Abstract
We evaluated the safety and efficacy of a novel protocol for haploidentical stem cell transplantation (haplo-SCT) in 312 patients with hematologic malignancies. The protocol evolved from the Beijing platform replacing ATG with ATLG; adding Fludarabine and removing cytarabine and Simustine. GVHD prophylaxis combined Basiliximab and low-dose cyclophosphamide post-transplant; overall, the conditioning duration was shortened. Median times to neutrophil and platelet recovery were both 11 days. Graft rejection occurred in 0.96% of patients. Cumulative incidences of grades II–IV and III–IV acute GVHD by day 200 were 35.3% and 8.9%, respectively. Probabilities of total and extensive chronic GVHD at 2 years were 40.7% and 14.7%. CMV viremia was observed in 35.6% of patients, with a 1.9% 100-day CMV pneumonia incidence and no CMV-related mortality. Cumulative incidences of non-relapse mortality at 100 days, 1 year, and 2 years were 2.9, 4.4, and 6.6%. The 4-year OS, RFS, and GRFS rates were 78.9, 70.7, and 47.3%. Older recipient age was associated with higher NRM, while positive pre-transplant MRD predicted worse OS, RFS, and higher relapse incidence. Our novel protocol for haplo-SCT is associated with low infection rates and acceptable risks of graft failure, severe GVHD, and mortality, representing a safe and effective haploidentical transplantation strategy.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains a potentially curative approach for acute leukemia. However, the limited availability of human leukocyte antigen (HLA)-matched donors has led to the increasing use of haploidentical HSCT (haplo-HSCT) as an alternative [1]. Compared to HLA-matched sibling donor-HSCT (sibling-HSCT), haplo-HSCT has been shown to have a lower long-term relapse rate, despite an increased risk of short-term complications. Importantly, studies have reported no significant difference in long-term overall survival (OS) between the two transplant modalities. Many transplant centers have demonstrated that if the short-term complications after haplo-HSCT are well-managed, long-term benefits of this approach can be achieved [2, 3].
Many centers in China employ the “Beijing Protocol” for haploidentical transplantation, which involves the use of rabbit anti-thymocyte globulin (ATG, Thymoglobulin®, Sanofi Genz Cambridge, MA, USA) for in vivo T-cell depletion to prevent graft-versus-host disease (GVHD) [4, 5]. The manufacturing process of ATG results in the production of antibodies not only against T-cells, but also against other immune cells, such as neutrophils, monocytes, NK cells, B cells, and even non-immune cells like endothelial cells. While GVHD is primarily driven by donor-derived alloreactive T cells, the excessive depletion of various immune cell subsets by ATG can significantly impair immune reconstitution and negatively impact clinical outcomes after HSCT [6].
Notably, the “Beijing Protocol” has been associated with a high incidence of infectious complications, particularly cytomegalovirus (CMV) infections, leading to increased non-relapse mortality (NRM) [7]. To address this issue, many centers have attempted to reduce the dose of ATG, which has been shown to decrease the rate of infections [8]. However, the optimal dosing of ATG remains challenging to determine.
Our center has adopted a different approach by using a polyclonal IgG antibody, specifically rabbit anti-human T-lymphocyte immunoglobulin (ATLG, Grafalon®, Neovii Biotech, Rapperswil, Switzerland) in reason to the distinct manufacturing process. It may lead to significant differences in immunosuppressive activities and impact on immune reconstitution after transplantation when compared to ATG [9, 10]. Previous studies have shown that the antibody profile of ATLG is primarily directed towards the clearance of T cells, with a weaker effect on other immune cells, in contrast to ATG. Consequently, the use of ATLG over ATG may facilitate the effective clearance of donor-derived alloreactive T cells in vivo and the prevention of acute graft-versus-host disease (aGVHD), while also promoting rapid immune system reconstitution after transplantation [11, 12]. The rationale for this choice is the difficulty in determining the optimal dosage of ATG, which led to the decision to utilize ATLG as an alternative.
Although ATLG can deplete activated T cells more precisely, its T-cell depletion capacity is weaker than that of ATG [13]. To enhance the lymphocyte depletion effect of ATLG and the antitumor activity, we incorporated Fludarabine and Basiliximab into our haplo-SCT protocol.
