Abstract
Internal tandem duplications (ITD) in fms-like tyrosine kinase 3 (FLT3) represent the most common genetic alteration in de novo acute myeloid leukemia (AML). Here, we identify ribosomal protein s6 kinase a1 (RSK1) as a core dependency in FLT3-ITD AML and unveil the existence of crucial bi-directional regulation. RSK1 perturbation resulted in marked apoptosis and abrogated phosphorylation of FLT3 and associated downstream signaling cascades in FLT3-ITD AML cell lines. Using cycloheximide, MG-132, and ubiquitination assays, we further demonstrate mechanistically that RSK1 regulates FLT3-ITD activity, and protein stability through deubiqutinase USP1, which we identify as a second dependency. Importantly, multivariate analysis revealed heightened expression of RPS6KA1 and USP1 to be associated with poor patient prognosis, and these effectors may serve as biomarkers predictive of patient survival and therapeutic response to FLT3-ITD inhibitors. Lastly, RSK1 inhibition utilizing a first-in-class RSK inhibitor, PMD-026, that is currently undergoing Phase 2 development for breast cancer, diminished leukemic disease burden in MV4-11 xenograft and syngeneic Flt3ITDTet2KO leukemia models. These findings illustrate an unconventional and promising therapeutic strategy targeting FLT3-ITD leukemia.
Similar content being viewed by others
Introduction
De novo acute myeloid leukemia (AML) is the most common adult myeloid cancer with incidence of 20,000 cases per year in the United States and is characterized by clonal expansion of immature myeloblasts that eventually lead to inadequate hematopoiesis, bone marrow failure, and blast crisis [1]. Rapid advancement in sequencing platforms in the last decade has unraveled the heterogeneous landscape of de novo AML, identifying numerous hotspot mutations in genes including NPM1, RUNX1, TP53, IDH1/2, and FLT3, with alterations of the latter being identified in up to one-third of patients and associated with poor prognosis [2,3,4,5].
FLT3 is a receptor tyrosine kinase expressed on hematopoietic stem/progenitor cells and upon ligand binding undergoes dimerization and trans-phosphorylation to propagate cell proliferation and differentiation [6]. Internal tandem duplication (ITD) in the juxtamembrane (JM) domain or point mutations in the tyrosine kinase domain (TKD) of FLT3 lead to constitutively active oncogenic signaling [6]. Currently, three inhibitors targeting mutant FLT3 have been FDA approved for use in AML either as monotherapy or in combination with conventional chemotherapy including midostaurin, quizartinib, and gilteritinib. However, several resistance mechanisms of FLT3 inhibitors such as onset of secondary resistance mutations in FLT3 and arising in other pathways such as RAS/MAPK have hindered the clinical impact for a subset of patients [7]. Thus, there remains a pressing need for evaluation of therapeutic agents that dampen this oncogenic pathway dependency through other mechanisms in both the treatment-naïve and treatment-resistant settings.
We previously identified RSK1 (RPS6KA1; p90RSK) to be an important signal transducer in the mTOR/AKT/S6 pathway and whose expression was essential for leukemia survival [8]. RSK1 has also been demonstrated to be activated by FLT3-ITD and inhibition by RSK inhibitors FMK and LJI308 inhibited cancer cell line proliferation in vitro in this genetic setting [9]. However, these pan-RSK inhibitors in addition to BI-D1870 have not been effectively utilized in animal studies due to poor pharmacokinetic profiles [9, 10]. Recently, a novel, first-in-class oral RSK1-4 inhibitor, PMD-026, has been developed for treatment of breast cancer and is currently undergoing phase 1/1b/2 clinical trials (NCT04115306) [11, 12]. Thus, we aimed to evaluate the therapeutic efficacy of targeting RSK1 in FLT3-ITD AML and repurposing this potential compound for the treatment of myeloid malignancies.
Results
RSK1 is elevated in treatment-naive and inhibitor-resistant FLT3-ITD AML
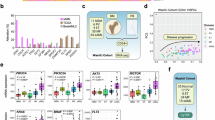
We demonstrated that targeting RSK1 could be therapeutic in myeloproliferative disease and chronic myelomonocytic leukemia models [13]. Thus, we further investigated if other myeloid malignancies exhibited similar dependencies. Patients positive for FLT3-ITD in the BeatAML2 cohort [14] displayed significantly elevated RPS6KA1 expression (Fig. 1A). To verify possible regulation of RSK1 by mutant FLT3, we ectopically expressed FLT3-ITD as well as FLT3 D835Y, a constitutively activating TKD hotspot mutant, in Ba/F3 cells. Indeed, both oncogenic mutations led to elevated phosphorylation of RSK1 and downstream effector S6 (Fig. 1B).
A RPS6KA1 expression in patients with positive (n = 158) or negative (n = 511) FLT3-ITD consensus calling from the BeatAML2 cohort. B Immunoblot of Ba/F3 cells transduced with FLT3-ITD, FLT3 D835Y, or GFP control plasmids. C scRNA-seq re-analysis of the myeloid population from serial gilteritinib treated FLT3-mutated AML patient samples from GSE199333. Samples were denoted as “sensitive” (n = 16,810 cells) or “resistant” (n = 30,993 cells) per source publication. D Venn diagram of shared DEGs upregulated in samples from three AML patients that developed resistance to gilteritinib compared to pre-treatment samples from (C). E Hallmark GSEA of shared 244 DEGs from gilteritinib-resistant patients from (D). F Expression dot plot showing expression changes of FLT3 and RPS6KA1 across gilteritinib treatment timepoints in patients who were sensitive or became resistant to treatment.
