Abstract
Anti-PD-1 based therapies and brentuximab vedotin (BV) have significantly improved survival in patients with classic Hodgkin lymphoma (cHL) and have been incorporated into earlier lines of therapy. However, there is insufficient data regarding the clinical outcomes in patients who develop refractory disease or who become intolerant of BV and anti-PD-1 therapies (double refractory/intolerant; DR/INT). Here, we evaluated outcomes in patients with DR/INT cHL from 15 US academic medical centers. A total of 173 patients were identified as DR/INT. The median overall survival from the time of cHL diagnosis (OS-1) was 14.8 years (95% CI: 10.9–20.9 years) and the 10-year OS-1 estimate was 62% (95% CI: 52–70%). After accounting for differences in age, patients who underwent autologous stem cell transplant prior to developing DR/INT had significantly longer OS-1 (HR 0.53, 95% CI: 0.29–0.96, p = 0.04). Median OS from time of DR/INT (OS-2) was 7.4 years (95% CI: 4.3-NR) and the 5-year OS-2 estimate was 57% (95% CI: 48-66%). Both anti-PD-1 and BV based therapy rechallenge were effective with median PFS of 237 days (95% CI: 155-357 days) and 183 days (95% CI: 108–273 days), respectively. Finally, advanced therapy options such as CD30 directed chimeric antigen receptor T-cell therapy and allogeneic stem cell transplant after DR/INT were associated with improved OS-2 (p < 0.001). To our knowledge, this represents the largest cohort of patients with DR/INT cHL. OS-2 will serve as a benchmark for future studies aiming to improve survival in DR/INT cHL.
Similar content being viewed by others
Introduction
Classic Hodgkin lymphoma (cHL) is a curable B-cell malignancy; however, approximately 10–15% of patients will experience refractory disease to front-line chemotherapy with adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) and an additional 20% will develop disease relapse [1,2,3]. The introduction of novel therapies such as brentuximab vedotin (BV) and anti-PD-1 agents (nivolumab and pembrolizumab) have significantly improved outcomes in relapsed/refractory (r/r) cHL [4,5,6,7,8,9,10].
Multiple studies have demonstrated improved clinical outcomes with moving these novel therapies into front-line treatment regimens [11]. For example, BV combined with adriamycin, vinblastine, and dacarbazine (BV-AVD) improved progression free survival (PFS) and overall survival (OS) compared to ABVD chemotherapy in front-line cHL (ECHELON-1) [12, 13]. Recently, nivolumab combined with AVD chemotherapy demonstrated improved PFS compared to BV-AVD in front-line therapy for cHL [14]. Additionally, multiple combinations of these novel agents with chemotherapy have demonstrated improved response rates and PFS when used for second-line therapy prior to autologous stem cell transplant (ASCT) consolidation [15,16,17,18,19].
As more patients are treated with BV and anti-PD-1 therapies in earlier lines of therapy, it has become important to understand the clinical course of patients who are refractory to these agents (double refractory, DR) or who become intolerant of these therapies due to side effects (intolerant, INT) [20]. There are currently no data characterizing outcomes for these patients, and the optimal management of DR/INT cHL has not been defined. Therefore, we conducted a large retrospective database analysis from 15 academic medical centers in the United States to better describe outcomes for these patients and elucidate potential therapeutic options after developing DR/INT cHL.
Patients and methods
Patient population
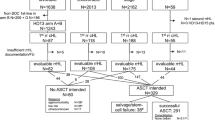
This is a retrospective study of patients with a history of cHL who developed DR cHL or became INT of BV or anti-PD-1 therapy. Patients were adults (age ≥ 18 years) at the time of DR/INT. Patients who were treated with BV and anti-PD-1 therapy between 1/1/2011 and 12/31/2021 were included from 15 U.S. academic medical centers. DR was defined as treatment failure (evidence of progression by imaging or biopsy) during therapy or within 3 months of the last dose of both BV and anti-PD-1 therapy. INT was defined as any toxicity limiting further cycles of BV or anti-PD-1 therapy. Patients with INT were either intolerant to both BV and anti-PD-1 therapy or intolerant of one therapy and refractory to the other.