Since 2015, we have progressively modified our protocol for haplo-SCT by: (1) replacing ATG with ATLG; (2) adding Fludarabine to the conditioning regimen and Basiliximab (a CD25-antibody) on day +3 after transplantation for GVHD prophylaxis; (3) reducing the dose of cyclophosphamide in the conditioning regimen and replacing low-dose methotrexate with low-dose cyclophosphamide after transplantation; (4) removing cytarabine and Simustine; (5) shortening the conditioning duration by 4 days. This report will focus on the clinical outcomes associated with this modified regimen.
Patients and methods
Patient eligibility
This retrospective study evaluated 312 patients with high-risk hematologic malignancies who underwent haplo-SCT at our institution between December 2015 and February 2023. Eligible diagnoses included poor-risk acute leukemia, advanced myelodysplastic syndrome (MDS), and chronic myeloid leukemia (CML) in second chronic phase. Prior to haplo-SCT, most acute leukemia patients had achieved complete hematologic remission (CHR) or complete molecular remission (CMR) following induction and consolidation chemotherapy. Detailed patient and donor characteristics are provided in Table 1. The study protocol was approved by the Ethics Committee of Sichuan Provincial People’s Hospital & Affiliated Hospital of the University of Electronic Science and Technology of China, and all participants or their legal guardians provided written informed consent in accordance with the Declaration of Helsinki.
Donor and stem cell harvesting
All patients in this study underwent haplo-SCT due to the lack of available HLA-matched siblings or unrelated donors. HLA typing was performed at high resolution for HLA-A, -B, -C, -DRB1, -DQB1, and -DPB1 loci. Donors were required to be matched for at least one allele at each of these loci to be considered haploidentical. Donors were selected based on the best HLA match, age, relationship, gender (male preferred), and health status. Donors were primed with granulocyte colony-stimulating factor (G-CSF, 5 μg/kg per day) administered subcutaneously for three consecutive days. On the fourth day, bone marrow cells were harvested. Peripheral blood stem cells (PBSCs) were collected on the 5th day using a Fresenius KABI Com. Tec Blood Cell Separator. The harvested bone marrow and PBSCs were infused into recipients on the same day of collection. The target cell doses were at least 6–8 × 108 mononuclear cells/kg and 3 × 106 CD34+ cells/kg of recipient weight. If the initial cell count was insufficient, an additional PBSCs collection was performed on the sixth day.
Conditioning regimen (Fig. 1)
Our conditioning regimen consisted of busulfan (3.2 mg/kg daily for 3 days; total dose 9.6 mg/kg), fludarabine (30 mg/m² daily for 5 days; total dose 150 mg/m²), cyclophosphamide (1.4 g/m² daily for 2 days; total dose 2.8 g/m²), and ATLG (5 mg/kg daily for 4 days; total dose 20 mg/m²). On days 0 and 1, patients received a combination of donor G-CSF-mobilized bone marrow and PBSCs, targeting a total nucleated cell dose of 8 × 108 cells/kg and a CD34+ cell dose of 3 × 106 cells/kg of recipient weight.
aGVHD prophylaxis included low-dose cyclophosphamide (0.6 g/m² on day +1, and 0.4 g/m² on days +3, +5, and +11 post-transplantation), basiliximab (20 mg IV on day +3), cyclosporine (1.25 mg/kg IV from day −5 to +11, then switched to oral administration), and mycophenolate mofetil (0.5 g every 12 h from day −10 before transplantation to day +30 after transplantation). Cyclosporine levels were monitored twice weekly to maintain the therapeutic range (100–200 ng/mL) for around 1 year. All patients received recombinant human thrombopoietin (300 U/kg/day) and G-CSF (5 μg/kg/day) starting on days 3 and 6, respectively, until platelet and neutrophil recovery.