We then examined a publicly available scRNA-seq dataset [15] of AML patients harboring FLT3-ITD mutations with serial samples obtained before and after treatment with FLT3 inhibitor gilteritinib. From this dataset, four patients were responsive to therapy (PTs 4, 17, 20, 22; sensitive) and three were unresponsive (PTs 2, 5, 21; resistant). We performed additional analysis on a subsetted myeloid population representing 47,803 cells (Fig. 1C). Resistant patients harbored 244 shared DEGs upregulated in the post-gilteritinib treatment samples relative to pre-treatment (Fig. 1D and Supplementary Table 1). The 244 shared DEGs included DUSP6 and KLF2, both of which we previously reported to be important in mediating MPN transformation to sAML, and in the regulation of the DUSP6-RSK1 signaling axis [8], and GSEA revealed enrichment of the PI3K/AKT/mTOR and mTORC1 signaling pathways (Fig. 1E). We also observed consistent upregulation of RPS6KA1 across resistant timepoints with increases in the myeloid population expressing RPS6KA1 and in RPS6KA1 expression on a per cell basis most evident in PT2 (Fig. 1F). Furthermore, consistent reduction of RPS6KA1 expression was identified across all patients who were responsive to gilteritinib. Of note, FLT3 expression followed a similar trend as RPS6KA1 in increased expression among resistant patients and decreased expression among sensitive patients, except for PT5. These observations further support a role of RSK1 and associated PI3K/AKT/mTOR pathway in mediating disease pathogenesis and potential utility of RSK1 as a clinical biomarker in predicting response to FLT3 inhibitors.
FLT3-ITD AML exhibit marked sensitivity to RSK1 inhibition
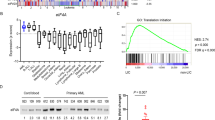
We aimed to further understand the relationship between RSK1 and FLT3. We investigated the top co-dependencies with RPS6KA1 knockout from the CRISPR DepMap database in 26 AML cell lines and observed FLT3 and FLT3LG (ranked 116 and 422 out of 17,385 genes, respectively) as top candidates (Supplementary Fig. 1A, Supplementary Table 2). Treatment with FLT3 inhibitors quizartinib and midostaurin suppressed RSK1 phosphorylation in FLT3-ITD mutant AML cell line MV4-11 (Supplementary Fig. 1B), which showed the opposite phenotype as FLT3-ITD ectopic expression (Fig. 1B), together suggesting that RSK1 could be a core dependency downstream of FLT3. Indeed, we also observed that FLT3-ITD cell lines MOLM-13 and MOLM-14 were most sensitive to RPS6KA1 knockout (Fig. 2A). This was further validated with shRNA knockdown of RPS6KA1 in MV4-11 and MOLM-13, which led to marked apoptosis (Fig. 2B). We then targeted RSK1 pharmacologically using a novel RSK inhibitor, PMD-026. FLT3-ITD mutant cell lines were preferentially sensitive to PMD-026 compared to cell lines carrying JAK2 V617F and BCR-ABL mutations (Fig. 2C). Furthermore, anti-proliferative effects of PMD-026 were comparable to those of the dual JAK2/FLT3 inhibitor fedratinib, while these cells were relatively resistant to the JAK1/JAK2 inhibitor ruxolitinib (Fig. 2D). Immunoblotting of cells following RPS6KA1 knockdown by shRNA and PMD-026 treatment revealed consistent suppression of pRSK1 and pS6 (Fig. 2E, F) and demonstrated on-target inhibition by PMD-026. We also observed dose-dependent suppression of RB phosphorylation and cyclin D1 expression by PMD-026 and a second RSK inhibitor, BI-D1870, suggesting anti-cell cycle consequences following RSK1 inhibition (Fig. 2G). Notably, there was also decreased phosphorylation of FLT3 and its downstream effector STAT5 (Fig. 2G).
A FLT3 and RPS6KA1 KO gene dependency score across 26 AML cell lines from DepMap. B Bi-axial flow cytometry plots of apoptosis and necrosis markers in FLT3-ITD cell lines following RPS6KA1 knockdown. C PMD-026 sensitivity across a myeloid cell line panel representative of various driver mutations. Cells were treated across various PMD-026 concentrations for 96 h. N = 6 biological replicates. D Cell viability assays of MV4-11 and MOLM-13 cells treated across various inhibitor combinations for 96 h. E Immunoblot of MV4-11 and MOLM-13 cells treated with indicated doses of PMD-026 for 24 h. F Immunoblot after knockdown with two RPS6KA1 shRNAs or a non-targeting pLKO control. G Immunoblot of MV4-11 cells following 24 h treatment with inhibitors at the indicated concentrations. H Venn diagram of shared DEGs between PMD-026 and quizartinib treatment relative to DMSO control. Cells were treated in duplicate with 500 nM PMD-026 or 50 nM quizartinib for 24 h. I Enrichment dot plot of top altered Hallmark pathways from top 500 DEGs from (H). J Heatmap showing genes of interest from (H).
Watanabe et al. [9] previously described that FLT3-ITD could activate RSK1 and suggested that two pre-clinical RSK1 inhibitors FMK and LJI308 suppressed FLT3 phosphorylation via an off-target drug effect, outlining a unidirectional signaling cascade from FLT3 to RSK1. Our findings indicate that the anti-proliferative effects of PMD-026 may be due to targeting RSK1 dependency rather than off-target FLT3 inhibition. While pFLT3 was suppressed by BI-D1870 and PMD-026 (Fig. 2G), a cell-free KINOMEscan across ~400 kinases did not reveal FLT3 or FLT3 D835Y as targets [11] (Supplementary Fig. 1C). Furthermore, shRNA targeting of RPS6KA1 resulted in consistent pFLT3 inhibition similar to PMD-026 (Fig. 2E, F). Thus, these results demonstrate novel, bi-directional regulation between FLT3 and RSK1, and suggest that RSK1 inhibitors may suppress FLT3 activity in a mechanistically distinct manner from current FLT3 ATP-competitive inhibitors.
