Abstract
The free light chain (FLC) ratio is a critical part of risk stratification for monoclonal gammopathy of undetermined significance (MGUS). Recently, revised FLC reference intervals developed using the iStopMM cohort, accounting for age and renal function, have reduced the rate of abnormal findings. Here, we examine the implications of the revision in an independent Danish MGUS cohort. Of 6993 MGUS individuals, 2641 had an abnormal FLC ratio by the original intervals, of whom 844 (32%) were reclassified as normal using the revised intervals. Reclassified individuals had no significantly increased risk of progression compared to those with a normal FLC ratio (hazard ratio (HR): 1.07, 95% confidence interval (CI) 0.74–1.57). Those with an abnormal FLC ratio by the revised reference intervals had an increased risk of progression (HR 2.23, 95% CI 1.79–2.78). Using the revised reference intervals, 490 individuals (16%) were reclassified to low-risk from a higher risk group. These individuals had a similar progression risk compared to others in the low-risk group. The findings validate the revised FLC reference intervals, enhancing prognostic accuracy and improving risk stratification to accurately identify MGUS individuals at risk of progression while reducing unnecessary classifications as high-risk.
Similar content being viewed by others
Background
Monoclonal gammopathy of undetermined significance (MGUS) is an asymptomatic precursor condition of multiple myeloma (MM) and other lymphoproliferative disorders [1]. The prevalence of MGUS increases with age and is found in approximately 4.2% of the population over 50 years of age [2]. Although all cases of MM are preceded by MGUS [3], only a minority (approximately 1% annually) of those with MGUS progress to malignant lymphoproliferative disease [1].
An early diagnosis of MM or a precursor state may decrease the risk of irreversible organ damage as seen in late-stage disease and improve treatment outcomes [4, 5]. Furthermore, MM patients with an acute disease presentation have a poorer prognosis compared to patients diagnosed in an outpatient setting [6]. Therefore, current guidelines recommend follow-up and/or more thorough examination (for example, bone marrow sampling) of individuals with MGUS at high risk of progression [7]. To identify high-risk patients, the Mayo Clinic risk stratification model is used to guide work-up and follow-up of MGUS [8].
An abnormal free light chain (FLC) ratio increases the risk of MGUS progression independently of other risk factors [9, 10] and is incorporated in the Mayo Clinic risk stratification model. Besides monoclonal gammopathies, the serum FLC is affected by age and renal function, which must be considered when interpreting FLC levels and ratios. The first reference intervals for FLC were defined in a smaller cohort of 282 individuals with normal kidney function [11] and later for patients with chronic kidney disease (CKD) with estimated glomerular rate (eGFR) < 60 mL/min/1.73 m2 [12]. However, these reference intervals have been revised as proposed by the Iceland Screens, Treats or Prevents MM (iStopMM) group [13, 14] based on a large cohort of 75,422 participants from the general population with and without CKD who were screened for MGUS by FLC assay allowing definition of reference intervals stratified by age and renal function [13].
The revised iStopMM reference intervals improve the diagnostics of light chain (LC) MGUS by reducing false positives (leading to an 82% reduction in prevalence) while still accurately detecting all cases that progressed to MM in the iStopMM cohort and have been externally validated [15]. Even so, the implications of the revised FLC reference intervals for MGUS risk stratification are unknown. This study aims to compare the original and revised (iStopMM) definition of abnormal FLC ratio as a risk factor of malignant progression for MGUS with an intact M-protein, both independently and as part of the Mayo Clinic risk stratification model. Optimizing MGUS risk stratification is important for the identification of clinically relevant individuals for work and follow-up, while avoiding unnecessary, costly, time-consuming, and potentially harmful scans and examinations of low-risk individuals. By improving this balance, the study contributes to the overall aim of early cancer detection while simultaneously minimizing overdiagnosis [16].
Methods
Study design and data
This nationwide cohort study was conducted using the Danish Lymphoid Cancer Research (DALY-CARE) data resource [17], containing data on all Danish adults with a lymphoid-linage cancer and lymphoid cancer precursors, registered from 2002 to 2024. The data were collected from 10 different Danish registries and linked using the Danish civil registration (CPR) number. This includes national registries from the Danish Health Data Authority and the Danish Clinical Quality Program—National Clinical Registries, and regional (region of Zealand and the Capital) data from electronic health records (EHR).