Data were retrospectively collected from the electronic medical record at each participating center after institutional review board (IRB) approval. The Ohio State University collected de-identified data from all participating centers after appropriate approvals and data use agreements were arranged. The study was performed in accordance with the Declaration of Helsinki.
Data collection
Patient and treatment characteristics recorded included baseline clinical characteristics, response to front-line therapy, characteristics surrounding initial BV and anti-PD-1 based therapy, details of progression or intolerance, timing of ASCT with respect to DR/INT, subsequent therapies and disease response, and overall survival (OS). Response was defined by either radiologic assessment or clinical documentation as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) [21].
Endpoints
The primary endpoints were assessment of OS from time of cHL diagnosis (OS-1) and from DR or INT (OS-2). Secondary endpoints included description of patient characteristics developing DR or INT cHL, estimate of OS comparing DR versus INT, and estimate of OS stratified by prior ASCT at the time of DR/INT.
Statistical analyses
Time-to-event analyses were evaluated by Kaplan-Meier methodology. Progression-free survival (PFS) was defined as the time from the initiation of treatment (with any specific therapy) to the date of radiologic evidence of disease progression or death. Patients alive and progression-free at last follow up were censored. INT patients were not included in PFS analysis for initial BV or anti-PD-1 therapy. OS-1 was defined as the time from diagnosis of cHL to death or last follow up. OS-2 was defined as the time from DR/INT to death. Patients alive at last follow up were censored at that time. Survival curves were compared between groups using log-rank tests. Cox proportional hazards models were used to estimate hazard ratios. Center effects were checked using random effects modeling. All analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Patient characteristics
A total of 173 patients were identified as having DR/INT cHL across 15 centers. The median age at initial diagnosis was 34 years (range 16–89), 61% were male, and 79% were White. At the time of diagnosis, the majority (76%) had advanced stage disease and nodular sclerosing subtype (68%), Table 1. Front-line treatment was ABVD/AVD in 134 patients (78%), BV-AVD in 15 patients (9%), BV-nivolumab in 5 patients (3%), other BV combination/monotherapy in 10 (5%) and other anthracycline-containing regimens in 9 patients (5%). The majority (58%) had primary refractory cHL after front-line therapy.
Initial BV and Anti-PD-1 Therapy
Initial BV treatment was after a median of 2 lines of therapy (LOT) (range 0–7). The majority (61%, n = 105) were treated with BV monotherapy, followed by BV with chemotherapy (25%, n = 43) and BV-nivolumab (14%, n = 25), Table 2. Seventeen percent (n = 30) developed intolerance to BV based therapy, limiting further cycles. The most common cause of BV intolerance was peripheral neuropathy (57%, n = 17). Regardless of LOT or treatment modality, the ORR with BV based therapy was 56% (21% CR, 35% PR). Excluding patients who discontinued therapy due to intolerance, the median PFS with BV based therapy was 166 days, (95% CI: 138–187 days). The majority (66%, n = 114) were refractory to or intolerant of BV prior to receiving an ASCT.
Initial anti-PD-1 therapy was after a median of 3 LOT (range 0–12). Nivolumab was more commonly utilized (72%, n = 124) compared to pembrolizumab (28%, n = 49), Table 2. The majority (78%, n = 134) were treated with anti-PD-1 monotherapy, followed by BV-nivolumab (15%, n = 25), anti-PD-1 therapy combined with investigational immunomodulatory therapy (7%, n = 12), and anti-PD-1 combined with chemotherapy (1%, n = 2). Fourteen percent (n = 24) developed intolerance to anti-PD-1 based therapy, limiting further cycles. The most common cause of anti-PD-1 intolerance was immune related adverse events (83%, n = 20). Regardless of LOT or therapy combination, the ORR with anti-PD-1 based therapies was 55% (19% CR, 36% PR). Excluding patients who discontinued therapy due to intolerance, the median PFS was 225 days, (95% CI: 179–265 days). Fifty-two percent (n = 90) were refractory to or intolerant of anti-PD-1 therapy prior to receiving an ASCT.
