation contributes to the benefits of environmental enrichment in neuropsychiatric models, where neuroinflammation is often dysregulated [68,69,70].
Building on our previous work showing that music alleviates stress-induced affective disturbances in adult mice via modulation of the HPA axis, oxidative stress, and neuroinflammation, the present study shifts focus to a critical early developmental window—starting at embryonic day 13—and examines long-term effects on social behavior. Unlike prior adult-targeted interventions, our findings reveal that early music exposure induces preventive neuroplasticity in key social brain regions, including enhanced dendritic architecture, increased synaptic gene expression, and reduced glial activation in the mPFC and amygdala. Transcriptomic profiling further supports a distinct mechanism of action, highlighting neurodevelopmental pathways such as synapse assembly and axon guidance, rather than the inflammatory signatures observed in adult models. These results underscore a mechanistically distinct and developmentally timed effect of music, offering translational potential as a noninvasive early-life intervention for disorders marked by social deficits.
While this study provides mechanistic insights, several limitations warrant consideration. First, although our study focuses on early-life passive exposure to music, we acknowledge that this approach does not fully replicate active musical engagement in humans, which involves motor, cognitive, and emotional components such as instrument playing or singing. Despite this limitation, passive auditory stimulation remains a valid and controllable model for investigating fundamental neural and behavioral effects of music in early development. To better simulate human music-based interventions, future studies could incorporate interactive or socially contingent auditory paradigms, particularly those engaging sensorimotor systems. Second, the translational relevance of mouse social behavior assays to human social cognition remains indirect; cross-species validation via electrophysiological or functional connectivity measures would strengthen these links. Finally, the precise temporal dynamics of MBI-induced molecular changes—particularly how acute versus chronic exposure differentially regulates gene networks—require further investigation to optimize therapeutic approaches. Third, although our study examined three early developmental time windows, the long-term or cumulative effects of musical exposure across the lifespan remain unexplored. Extending MBI into adolescence, adulthood, or even aging periods may offer richer insight into how timing influences the durability and reversibility of neurobehavioral adaptations.
In conclusion, early music exposure enhances social behavior in mice through multilevel neuroadaptations, including transcriptional reprogramming, synaptic remodeling, neuronal maturation, and neuroimmune modulation. These findings underscore the potential of MBI as a noninvasive strategy to promote neurodevelopmental resilience, with implications for individuals with disorders characterized by social dysfunction, such as ASD and schizophrenia [33, 71, 72]. Future research should explore the translational efficacy of music-based therapies in at-risk populations, leveraging our mechanistic insights to optimize timing, duration, and delivery modalities to maximize therapeutic benefit. In parallel, future work will incorporate protein-level validation and circuit-level interrogation—such as Western blotting, ELISA, and calcium imaging—to further elucidate the molecular and systems-level mechanisms underlying music-induced neuroplasticity. Additionally, introducing interactive paradigms that model active musical engagement, expanding validation across multiple species, and investigating individual variability will be critical to advancing personalized and clinically relevant music-based interventions. These approaches will strengthen causal links between gene expression changes and functional outcomes, ultimately enhancing our understanding of how music promotes neurodevelopmental resilience.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Leblanc H, Ramirez S. Linking social cognition to learning and memory. J Neurosci. 2020;40:8782–98. https://doi.org/10.1523/JNEUROSCI.1280-20.2020.
Rokita KI, Dauvermann MR, Donohoe G. Early life experiences and social cognition in major psychiatric disorders: a systematic review. Eur Psychiatry. 2018;53:123–33. https://doi.org/10.1016/j.eurpsy.2018.06.006.
Hiura LC, Ophir AG. Interactions of sex and early life social experiences at two developmental stages shape nonapeptide receptor profiles. Integr Zool. 2018;13:745–60. https://doi.org/10.1111/1749-4877.12338.
Chanda ML, Levitin DJ. The neurochemistry of music. Trends Cogn Sci. 2013;17:179–93. https://doi.org/10.1016/j.tics.2013.02.007.
Huang WC, Zucca A, Levy J, Page DT. Social behavior is modulated by valence-encoding mPFC-amygdala sub-circuitry. Cell Rep. 2020;32:107899. https://doi.org/10.1016/j.celrep.2020.107899.