The cohort
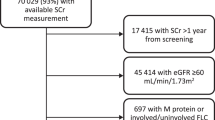
The cohort was identified in DALY-CARE using International Classification of Diseases, 10th revision (ICD10) coded MGUS diagnoses, originally registered in the Danish National Patient Registry (DNPR), the Danish Myeloma Database (DaMyDa), the National Pathology Data Bank, or EHR data. Similarly, MM, non-Hodgkin’s lymphomas (NHL), and amyloid light-chain (AL) amyloidosis diagnoses were identified in DALY-CARE using ICD10 codes (Supplementary Table 1). Only individuals with relevant laboratory data were included in the study. Specifically, this included FLC measurements and a detectable M protein, obtained within a 90-day interval. The laboratory results were extracted using the Nomenclature, Properties and Units (NPU) coding system, and originally retrieved from EHRs and the Danish Laboratory Database (Supplementary Table 2). FLC results from laboratories using other FLC assays than Freelite® (Binding Site by Thermo Fisher Scientific) were excluded, since the revised iStopMM reference intervals were developed using this assay. Individuals without creatinine measurement within 30 days of FLC measurement were excluded. Finally, individuals with a concomitant diagnosis of MM, non-Hodgkin lymphoma (NHL), including chronic lymphoid leukemia (CLL), or AL amyloidosis prior to or within 90 days of MGUS diagnosis were excluded. The date of MGUS diagnosis was defined as the date of first detectable M protein. The inclusion of patients is summarized in Fig. 1.
The diagram outlines the total number of MGUS individuals in the Danish Lymphoid Cancer Research (DALY-CARE) data resource, and individuals excluded (orange) and included (green) in the final analyses. Abbreviations: MGUS monoclonal gammopathy of undetermined significance, FLC free light chain, AL amyloid light chain, M protein monoclonal protein.
Exposures and outcomes
Using the original and revised reference intervals, respectively, the exposure for the study was 1) abnormal FLC ratio, and 2) risk group stratification. The FLC measurement closest in time to the M protein measurement was used. Using the original and revised reference intervals, respectively, the FLC ratio (kappa/lambda) was considered abnormal if outside the reference ranges listed in Table 1.
The primary outcomes for the study were (1) progression to MM, or (2) progression to MM (including smoldering MM), B-cell NHL (including CLL), or AL amyloidosis. These diagnoses were identified in DALY-CARE using ICD10 codes (listed in supplementary Table 2), originally registered in the Danish Lymphoma Database or in the previously mentioned databases. Patients were followed from the set MGUS diagnosis date (defined in the section above) until progression, death, or end of follow-up. As data on progression may have a delay compared to data on vital status, follow-up was terminated on December 31, 2023. For some individuals, the date of MGUS diagnosis and/or relevant laboratory data was later than or equal to the end of the follow-up date. These individuals were excluded from the study, including those diagnosed after December 31, 2023.
Statistical analysis
Descriptive analysis
The median follow-up was calculated using the reversed Kaplan–Meier. Characteristics of the MGUS cohort were presented overall and stratified into (1) normal FLC ratio using any reference interval, (2) FLC ratio reclassified from abnormal by the original reference to normal by the revised, and (3) abnormal FLC ratio according to the revised reference intervals. The descriptive analysis was generated in R using the gtsummary [18] and tidyverse [19] packages. Overall cumulative incidence was calculated using the Aalen–Johansson estimator, considering death as a competing risk. Additionally, overall cumulative incidences were calculated using Kaplan–Meier estimation, not accounting for death as a competing risk. This was added to enable data comparability with existing studies that did not account for death as a competing risk.
Abnormal free light chains measures and ratios
The estimated cumulative incidence of (1) MM and (2) MM, NHL, or AL amyloidosis was calculated and visualized graphically using the Aalen–Johansson estimator, considering death as a competing risk. Differences in cumulative incidence were evaluated using Gray’s test. Cause-specific hazard ratios (HR) were calculated using Cox regression, censoring at death or end of follow-up. The cumulative martingale residuals test was used to assess the Cox proportional hazard assumption and computed using the mets package [20] in R. The proportionality assumption was not violated. HRs were presented crude and adjusted for age, sex, isotype, and M protein concentration. A total of 112 (1.6%) individuals had missing M protein concentration, for which the median was imputed in this analysis. Age, as a continuous variable, violated the linearity assumption and was therefore modeled non-linearly using four knotted restricted cubic splines from the rms package [21]. Potential interactions were examined between exposure (FLC group) and both covariates, age, and sex, using a likelihood ratio test. All analyses were performed in R using the prodlim [22] and survival [23] packages.
