Abstract
Background
Autologous platelet concentrates (APCs) have played a significant role in regenerative dentistry, with clinical evidence suggesting its benefits over controls. Particularly, APCs could reduce postoperative pain following tooth extractions.
Aim
To compare patient reported pain after tooth extractions using different autologous platelet concentrates (APCs) such as platelet-rich plasma (PRP) and platelet-rich fibrin (PRF).
Method
A search on Pubmed, Scopus, Embase and Google Scholar databases was conducted to identify human studies using APC(s) in extraction sockets between January 2014 and June 2024. This review followed the PRISMA guidelines. The inclusion criteria involved comparative human studies ranging from evidence levels II to III (Oxford Centre for Evidence-Based Medicine Levels of Evidence). For assessing bias in the included studies, the Cochrane Risk of Bias tools were used. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to determine the quality of evidence available.
Results
This review identified 8 studies; with 338 extraction sites in total and 1–15 days pain follow up. Four studies showed no statistically significant difference in postoperative pain reduction between PRP and PRF. One study observed no statistically significant difference between leukocyte-rich PRF (L-PRF) and titanium-prepared PRF (T-PRF). One study indicated that advanced platelet-rich fibrin (A-PRF) is superior to PRF in reducing postoperative pain on day 2 postoperatively. In addition, two studies reported that A-PRF is more effective than L-PRF on day 2. Moderate-to-high risk of bias was identified within 75% of the selected papers. GRADE score for evidence quality assessment was ‘Low’.
Conclusion
A-PRF was favoured to reduce postoperative pain on day 2 among the investigated APCs, although the GRADE criteria rate the evidence as “Low”. Future trials should directly compare A-PRF with PRF and L-PRF using high-quality randomized controlled designs.
Similar content being viewed by others
Introduction
Autologous platelet concentrates (APCs)
In recent years, autologous platelet concentrates (APCs) have played a significant role in regenerative dentistry, particularly in applications such as sinus floor elevation, peri-implantitis, medication-related osteonecrosis of the jaw (MRONJ), bone regeneration, and socket preservation [1, 2].
The first APC, platelet-rich plasma (PRP), was introduced in the field of haematology for transfusion purposes [3]. In 1990s, PRP was firstly used in oral and maxillofacial surgery [4]. It took approximately a decade for Choukroun to introduce the platelet-rich-fibrin (PRF) [5], leading to the development of various APC subcategories with and without anticoagulants, as well as formulations rich or poor in leucocytes [6]. These subcategories are further differentiated into unique formulations based on distinct preparation protocols [7, 8] (Fig. 1).
The literature supports that the main components of platelet concentrates for facilitating healing and repair processes are leucocytes and growth factors [9]. Upon activation, these growth factors, embedded within the fibrin matrix, have been shown to stimulate a mitogenic response in periosteal cells, promoting bone healing [10].
Socket preservation
Following tooth extraction, the supporting bone undergoes resorption [11], with two-thirds of the surrounding bone affected within the first three months [12]. Adequate bone levels are essential for the survival and long-term stability of dental implants [13, 14]. To mitigate bone loss, socket preservation strategies with biomaterials such as bone grafts and membranes are employed [15]. Numerous approaches to socket preservation have been developed over time [16], including the use of APCs like PRP and PRF [17]. Systematic reviews have demonstrated that APCs are effective in reducing vertical bone resorption following tooth extraction [17,18,19].
Pain following tooth extraction
Postoperative pain following tooth extraction is a common risk, persisting for up to seven days [20]. Key risk factors include oral hygiene, procedural difficulty, operator skill level, smoking status, age and the use of oral contraceptives [21]. A recent systematic review of 82 studies identified that the combination of ibuprofen and acetaminophen or naproxen, as well as oxycodone and acetaminophen, are the most effective oral analgesics for managing pain post-tooth extraction [22]. Nevertheless, oral analgesics may be insufficient for some patients [23], prompting the investigation of adjunctive interventions to further alleviate postoperative pain.