Double Refractory and Intolerant cHL
The majority of the cohort (73%, n = 127) developed DR disease, while a minority (27%, n = 46) were intolerant of either BV or anti-PD-1 therapy. There were 8 patients (5%) intolerant of both BV and anti-PD-1 therapy (included in the INT subgroup), Table 3. The median years from diagnosis to DR/INT was 3.4 years (range 0.5–23.1 years). The median age at the time of DR/INT was 40 years (range 19–90 years). Median prior LOT at the time of DR/INT was 5 (range 1–13). The majority (88%, n = 153) received additional therapy after becoming DR/INT.
Impact of ASCT on OS from diagnosis in DR/INT cHL (OS-1)
The median OS from the time of cHL diagnosis (OS-1) was 14.8 years (95% CI: 10.9–20.9 years) and the 10-year OS estimate was 62% (95% CI: 52-70%), Fig. 1A. OS-1 was not different between patients who were DR (median OS-1: 15.0 years) or INT (median OS-1: 14.8 years), log rank, p = 0.99, Fig. 1B. We then evaluated the impact on OS-1 in patients who developed DR/INT cHL but had not received an ASCT (87 patients, 50.3%) compared to those who developed DR/INT cHL at any time after an ASCT (86 patients, 49.7%). Patients who had not received an ASCT at the time of DR/INT were older at initial diagnosis (median 42 vs 31 years, p < 0.001), were more likely to have cHL subtype other than nodular sclerosing type (p < 0.001), had a shorter time from diagnosis to DR/INT (median 2.1 vs 4.8 years, p < 0.001), and had a lower median LOT at the time of DR/INT (3 vs 6 LOT, p < 0.001). There was no association between primary refractory cHL and receipt of ASCT (p = 1.0).
Patients who developed DR/INT at any time after ASCT had a significantly longer OS-1 compared to DR/INT who had not received ASCT, with a median OS-1 of 19.1 years (95%CI 14.4-NR) versus 8.6 years (95%CI 5.4-15.0), respectively (HR 0.33, 95% CI: 0.19–0.57, log-rank p < 0.001, Fig. 1C). Adjusting for age at DR/INT did not impact the association between prior ASCT and improved OS-1 (HR 0.53, 95% CI: 0.29–0.96, p = 0.04). The 5 and 10-year OS-1 estimates for patients with prior ASCT compared to no prior ASCT were 91% vs 65% and 73% vs 48%, respectively. There was no impact of random effect modeling by institution on the association. In a sensitivity analysis, excluding patients > 65 years old at the time of DR/INT, ASCT remained associated with improved OS-1 (HR 0.46, 95% CI: 0.24–0.89, p = 0.02), Supplemental Fig. 1.
Impact of subsequent therapy on OS from time of DR/INT (OS-2)
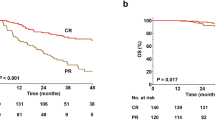
The median OS from the time of DR/INT (OS-2) was 7.4 years (95% CI: 4.3-NR) and the 5-year OS estimate was 57% (95% CI: 48–66%), Fig. 2A. OS-2 was not different between DR and INT groups (log rank, p = 0.33), Fig. 2B. The median LOT after DR/INT was 2 (range 0-14). Sixty-three patients (36%) were rechallenged with anti-PD-1 based therapy and 42 patients (24%) were rechallenged with BV based therapies after DR/INT. The ORR with anti-PD-1 based rechallenge was 55% (27% CR, 29% PR). The median PFS with anti-PD-1 based rechallenge was 237 days (95% CI: 155–357 days). The ORR with BV based rechallenge was 62% (31% CR, 31% PR). The median PFS with BV based rechallenge was 183 days (95% CI: 108–273 days). There was no difference in PFS between anti-PD-1 or BV based rechallenge (log rank, p = 0.25), Fig. 2C.
