Abstract
Autologous stem cell transplantation (ASCT) is a key therapeutic strategy for many patients diagnosed with multiple myeloma (MM), yet early relapses post-transplant remains a major clinical challenge. The plasma cell proliferation (PCPRO) test, which quantifies the proportion of clonal plasma cells in the bone marrow in S-phase (S-phase%) offers a scalable alternative to measuring their proliferation rate compared to the older plasma cell labeling index (PCLI) assay. The impact of the S-phase% in residual clonal plasma cells at the time of ASCT is not clear. We retrospectively analyzed MM patients undergoing an ASCT within one year of diagnosis at Mayo Clinic between January 2013-August 2024. The S-phase% was determined by multiparametric flow cytometry. Patients were grouped into S-phase <2%, ≥2%, or non-assessable, reflecting low numbers of clonal plasma cells at time of ASCT. Among 1,136 patients, 372 had an S-phase <2%, 142 had an S-phase of ≥2% and 622 had non-assessable S-phase. Patients with S-phase ≥2% had higher rates of high-risk cytogenetics, ISS stage III, and elevated creatinine. Median progression-free survival (PFS) and overall survival (OS) from time of ASCT were 26 months and 57 months for patients with S-phase ≥2%, compared to 47 months and not reached for those with S-phase <2%. (P < 0.0001 for both PFS and OS). Patients with non-assessable S-phase, had the most favorable outcomes. In conclusion, our results show that S-phase% at the time of ASCT is a significant prognostic marker in MM. Notably, patients with S-phase ≥5%, and especially ≥10%, had extremely poor outcomes (median PFS of 13 and 3.5 months, respectively), identifying a functionally high-risk group that may derive little or no benefit from standard ASCT. This poor prognostic factor should lead to consideration of alternative strategies including enrollment in clinical trials evaluating novel immunotherapies such as CAR T-cells or T-cell engagers as part of first-line therapy.
Similar content being viewed by others
Introduction
autologous stem cell transplant (ASCT) remains an important element of the treatment paradigm for patients with multiple myeloma (MM), offering deeper responses and improved progression free survival (PFS) [1,2,3,4]. However, some patients experience suboptimal outcomes following ASCT. Studies have shown that early relapses, occurring within one to two years post-ASCT, are associated with inferior outcomes [5]. Some pre and post- transplant prognostic factors that have been associated with earlier relapses following ASCT include the baseline presence of high-risk fluorescence in situ hybridization (HR-FISH) cytogenetics, higher international staging system (ISS) stage, elevated LDH (Lactate dehydrogenase) levels, higher number of prior lines of therapy and achieving less than a complete response (CR) after ASCT [6, 7]. However, there are limited studies addressing factors present at the time of ASCT that may influence relapse risk and survival outcomes in patients with MM. Our group has previously demonstrated that a higher percentage of plasma cell labeling index (PCLI) at the time of ASCT is associated with earlier relapses following ASCT [8]. The PCLI measures the percentage of plasma cells actively synthesizing DNA, reflecting their proliferative activity and providing insight into the biology of plasma cells. However, the use of PCLI has been limited due to its labor-intensive nature. As a result, the plasma cell proliferation (PCPRO) test, a multiparametric flow cytometry-based assay, has replaced PCLI in clinical practice. The PCPRO test offers a more convenient method to quantify the proportion or percentage of monotypic plasma cells in the S-phase, effectively capturing their proliferation rate [9]. Recently, our group evaluated the impact of S-phase% at the time of MM diagnosis on outcomes, considering more recent prognostic stratification and treatment strategies. It was observed that a higher S-phase% (i.e., S phase% ≥2) at diagnosis was associated with an inferior PFS and overall survival (OS) in multivariate analysis containing conventional prognostic features [10].
In this study, we aim to investigate whether the S-phase% of the residual plasma cells in the bone marrow at the time of ASCT retains its prognostic value in predicting for a shorter PFS and OS outcomes after ASCT. We seek to identify a subset where the benefit of ASCT is so limited that alternative strategies should be considered in the first line.