Various methods to mitigate postoperative pain have been studied, including photobiomodulation [24], submucosal injection of dexamethasone [25], and low-level laser therapy [26]. However, these interventions do not provide the additional benefits of enhanced healing and socket preservation observed with the use of APCs [27, 28].
Rationale behind this review
Conventionally, oral analgesics are routinely used to reduce postoperative pain following tooth extraction. However, given that oral analgesics are not always sufficient and considering the added benefits of APCs, such as promoting healing and socket preservation, it is reasonable to consider APCs as adjunct interventions for postoperative pain management. Therefore, identifying the most effective APC for this application is warranted.
The aim of this evidence-based review was to evaluate the current evidence to identify the best APC for reducing postoperative pain following tooth extraction.
Methods
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) was followed for reporting this review (Supplement 1) [29]. The PICO framework was used to structure the reporting of eligibility criteria [30]:
(P) Population: Controlled clinical studies involving tooth extractions
(I) Intervention: Application of an APC in extraction sockets
(C) Comparison: Application of another APC in extraction sockets
(O) Outcomes: Patient self-reported pain
Search strategy
PubMed, Scopus, Embase and Google Scholar were the main databases used for conducting the search for articles in this review. Table 1 presents the key search terms used for articles retrieval. The databases were searched between 1st of January 2014 and June 24th, 2024. The search was confined to peer-reviewed journal articles indexed in the selected electronic databases; grey-literature sources were not explored.
Study selection
The eligibility criteria (Supplement 1b) ensured that the selected studies focused on comparing different APCs in reducing postoperative pain following tooth extraction.
Study selection was conducted by two independent reviewers (HH, RS) in the following stages: (1) Initial screening of potentially suitable titles and abstracts against the inclusion criteria to identify potentially relevant papers. (2) Screening of the full papers identified as possibly relevant in the initial screening. (3) Studies were excluded if not meeting the inclusion criteria. Following the screening of titles and abstracts, the studies included by both reviewers were compared. In case of a disagreement between reviewers, the decision about study eligibility was made by trying to reach a consensus between the two reviewers. In case of continued disagreement, a third reviewer (INP) judged study inclusion.
Data collection
In relation to each investigated study, data collection was completed independently by two reviewers (HH, RS). Another author (INP) reviewed extracted data and resolved any discrepancies.
(1) Study Publication Details: Authorship, year of publication, and country of origin. (2) Study Characteristics: Demographic variables including extraction sites, sex and age. (3) Study Methodology: APCs employed, APC preparation protocols and investigated outcomes. The selected studies were categorised using the Oxford Centre for Evidence-Based Medicine Levels of Evidence (OCEBM) classification system [31]. (4) Study Outcomes: Patient reported pain.
Data preparation
In instances where a specific data point was entirely absent, we systematically documented and presented this absence as “Not Reported” (NR) in our analysis. Regarding continuous variables, such as age, when distinct values were provided for each respective group and there were no statistically significant difference between the groups, we combined mean and standard deviation values into our table.
Risk of bias
The Risk of Bias 2 (RoB2) assessment tool used to assess risk of bias for RCTs (Level II) [32], and the Risk of Bias tool of Non-randomised Studies - of Interventions (ROBINS-I) for cohort studies (III) [33]. The bias category of ‘Some concerns’ was labelled as ‘Moderate’ for the purpose of font size clarity in the generated tables.
For each study the overall bias was given based on the highest bias score for each decision category. For example, if the highest score of ‘high’ was estimated for one or more decision categories, then the overall bias was considered ‘high’.
Data analysis
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach [34] was used to determine the quality of evidence available in the identified studies for this evidence-based review [34]. Each component of the GRADE approach was independently assessed by two reviewers (RS, HH). In cases of disagreement, another author (INP) facilitated a discussion to ensure consensus was reached. It was anticipated that the nature of patient-reported outcome measurement would lead to high heterogeneity in results and methodologies, hence, a quantitative meta-analysis would not be feasible. Therefore, a qualitative comparison network was employed alternatively.