LaGasse AB. Effects of a music therapy group intervention on enhancing social skills in children with autism. J Music Ther. 2014;51:250–75. https://doi.org/10.1093/jmt/thu012.
Van't Hooft JJ, Pijnenburg YAL, Sikkes SAM, Scheltens P, Spikman JM, Jaschke AC, et al. Frontotemporal dementia, music perception and social cognition share neurobiological circuits: a meta-analysis. Brain Cogn. 2021;148:105660 https://doi.org/10.1016/j.bandc.2020.105660.
Yao TT, Chen L, Du Y, Jiang ZY, Cheng Y MicroRNAs as regulators, biomarkers, and therapeutic targets in autism spectrum disorder. Mol Neurobiol. 2024. https://doi.org/10.1007/s12035-024-04582-x.
Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15:669–74. https://doi.org/10.1038/nn.3086.
Hodgetts S, Gallagher P, Stow D, Ferrier IN, O’Brien JT. The impact and measurement of social dysfunction in late-life depression: an evaluation of current methods with a focus on wearable technology. Int J Geriatr Psychiatry. 2017;32:247–55. https://doi.org/10.1002/gps.4632.
Gassner L, Geretsegger M, Mayer-Ferbas J. Effectiveness of music therapy for autism spectrum disorder, dementia, depression, insomnia and schizophrenia: update of systematic reviews. Eur J Public Health. 2022;32:27–34. https://doi.org/10.1093/eurpub/ckab042.
Speranza L, Pulcrano S, Perrone-Capano C, di Porzio U, Volpicelli F. Music affects functional brain connectivity and is effective in the treatment of neurological disorders. Rev Neurosci. 2022;33:789–801. https://doi.org/10.1515/revneuro-2021-0135.
Schlaug G. Musicians and music making as a model for the study of brain plasticity. Prog Brain Res. 2015;217:37–55. https://doi.org/10.1016/bs.pbr.2014.11.020.
Koelsch S. Brain correlates of music-evoked emotions. Nat Rev Neurosci. 2014;15:170–80. https://doi.org/10.1038/nrn3666.
Shahbazi F, Fattahi-Darghlou M, Moslehi S, Dabiri-Golchin M, Shahbazi M. Effect of music therapy on behavioral and physiological neonatal outcomes: a systematic review and dose-response meta-analysis. PLoS One. 2025;20:e0316674 https://doi.org/10.1371/journal.pone.0316674.
Massimello F, Billeci L, Canu A, Montt-Guevara MM, Impastato G, Varanini M, et al. Music modulates autonomic nervous system activity in human fetuses. Front Med (Lausanne). 2022;9:857591. https://doi.org/10.3389/fmed.2022.857591.
Gallazzi M, Pizzolante M, Biganzoli EM, Bollati V. Wonder symphony: epigenetics and the enchantment of the arts. Environ Epigenet. 2024;10:dvae001. https://doi.org/10.1093/eep/dvae001.
Bieleninik L, Kvestad I, Gold C, Stordal AS, Assmus J, Arnon S, et al. Music therapy in infancy and neurodevelopmental outcomes in preterm children: a secondary analysis of the LongSTEP randomized clinical trial. JAMA Netw Open. 2024;7:e2410721. https://doi.org/10.1001/jamanetworkopen.2024.10721.
van der Steen JT, Smaling HJ, van der Wouden JC, Bruinsma MS, Scholten RJ, Vink AC. Music-based therapeutic interventions for people with dementia. Cochrane Database Syst Rev. 2018;7:CD003477. https://doi.org/10.1002/14651858.CD003477.pub4.
Sihvonen AJ, Sarkamo T, Leo V, Tervaniemi M, Altenmuller E, Soinila S. Music-based interventions in neurological rehabilitation. Lancet Neurol. 2017;16:648–60. https://doi.org/10.1016/S1474-4422(17)30168-0.
Bowling DL. Biological principles for music and mental health. Transl Psychiatry. 2023;13:374. https://doi.org/10.1038/s41398-023-02671-4.
Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. 2011;14:257–62. https://doi.org/10.1038/nn.2726.
Molnar-Szakacs I, Overy K. Music and mirror neurons: from motion to 'e'motion. Soc Cogn Affect Neurosci. 2006;1:235–41. https://doi.org/10.1093/scan/nsl029.