Patients treated with an advanced therapy option such as allogeneic stem cell transplantation (AlloSCT) or CD30 directed chimeric antigen receptor T-cell therapy (CD30.CAR-T) had improved OS-2, Fig. 3. Fifty-five patients (32%) received either AlloSCT or CD30.CAR-T following DR/INT. Two patients received both an AlloSCT and CD30.CAR-T cell therapy and were grouped for survival based on their last line of therapy (1 for AlloSCT, 1 for CD30.CAR-T). Thirty-one patients were included in survival analysis for AlloSCT, 24 patients with CD30.CAR-T, and 118 patients did not receive either therapy. The median time from DR/INT to AlloSCT and CD30.CAR-T was 183 days (95% CI: 38-1801 days) and 255 days (95% CI: 79-1540 days), respectively.
Disease status at AlloSCT included CR (61%), PR (23%), and SD (16%), Supplemental Table 2. The majority (87%, n = 27) received reduced intensity conditioning. Donor specific details included peripheral blood donor source in all patients, 67% (n = 21) had >8/8 HLA match, 23% (n = 7) haplo-identical match, and 10% (n = 3) < 8/8 HLA match. The majority of patients (68%, n = 21) received post-transplant cyclophosphamide graft versus host disease (GVHD) prophylaxis. Eleven patients (35%) developed acute GVHD, and 18 (58%) patients developed chronic GVHD, Supplemental Table 3.
The median age at DR/INT across the three treatment cohorts (AlloSCT vs CD30.CAR-T vs Neither) was 32 years (95% CI: 20-65 years) vs 36 years (95% CI: 24-62 years) vs 44 years (95% CI: 21–89). The three-year OS-2 estimates for AlloSCT vs CD30.CAR-T vs Neither were 92.9% (95%CI: 74.3–98.2%), 85.2% (95%CI: 60.5–95%), and 52.2% (95% CI: 41.1–62.2%), respectively, p < 0.001. Adjusting for age and prior LOT at the time of DR/INT did not impact the association of AlloSCT and CD30.CAR-T cell therapy with improved OS-2 (age and LOT adjusted p = 0.02).
Discussion
To our knowledge, this is the largest cohort of patients refractory to, or intolerant of BV and anti-PD-1 therapy. This analysis provides several new survival benchmarks in cHL patients who are DR/INT. Both OS-1 (survival from diagnosis) and OS-2 (survival from DR/INT) were longer than anticipated. Patients who developed DR/INT post-ASCT had a significantly longer OS-1 as compared to patients who developed DR/INT and had not received an ASCT. Rechallenge with both anti-PD-1 based therapies and BV based therapies induced clinical responses and remissions; however, median PFS was less than a year from re-challenge. OS-2 serves as an important benchmark of survival for patients with DR/INT cHL. Finally, advanced therapies such as alloSCT and CAR-T cell therapy were associated with improved OS after developing DR/INT cHL (OS-2).
Several studies have evaluated OS trends in patients with cHL who progress after ASCT [22,23,24]; however, this is the first study to specifically identify and describe the highest-risk subset of patients (i.e., patients with DR/INT cHL). Desai et al evaluated 1158 patients with r/r cHL with disease recurrence following ASCT and found a median OS of 9.5 years in the modern era of BV and anti-PD-1 therapy; however, many of these patients were BV and/or anti-PD-1 therapy naïve [23]. In the present study, we demonstrated that patients developing DR/INT continue to have the opportunity for long survival with a median OS-2 of 7.4 years. This new benchmark of survival after DR/INT (OS-2) is critical to evaluating the benefit of novel therapies in the future.