To further investigate the inhibition profiles by PMD-026 and the bonafide FLT3-ITD inhibitor quizartinib, we performed RNA-seq of MV4-11 cells after 24-hours treatment which showed alteration of 1161 shared candidates, 695 unique targets by PMD-026, and 1964 unique targets by quizartinib (Fig. 2H and Supplementary Table 3). Top pathways altered by PMD-026 were mTORC1, IL2/STAT5, and TNF/NFκB, whereas quizartinib demonstrated a more profound anti-cell cycle phenotype with milder effects on mTORC1 signaling (Fig. 2I, J). Of note, both inhibitors suppressed an abundance of tRNA synthetases, and dynamic feedback response to quizartinib resulting in upregulation of potent oncogenes including FLT3 was observed. These results reveal both overlapping and unique inhibition profiles by PMD-026 and quizartinib and further suggest RSK1 inhibition as a novel strategy in treating FLT3-mutant AML.
PMD-026 alleviates disease burden in FLT3-ITD in vivo models
Following, we assessed the potency of PMD-026 in FLT3-ITD AML mouse models. We first utilized a MV4-11 xenograft model and observed significant reduction of leukemic cells after 4-week PMD-026 treatment with a ~11-fold mean reduction at treatment endpoint compared to vehicle (20.64% vs 1.94%, respectively; Fig. 3A). PMD-026 treated mice were then taken off therapy and followed for another two weeks. At experiment endpoint, there was evident suppression of bone marrow leukemic engraftment by PMD-026, and significantly prolonged survival in treated mice (Fig. 3B, C). We also evaluated PMD-026 efficacy in a syngeneic Flt3ITDTet2KO leukemia model [16]. CD45.2+ Flt3ITDTet2KO cells were transplanted into sub-lethally irradiated WT CD45.1+ mice, and following demonstration of ~50% CD45.2 engraftment, mice were started on PMD-026 treatment for four weeks. We observed a significant reduction of CD45.2+ cells in the peripheral blood and bone marrow at treatment endpoint (Fig. 3D, E). Four out of six vehicle treated mice had near 100% CD45.2+ cell engraftment in the bone marrow, whereas in contrast four out of six mice in the PMD-026 group had less than 50% CD45.2 cell engraftment, of which two were below 20%. Hallmarks of this model including leukocytosis and splenomegaly were significantly reduced, with trending reduction of hepatomegaly with PMD-026 treatment (Fig. 3F–H). We also observed a trend of survival benefit, with all (6/6) PMD-026 treated animals remaining alive at endpoint (Fig. 3I). Taken together, these findings highlight RSK1 targeting as a promising therapeutic strategy against FLT3-ITD AML.
A Efficacy in the MV4-11 xenograft model. Percentage of hCD45+ cells in the PB of mice transplanted with MV4-11 cells. Mice were treated with vehicle (n = 5) or PMD-026 (n = 5) for four weeks. %PB hCD45+ statistics were assessed by two-way ANOVA. B Percentage of hCD45+ cells in the BM of xenografted animals at endpoint. Vehicle (n = 5); PMD-026 (n = 3). Statistics were assessed by two-tailed Student’s t-test. C Kaplan–Meier survival analysis of xenografted animals assessed by log-rank test. D–I Efficacy in the syngeneic Vav-Cre Flt3ITDTet2KO model. Chimerism in the peripheral blood (D) and bone marrow (E) following vehicle (n = 6 at Start, n = 4 at Endpoint) or 100 mg/kg PMD-026 (n = 6) treatment at endpoint. F White blood cells in vehicle (n = 4) and PMD-026 (n = 6) treated mice at endpoint. Gray bars denote normal reference ranges. Spleen (G) and liver (H) weights (n = 6 per group) were normalized to animal body weight. Statistics were assessed by two-tailed Student’s t-test. (I) Kaplan-Meier survival analysis assessed by log-rank test (n = 6 per group, gray bars represent treatment).
RSK1 alters FLT3 protein dynamics through deubiquitinase USP1
While inhibition of RSK1 by PMD-026 and shRNA knockdown potently suppressed FLT3 phosphorylation, we concurrently observed downregulation of total FLT3 protein expression in FLT3-ITD mutant lines after RSK1 perturbation (Fig. 4A, B). In contrast, quizartinib led to FLT3 mRNA and protein upregulation (Fig. 2J, Supplementary Fig. 2A), suggestive of acute adaptive feedback. Furthermore, RSK1 inhibition by PMD-026 or shRNA knockdown in THP-1 cells, which harbor wild-type FLT3, did not lead to FLT3 protein downregulation or dephosphorylation (Supplementary Fig. 2B, C), suggesting preferential dependency of RSK1 in FLT3-mutated cancers.