Methods
This retrospective study included patients diagnosed with MM between January 1, 2013, and August 31, 2024, who underwent ASCT within one year of diagnosis at the Mayo Clinic in Rochester, MN. The study was approved by the institutional review board (IRB #24-009552). As shown in (Fig. 1), of 2,348 patients identified, 1,157 met the inclusion criteria. After excluding 21 patients without available S-phase data, 1,136 patients were included in the final analysis.
From a total of 2348 patients with multiple myeloma, exclusions were made for those not undergoing ASCT within 1 year of diagnosis (n = 1191) and those lacking S-phase data (n = 21). The final cohort of 1136 was categorized as S-phase <2% (n = 372), S-phase ≥2% (n = 142), and S-phase not assessable (n = 622). ASCT autologous stem cell transplantation.
Flow cytometric immunophenotyping was performed using the following antibodies: CD19, CD38, CD45, CD138, cytoplasmic kappa and lambda immunoglobulin, and DAPI (4’,6-diamidino-2-phenylindole) as previously described [9]. The plasma cell clonality was determined through demonstrating CD38 and CD138 positivity, absence of CD19/CD45 expression, immunoglobulin light chain restriction, and/or ploidy difference by DAPI staining. The percentage of clonal plasma cells in S-phase was determined by measuring the proportion of cells with DNA content between the G0/G1 and G2/M peaks.
A minimum of 300 abnormal plasma cells is required for reliable S-phase assessment using the PCPRO assay; samples with fewer events were considered non-assessable [11], which usually reflects patients with a CR or better after induction therapy.
Following previously established cutoffs [12], patients were stratified into three groups: S-phase <2%, S-phase ≥2%, and non-assessable. A secondary analysis further divided assessable cases into four subgroups: <2%, 2–4.9%, 5–9.9%, and ≥10%.
High-risk cytogenetic abnormalities (HRCA) by FISH included del(17p), t(4;14), t(14;16), t(14;20), and 1q21 gain/amplification. PFS was defined from the date of transplant to relapse, progression [13], or death, OS from transplant to death from any cause, with censoring at the date of last follow-up.
Categorical variables were compared using the chi-square or Fisher’s exact test, and continuous variables using the Wilcoxon rank-sum test. PFS and OS were estimated using the Kaplan–Meier method and compared using log-rank tests. Univariable and multivariable Cox proportional hazards models were used to assess the prognostic impact of S-phase%, while adjusting for age, ISS stage, serum creatinine, high-risk cytogenetics, depth of response at ASCT, and post-transplant maintenance therapy. All analyses were performed using JMP software (version 10; SAS Institute) and R software (version 4.2.2; R Foundation for Statistical Computing).
Results
A total of 1136 patients who underwent ASCT within one year of MM diagnosis were identified. Baseline patient characteristics are summarized in Table 1. Compared to patients with an S-phase <2%, those with an S-phase ≥2% were more likely to have high-risk cytogenetic abnormalities (59.8% vs 31.9%, P < 0.0001), ISS stage III disease (31.6% vs 19.3%, P = 0.0048), and creatinine ≥2 mg/dL (18.3% vs 8.6%, P = 0.0044). Additionally, patients with an S-phase ≥2% had a higher rate of achieving very good partial response (VGPR) or better at the time of ASCT (58.4% vs 36.8%, P < 0.0001) with no difference in the likelihood to receive maintenance therapy post-transplant (13.4% vs 11.3, P = 0.5339).