Results
Studies included
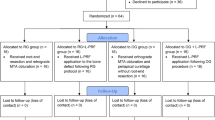
Figure 2 shows the PRISMA flowchart representing study selection and inclusion [29]. The initial search resulted in 8321 papers for all databases combined. This was trimmed down to 2412 after duplicates were removed. Following the first-stage screening of titles and abstracts, 49 articles (considered potentially suitable by at least one reviewer) qualified for full-text screening. After full-text reading; 8 studies, with 338 extraction sites in total and 1–15 days pain follow up, met the inclusion criteria, and 41 papers were excluded.
Study characteristics
Table 1 reports the studies and their characteristics, 4 RCTs and 4 cohort studies [35,36,37,38,39,40,41,42]. The table shows the authors, year of publication, country of publication, study type, level of evidence, risk of bias, APC preparation protocol, groups characteristics, outcomes measured and pain results. Studies were classified as level of evidence II for RCTs and level III for cohort studies (Table 2).
Risk of bias
The risk of bias assessment focused exclusively on patient-reported pain, excluding bias considerations related to other outcomes such as trismus, swelling or bone levels. Two studies exhibited a low risk of bias, three studies had a moderate risk of bias, and three studies showed a high risk of bias (Fig. 3). Despite the varying levels of bias, there was no evidence suggesting that any study was biased in favour of a particular protocol regarding postoperative pain scores.
Pain
Owing to the small number of eligible studies (n = 8), the heterogeneity in pain measurement methodologies and the subjective nature of patient-reported pain; subgroup, sensitivity, meta-, or meta-regression analyses were not undertaken. Consequently, results of the studies were synthesised narratively, without statistical pooling, with a qualitative comparative network analysis.
Four studies, with varying levels of bias (low, moderate, and high), have demonstrated no statistically significant difference in postoperative pain between platelet-rich plasma (PRP) and platelet-rich fibrin (PRF). One study indicated that advanced platelet-rich fibrin (A-PRF) is superior to PRF in reducing postoperative pain, with high risk of bias. Additionally, two studies reported that A-PRF is more effective than leukocyte-rich PRF (L-PRF) in alleviating postoperative pain on day 2, with moderate to high risk of bias. Another study found A-PRF+ to be more effective than L-PRF in managing postoperative pain with a moderate risk of bias, although no statistically significant difference was observed between A-PRF and A-PRF + . One study with a low risk of bias found no statistically significant difference in postoperative pain between leukocyte-rich platelet-rich fibrin (L-PRF) and titanium-prepared platelet-rich fibrin (T-PRF). (Fig. 4).
GRADE
Table 3 reflects the summary of the GRADE assessment carried in this review. 6 out of 8 studies were assessed as having a moderate to high risk of bias. Nevertheless, there is no evidence to suggest that biases either reduce or increase the effect size between APCs. Additionally, no study presented results that contradicted those of another study and no study demonstrated a large effect size. Statistical test for heterogeneity could not be observed as results were quantitatively incomparable.
Furthermore, 7 out of 8 of the studies focused exclusively on third molar extractions, highlighting a narrow scope in the research. A significant gap in the literature is the lack of direct comparison studies between L-PRF and PRF on postoperative pain reduction following tooth extraction.