A Immunoblot of MV4-11 and MOLM-13 cells treated with 2 µM PMD-026 for 24 h. B Immunoblot of MV4-11 and MOLM-13 cells after knockdown with two RPS6KA1 shRNAs or a non-targeting pLKO control. C Immunoblot of MV4-11 cells following knockdown with shRPS6KA1 #1 or control vector and treated with 50 µg/mL CHX for the indicated duration. Line plot shows the half-life of FLT3 following RPS6KA1 inhibition normalized to FLT3 expression from each genetically altered group at time 0 and normalized to HSP90 control. D Pearson correlation of gene KO gene dependency scores with FLT3 KO gene dependency scores across 26 AML cell lines from DepMap. E USP1 KO gene dependency score across 26 AML cell lines from DepMap. F Immunoblot following immunoprecipitation (IP) of HA in 293 T cells co-transfected with FLT-ITD and HA-Ub. Following 36 h co-transfection, cells were treated with 10 µM PMD-026 and 10 µM MG-132 for 18 h. G Immunoblot following IP of FLT3 in Ba/F3 cells transduced with FLT3-ITD and treated with 10 µM PMD-026 for 18 h. H Immunoblot of MV4-11 and MOLM-13 cells treated with 2 µM PMD-026 for 24 h. I Immunoblot of MV4-11 cells after knockdown with two RPS6KA1 shRNAs or a non-targeting pLKO control. J Immunoblot of MV4-11 and MOLM-13 cells treated with the indicated doses of SJB3-019A for 24 h. K Immunoblot of MV4-11 and MOLM-13 cells after knockdown with two USP1 shRNAs or a non-targeting pLKO control. L Cell viability assay upon knockdown of USP1. Cells were plated at n = 6 for each condition and grown for 96 h with viability normalized to the control. Data are presented as mean values ± SD. Statistics were assessed by two-tailed Student’s t-test. M Immunoblot of MV4-11 cells following knockdown with shUSP1 #1, shUSP1 #2, or control vector and treated with 50 µg/mL CHX for the indicated duration. Line plot shows the half-life of FLT3 following USP1 inhibition normalized to FLT3 expression from each genetically altered group at time 0 and normalized to actin control. N Time course immunoblot of Ba/F3 FLT3-ITD cells treated with 5 µM SJB3-019A. O Immunoblot following IP of HA in Ba/F3 FLT3-ITD HA-Ub cells (left) or IP for FLT3-ITD in Ba/F3 FLT3-ITD cells (right) following treatment with 5 µM SJB3-019A for 2 h. P Immunoblot of Ba/F3 FLT3-ITD cells treated with 10 µM SJB3-019A and/or 10 µM MG-132 for 18 h.
Decreased FLT3 protein expression following RSK1 perturbation suggests that RSK1 may exert post-transcriptional regulation of FLT3 and its protein dynamics. Following RPS6KA1 knockdown, we inhibited protein synthesis with cycloheximide (CHX), which revealed a shortened half-life of FLT3 in RSK1-deficient cells (Fig. 4C). These findings suggest that RSK1 plays a role in mediating FLT3 protein stability. We then interrogated the AML cell lines DepMap CRISPR-KO dataset to screen co-dependent candidates with FLT3 that also harbor canonical post-translational regulatory function. We identified deubiquitinating enzyme (DUB) USP1, which was ranked 215 out of 17,385 genes, whose inhibition correlated with FLT3 inhibition, and was a preferential dependency in FLT3-ITD AML mutant lines (Fig. 4D, E).
To determine if RSK1 played a role in mediating ubiquitination of FLT3-ITD, we performed immunoprecipitation assays for HA-Ubiquitin (HA-Ub) or FLT3-ITD following their ectopic expression under PMD-026 treatment. Indeed, PMD-026 led to enhanced FLT3-ITD ubiquitination, specifically K48-linkage which targets proteins for proteasome-dependent protein degradation (Fig. 4F, G). We further found that RSK1 inhibition by PMD-026 and shRNAs resulted in concomitant decrease of USP1 but not its regulator UAF1 (Usp1-associated factor 1; WDR48; 4H, I). To validate a novel role of USP1 along this RSK1-FLT3 axis, we treated MV4-11 and MOLM-13 cell lines with USP1 inhibitor SJB3-019A which reduced expression of FLT3 and USP1-UAF1 complex and suppressed FLT3 and S6 phosphorylation (Fig. 4J). FLT3-ITD leukemia cell lines were also more sensitive to SJB3-019A compared to FLT3-WT cells, albeit with potency observed across all AML lines tested (Supplementary Fig. 2D). USP1 knockdown also resulted in concomitant downregulation of total FLT3 protein, significantly inhibited cell proliferation, and destabilized FLT3 protein (Fig. 4K-M), collectively phenocopying RPS6KA1 inhibition. Treatment with SJB3-019A led to time-dependent degradation of FLT3 and USP1, with increasing abundance of total K48-Ubiquitin accumulation (Fig. 4N). Importantly, we identified increased polyubiquitination of FLT3-ITD following USP1 inhibition (Fig. 4O). In contrast, treatment of Ba/F3 FLT3-ITD cells with proteasome inhibitor MG-132 rescued FLT3-ITD protein expression following SJB3-019A treatment (Fig. 4P). These mechanistic findings indicate that RSK1 inhibition leads to the loss of deubiquitinase USP1 and consequently enhances proteasome-mediated degradation of FLT3.