Hung PL, Wu KLH, Chen CJ, Siu KK, Hsin YJ, Wang LJ, et al. Music-based intervention ameliorates Mecp2-loss-mediated sociability repression in mice through the prefrontal cortex FNDC5/BDNF pathway. Int J Mol Sci. 2021;22. https://doi.org/10.3390/ijms22137174.
Sakamoto M, Ando H, Tsutou A. Comparing the effects of different individualized music interventions for elderly individuals with severe dementia. Int Psychogeriatr. 2013;25:775–84. https://doi.org/10.1017/S1041610212002256.
Gaden TS, Ghetti C, Kvestad I, Bieleninik L, Stordal AS, Assmus J, et al. Short-term music therapy for families with preterm infants: a randomized Trial. Pediatrics 2022;149. https://doi.org/10.1542/peds.2021-052797.
Witusik A, Pietras T. Music therapy as a complementary form of therapy for mental disorders. Pol Merkur Lekarski. 2019;47:240–3.
Jang S, Park I, Choi M, Kim J, Yeo S, Huh SO, et al. Impact of the circadian nuclear receptor REV-ERBalpha in dorsal raphe 5-HT neurons on social interaction behavior, especially social preference. Exp Mol Med. 2023;55:1806–19. https://doi.org/10.1038/s12276-023-01052-7.
Zhong XL, Huang Y, Du Y, He LZ, Chen YW, Cheng Y, et al. Unlocking the therapeutic potential of exosomes derived from nasal olfactory mucosal mesenchymal stem cells: restoring synaptic plasticity, neurogenesis, and neuroinflammation in schizophrenia. Schizophr Bull. 2024;50:600–14. https://doi.org/10.1093/schbul/sbad172.
Fu Q, Qiu R, Chen L, Chen Y, Qi W, Cheng Y. Music prevents stress-induced depression and anxiety-like behavior in mice. Transl Psychiatry. 2023;13:317. https://doi.org/10.1038/s41398-023-02606-z.
Liu S, Chen L, Guo M, Li Y, Liu Q, Cheng YJR. Targeted delivery of engineered RVG-BDNF-exosomes: a novel neurobiological approach for ameliorating depression and regulating neurogenesis. Research. 2024;7:0402.
Lieberman MD, Straccia MA, Meyer ML, Du M, Tan KM. Social, self, (situational), and affective processes in medial prefrontal cortex (MPFC): causal, multivariate, and reverse inference evidence. Neurosci Biobehav Rev. 2019;99:311–28. https://doi.org/10.1016/j.neubiorev.2018.12.021.
Angelopoulou E, Stanitsa E, Karpodini CC, Bougea A, Kontaxopoulou D, Fragkiadaki S, et al. Pharmacological and non-pharmacological treatments for depression in Parkinson’s disease: an updated review. Medicina (Kaunas). 2023;59, https://doi.org/10.3390/medicina59081454.
Feng J, Xu N, Wang L, Wang H, Zhou Y, Shen Q. Synaptic structure and transcriptomic profiling of reward and sensory brain areas in male mice of fentanyl addiction. Subst Abus Rehabil. 2024;15:233–45. https://doi.org/10.2147/SAR.S484167.
Smith NK, Kondev V, Hunt TR, Grueter BA. Neuropeptide Y modulates excitatory synaptic transmission and promotes social behavior in the mouse nucleus accumbens. Neuropharmacology. 2022;217:109201. https://doi.org/10.1016/j.neuropharm.2022.109201.
Basu J, Siegelbaum SA The corticohippocampal circuit, synaptic plasticity, and memory. Cold Spring Harb Perspect Biol. 2015;7. https://doi.org/10.1101/cshperspect.a021733.
Walsh JJ, Christoffel DJ, Malenka RC. Neural circuits regulating prosocial behaviors. Neuropsychopharmacology. 2023;48:79–89. https://doi.org/10.1038/s41386-022-01348-8.
Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. https://doi.org/10.1146/annurev.psych.56.091103.070234.
Meisner OC, Nair A, Chang SWC. Amygdala connectivity and implications for social cognition and disorders. Handb Clin Neurol. 2022;187:381–403. https://doi.org/10.1016/B978-0-12-823493-8.00017-1.
Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. https://doi.org/10.1146/annurev.neuro.24.1.677.
Shahraki A, Stone TW. Interactions between adenosine and metabotropic glutamate receptors in the rat hippocampal slice. Br J Pharm. 2003;138:1059–68. https://doi.org/10.1038/sj.bjp.0705083.
Schmandke A, Schmandke A, Strittmatter SM. ROCK and Rho: biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist. 2007;13:454–69. https://doi.org/10.1177/1073858407303611.
Maruska KP. Social transitions cause rapid behavioral and neuroendocrine changes. Integr Comp Biol. 2015;55:294–306. https://doi.org/10.1093/icb/icv057.
Froemke RC, Young LJ. Oxytocin, neural plasticity, and social behavior. Annu Rev Neurosci. 2021;44:359–81. https://doi.org/10.1146/annurev-neuro-102320-102847.
Love TM. Oxytocin, motivation and the role of dopamine. Pharmacol Biochem Behav. 2014;119:49–60. https://doi.org/10.1016/j.pbb.2013.06.011.
Yang Y, Wang ZH, Jin S, Gao D, Liu N, Chen SP, et al. Opposite monosynaptic scaling of BLP-vCA1 inputs governs hopefulness- and helplessness-modulated spatial learning and memory. Nat Commun. 2016;7:11935. https://doi.org/10.1038/ncomms11935.
McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. https://doi.org/10.1038/npp.2015.171.
Kim Y, Jang YN, Kim JY, Kim N, Noh S, Kim H, et al. Microtubule-associated protein 2 mediates induction of long-term potentiation in hippocampal neurons. FASEB J. 2020;34:6965–83. https://doi.org/10.1096/fj.201902122RR.
Liu Y, Shen X, Zhang Y, Zheng X, Cepeda C, Wang Y, et al. Interactions of glial cells with neuronal synapses, from astrocytes to microglia and oligodendrocyte lineage cells. Glia. 2023;71:1383–401. https://doi.org/10.1002/glia.24343.
Nagata S, Yamasaki R The involvement of glial cells in blood-brain barrier damage in neuroimmune diseases. Int J Mol Sci. 2024;25. https://doi.org/10.3390/ijms252212323.
Loeb JA. Neuroprotection and repair by neurotrophic and gliotrophic factors in multiple sclerosis. Neurology. 2007;68:S38–42. discussion S43-54. https://doi.org/10.1212/01.wnl.0000275231.97764.43.
Muller HW, Junghans U, Kappler J. Astroglial neurotrophic and neurite-promoting factors. Pharmacol Ther. 1995;65:1–18. https://doi.org/10.1016/0163-7258(94)00047-7.
Murphy-Royal C, Ching S, Papouin T. A conceptual framework for astrocyte function. Nat Neurosci. 2023;26:1848–56. https://doi.org/10.1038/s41593-023-01448-8.
Colardo M, Petraroia M, Lerza L, Pensabene D, Martella N, Pallottini V, et al. NGF modulates cholesterol metabolism and stimulates ApoE secretion in glial cells conferring neuroprotection against oxidative stress. Int J Mol Sci. 2022;23. https://doi.org/10.3390/ijms23094842.
Woodburn SC, Bollinger JL, Wohleb ES. The semantics of microglia activation: neuroinflammation, homeostasis, and stress. J Neuroinflammation. 2021;18:258. https://doi.org/10.1186/s12974-021-02309-6.
Schafer ST, Mansour AA, Schlachetzki JCM, Pena M, Ghassemzadeh S, Mitchell L, et al. An in vivo neuroimmune organoid model to study human microglia phenotypes. Cell. 2023;186:2111–2126 e2120. https://doi.org/10.1016/j.cell.2023.04.022.
Freeman MR. Specification and morphogenesis of astrocytes. Science. 2010;330:774–8. https://doi.org/10.1126/science.1190928.
Santello M, Toni N, Volterra A. Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci. 2019;22:154–66. https://doi.org/10.1038/s41593-018-0325-8.
Galland F, Seady M, Taday J, Smaili SS, Goncalves CA, Leite MC. Astrocyte culture models: molecular and function characterization of primary culture, immortalized astrocytes and C6 glioma cells. Neurochem Int. 2019;131:104538 https://doi.org/10.1016/j.neuint.2019.104538.
Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64:300–16. https://doi.org/10.1002/glia.22930.
Nguyen T, Flaten E, Trainor LJ, Novembre G. Early social communication through music: state of the art and future perspectives. Dev Cogn Neurosci. 2023;63:101279 https://doi.org/10.1016/j.dcn.2023.101279.
Bernier A, Ratcliff K, Hilton C, Fingerhut P, Li CY Art interventions for children with autism spectrum disorder: a scoping review. Am J Occup Ther. 2022;76. https://doi.org/10.5014/ajot.2022.049320.
Geretsegger M, Elefant C, Mossler KA, Gold C. Music therapy for people with autism spectrum disorder. Cochrane Database Syst Rev. 2014;2014:CD004381 https://doi.org/10.1002/14651858.CD004381.pub3.
Gangopadhyay P, Chawla M, Dal Monte O, Chang SWC. Prefrontal-amygdala circuits in social decision-making. Nat Neurosci. 2021;24:5–18. https://doi.org/10.1038/s41593-020-00738-9.
Isaac J, Karkare SC, Balasubramanian H, Schappaugh N, Javier JL, Rashid M, et al. Sex differences in neural representations of social and nonsocial reward in the medial prefrontal cortex. Nat Commun. 2024;15:8018. https://doi.org/10.1038/s41467-024-52294-6.
Chen H, Xiong XX, Jin SY, He XY, Li XW, Yang JM, et al. Dopamine D2 receptors in pyramidal neurons in the medial prefrontal cortex regulate social behavior. Pharmacol Res. 2024;199:107042. https://doi.org/10.1016/j.phrs.2023.107042.
Xiong Y, Chen J, Li Y. Microglia and astrocytes underlie neuroinflammation and synaptic susceptibility in autism spectrum disorder. Front Neurosci. 2023;17:1125428. https://doi.org/10.3389/fnins.2023.1125428.
Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here?. Nat Rev Neurol. 2021;17:157–72. https://doi.org/10.1038/s41582-020-00435-y.
Zheng ZH, Tu JL, Li XH, Hua Q, Liu WZ, Liu Y, et al. Neuroinflammation induces anxiety- and depressive-like behavior by modulating neuronal plasticity in the basolateral amygdala. Brain Behav Immun. 2021;91:505–18. https://doi.org/10.1016/j.bbi.2020.11.007.
Hughes HK, Moreno RJ, Ashwood P. Innate immune dysfunction and neuroinflammation in autism spectrum disorder (ASD). Brain Behav Immun. 2023;108:245–54. https://doi.org/10.1016/j.bbi.2022.12.001.
Maratos AS, Gold C, Wang X, Crawford MJ Music therapy for depression. Cochrane Database Syst Rev. 2008:CD004517. https://doi.org/10.1002/14651858.CD004517.pub2.
Geretsegger M, Mossler KA, Bieleninik L, Chen XJ, Heldal TO, Gold C. Music therapy for people with schizophrenia and schizophrenia-like disorders. Cochrane Database Syst Rev. 2017;5:CD004025. https://doi.org/10.1002/14651858.CD004025.pub4.
Author information
Authors and Affiliations
Contributions
YC and LL conceived and designed the study. RQ conducted the behavioral tests and data collection. LL was responsible for the analysis of sex differences in social behavior following music exposure. YS analyzed the relationship between music exposure duration and social ability. QF and ZH performed the molecular and biochemical assays. RQ, TY, HC and HZ conducted the immunofluorescence and histological analyses. YC analyzed and interpreted the data. WQ designed and provided the music intervention. RQ drafted the manuscript with critical revisions from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors have no biomedical financial interests or potential conflicts of interest to declare.
Ethics approval and consent to participate
All animal experiments within this investigation adhered strongly to the guidelines outlined by the National Institutes of Health for the Care and Use of Laboratory Animals (NIH Publication No. 80-23), receiving full endorsement from the Animal Care and Use Committee at Minzu University of China.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qiu, R., Li, L., Su, Y. et al. The impact of musical intervention during fetal and infant stages on social behavior and neurodevelopment in mice. Transl Psychiatry 15, 408 (2025). https://doi.org/10.1038/s41398-025-03645-4
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03645-4