We observed a considerable difference in OS-1 in patients who developed DR/INT cHL after ASCT compared to those developing DR/INT and not previously treated with ASCT. The majority of data supporting the use of ASCT in r/r cHL as consolidation after high dose chemotherapy comes from the pre-novel agent era [25,26,27,28]. Schmitz et al published the only randomized data for ASCT in r/r cHL demonstrating an improvement in time to treatment failure, but interestingly, no OS benefit. Despite the lack of clear OS benefit, ASCT consolidation has been the standard of care following second-line chemotherapy for r/r cHL in the United States and many other countries for the past two decades. Here, we demonstrated that in patients with DR/INT cHL, those patients able to undergo an ASCT prior to DR/INT had a significantly longer OS-1. This finding is potentially confounded by disease biology, chemo-sensitive nature of disease, and comorbidities of individual patients. However, this finding is still of interest as BV and anti-PD-1 therapies have moved earlier in lines of therapy both in the front-line (ECHELON-1 and SWOG 1826) and at first relapse. Additionally, the role of ASCT consolidation is being challenged in clinical trials incorporating anti-PD-1 based therapies for salvage therapy (NCT03618550). Future randomized prospective clinical trials will be needed to evaluate the role of ASCT following anti-PD-1 and BV based therapies.
Limited data exists supporting the role of BV and/or anti-PD-1 therapy rechallenge in patients with DR/INT cHL. Bartlett et al. reported a cohort of 21 patients with r/r cHL rechallenged with BV, demonstrating an ORR of 60% and CR rate of 30% [29]. Manson et al reported a small cohort of 7 patients with r/r cHL who stopped anti-PD-1 therapy in a CR, relapsed, and were re-challenged with anti-PD-1 therapy demonstrating five complete remissions and 1 partial remission [30, 31]. Finally, Armand et al reported a cohort of 20 patients from KEYNOTE-87 who achieved CR with their first pembrolizumab course and were later re-challenged with pembrolizumab at relapse, observing an ORR of 74% and CR rate of 37% [6]. Notably, these studies did not evaluate patients re-challenged after prior refractory disease or intolerance. The current study includes the largest cohort of patients rechallenged with either BV or anti-PD-1 based therapies. We observed an ORR of 62% with BV based rechallenge and an ORR of 55% with anti-PD-1 based rechallenge. Median PFS was similar with either BV or anti-PD-1 based rechallenge, 183 and 237 days, respectively. This data supports a role for either BV or anti-PD-1 based therapy rechallenge as a bridge to other therapies, though few long-term responses were observed with either BV or anti-PD-1 based rechallenge.
Patients with multiply relapsed DR/INT cHL have limited therapeutic options for long-term disease control and potential cure. AlloSCT has been well-studied in r/r cHL, demonstrating promising disease control, though historically complicated by high non relapse mortality (NRM) [32]. Recently, multiple studies have demonstrated the ability to decrease NRM with non-myeloablative conditioning regimens and improved GVHD prophylaxis [33,34,35,36,37]. Here, we confirm excellent long-term disease control with AlloSCT with a 2-year OS of 93% in patients with DR/INT cHL including patients entering AlloSCT having not achieved a complete remission (12/31 patients). Additionally, CD30.CAR-T cell therapy is an exciting option for patients with r/r cHL. Ramos et al., demonstrated high disease response rates, ORR 72% and CR rate 59% with CD30.CAR-T [38]. Evaluating patients with DR/INT cHL, response rates and overall survival of patients treated with CD30.CAR-T were excellent with a 2-year OS estimate of 85%. There was no statistical difference in OS by treatment with AlloSCT or CD30.CAR-T, though both groups were superior to patients unable to be treated with either therapy option. While AlloSCT is a standard of care approach for patients with r/r cHL, CD30.CAR-T is not FDA approved or commercially available, limiting its clinical utility at this time.