Prognostic value of the RSK1-USP1 axis in AML patients
Given the dependency of this RSK1-USP1-FLT3 axis, we next investigated the impact of their expression on disease severity and patient outcomes. Patients stratified into higher risk groups following European LeukemiaNet (ELN) 2017 guidelines exhibited significantly elevated USP1 in both TCGA and BeatAML cohorts (Fig. 5A). AML patients with high USP1 copy number variation and elevated RNA expression had significantly poorer survival outcomes from TCGA, BeatAML, and Leucegene cohorts (Supplementary Fig. 3A–C). We then performed multivariate analysis of the RSK1-USP1-FLT3 cascade on pooled TCGA and BeatAML cohorts (n = 568 patients) considering shared clinical features (sex, race, and ELN 2017 risk) from both cohorts. Elevated expression of USP1 (Hazard ratio; HR = 1.26, 95% confidence interval; CI: 1.02–1.56) and RPS6KA1 (HR = 1.42, 95% CI = 1.15–1.76) but not FLT3 (HR = 1.09, 95% CI = 0.89–1.35) was significantly associated with worse patient outcome (Table 1). This was further supported by Kaplan-Meier analysis when stratifying patients by median expression of USP1 and RPS6KA1, in which patients with high expression of either gene had poor survival (Fig. 5B). Importantly, patients with elevated expression of both genes had the worst outcome. Overall, we demonstrate RSK1 playing a critical role in regulating both the activity and protein dynamics of FLT3 via deubiquitinate USP1. These findings highlight a RSK1-USP1-FLT3 cascade in which perturbation along any node results in AML lethality and may act as novel biomarkers predictive of patient outcome.
A USP1 expression across patient risk stratification following ELN 2017 classification. TCGA: Favorable (Fav; n = 51), Intermediate (Int; n = 42), Adverse (Adv; n = 61). Statistics assessed by two-tailed Student’s t-test. BeatAML: Fav (n = 118), Fav/Int (n = 14), Int (n = 150), Int/Adv (n = 7), Adv (n = 162). Statistics comparing pooled group of Fav and Fav/Int against pooled group of Int, Int/Adv, and Adv assessed by two-tailed Student’s t-test. B Kaplan–Meier survival analysis of combined TCGA LAML and BeatAML cohorts. Patients were stratified into USP1 and RPS6KA1 high and low groups by median gene expression. Statistics were assessed by log-rank test compared to the USP1lowRPS6KA1low group.
Discussion
We demonstrated that targeting RSK1 was efficacious in chronic myeloproliferative disease through inhibition of S6 signaling and dampening of hyperinflammation [13]. Here, we extend our findings to another distinct myeloid leukemia subtype and demonstrate an unconventional strategy of targeting FLT3-ITD mutant AML through inhibition of RSK1, actionable through the first-in-class oral RSK inhibitor, PMD-026. The unexpected bi-directional regulatory effects between mutant FLT3 and RSK1 in these leukemias highlight convergence of signaling pathway and dependency. As functional mutations in RPS6KA1 have not been reported in the leukemia literature, its elevated expression in patients harboring FLT3-ITD mutations as observed in the BeatAML2 cohort may be necessary to sustain oncogenesis in this genetic context. Consequently, RSK1 inhibition led to marked apoptosis in vitro and targeting RSK1 in FLT3-ITD leukemic mouse models revealed evident therapeutic benefit. Moreover, genetic knockdown experiments phenocopying PMD-026 and KINOMEscan data strongly suggest that the anti-proliferative phenotypes by PMD-026 are largely due to on target inhibition of RSK1 in the context of the hematologic malignancies studied here. The potent and consistent disease-ameliorating effects across numerous leukemia mouse models demonstrates promise for repurposing PMD-026 for the treatment of myeloid malignancies.
We further define a mechanism in which RSK1 modulates FLT3 stability through USP1 via proteosome-mediated ubiquitin degradation and identify an additional core dependency in USP1, actionable through pre-clinical agents such as SJB3-019A. Strong co-dependency between FLT3 and USP1 were observed, and it is plausible that RSK1 mediates direct phosphorylation of USP1 or other interacting partners along the ubiquitin-proteasome system. Across clinical samples, RPS6KA1 expression was also elevated in FLT3-ITD AML patients who developed resistance to gilteritinib. Thus, RPS6KA1 and its regulatory and signaling axes may serve as relevant biomarkers that may predict patient outcome and response to targeted therapies. Future studies aiming to assess whether RSK1 inhibition could overcome resistance to FLT3-ITD inhibitors would bring further impact in the clinical setting and possibility as a therapeutic option in refractory disease.
Adverse safety profiles including intolerable constitutional effects, myelosuppression, and pancytopenia contribute to a quarter of failed phase 2 and phase 3 studies [17]. Treatment-induced anemia, in addition to thrombocytopenia and myelosuppression, are the most common grade 3/4 adverse events (AE) for approved FLT3-ITD inhibitors such as quizartinib and gilteritinib [18, 19]. Interim reporting of PMD-026 safety in the Phase 1/1b/2 breast cancer trial (NCT04115306) demonstrated no sustained suppressive effect on hematological parameters and treatment was well-tolerated [20]. None of the 41 patients came off study due to hematological AEs and there were no hematological PMD-026-related toxicities that met the criteria for dose modifications during the trial period [20]. These results suggest that PMD-026 treatment in patients with hematologic malignancies may not result in limiting myelosuppression, although this will need to be formally investigated.
Overall, our findings here reveal durable anti-leukemic efficacy and favorable safety profiles of targeting RSK1 in humans, which taken together support emergence of clinical studies of repurposing RSK inhibitors for liquid tumors.
Methods
Ethics approval
All research and methods were performed in accordance with the relevant guidelines and regulations. All in vivo procedures were conducted in accordance with and approved by the Institutional Animal Care and Use Committee of Washington University (no. 20-0463). The maximal disease burden denoted by the ethics committee/institutional review board (e.g., moribund and sluggish appearance, hunched and with ruffled coats, harboring visible lesions) was not exceeded. Animals were humanely euthanized upon immobility or appearance with signs of distress.