Owing to the large heterogeneity described earlier—particularly differences in APC preparation protocols, subjective pain scales, follow-up periods and third-molar–only study populations—pooling data was neither statistically nor clinically appropriate. Instead, we qualitatively mapped the network of available comparisons and judged consistency by narrative synthesis. While this approach preserves clinical nuance, it inevitably yields wider uncertainty around the true magnitude of differences between any APCs. Consequently, the variability of individual results was assessed to categorise imprecision as low, moderate, or high. This categorisation was based on thresholds corresponding to how wide were the estimates of pain Visual Analogue Scale (VAS) results. Specifically, variability corresponding to a 95% confidence interval of 4 points or higher was considered high, variability within 2–4 points was deemed moderate, and variability of 2 points or lower was categorised as low. 13 out of 15 (87%) of the individual results, which demonstrated a statistically significant difference in pain scores on days 2 and 3, exhibited low to moderate imprecision/variability (Fig. 5).
The top left plot categorises pain scores into three imprecision categories—low, moderate, and high—based on the standard deviation (SD) and confidence intervals (CI). The top right plot shows the distribution of imprecision/variability across different days (Day 1, Day 2, Day 3, Day 7) for each intervention. The bottom plot displays the frequency of these imprecision categories by day.
There were insufficient comparative pairs of APCs data to construct a meaningful funnel plot or to conduct Egger’s test to assess for publication bias. Nevertheless, 88% (7/8) of the studies reported that the authors did not have any relevant financial relationships with commercial interests. In contrast, 12% (1/8) of the studies did not provide information regarding the presence of external sources of funding. Based on the evidence from the studies included in this review, the overall GRADE assessment of the quality of evidence has been determined to be ‘Low’.
Discussion
The current literature on APCs supports their added benefits in socket preservation [17, 18]. However, in maxillary sinus augmentations, studies have demonstrated no additional benefits of APCs when combined with bone grafts [43,44,45]. Furthermore, Alrayyes (2022) highlights the lack of standardised preparation protocols for specific APC formulations [19].
Patient-reported outcome measures (PROMs), such as self-reported pain, are valuable tools for monitoring treatment responses and patient quality of life, particularly when collected soon after treatment. These measures aid in investigating clinical factors that enhance patient communication and satisfaction, ultimately improving patient engagement with the care plan provided [46,47,48].
Since pain is likely to be the first postoperative complication, starting from day 0 and lasting up to day 7 [20], it is valuable for patient satisfaction and quality of life to determine strategies to reduce it.
Research by Mourão (2020) shows that L-PRF improves healing and decreases pain following tooth extraction compared to controls [49]. A systematic review by Al-Maawi (2021) confirms the efficacy of PRF over control in reducing postoperative pain [50].
Our review explored comparative studies between different APCs regarding their efficacy in postoperative pain reduction, revealing that A-PRF and A-PRF+ offer additional benefits over L-PRF and PRF, with no significant difference between PRF and PRP.
Implications for clinical practice
For tooth extractions, APCs can be applied to extraction sites to promote faster healing and reduce postoperative pain. Nonetheless, there are practical limitations for using APCs in dental practice:
-
1.
The preparation of APCs requires specialised equipment, which can be costly.
-
2.
Dental practitioners need specialised training to effectively use APCs. This includes understanding the preparation, handling, and application of APCs, since different centrifugation speeds, times, and techniques can produce varying qualities and quantities of APCs [8].
-
3.
Patients with blood disorders such as thrombocytopenia may not be suitable candidates for APCs therapy since their platelets are compromised. Likewise, conditions such as diabetes, immunocompromising diseases and chronic infections can impact the body’s healing response [51, 52] and as a result could reduce the effectiveness of APCs treatments.
-
4.
Medications such as anticoagulant and antiplatelet (e.g., warfarin, aspirin) and nonsteroidal anti-inflammatory drugs (NSAIDs) can affect platelet function [53] and hence could reduce the overall effectiveness of APCs. Furthermore, the use of corticosteroids may interfere with the body’s healing process [52] and thus may affect the efficacy of APCs.
-
5.
Patients must be fully informed of the relative novel nature of APCs and the potential efficacy and benefits involved.