There are several limitations to the current study. This was a retrospective, real-world analysis from multiple academic institutions, which could lead to recall bias, missing data, and/or the presence of unmeasured confounders. Treatment was not standardized across centers, and certain therapeutic pathways may have been favored in one institution over others. However, we found no center effect and attempted to control for confounding variables. Finally, we did not collect the number of cycles of BV or anti-PD-1 therapies prior to DR/INT.
In conclusion, we report the largest dataset of outcomes for patients with DR/INT cHL. Despite DR/INT disease, survival is longer than anticipated for patients, both from diagnosis as well as from the time of DR/INT. We found that subsequent rechallenge with BV or anti-PD-1 based therapies after DR/INT did lead to high clinical response rates, though few long-term remissions. We demonstrated excellent survival outcomes with patients proceeding to AlloSCT and/or CD30.CAR-T cell therapy after developing DR/INT cHL. Finally, the median OS-2 of 7.4 years serves as an important benchmark for therapies being evaluated and tested in patients with DR/INT cHL.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due confidentiality agreements but are available from the corresponding author on reasonable request.
References
Johnson P, Federico M, Kirkwood A, Fosså A, Berkahn L, Carella A, et al. Adapted Treatment Guided by Interim PET-CT Scan in Advanced Hodgkin’s Lymphoma. N. Engl J Med. 2016;374:2419–29.
Ansell SM. Hodgkin lymphoma: 2018 update on diagnosis, risk‐stratification, and management. Am J Hematol. 2018;93:704–15.
Voorhees TJ, Beaven AW. Therapeutic Updates for Relapsed and Refractory Classical Hodgkin Lymphoma. Cancers. 2020;12:2887.
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl J Med. 2015;372:311–9.
Armand P, Shipp MA, Ribrag V, Michot J-M, Zinzani PL, Kuruvilla J, et al. Programmed Death-1 Blockade With Pembrolizumab in Patients With Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure. J Clin Oncol. 2016;34:3733–9.
Armand P, Zinzani PL, Lee HJ, Johnson NA, Brice P, Radford J, et al. Five-year follow-up of KEYNOTE-087: pembrolizumab monotherapy for relapsed/refractory classical Hodgkin lymphoma. Blood. 2023;142:878–86.
Chen R, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128:1562–6.
Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J Clin Oncol. 2017;35:2125–32.
Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283–94.
Bair SM, Strelec L, Nagle SJ, Nasta SD, Landsburg DJ, Mato AR, et al. Outcomes of patients with relapsed/refractory Hodgkin lymphoma progressing after autologous stem cell transplant in the current era of novel therapeutics: A retrospective analysis. Am J Hematol. 2017;92:879–84.
Epperla N, Herrera AF. How I incorporate novel agents into the treatment of classical Hodgkin lymphoma. Blood. 2021;138:520–30.
Ansell SM, Radford J, Connors JM, Długosz-Danecka M, Kim WS, Gallamini A, et al. Overall Survival with Brentuximab Vedotin in Stage III or IV Hodgkin’s Lymphoma. N. Engl J Med. 2022;387:310–20.
Connors JM, Jurczak W, Straus DJ, Ansell SM, Kim WS, Gallamini A, et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N. Engl J Med. 2018;378:331–44.
Herrera AF, LeBlanc M, Castellino SM, Li H, Rutherford SC, Evens AM, et al. Nivolumab+AVD in Advanced-Stage Classic Hodgkin’s Lymphoma. N. Engl J Med. 2024;391:1379–89.
Advani RH, Moskowitz AJ, Bartlett NL, Vose JM, Ramchandren R, Feldman TA, et al. Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3-year study results. Blood. 2021;138:427–38.
LaCasce AS, Bociek RG, Sawas A, Caimi P, Agura E, Matous J, et al. Brentuximab vedotin plus bendamustine: a highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood. 2018;132:40–8.