Cell culture
HEL (ATCC), SET2 (ATCC), UKE-1 (Coriell Institute), MOLM-13 (DSMZ), MEG-01 (ATCC) cells were cultured in RPMI 1640 ATCC modification (ThermoFisher, Waltham, MA). MV4-11 (ATCC) cells were cultured in IMDM. THP-1 (ATCC) cells were cultured in RPMI 1640 supplemented with 0.05 mM beta-mercaptoethanol. Ba/F3 (DSMZ) cells were cultured in RPMI 1640 supplemented with 1 ng/mL murine IL-3, while Ba/F3 transduced with FLT3-ITD and FLT3-D835Y were cultured in the absence of murine IL-3. HEK293T (ATCC) cells were cultured in DMEM. All cell lines were maintained at 37 °C and 5% CO2, grown in media supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin, and regularly tested for mycoplasma.
Compounds, immunoblot and flow cytometry antibodies
PMD-026 was provided by Phoenix Molecular Designs. Ruxolitinib, fedratinib, midostaurin, and quizartinib were purchased from Selleck Chemicals (Houston, TX). BI-D1870, MG-132, and SJB3-019A were purchased from MedChemExpress (Monmouth Junction, NJ). Murine IL-3 was purchased from Peprotech (Rocky Hill, NJ). Cycloheximide was purchased from Sigma-Aldrich (St. Louis, MO).
Immunoblotting antibodies were used as follows: pRB S795 (#9301), cyclin D1 (92G2, #2978), RSK1 (D6D5, #8408), p90RSK T359/S363 (#9344), pS6 S235/236 (D57.2.2E, #4858), pFLT3 Y591 (54H1, #3466), FLT3 (8F2, #3462), USP1 (D37B4, #8033), HA (C29F4, #3724), Ubiquitin (P37; #58395), and K48-linkage Specific Polyubiquitin (D9D5, #8081) from Cell Signaling Technology (Danvers, MA). HSP90 (sc-13119) and WDR48 (E-4, sc-514473) from Santa Cruz Biotechnology (Dallas, TX). Flow cytometry antibodies were used as follows: anti-mouse CD45.1-PE (110708) and anti-mouse CD45.2-APC (109814) from BioLegend (San Diego, CA).
Plasmid constructs and transduction
Lentiviral and retroviral transduction with low multiplicity of infection (MOI) was performed following protocol described at http://www.broadinstitute.org/rnai/public/resources/protocols. Transduced cells were selected in puromycin for 2–3 days and plated for downstream assays immediately after selection.
Individual shRNA vectors used were from the Mission TRC library (Sigma) provided by the McGill Platform for Cellular Perturbation (MPCP) of Rosalind and Morris Goodman Cancer Research Centre at McGill University as follows: control vector pLKO.5, shRPS6KA1 #1 (TRCN0000001386), shRPS6KA1 #2 (TRCN0000001388). The following shRNAs were purchased from Sigma: shUSP1 #1 (TRCN0000004043), shUSP1 #2 (TRCN0000004046). FLT3-ITD-MSCV-IRES-GFP and FLT3-D835Y-MSCV-IRES-GFP were gifts from Timothy Ley (Addgene plasmid # 184779; http://n2t.net/addgene:184779; RRID:Addgene_184779, Addgene plasmid # 184780; http://n2t.net/addgene:184780; RRID:Addgene_184780); pLenti puro HA-Ubiquitin was a gift from Melina Fan (Addgene plasmid # 74218; http://n2t.net/addgene:74218; RRID:Addgene_74218).
Cell viability assays
Cell lines were seeded into 96-well plates upon gene perturbation or treated with indicated doses of inhibitors and cultured for 72–96 h. At endpoint, resazurin viability reagent was added and absorbance was read in a BioTek microplate reader.
Immunoblotting
Cells were collected and lysed with Laemmli Sample Buffer (BioRad, Hercules, CA) with beta-mercaptoethanol and heated at 95 °C. Samples were processed with NuPAGE Novex Gel Electrophoresis Systems (ThermoFisher) followed by standard immunoblotting procedure.
Immunoprecipitation (IP) and ubiquitin assay
Co-IPs were performed using the Pierce Classic Magnetic IP/Co-IP Kit (Thermofisher). In brief, cells were pelleted, washed with PBS and lysed with IP lysis/wash buffer (pH 7.4, 0.025 M Tris, 0.15 M NaCl, 0.001 M EDTA, 1% NP40, and 5% glycerol). Cells were then centrifuged, an input fraction was collected, and the supernatant was incubated with 1 μg of RSK1 primary antibody or IgG control (Cell Signaling #2729) for 2 h at room temperature to form the immune complex. Immune complexes were then incubated with protein A/G magnetic beads, eluted, and collected following heating under reducing conditions.
For the ubiquitin assays, 293 T cells were transfected with HA-Ubiquitin and FLT3-ITD. Following 36 h, cells were treated with PMD-026 and MG-132 for 16 h. IP was then performed following protocol above with 1 ug of HA antibody. Ba/F3 cells were transduced with FLT3-ITD and IP was performed following 16 h treatment with PMD-026 and using 1 ug of FLT3 antibody. Two sets of SJB3-019A treatment experiments were performed: Ba/F3 cells transduced with 1) FLT3-ITD, and 2) FLT3-ITD and HA-Ub. IP was performed following 2 h treatment with SJB3-019A and using 1 μg of HA or FLT3 antibody.
Cycloheximide (CHX) assay
Transduced MV4-11 cells were treated for the indicated hours in the presence of cycloheximide before collection and immunoblotting. Line plots showing protein normalization were performed through densitometry with ImageJ (NIH) where bands were normalized by the internal loading control and to the 0 CHX treatment condition within each group.