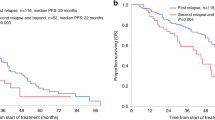
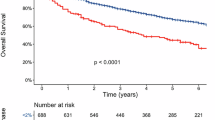
At a median follow-up of 41 months (interquartile range [IQR], 21–63); the median PFS was 26 months (95% CI: 17–35) in the S-phase ≥2% group compared to 47 months (95% CI: 39–58) in the S-phase <2% group (HR 1.7; 95% CI:1.3-2.3) (P < 0.0001) (Fig. 2A). The median OS was 57 months (95% CI: 42–NR) in the S-phase ≥2% group, while it was NR (95% CI: 110–NR) in the S-phase <2% group (HR 3.3; 95% CI: 2.3–4.8) (P < 0.0001) (Fig. 2B). In multivariate analysis, adjusting for age, ISS stage, serum creatinine level, high-risk FISH, response at ASCT, and maintenance therapy, S-phase ≥2% remained an independent predictor of inferior PFS (HR, 1.4; 95% CI: 1.0–2.3; P = 0.0266) (Table 2). Similarly; OS remained inferior in the group with S-phase ≥2% (HR, 1.8; 95% CI: 1.1–2.9; P = 0.0191) in multivariate analysis (Table 3).
We also compared outcomes between patients with an S-phase <2%, ≥2% and those with non-assessable S-phase. Median PFS was 26 months (95% CI: 17–35) for patients with S-phase ≥2%, 47 months (95% CI: 39–58) for patients with S-phase <2%, and 104 months (95% CI: 95–NR) for patients with non-assessable S-phase (P < 0.0001). The median OS was 57 months (95% CI: 42–NR) for patients with S-phase ≥2%, NR with a lower bound of 110 months (95% CI: 110–NR) for patients with S-phase <2%, and NR (95% CI: NR–NR) for patients with non-assessable S-phase (P < 0.0001) (Supplementary Figs. 1 and 2).
After subcategorizing patients with assessable S-phase results into groups with S-phase <2% (n = 372), 2–4.9% (n = 105), 5–9.9% (n = 27), and ≥10% (n = 10), PFS and OS were most inferior in the subgroup with an S-phase% of ≥10%. The median PFS and OS were 13 months (95% CI: 7–35) and 34 months (95% CI: 13–NR) respectively in the group with a S-phase% of 5–9.9% (Fig. 3A, B). The group with an S-phase ≥10% had a median PFS and OS of 3.5 months (95% CI: 1–6) and 9.5 months (95% CI: 3–26), respectively.
Discussion
Under normal physiological conditions, polyclonal plasma cells are terminally differentiated and non-proliferative [14]. However, in MM, clonal plasma cells may re-acquire proliferative capacity through molecular alterations, leading to more aggressive disease behavior [15]. Increased proliferative activity, as captured by the S-phase% measurement, has been associated with worse clinical outcomes [16]. We previously showed that S-phase% assessment by PCPRO test at MM diagnosis carries prognostic significance in this population [10]. Our study extended the utility of assessing the S-phase% of monoclonal plasma cells measured by the PCPRO test at the time of ASCT as a prognostic tool to predict post-ASCT outcomes in MM patients, as we demonstrated inferior PFS and OS outcomes in patients who had high S-phase% at the time of ASCT. These findings align with an earlier study utilizing the PCLI test, a slide-based, labor-intensive method for estimating plasma cell proliferation, which also demonstrated prognostic relevance at the time of ASCT [8]. Importantly, the PCPRO utilized to measure S-phase% offers a more accessible and scalable alternative to PCLI [17,18,19]. Our results reinforce the clinical value of S-phase analysis in the modern therapeutic landscape, which has been transformed by the integration of triplet and quadruplet induction regimens and the widespread use of CD38-targeted monoclonal antibodies [20,21,22]. Also, it shows its significance in the current understanding of the prognostic impact of cytogenetic abnormalities [23].
While the PCPRO assay is not yet widely adopted in general practice, its flow-based methodology makes it considerably more practical than older techniques such as PCLI. This highlights the importance of incorporating S-phase assessment into prospective studies, which could support broader use in routine pre-transplant evaluations.