MGUS risk stratification
The MGUS cohort was stratified into Mayo Clinic risk groups [9] by scoring each individual based on the following risk factors: abnormal FLC ratio, M protein ≥ 15 mg/L, and non-IgG M-protein. Individuals were categorized into low-, intermediate-low-, intermediate-high-, and high-risk groups according to the presence of zero, one, two, or three factors, respectively [8]. Individuals with missing quantification of M protein were excluded from these analyses. Cumulative incidence and cause-specific HR were calculated as described in the section above using FLC ratio abnormality for original and revised reference intervals, respectively.
Finally, a subgroup analysis was conducted for the individuals with low-risk MGUS using the revised reference intervals. In this subgroup, we compared individuals who reclassified to low-risk from a higher risk group according to the revised reference intervals to individuals who were classified as low-risk regardless of reference interval. As all cases in the low-risk group were obligate IgG MGUS, we only considered MM and AL amyloidosis as outcomes in this analysis. For each group, incidence rate and cumulative incidences with death as competing risk were calculated with the same methodology as described above. Lastly, a Cox regression was performed, comparing the reclassified low-risk individuals to the consistently low-risk individuals. Cause-specific HR are presented unadjusted and adjusted for age and sex.
Results
The study population
A total of 18150 individuals with MGUS were identified in the DALY-CARE cohort, and 6993 met the inclusion criteria (Fig. 1). The FLC ratio of 844 individuals reclassified from abnormal to normal, and 273 reclassified from normal to abnormal, when changing from the original to the revised intervals. This resulted in an overall reduction of 22% in the number of individuals with an abnormal FLC ratio, from 2641 (38%) to 2070 (30%). The included individuals had a median age of 72 years (interquartile range (IQR): 64–79), which did not vary between those with normal FLC ratio and those with abnormal FLC ratio in either the original or revised reference interval. In total, 47% of the MGUS individuals were female, which was similar among strata, except for the group with abnormal FLC ratio using revised reference interval, where the proportion of females was 41% (Table 2). Overall, the individuals were followed for 26763 person-years with a median follow-up time of 4.4 years (IQR 2.1–7.0). During follow-up, 258 (3.7%), 91 (1.3%), and 20 (0.3%) progressed to MM, NHL, and AL amyloidosis, respectively. A total of 1168 (17%) died prior to progression to MM/NHL/AL amyloidosis. Of all MGUS individuals, 4374 (63%) had IgG isotype, 936 (13%) had IgA isotype, and 1332 (19%) had IgM isotype. The remaining (5%) had IgD, IgE, or biclonal isotype. For all individuals with quantification of M protein available (N = 6881), the median M protein concentration was 2.0 g/L (IQR 1.0–5.1) and was higher for those with abnormal FLC ratio using revised reference interval (4.4 g/L (IQR: 1.8–8.0)).
For the entire cohort, accounting for death as a competing risk, the 5-year cumulative incidence of progression to MM was 2.8% (95% confidence interval (CI) 2.4–3.2), and 3.9% (95% CI 3.4–4.3) for progression to MM, NHL, or AL amyloidosis. The 10-year cumulative incidence was 3.6% (95% CI 3.2–4.0) for progression to MM, and 5.0% (95% CI 4.5–5.5) for progression to MM, NHL, or AL amyloidosis.
Not accounting for death as a competing risk, the overall cumulative incidence of progression to MM was 4.9% (95% CI 4.2–5.6) after 5 years and 10.5% (95% CI 8.9–12.3) after 10 years. Again, not accounting for death as a competing risk, the overall cumulative incidence of progression to MM, NHL, or AL amyloidosis was 6.5% (95% CI 5.7–7.3) after 5 years and 14.4% (95% CI 12.5–16.5) after 10 years.
Free light chains ratio and progression risk
The incidence rate of progression to MM/NHL/AL amyloidosis was 13.5 cases per 1000 person-years for MGUS individuals overall. The rate was similar for those with normal FLC ratio (9.5 cases per 1000 person-years) and for those who reclassified from abnormal to normal FLC ratio (10.2 cases per 1000 person-years). For the individuals with abnormal FLC ratio according to the revised reference interval, the incidence rate of progression was significantly higher (21.8 cases per 1000 person-years).