Evidence identified in this review supports A-PRF as the most effective APC for postoperative pain reduction following tooth extraction, however, with a ‘Low’ GRADE recommendation level. This means that the estimated effect of an intervention may be substantially different from the true effect. Consequently, further clinical research is necessary to support a strong recommendation for the general practice use of A-PRF.
Limitations
The inclusion criteria limited the selection to studies available exclusively in English, potentially excluding articles that might either support or contradict the conclusions of this review. Additionally, the temporal range of considered articles, from January 2014 to June 2024, may not consider all relevant developments in the field. In addition, only eight studies met the eligibility criteria, thereby limiting the generalisability and statistical power of the findings. Also, seven out of eight studies focused exclusively on third molar extractions, which further narrows the clinical applicability of the results to other types of tooth extractions. Furthermore, the lack of direct comparisons between PRF and L-PRF highlights a gap in the literature that reduces the comprehensiveness of the review. Another important limitation is the inherent heterogeneity among the selected papers, possibly arising from variations in methodologies, populations, or APC preparations, introducing potential inconsistencies: centrifugation speeds ranged from 1300 rpm (single-spin PRF) to 3000 rpm (second spin in two-step PRP protocols) with total spin times of 8–15 min; several studies employed sequential double-spin methods. Preparations differed in both leukocyte content (e.g., L-PRF vs. PRF) and anticoagulant use (present in all PRP arms but absent from PRF-type concentrates). Pain was measured with either 0–10 or 0–100 visual-analogue scales at postoperative intervals spanning 1 to 15 days. Surgical difficulty and operator experience were seldom reported. These discrepancies limit direct comparability and likely underlie the dispersion of point estimates observed in Fig. 4. Moreover, a notable constraint is the presence of moderate-to-high risk of bias within 75% of the selected papers, coupled with a ‘Low’ GRADE score for evidence quality assessment, which could affect the overall validity and reliability of the synthesised findings. The inability to conduct a meta-analysis impeded a robust evaluation of imprecision using a combined confidence interval and hindered a robust assessment of publication bias through funnel plots or Egger’s Test. Finally, the present review was not previously registered, which increases the risk of an unplanned duplication.
Recommendations for future trials
The current literature clearly demonstrates the benefits of autologous platelet concentrates (APCs) in pain management, socket preservation, bone regeneration, and promoting healing compared to controls. Future trials should focus on comparing different APCs to each other to identify the most effective APC for achieving specific benefits in particular clinical settings. Furthermore, it would be beneficial to investigate which patients could derive the most benefit from the procedure by identifying specific patient characteristics predictive of a favourable or unfavourable response. This knowledge would aid practitioners in case selection for implementing APCs.
In the context of tooth extraction, this review identified limited evidence suggesting that A-PRF and A-PRF+ are more effective in reducing postoperative pain compared to L-PRF and PRF directly, and PRP indirectly. Notably, none of the eight eligible trials offered a direct head-to-head comparison between the conventional PRF and its leukocyte-rich variant (L-PRF). This omission represents a gap in the literature.
Conclusion
Between January 2014 and June 2024, 8 clinical studies have been identified that compare the efficacy of different APCs for postoperative pain management following tooth extraction. A-PRF and A-PRF+ were favoured to reduce postoperative pain on day 2 among the investigated APCs, although the GRADE criteria rate the evidence as “Low”. This conclusion must be interpreted cautiously in view of large heterogeneity in the studies’ methods and follow-up periods (1–15 days). Future trials should directly compare A-PRF or A-PRF+ with PRF and L-PRF using high-quality randomised-controlled designs.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Mijiritsky E, Assaf HD, Peleg O, Shacham M, Cerroni L, Mangani L. Use of PRP, PRF and CGF in periodontal regeneration and facial rejuvenation—a narrative review. Biology. 2021;10.