In the modern era, despite the ongoing and rapidly evolving treatment options, the role of ASCT in upfront consolidation after induction therapy remains the standard of care irrespective of baseline poor prognostic risk factors for early relapse. However, the most striking and clinically relevant finding of our study is that patients with an S-phase ≥5%, and especially those with ≥10%, experienced dramatically inferior outcomes after ASCT, with median PFS of only 13 and 3.5 months, respectively. This identifies a functionally high-risk group that may derive little or no benefit from standard ASCT consolidation. Recognizing such patients before transplant could profoundly impact treatment planning. Many patients confronted with median PFS < 1 year may elect to forgo ASCT, concluding that the impact on quality of life and geographic relocation to a transplant center for up to 6 weeks would not be justified for this short period of PFS. In the context of expanding options, including quadruplet induction regimens and novel immunotherapies, these findings support considering alternative frontline strategies or enrollment in clinical trials for patients identified with highly proliferative disease at the time of ASCT evaluation.
Indeed, chimeric antigen receptor (CAR) T-cell therapy and T-cell engagers are being studied at the frontline treatment of patients with newly diagnosed MM (NCT05257083, NCT05695508) and could potentially be standard options for these difficult to treat subgroups of patients.
A major strength of our study is the large patient cohort. Although a significant proportion of patients did not have assessable S-phase at the time of ASCT, primarily due to an insufficient number of abnormal plasma cells (fewer than 300 events required for PCPRO analysis at Mayo Clinic), we addressed this subset of patient by comparing their clinical outcomes to those with both low ( < 2%) and high ( ≥ 2%) S-phase fractions. Outcomes in these patients with no assessable S-Phase% were the most favorable, supporting the notion that they represent a biologically favorable group. This would be expected given the fact that fewer than 300 plasma cells by flowcytometry reflects the deepest response to anti-myeloma induction therapy and one would anticipate a better outcome than patients with greater than 300 plasma cells presenting for consideration for ASCT.
This study’s limitations include its retrospective nature. Additionally, the relatively small number of patients with extremely high S-phase values (≥10%) limited the statistical power for some subgroup analyses.
In conclusion, incorporating flow-based S-phase% assessment of residual clonal plasma cells in the bone marrow at the time of ASCT into routine pre-transplant evaluation could enhance individualized treatment planning. Notably, identifying patients with S-phase ≥5% — who had exceptionally poor outcomes and represent a functionally high-risk group — could directly inform decisions to consider alternative upfront strategies, deferring stem cell transplantation given the short duration of PFS. Future prospective studies are warranted to validate these observations and to define optimal management approaches for patients with highly proliferative disease.
Data availability
Data are available upon reasonable request.
References
Richardson PG, Jacobus SJ, Weller EA, Hassoun H, Lonial S, Raje NS, et al. Triplet therapy, transplantation, and maintenance until progression in myeloma. N Engl J Med. 2022;387:132–47.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20.
Mo CC, Hartley-Brown MA, Midha S, Richardson PG. Upfront or deferred autologous stem cell transplantation for newly diagnosed multiple myeloma in the era of triplet and quadruplet induction and minimal residual disease/risk-adapted therapy. Cancers (Basel). 2023;15:5709.
Ebraheem M, Kumar SK, Dispenzieri A, Jevremovic D, Buadi FK, Dingli D, et al. Deepening responses after upfront autologous stem cell transplantation in patients with newly diagnosed multiple myeloma in the era of novel agent induction therapy. Transplant Cell Ther. 2022;28:760.e1–e5.
Corre J, Montes L, Martin E, Perrot A, Caillot D, Leleu X, et al. Early relapse after autologous transplant for myeloma is associated with poor survival regardless of cytogenetic risk. Haematologica. 2020;105:e480–3.
Zhou H, Jian Y, Du J, Liu J, Zhang Z, Yang G, et al. Prognostic nomogram for multiple myeloma early relapse after autologous stem cell transplant in the novel agent era. Cancer Med. 2023;12:9085–96.
Martinez-Lopez J, Blade J, Mateos MV, Grande C, Alegre A, García-Laraña J, et al. Long-term prognostic significance of response in multiple myeloma after stem cell transplantation. Blood. 2011;118:529–34.