Similarly, cumulative incidence of progression was similar for individuals with normal FLC ratio compared to those with reclassified FLC ratio compared after 5 years (2.7% vs. 2.4%) and after 10-years (3.4% vs. 3.8%). For individuals with abnormal FLC ratio according to the revised reference intervals, the cumulative incidence was higher after 5 years (6.8, 95% CI 5.5–7.9) and 10 years (8.7%, 95% CI 7.5–10.0).
In the Cox regression model, we found no difference in progression risk between individuals with reclassified FLC ratio (from abnormal to normal) compared to those with normal FLC ratio in the unadjusted analysis (HR: 1.07, 95% CI 0.74–1.57) or adjusted for age, sex, isotype, and M protein concentration (HR: 1.03, 95% CI 0.71–1.51). For individuals with abnormal FLC according to the revised reference intervals, we found a significantly higher risk of progression compared to those with normal FLC ratio in the unadjusted analysis (HR: 2.23, 95% CI 1.79–2.78) and adjusted (HR: 1.85, 95% CI 1.47–2.33). The results are summarized in Fig. 2 and Table 3.
The individuals were stratified into normal FLC ratio using any reference interval (black), FLC ratio reclassified from abnormal to normal (blue), and abnormal FLC ratio using the revised reference intervals (red). Abbreviations: MGUS monoclonal gammopathy of undetermined significance, FLC free light chain, MM multiple myeloma, NHL non-Hodgkin’s lymphoma, AL amyloid light chain .
The cumulative incidence of death prior to progression to lymphoproliferative malignancy is visualized in Supplementary Fig. 1 for the three groups. We found no difference between the three groups (Grays test: p = 0.36). The 5- and 10-year cumulative incidences are listed in Supplementary Table 3 for all three groups.
Risk group stratification
A total of 6881 individuals with MGUS had all the required laboratory data for risk group stratification according to the Mayo Clinic risk stratification model. The quantitative distribution of individuals in the risk groups differed when using the original and revised reference intervals. The fraction of individuals with low-risk MGUS increased by 12% from 2751 (40%) to 3113 (45%) when incorporating the revised reference interval. The incidence rate and cumulative incidence of progression to MM, NHL, and AL amyloidosis did not differ significantly for the low-risk group when changing the reference interval (Table 4). For the individuals, who reclassified to low-risk from a higher risk group, when incorporating the revised reference intervals, we found no difference in risk of progression compared to those, who were classified low-risk independently of reference interval in neither cumulative incidence (Gray’s test, p = 0.95) (Supplementary Fig. 2) nor the regression model (adjusted HR: 0.94, 95% CI 0.52–1.70) (Table 5).
Subgroup analysis
In 273 individuals, the FLC ratio reclassified from normal to abnormal when applying the revised reference intervals. They had a similar age (median of 72 years) and sex distribution (48% females) compared to the cohort overall. The majority (87%) had an FLC ratio below the reference interval. A total of 9 individuals (3%) progressed to MM, NHL, or AL amyloidosis during follow-up. Finally, 128 (47%) moved from the low-risk to a higher risk group. Of these, 6 (5%) individuals progressed to MM, NHL, or AL amyloidosis during follow-up.
Discussion
This nationwide cohort study demonstrates that the revised FLC reference intervals by iStopMM lead to improved prognostic value of FLC testing in individuals with MGUS with an intact M protein. The revised reference intervals resulted in lower rates of abnormal FLC testing, outlining a smaller subgroup at higher risk of progression to MM, NHL, or AL amyloidosis. Individuals whose FLC ratio was reclassified from abnormal to normal had a similar risk of progression compared to those with a normal FLC ratio, regardless of the reference interval. Furthermore, using the revised reference intervals, the number of individuals classified as low-risk MGUS increased without compromising the risk of progression to MM or AL amyloidosis. Thus, the findings show that the revised FLC intervals improve the prognostic value of FLC testing while increasing the proportion of low-risk MGUS.