Flow cytometry and apoptosis assay
Cells were stained in live cell buffer and run on the BD FACSCanto II Cell Analyzer utilizing unstained controls, and downstream analysis was performed with FlowJo (Ashland, OR). Cells were collected and stained per the PE Annexin V Apoptosis Detection Kit I (BD Biosciences). In brief, post-treatment, cells were washed with PBS and resuspended in binding buffer, incubated with PE-Annexin V, and stained with 7-AAD viability staining solution. Cells were then analyzed by flow cytometry, in which AnnexinV + /7-AAD+ cells were identified as necrotic, AnnexinV + /7-AAD− as apoptotic, and AnnexinV − /7-AAD− as healthy.
Sequencing and downstream analyses
RNA-sequencing (RNA-seq)
Cells were treated in duplicate and RNA was extracted using the RNeasy Mini Kit (Qiagen) with Dnase treatment. cDNA synthesis was performed using PolyA selection. Samples were then indexed, pooled, and sequenced by Illumina NovaSeq 6000. Basecalls and demultiplexing were performed with Illumina’s bcl2fastq software and a custom python demultiplexing program with a maximum of one mismatch in the indexing read. RNA-seq reads were then aligned to the Ensembl release 76 primary assembly with STAR version 2.5.1a [21]. TMM normalization size factors in EdgeR [22] were calculated to adjust for samples for differences in library size. Ribosomal genes and genes not expressed in the smallest group size minus one samples greater than one count-per-million were excluded from further analysis. The TMM size factors and the matrix of counts were then imported into the R/Bioconductor package Limma [23]. Additional analysis was performed using DEseq2 [24], using GSEA [25] software with the Hallmark gene set, or pathway analysis at https://www.gsea-msigdb.org/.
Animal models
MV4-11 xenograft
1 × 106 cells were engrafted into sub-lethally irradiated (200 cGy) female 6–9 week-old NSGS mice by tail-vein injection. Two weeks post-transplantation animals were randomized and vehicle or 100 mg/kg PMD-026 was administered by oral gavage twice a day for 28 days. Human CD45+ cells in the peripheral blood were measured every week and bone marrow at endpoint by flow cytometry. Measurements and assessments were performed blindly.
Vav-Cre Flt3 ITD Tet2 KO model
1 × 106 Vav-Cre Vav-Cre Flt3ITDTet2KO CD45.2 cells were injected into the tail veins of sub-lethally irradiated CD45.1 female mice. Three weeks post-transplantation animals were randomized, PMD-026 was administered by oral gavage (100 mg/kg/BID) for 28 days. Hematological parameters were analyzed by flow cytometry and Hemavet. Measurements and assessments were performed blindly.
Multivariate and Kaplan–Meier survival analysis
For multivariate analysis, patient clinical information was retrieved from TCGA LAML [4] and BeatAML [5] from vizome.org. Between these two cohorts, overlapping clinical features included sex, race, and ELN 2017 risk, and samples with these factors, survival data, and expression data of FLT3, RPS6KA1, and USP1 were pooled together. Multivariate analysis was performed, where expression was stratified by greater or lesser than the median gene expression. Kaplan-Meier analysis was performed on the pooled TCGA and BeatAML cohorts and patients were stratified into four groups based on USP1 and RPS6KA1 gene expression (singular high or low, double high, or double low, where high and low represent comparison to the median expression).
Public databases
DepMAP
Gene effect scores (CERES) [26] from CRISPR (Avana) public 21Q1 were accessed from the DepMap portal by the Broad Institute.
BeatAML2
OHSU BeatAML2 [14] clinical and expression data were obtained from http://vizome.org.
TCGA LAML
TCGA LAML mutational and clinical data were accessed from cBioPortal [27].
Leucegene cohort
Survival data were obtained from data.leucegene.iric.ca.
scRNA-seq of gilteritinib-treated AML patients (GSE199333)
Feature files and clinical information were assessed from GSE199333 and source publication [15]. Data were processed following scRNA-seq pipelines as previously described [8]. Following population annotation, myeloid cells were subsetted for downstream gene expression analyses. No new code was generated. R scripts used in this study are available from corresponding author upon request.
Other statistical analysis and figures
Statistical analyses were performed using GraphPad Prism 9 (San Diego, CA) and R software. All measurements were taken from distinct samples. All relevant assays were performed independently at least 3 times. Figures were generated with GraphPad Prism 9, and R software.
Data availability
Sequencing data is deposited in the Gene Expression Omnibus (GEO) with accession number GSE228760.
References
De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6:e441.
Consortium APG. AACR project GENIE: powering precision medicine through an international consortium. Cancer Discov. 2017;7:818–31.
Kennedy VE, Smith CC. FLT3 mutations in acute myeloid leukemia: key concepts and emerging controversies. Front Oncol. 2020;10:612880.
Cancer Genome Atlas Research N, Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–74.
Tyner JW, Tognon CE, Bottomly D, Wilmot B, Kurtz SE, Savage SL, et al. Functional genomic landscape of acute myeloid leukaemia. Nature. 2018;562:526–31.
Kiyoi H, Kawashima N, Ishikawa Y. FLT3 mutations in acute myeloid leukemia: therapeutic paradigm beyond inhibitor development. Cancer Sci. 2020;111:312–22.
Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33:299–312.
Kong T, Laranjeira ABA, Yang K, Fisher DAC, Yu L, Poittevin De La Fregonniere L, et al. DUSP6 mediates resistance to JAK2 inhibition and drives leukemic progression. Nat Cancer. 2023;4:108–27.