Liu Y, Sun X, Yu J, Wang J, Zhai P, Chen S, et al. Platelet-Rich Fibrin as a Bone Graft Material in Oral and Maxillofacial Bone Regeneration: Classification and Summary for Better Application. Biomed Res Int. 2019;2019. https://pubmed.ncbi.nlm.nih.gov/31886202/
Kingsley CS. Blood Coagulation: Evidence of an Antagonist to Factor VI in Platelet-Rich Human Plasma. Nature 1954 173:4407 [Internet]. 1954;173:723–4. https://www.nature.com/articles/173723a0
Whitman DH, Berry RL, Green DM. Platelet gel: An autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997;55:1294–9.
Choukroun J, Adda F, Schoeffler C, Vervelle A. The opportunity in perio-implantology: The PRF. 2001.
Dohan Ehrenfest DM, Sammartino G, Shibli JA, Wang HL, Zou DR, Bernard JP. POSEIDO. 2013;1(1) Classification of platelet concentrates Special Review: Consensus Conference Guidelines for the publication of articles related to platelet concentrates (Platelet-Rich Plasma-PRP, or Platelet-Rich Fibrin-PRF): The International Classification of the POSEIDO.
Saini K, Chopra P, Sheokand V. Journey of platelet concentrates: a review. Biomed Pharmacol J. 2020;13:185–91.
Calciolari E, Dourou M, Akcali A, Donos N. Differences between first- and second-generation autologous platelet concentrates. Periodontol 2000. 2024; https://onlinelibrary.wiley.com/doi/full/10.1111/prd.12550
Bartold M, Ivanovski S. Biological processes and factors involved in soft and hard tissue healing. Periodontol 2000. 2024; https://onlinelibrary.wiley.com/doi/full/10.1111/prd.12546
Gassling V, Douglas T, Warnke PH, Açil Y, Wiltfang J, Becker ST. Platelet-rich fibrin membranes as scaffolds for periosteal tissue engineering. Clin Oral Implants Res. 2010;21:543–9.
Hansson S, Halldin A. Alveolar ridge resorption after tooth extraction: A consequence of a fundamental principle of bone physiology. J Dent Biomech. 2012;3:1–8.
Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restor Dent. 2003;23:313–23.
Meffert RM, Langer B, Fritz ME. Dental implants: a review. J Periodontol. 1992;63:859–70.
Heinemann F, Hasan I, Bourauel C, Biffar R, Mundt T. Bone stability around dental implants: Treatment related factors. Ann Anat Anatomischer Anz. 2015;199:3–8.
Wang RE, Lang NP. Ridge preservation after tooth extraction. Clin Oral Implants Res. 2012;23:147–56.
Kim S, Kim SG. Advancements in alveolar bone grafting and ridge preservation: a narrative review on materials, techniques, and clinical outcomes. Maxillofac Plast Reconstr Surg. 2024;46:1–13.
Zhang Y, Du R, Yang B, Tao J, Jing W. Efficacy of autologous platelet concentrate products for alveolar preservation: a meta-analysis. Oral Dis. 2024; https://onlinelibrary.wiley.com/doi/full/10.1111/odi.14874
Caponio VCA, Baca-González L, González-Serrano J, Torres J, López-Pintor RM. Effect of the use of platelet concentrates on new bone formation in alveolar ridge preservation: a systematic review, meta-analysis, and trial sequential analysis. Clin Oral Investig. 2023;27:4131–46.
Alrayyes Y, Al-Jasser R. Regenerative Potential of Platelet Rich Fibrin (PRF) in Socket preservation in comparison with conventional treatment modalities: a systematic review and meta-analysis. Tissue Eng Regen Med. 2022;19:463–75.
Seymour RA, Blair GS, Wyatt FAR. Post-operative dental pain and analgesic efficacy. Part I. Br J Oral Surg. 1983;21:290–7.
Rakhshan V. Common risk factors for postoperative pain following the extraction of wisdom teeth. J Korean Assoc Oral Maxillofac Surg. 2015;41:59.