Current guidelines do not recommend bone marrow examination and skeletal imaging in asymptomatic patients with biochemical data compatible with low-risk MGUS [24]. In addition, individuals with low-risk MGUS are followed less frequently [24, 25]. Bone marrow examination and skeletal imaging are expensive, labor-intensive, and potentially harmful analyses, and should therefore be reserved for individuals with a higher risk of malignancy. In this study, we show that using the revised reference intervals, the number of individuals with low-risk MGUS increases by 12% without resulting in an increased progression risk for individuals that reclassified to low-risk from a higher-risk group compared to those originally classified as low-risk. Hence, incorporation of the revised reference intervals can reduce resource use and patient stress by preventing unindicated examinations for individuals and health care systems.
Since FLC levels and ratio are associated with age, renal function, and poor outcomes for lymphoid malignancies [26,27,28], we must consider whether an abnormal FLC ratio is a marker of ageing and/or comorbidity in general, which could introduce a biased result. Furthermore, the individuals with abnormal FLC using the revised reference intervals have a higher M protein concentration, which has previously been associated with increased infection risk [29] and could cause bias if these individuals died earlier and potentially before progression to lymphoproliferative malignancy. However, we did not find an increased risk of death from causes other than MM, NHL, and AL amyloidosis (illustrated and tabulated in Supplementary Tables 3 and 4). Importantly, age did not differ between FLC strata. We thus conclude that our results are not driven or biased by either age or survival difference in the strata and that FLC abnormalities in individuals with MGUS do not appear to be associated with mortality unaccompanied by hematological malignancy.
The strengths of this study include the large population-based dataset with high-quality data from multiple data sources. The linkage of data from different registries and databases in the DALY-CARE data resource makes it possible to extract information on diagnoses, laboratory data, and vital status from individuals not registered in a clinical database or followed systematically in a clinical setting. Hence, this captures both high- and low-risk individuals with MGUS. However, this study has several limitations. We excluded FLC measures from laboratories using assays other than the Freelite® assay, because the revised FLC reference intervals from the iStopMM were based on blood tests analyzed with this laboratory method only. The results from different FLC assays are not interchangeable [30], which limits the generalizability of the study to some extent, but the Freelite® assay is one of the most widely used assays in the world. Due to this, the exclusion of laboratories using other assays resulted in the loss of a substantial part of the total MGUS cases. Furthermore, the clinical use of FLC measurements was scarce prior to 2010, limiting the follow-up time (median 4.4 years). The study cohort is limited by the absence of universal M protein screening in Denmark, leaving an unknown part of individuals with MGUS undiagnosed. Information on the indication for measuring M protein was not available, and a potential correlation to progression risk could not be further investigated in this study. The individuals diagnosed with MGUS are likely to be more morbid than the undiagnosed MGUS [2] as examinations usually occur when investigating, e.g., nonspecific symptoms, bone-related disease, or any suspicion of lymphoid cancer. However, the Danish MGUS cohort likely shares similar characteristics with other clinically diagnosed MGUS populations. Accordingly, the findings of this study show the clinical utility of results derived from studies conducted in screened MGUS cohorts such as the iStopMM cohort. Since the study is limited by the lack of data on ethnicity and the Danish population is primarily Caucasian, extrapolation of the results to other populations may not be possible. However, the revised reference ranges have been shown to perform well in another population that is genetically/ethnically different from the Nordic countries [15]
The clinical implications of these findings are significant. The revised FLC reference intervals enhance risk stratification for MGUS by reducing false-positive classifications and increasing the proportion of individuals in the low-risk category without compromising prognostic accuracy. This refinement minimizes unnecessary diagnostic evaluations and follow-up (i.e., overdiagnosis), enabling healthcare providers to focus resources on patients with a higher risk of progression. By streamlining care pathways and reducing patient burden, the implementation of these intervals in clinical practice has the potential to improve both patient outcomes and healthcare efficiency.
In conclusion, we demonstrate that the revised reference intervals for FLC from the iStopMM study improve risk stratification of individuals with MGUS in a population-based cohort while increasing the total number of individuals in the low-risk MGUS group. The incorporation of the revised reference did not significantly affect the progression risk of either low-risk MGUS individuals overall or the subgroup that reclassified to low-risk from the higher risk group. Consequently, fewer MGUS individuals would require extensive diagnostic work and follow-up under current guidelines. The findings validate the revised reference intervals for FLC, demonstrating improved prognostic accuracy and more focused care for individuals with MGUS. We recommend implementing these revised FLC reference intervals in clinical practice as summarized in Table 1.