Mei MG, Lee HJ, Palmer JM, Chen R, Tsai NC, Chen L, et al. Response-adapted anti-PD-1-based salvage therapy for Hodgkin lymphoma with nivolumab alone or in combination with ICE. Blood. 2022;139:3605–16.
Moskowitz AJ, Shah G, Schöder H, Ganesan N, Drill E, Hancock H, et al. Phase II Trial of Pembrolizumab Plus Gemcitabine, Vinorelbine, and Liposomal Doxorubicin as Second-Line Therapy for Relapsed or Refractory Classical Hodgkin Lymphoma. J Clin Oncol. 2021;39:3109–17.
Bryan LJ, Casulo C, Allen PB, Smith SE, Savas H, Dillehay GL, et al. Pembrolizumab Added to Ifosfamide, Carboplatin, and Etoposide Chemotherapy for Relapsed or Refractory Classic Hodgkin Lymphoma: A Multi-institutional Phase 2 Investigator-Initiated Nonrandomized Clinical Trial. JAMA Oncol. 2023;9:683–91.
Epperla N, Hamadani M. Double-refractory Hodgkin lymphoma: tackling relapse after brentuximab vedotin and checkpoint inhibitors. Hematol Am Soc Hematol Educ Program. 2021;2021:247–53.
Cheson BD, Ansell S, Schwartz L, Gordon LI, Advani R, Jacene HA, et al. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 2016;128:2489–96.
Badar T, Epperla N, Szabo A, Borson S, Vaughn J, George G, et al. Trends in postrelapse survival in classic Hodgkin lymphoma patients after experiencing therapy failure following auto-HCT. Blood Adv. 2020;4:47–54.
Desai SH, Spinner MA, Evens AM, Sykorova A, Bachanova V, Goyal G, et al. Overall survival of patients with cHL who progress after autologous stem cell transplant: results in the novel agent era. Blood Adv. 2023;7:7295–303.
Epperla N, Huang Y, Cashen AF, Vaughn JL, Hanel W, Badar T, et al. Evaluation of prognostic factors in patients with high-risk classical Hodgkin lymphoma undergoing autologous transplantation. Blood Adv. 2024;8:5458–66.
Crump M, Smith AM, Brandwein J, Couture F, Sherret H, Sutton DM, et al. High-dose etoposide and melphalan, and autologous bone marrow transplantation for patients with advanced Hodgkin’s disease: importance of disease status at transplant. J Clin Oncol. 1993;11:704–11.
Lavoie JC, Connors JM, Phillips GL, Reece DE, Barnett MJ, Forrest DL, et al. High-dose chemotherapy and autologous stem cell transplantation for primary refractory or relapsed Hodgkin lymphoma: long-term outcome in the first 100 patients treated in Vancouver. Blood. 2005;106:1473–8.
Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–4.
Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359:2065–71.
Bartlett NL, Chen R, Fanale MA, Brice P, Gopal A, Smith SE, et al. Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J Hematol Oncol. 2014;7:24.
Manson G, Brice P, Herbaux C, Bouabdallah K, Antier C, Poizeau F, et al. Efficacy of anti-PD1 re-treatment in patients with Hodgkin lymphoma who relapsed after anti-PD1 discontinuation. Haematologica. 2020;105:2664–6.
Manson G, Brice P, Herbaux C, Silva M, Bouabdallah K, Deau-Fisher B, et al. Risk of Relapse after Anti-PD1 Discontinuation and Efficacy of Anti-PD1 Re-Treatment in Patients with Hodgkin Lymphoma. Blood. 2019;134:1549.
Sureda A, Robinson S, Canals C, Carella AM, Boogaerts MA, Caballero D, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26:455–62.