Miroshnychenko A, Ibrahim S, Azab M, Roldan Y, Martinez JPD, Tamilselvan D, et al. Acute postoperative pain due to dental extraction in the adult population: a systematic review and network meta-analysis. J Dent Res. 2023;102:391–401.
Savin J, Ogden GR. Third molar surgery—a preliminary report on aspects affecting quality of life in the early postoperative period. Br J Oral Maxillofac Surg. 1997;35:246–53.
Isolan C, Kinalski MD, Leão OA, Post LK, Isolan TM, Dos Santos MB. Photobiomodulation therapy reduces postoperative pain after third molar extractions: A randomized clinical trial. Med Oral Patol Oral Cir Bucal. 2021;26:e341.
Chen Q, Chen J, Hu B, Feng G, Song J. Submucosal injection of dexamethasone reduces postoperative discomfort after third-molar extraction: A systematic review and meta-analysis. J Am Dent Assoc. 2017;148:81–91.
Landucci A, Wosny AC, Uetanabaro LC, Moro A, Araujo MR. Efficacy of a single dose of low-level laser therapy in reducing pain, swelling, and trismus following third molar extraction surgery. Int J Oral Maxillofac Surg. 2016;45:392–8.
Sharma A, Ingole S, Deshpande M, Ranadive P, Sharma S, Kazi N, et al. Influence of platelet-rich fibrin on wound healing and bone regeneration after tooth extraction: A clinical and radiographic study. J Oral Biol Craniofac Res. 2020;10:385–90.
Fabbro M, Del, Corbella S, Taschieri S, Weinstein R, Francetti L. Autologous platelet concentrate for post-extraction socket healing: A systematic review. Review J Oral Implantol. 2014;7:333–44.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372. https://pubmed.ncbi.nlm.nih.gov/33781993/
Richardson W, Wilson M, Nishikawa J, Hayward R. The well-built clinical question: a key to evidence-based decisions - PubMed. ACP J Club. 1995;123:12.
Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, et al. OCEBM Levels of Evidence Working Group. The Oxford Levels of Evidence 2. Oxford Centre for Evidence-Based Medicine. 2011; https://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366. https://www.bmj.com/content/366/bmj.l4898
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355. https://www.bmj.com/content/355/bmj.i4919
Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–2.
Dutta S, Passi D, Singh P, Sharma S, Singh M, Srivastava D. A randomized comparative prospective study of platelet-rich plasma, platelet-rich fibrin, and hydroxyapatite as a graft material for mandibular third molar extraction socket healing. Natl J Maxillofac Surg. 2016;7:45.
Unakalkar S, Bhushan K, Sahu R. Comparison of the efficacy of platelet-rich fibrin with platelet-rich plasma in third molar extraction socket-a prospective clinical study. Int J Oral Care Res. 2024;6:1–6. https://www.researchgate.net/publication/358571108
Caymaz M, Uyanik L. Comparison of the effect of advanced platelet-rich fibrin and leukocyte- and platelet-rich fibrin on outcomes after removal of impacted mandibular third molar: A randomized split-mouth study. Niger J Clin Pr. 2019;22:546–52.
Bhujbal R, Veerabhadrappa SK, Yadav S, Chappi M, Patil V. Evaluation of platelet-rich fibrin and platelet-rich plasma in impacted mandibular third molar extraction socket healing and bone regeneration: A split-mouth comparative study. Eur J Gen Dent. 2020;9:96–102.
Ustaoğlu G, Göller Bulut D, Gümüş K. Evaluation of different platelet-rich concentrates effects on early soft tissue healing and socket preservation after tooth extraction. J Stomatol Oral Maxillofac Surg. 2020;121:539–44.
Kumar Verma V, Sublok K, Kushwaha AK, Patra D, Khare A, Singh J. Original Research A prospective study to evaluate post operative clinical parameters of platelet rich fibrin and platelet rich plasma in third molar extraction sockets: An original research study. J Adv Med Dental Sci Res. 2021;1. www.jamdsr.com
Riaz R, Radhakrishnan M, Perumal J. Comparative study of the efficacy of advanced platelet-rich fibrin and standard platelet-rich fibrin in mandibular third molar surgery. J Pharm Bioallied Sci. 2022;14:S781.