Data availability
Data are not publicly available to preserve the privacy of individuals in the study cohort but can be accessed through the Danish Lymphoid Cancer Research (DALY-CARE) data resource with the necessary approvals.
Code availability
R scripts with codes of statistical analyses are available upon request from the corresponding author.
Change history
16 May 2025
The original online version of this article was revised: Tab. 3 has been updated has been updated due to an incorrect formatting occurred during the correction process.
27 May 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41408-025-01307-8
References
Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–9.
Sigurbergsdóttir AÝ, Rögnvaldsson S, Thorsteinsdóttir S, Sverrisdóttir I, Sigurðardóttir GÁ, Viðarsson B, et al. Disease associations with monoclonal gammopathy of undetermined significance can only be evaluated using screened cohorts: results from the population-based iStopMM study. Haematologica. 2023;108:3392–8.
Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–7.
Xu H, Tian J, Li L, Liu F, Ren Y, Guo G, et al. Risk factors for the outcome and prognosis of multiple myeloma patients with pathological fractures undergoing percutaneous vertebroplasty. Am J Cancer Res. 2024;14:2946–56.
Courant M, Orazio S, Monnereau A, Preterre J, Combe C, Rigothier C. Incidence, prognostic impact and clinical outcomes of renal impairment in patients with multiple myeloma: a population-based registry. Nephrol Dial Transplant. 2021;36:482–90.
Rasmussen LA, Vedsted P, Jensen H, Frederiksen H, El-Galaly TC, Kristensen IB, et al. Multiple myeloma: unplanned diagnostic pathways and association with risk factors and survival - a nationwide register-based cohort study in Denmark. BMC Cancer. 2024;24:998.
Danish Myeloma Study Group (DMSG). MGUS—udredning og opfølgning af monoklonal gammopati med ubestemt signifikans. (2025).
Rajkumar SV, Kyle RA, Buadi FK. Advances in the Diagnosis, Classification, Risk Stratification, and Management of Monoclonal Gammopathy of Undetermined Significance: Implications for Recategorizing Disease Entities in the Presence of Evolving Scientific Evidence. 10th ed. Mayo Clinic Proceedings, editor. Vol. 85. 945–8 p. (2010).
Rajkumar SV, Kyle RA, Therneau TM, Melton LJ, Bradwell AR, Clark RJ, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106:812–7.
Shpitzer D, Cohen YC, Perry C, Melamed G, Alapi H, Reiner-Benaim A, et al. Clinical significance of FLC tests in patients without other evidence of hematologic disorder. Clin Exp Med. 2024;24:198.
Katzmann JA, Clark RJ, Abraham RS, Bryant S, Lymp JF, Bradwell AR, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48:1437–44.
Hutchison CA, Harding S, Hewins P, Mead GP, Townsend J, Bradwell AR, et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1684–90.
Long TE, Indridason OS, Palsson R, Rognvaldsson S, Love TJ, Thorsteinsdottir S, et al. Defining new reference intervals for serum free light chains in individuals with chronic kidney disease: Results of the iStopMM study. Blood Cancer J. 2022;12:133.
Einarsson Long T, Rögnvaldsson S, Thorsteinsdottir S, Sverrisdottir I, Eythorsson E, Indridason O, et al. Revised definition of free light chains in serum and light chain monoclonal gammopathy of undetermined significance: results of the istopmm study. Blood. 2023;142:535.
Shpitzer D, Cohen YC, Shragai T, Melamed G, Reiner-Benaim A, Avivi I. Adopting the new iStopMM-based criteria for light-chain monoclonal gammopathy of undetermined significance: an ongoing debate. Haematologica. 2024.
Welch HG, Black WC. Overdiagnosis in Cancer. JNCI J Natl Cancer Inst. 2010;102:605–13.
Brieghel C, Werling M, Frederiksen CM, Parviz M, Lacoppidan T, Faitova T, et al. The Danish Lymphoid Cancer Research (DALY-CARE) Data Resource: The Basis for Developing Data-Driven Hematology. Clin Epidemiol. 2025.
Sjoberg DD, Whiting K, Curry M, Lavery JA, Larmarange J. Reproducible Summary Tables with the gtsummary Package. R J. 2021;13:570.
Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4:1686.