Watanabe D, Nogami A, Okada K, Akiyama H, Umezawa Y, Miura O. FLT3-ITD activates RSK1 to enhance proliferation and survival of AML cells by activating mTORC1 and eIF4B cooperatively with PIM or PI3K and by inhibiting Bad and BIM. Cancers. 2019;11:1827.
Chae HD, Dutta R, Tiu B, Hoff FW, Accordi B, Serafin V, et al. RSK inhibitor BI-D1870 inhibits acute myeloid leukemia cell proliferation by targeting mitotic exit. Oncotarget. 2020;11:2387–403.
Dunn SE, Jayanthan A, Huynh M-M, Flahive E, Pambid MR, Dorr A, et al. Abstract P3-10-10: PMD-026, a first-in-class oral p90 ribosomal S6 kinase (RSK) inhibitor for triple negative breast cancer (TNBC). Cancer Res. 2020;80:P3–10.
Jayanthan A, Huynh M-M, Lee J, Los G, Yue L, Pambid MR, et al. Abstract 1038: PMD-026, a first in class oral RSK inhibitor, demonstrates synergy when combined with standard of care in breast cancer tumor models. Cancer Res. 2021;81:1038.
Kong T, Laranjeira ABA, Letson CT, Yu L, Lin S, Fowles JS, et al. RSK1 is an exploitable dependency in myeloid malignancies. Nat Commun. 2024.
Bottomly D, Long N, Schultz AR, Kurtz SE, Tognon CE, Johnson K, et al. Integrative analysis of drug response and clinical outcome in acute myeloid leukemia. Cancer Cell. 2022;40:850–64.e9.
Buelow DR, Bhatnagar B, Orwick SJ, Jeon JY, Eisenmann ED, Stromatt JC, et al. BMX kinase mediates gilteritinib resistance in FLT3-mutated AML through microenvironmental factors. Blood Adv. 2022;6:5049–60.
Ostrander EL, Kramer AC, Mallaney C, Celik H, Koh WK, Fairchild J, et al. Divergent effects of Dnmt3a and Tet2 mutations on hematopoietic progenitor cell fitness. Stem Cell Rep. 2020;14:551–60.
Harrison RK. Phase II and phase III failures: 2013–2015. Nat Rev Drug Discov. 2016;15:817–8.
Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N. Engl J Med. 2019;381:1728–40.
Levis M. Quizartinib for the treatment of FLT3/ITD acute myeloid leukemia. Future Oncol. 2014;10:1571–9.
Wang JS, Beeram M, Chalasani P, Mina L, Shatsky RA, Hurvitz S, et al. High levels of RSK2 in breast cancer patients is associated with longer PFS in patients treated with PMD-026, a first in class RSK inhibitor. SABCS. Cancer Res. 2023;83:P4-01-16. https://aacrjournals.org/cancerres/article/83/5_Supplement/P4-01-16/718165/Abstract-P4-01-16-High-levels-of-RSK2-in-breast.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Meyers RM, Bryan JG, McFarland JM, Weir BA, Sizemore AE, Xu H, et al. Computational correction of copy number effect improves specificity of CRISPR-Cas9 essentiality screens in cancer cells. Nat Genet. 2017;49:1779–84.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4.
Acknowledgements
This work was supported by NIH grants R01HL134952 (STO), a Canadian Institutes of Health Research (CIHR) Doctoral Foreign Study Award (TK), and the MPN Research Foundation (STO). Additional support was provided by NIH grants R01HL147978 (GAC), the Leukemia and Lymphoma Society Translational Research Program (STO), When Everyone Survives Foundation (STO), Edward P. Evans Foundation (GAC), and Gabrielle’s Angel Foundation (GAC). GAC is a scholar of the Leukemia and Lymphoma Society. We thank Feng Gao for assistance with biostatistical analysis. We thank the Genetic Perturbation Service of Goodman Cancer Research Centre at McGill University for access to and preparation of functional genetic tools.
Author information
Authors and Affiliations
Contributions
TK, ABAL, CTL, LY, and FH performed experiments and data analysis. AJ, GL, SED, and GAC provided technical and/or clinical support. TK and STO designed and supervised the experiments. TK and STO wrote the manuscript. All authors read and approved of the manuscript.
Corresponding author
Ethics declarations
Competing interests
STO has served as a consultant for Kartos Therapeutics, CTI BioPharma, Celgene/Bristol Myers Squibb, Disc Medicine, Protagonist, Blueprint Medicines, Cogent, PharmaEssentia, Constellation, Geron, Abbvie, Sierra Oncology, and Incyte. MH, GL, and SED, are employees of Phoenix Molecular Designs. GAC has received funding for consulting and/or sponsored research from Incyte, Ajax Therapeutics, and Renagade Therapeutics. All other authors disclose no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kong, T., Laranjeira, A.B.A., Letson, C.T. et al. RSK1 dependency in FLT3-ITD acute myeloid leukemia. Blood Cancer J. 14, 207 (2024). https://doi.org/10.1038/s41408-024-01187-4
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41408-024-01187-4
This article is cited by
-
Integrated Multi-Omics Analysis Reveals Cytokine Network Dynamics and Prognostic Signatures in Hepatitis B Virus-Associated Hepatocellular Carcinoma
Applied Biochemistry and Biotechnology (2026)
-
Elucidating the risk factors and oncogene drivers of acute myeloid leukemia
Human Cell (2025)
-
RSK1 is an exploitable dependency in myeloproliferative neoplasms and secondary acute myeloid leukemia
Nature Communications (2025)