Yari A, Fasih P, Ghotbi N, Badkoobeh A, Goodarzi A, Hosseini Hooshiar M. Do Platelet-rich concentrates improve the adverse sequelae of impacted mandibular third molar removal?. J Oral Maxillofac Surg. 2024;82:671–83.
Idiri K, Bandiaky O, Soueidan A, Verner C, Renard E, Struillou X. The effectiveness of the addition of platelet-rich fibrin to bovine xenografts in sinus and bone ridge augmentation: a systematic review. J Funct Biomater. 2023. https://pubmed.ncbi.nlm.nih.gov/37504884/
Dragonas P, Katsaros T, Avila-Ortiz G, Chambrone L, Schiavo JH, Palaiologou A. Effects of leukocyte–platelet-rich fibrin (L-PRF) in different intraoral bone grafting procedures: a systematic review. Int J Oral Maxillofac Surg. 2019;48:250–62.
Lemos CAA, Mello CC, Dos Santos DM, Verri FR, Goiato MC, Pellizzer EP. Effects of platelet-rich plasma in association with bone grafts in maxillary sinus augmentation: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2016;45:517–25.
Wartolowska K, Dieppe P, Kong J. The nocebo effect as a source of bias in the assessment of treatment & effects. F1000Research. 2019;8:5. https://f1000research.com/articles/8-5
Bradley C, Eschwège E, De Pablos-Velasco P, Parhofer KG, Simon D, Vandenberghe H, et al. Predictors of Quality of Life and Other Patient-Reported Outcomes in the PANORAMA Multinational Study of People With Type 2 Diabetes. Diab Care [Internet]. 2018;41:267–76. https://doi.org/10.2337/dc16-2655.
Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013;13:1–24.
de Almeida Barros Mourão CF, de Mello-Machado RC, Javid K, Moraschini V. The use of leukocyte- and platelet-rich fibrin in the management of soft tissue healing and pain in post-extraction sockets: A randomized clinical trial. J Cranio-Maxillofac Surg. 2020;48:452–7.
Al-Maawi S, Becker K, Schwarz F, Sader R, Ghanaati S. Efficacy of platelet-rich fibrin in promoting the healing of extraction sockets: a systematic review. Int J Implant Dent. 2021;7:1–27.
Dasari N, Jiang A, Skochdopole A, Chung J, Reece EM, Vorstenbosch J, et al. Healing, Inflammation, and Fibrosis: Updates in Diabetic Wound Healing, Inflammation, and Scarring. Semin Plast Surg. 2021;35:153.
Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–29.
Villa Zapata L, Hansten PD, Panic J, Horn JR, Boyce RD, Gephart S, et al. Risk of bleeding with exposure to warfarin and nonsteroidal anti-inflammatory drugs: a systematic review and meta-analysis. Thromb Haemost. 2020;120:1066.
Funding
This study received no funding. One of the authors (INP) is supported by a grant from Fundação para a Ciência e a Tecnologia (FCT) (2023.00543.BD) as a Doctoral Scholarship.
Author information
Authors and Affiliations
Contributions
Haidar Hassan and Rawand Shado contributed to data collection, data analysis. All authors contributed critical revisions of the article. All authors read and approved the final version of the submitted article and title page.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests or personal relationships that could have influence this paper. This study is a review of previously published literature and did not involve the collection of primary data from human participants, hence no ethical approval or consent was required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassan, H., Shado, R., Novo Pereira, I. et al. Patient reported pain following tooth extraction with different autologous platelet concentrates. Systematic review. BDJ Open 11, 66 (2025). https://doi.org/10.1038/s41405-025-00348-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41405-025-00348-2