Holst KK mets: Analysis of Multivariate Event Times. CRAN: Contributed Packages. 2012.
Harrell FE Jr. rms: Regression Modeling Strategies. CRAN: Contributed Packages. 2009.
prodlim: Product-Limit Estimation for Censored Event History Analysis. CRAN: Contributed Packages. 2008.
Therneau TM. survival: Survival Analysis. CRAN: Contributed Packages. 2001.
Kyle RA, Durie BGM, Rajkumar SV, Landgren O, Blade J, Merlini G, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–7.
Dansk Myelomatose Studie Gruppe (DMSG). Diagnostik og opfølgning af MGUS—Retningslinje 2018. 2018.
Dispenzieri A, Kyle R, Merlini G, Miguel JS, Ludwig H, Hajek R, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23:215–24.
Kyle RA, Therneau TM, Rajkumar SV, Remstein ED, Offord JR, Larson DR, et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood. 2003;102:3759–64.
Maurer MJ, Cerhan J, Katzmann JA, Link B, Micallef IN, Colgan J, et al. Elevation of serum free light chains are common in lymphoma and associated with poor event free and overall survival. Blood. 2010;116:4136.
Kristinsson SY, Tang M, Pfeiffer RM, Bjorkholm M, Goldin LR, Blimark C, et al. Monoclonal gammopathy of undetermined significance and risk of infections: a population-based study. Haematologica. 2012;97:854–8.
Daves M, Piccin A, Roccaforte V, Lippi G. Comparison of Freelite and N-Latex serum free light chain assays. Biochem Med (Zagreb). 2021;31:431–8.
Acknowledgements
Cecilie Velsoe Maeng was supported by the Danish Cancer Institute. The study was supported by the Nordic Cancer Union (grant reference number: R280-A16025) and the Nordic Myeloma Study Group.
Author information
Authors and Affiliations
Contributions
CVM was responsible for data cleaning, data analysis, and drafting the paper. CB and CUN were responsible for data collection and facilitated data approvals for the DALY-CARE data resource. SYK, SR, TL, and ST created the study design and ST was responsible for protocol development. CB, SR, and ST supported decision-making in data filtering and choice of statistical analyses. CVM, SR, TL, CB, CUN, KG, EH, SYK, and ST provided critical feedback on the manuscript, contributed to the interpretation and presentation of results, and suggested improvements for tables and figures within their respective fields of expertise.
Corresponding author
Ethics declarations
Competing interests
CVM has participated in a conference supported by Daiichi Sankyo. SR has received honoraria for scientific talks from Siemens Healthineers and Johnson & Johnson. TL has no conflicts of interest to declare. CB has received an honorarium from AbbVie. EH reports honoraria for lectures and educational materials from Amgen, Bristol Myers Squibb, Johnson & Johnson, Pfizer, Sanofi, and Takeda, research cooperation with Bristol Myers Squibb, Johnson & Johnson, and Sanofi, participation in conferences supported by Bristol Myers Squibb, Pfizer, Roche, and Takeda, and advisory board consultancy to Johnson & Johnson, Sanofi, and Oncopeptides. CUN has received research funding from AbbVie, Johnson & Johnson, AstraZeneca, Novo Nordisk Foundation, Genmab, and Octapharma, and has provided consultancy for AbbVie, BeiGene, Janssen, Roche, AstraZeneca, CSL Behring, Genmab, Takeda, Octapharma, MSD, Synamics, and Lilly. KG has received research funding from Janssen. SYK reports research funding from Amgen and Celgene, and an Independent Data Monitoring Committee for Johnson & Johnson. ST has received honoraria for scientific talks from AbbVie and Thermo Fisher Scientific.
Ethics approval and consent to participate
The study was conducted under the approval of Danish Lymphoid Cancer Research (DALYCARE) protocol by the Danish National Ethics Committee (1804410) and Data Protection Agency (P-2020-561). All methods were performed in compliance with all relevant guidelines and regulations for use of Danish data, including legal exemption from informed consent as a nationwide epidemiological study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Maeng, C.V., Rögnvaldsson, S., Einarsson Long, T. et al. Revised free light chain reference intervals enhance risk stratification in monoclonal gammopathy of undetermined significance and reduce overdiagnosis. Blood Cancer J. 15, 80 (2025). https://doi.org/10.1038/s41408-025-01289-7
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41408-025-01289-7