Kumar S, Mahmood ST, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, et al. Impact of early relapse after auto-SCT for multiple myeloma. Bone Marrow Transplant. 2008;42:413–20.
Aljama MA, Sidiqi MH, Lakshman A, Dispenzieri A, Jevremovic D, Gertz MA, et al. Plasma cell proliferative index is an independent predictor of progression in smoldering multiple myeloma. Blood Adv. 2018;2:3149–54.
Zanwar S, Jevremovic D, Kapoor P, Olteanu H, Buadi F, Horna P, et al. Clonal plasma cell proportion in the synthetic phase identifies a unique high-risk cohort in multiple myeloma. Blood Cancer J. 2025;15:20.
Laboratories MC. Plasma cell proliferation, bone marrow. 2024. https://www.mayocliniclabs.com/test-catalog/overview/61654.
Mellors PW, Binder M, Ketterling RP, Greipp PT, Baughn LB, Peterson JF, et al. Metaphase cytogenetics and plasma cell proliferation index for risk stratification in newly diagnosed multiple myeloma. Blood Adv. 2020;4:2236–44.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–46.
Calame KL. Plasma cells: finding new light at the end of B cell development. Nat Immunol. 2001;2:1103–8.
Bergsagel PL, Kuehl WM. Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol. 2005;23:6333–8.
Greipp PR, Lust JA, O’Fallon WM, Katzmann JA, Witzig TE, Kyle RA. Plasma cell labeling index and beta 2-microglobulin predict survival independent of thymidine kinase and C-reactive protein in multiple myeloma. Blood. 1993;81:3382–7.
Greipp PR, Kumar S. Plasma cell labeling index. Methods Mol Med. 2005;113:25–35.
Kumar S, Rajkumar SV, Greipp PR, Witzig TE. Cell proliferation of myeloma plasma cells: comparison of the blood and marrow compartments. Am J Hematol. 2004;77:7–11.
Steensma DP, Gertz MA, Greipp PR, Kyle RA, Lacy MQ, Lust JA, et al. A high bone marrow plasma cell labeling index in stable plateau-phase multiple myeloma is a marker for early disease progression and death. Blood. 2001;97:2522–3.
Durie BGM, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389:519–27.
Sonneveld P, Dimopoulos MA, Boccadoro M, Quach H, Ho PJ, Beksac M, et al. Daratumumab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2024;390:301–13.
Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136:936–45.
Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127:2955–62.
Acknowledgements
Research reported in this publication was supported by Mayo Clinic Hematological Malignancies Program and in part by grants from the National Cancer Institute of the National Institutes of Health under Award Number R01 CA254961 (WIG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
TH, MAG, and WIG came up with the conception and design of the study. TH, SAAY, DGP, and AMO collected and assembled data. TH and SS analyzed the data. TH drafted the paper. TH, DGP, SAAY, AMO, SS, SKK, AD, SZ, DJ, HO, PH, GO, FKB, DD, SRH, PK, NL, JC, NA, MB, EM, RW, TVK, RSG, YL, SVR, WIG, and MAG contributed to data interpretation, provided expert input, and approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
MAG reports personal fees from Ionis/Akcea, honorarium from Alnylam, personal fees from Prothena, personal fees from Sanofi, personal fees from Janssen, personal fees for Data Safety Monitoring board from AbbVie and Arcellx, fees from Johnson & Johnson, Honoraria from Astra Zeneca, Medscape, Dava Oncology, Alexion. All other authors declare no competing interests.
Ethics approval and consent to participate
All methods were performed in accordance with relevant guidelines and regulations. This study was approved by the Mayo Clinic Institutional Review Board (IRB #24-009552). Informed consent was obtained from all participants in the cohort.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hellou, T., Packard, D.G., Atallah-Yunes, S.A. et al. Impact of residual clonal plasma cells in S-phase at the time of autologous stem cell transplantation on clinical outcomes. Blood Cancer J. 15, 152 (2025). https://doi.org/10.1038/s41408-025-01349-y
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41408-025-01349-y