Ahmed S, Ghosh N, Ahn KW, Khanal M, Litovich C, Mussetti A, et al. Impact of type of reduced-intensity conditioning regimen on the outcomes of allogeneic haematopoietic cell transplantation in classical Hodgkin lymphoma. Br J Haematol. 2020;190:573–82.
Ahmed S, Kanakry JA, Ahn KW, Litovich C, Abdel-Azim H, Aljurf M, et al. Lower Graft-versus-Host Disease and Relapse Risk in Post-Transplant Cyclophosphamide-Based Haploidentical versus Matched Sibling Donor Reduced-Intensity Conditioning Transplant for Hodgkin Lymphoma. Biol Blood Marrow Transpl. 2019;25:1859–68.
Faisal MS, Hanel W, Voorhees T, Li R, Huang Y, Khan A, et al. Outcomes associated with allogeneic hematopoietic stem cell transplantation for relapsed and refractory Hodgkin lymphoma in the era of novel agents. Cancer Med. 2023;12:8228–37.
Ghosh N, Ahmed S, Ahn KW, Khanal M, Litovich C, Aljurf M, et al. Association of Reduced-Intensity Conditioning Regimens With Overall Survival Among Patients With Non-Hodgkin Lymphoma Undergoing Allogeneic Transplant. JAMA Oncol. 2020;6:1011–8.
Martínez C, Gayoso J, Canals C, Finel H, Peggs K, Dominietto A, et al. Post-Transplantation Cyclophosphamide-Based Haploidentical Transplantation as Alternative to Matched Sibling or Unrelated Donor Transplantation for Hodgkin Lymphoma: A Registry Study of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation. J Clin Oncol. 2017;35:3425–32.
Ramos CA, Grover NS, Beaven AW, Lulla PD, Wu MF, Ivanova A, et al. Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma. J Clin Oncol. 2020;38:3794–804.
Acknowledgements
This work was supported by the Lymphoma Research Foundation Career Development Award (TJV).
Author information
Authors and Affiliations
Contributions
TJV and NE designed the research. All authors collected the data. TJV, NE and EM analyzed the data and discussed the results with PT, JF, NK, TM, HR, NS, SD, AR, CD, MF, SH, SS, SA, LS, MC, KP, AM, HS, JS, SD, PG, MH, NG. TJV wrote the first draft of the manuscript; and all authors critically revised and modified the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
NE: Research funding: Beigene, Lilly, and Incyte; Speakers Bureau for Beigene and Genentech, Ad boards for ADC Therapeutics, Lilly, and Ipsen; Honorarium from Novartis. TJV: Advisory Board: Genmab/AbbVie, ADC Therapeutics. Consultancy: Novartis, Recordati, Genmab. Research Funding: Kite, Viracta, Incyte/Morphosys, Genmab/AbbVie, Recordati. TKM: Advisory Board: Kite. Research funding: Genmab, Century Therapeutics, Johnson & Johnson. PRG: Consultancy: Kite/Gilead Pharma, Bristol Myers Squibb (BMS). Advisory Board ADC Therapeutics, Bristol Myers Squibb (BMS), Cellectar Biosciences, Ono Pharma, Ipsen Biopharma and Regeneron Pharma. SS: Advisory Board ADC Therapeutics. CD: Advisory Board: EMD Serono Advisory Board, Cogent Biosciences.
Ethics approval
This retrospective study approved through The Ohio State University institutional review board (Study Number: 2022C0159). Given the retrospective nature of this study, individual patient consent was waived and de-identified data was utilized for analysis. The study was conducted in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Voorhees, T.J., McLaughlin, E.M., Torka, P. et al. Outcomes in patients with classic Hodgkin lymphoma refractory or intolerant to brentuximab vedotin and anti-PD-1 therapy: a real world analysis from 15 U.S. academic centers. Blood Cancer J. 15, 45 (2025). https://doi.org/10.1038/s41408-025-01257-1
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41408-025-01257-1
This article is cited by
-
Brentuximab-vedotin
Reactions Weekly (2025)