Supportive care
Antimicrobial prophylaxis was administered according to the Chinese expert consensus, including oral trimethoprim-sulfamethoxazole, quinolone, caspofungin, and acyclovir [14]. If invasive fungal infections occurred during induction and consolidation chemotherapy, antifungal prophylaxis with posaconazole or voriconazole was adopted based on previous effective treatments. Patients were monitored for CMV reactivation by weekly serum PCR until day 100 post-transplant. Preemptive therapy with ganciclovir (5 mg/kg IV twice daily) was initiated when CMV copy number reached ≥103 copies/mL, which was escalated to combined ganciclovir and phosphonate (90 mg/kg once daily) when ≥104 copies/mL were detected. Treatment was discontinued after two negative surveillance tests. All blood products were irradiated before transfusion. Intravenous immunoglobulin (400 mg/kg) was administered on day +1 after transplantation. The thresholds for red blood cell and platelet transfusions were hemoglobin <60 g/L or platelet count <20 ×109/L, respectively.
Engraftment and chimerism evaluation
Engraftment of neutrophils was defined as the maintenance of an absolute neutrophil count above 0.5 × 109/L for three consecutive days after the nadir. Platelet recovery was defined as a platelet count above 20 × 109/L without a platelet transfusion in the preceding 7 days [15]. Donor chimerism was analyzed at +30 and +60 days post-transplant. Nucleated cells were isolated from the marrow or peripheral blood, and T cells (CD3-positive), B cells (CD19-positive), NK cells, and CD34-positive cells were sorted from peripheral blood using magnetic beads. Donor-host chimerism percentages were determined by multiplex PCR [16]. Specifically, DNA was extracted using the GenMagBio Blood DNA Purification Kit (Changzhou GenMagBio Biological Technology Co., LTD). The SifaSTR™23 plex Amplification Kit was used to amplify 22 autosomal STRs and the gender marker amelogenin in a 25.0 μl total reaction volume. The amplified products were analyzed using an ABI 3130 genetic analyzer (Applied Biosystems, Foster City, CA, USA), and the data was analyzed using GeneMapper v3.2 software [17].
GVHD grading and therapy
aGVHD was graded according to the Keystone Criteria [18], while chronic GVHD (cGVHD) was diagnosed and staged based on the National Institutes of Health (NIH) consensus criteria [19]. For the treatment of clinically significant aGVHD, a combination therapy approach is commonly adopted, typically consisting of methylprednisolone and calcineurin inhibitors, such as cyclosporine. Additional agents, including ruxolitinib, mycophenolate mofetil, and sirolimus, may also be incorporated into the treatment regimen.
Statistics
Patient outcomes were analyzed as of June 1, 2023 OS and relapse-free survival (RFS) probabilities were estimated using the Kaplan–Meier method. Cumulative incidences of engraftment, GVHD, CMV infection, relapse, and non-relapse mortality (NRM) were calculated using competing risk analysis, and differences were assessed using the chi-square test. In the total population, multivariate hazard ratios for OS, RFS, relapse, and GVHD were estimated using Cox proportional hazards regression. The regression model included the following factors: age, disease type, disease risk stratification, degree of disease remission before transplantation, donor gender, donor type, HLA disparity, donor-recipient sex match, total nucleated cell count of the graft, CD34+ cell count of the graft, grade II–IV GVHD, and grade III–IV GVHD. All reported p values were based on two-sided hypothesis tests, and p values < 0.05 were considered statistically significant. Data analyses were primarily conducted using SPSS (version 13.0) and GraphPad Prism 5 (version 5.01) software.
Results
Patient, disease, and donor characteristics
Patients were aged 3–60 years and had high-risk hematologic malignancies, as defined in the Materials and methods (Table 1). Most patients with acute leukemia (97%, 279/288) achieved complete remission (CR) prior to transplantation. Donor and graft characteristics are presented in Table 2. Most donors were either parents (160/312) or children (113/312) of the patients, while approximately one-quarter were siblings (39/312). Over 70% of donor-recipient pairs were mismatched for at least 6 HLA loci in the graft-versus-host (GVH) direction.
Engraftment and chimerism
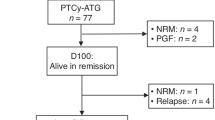
In this cohort, the median time to reach an absolute neutrophil count above 0.5 × 109 cells/L was 11 days (range: 9–22 days), and the median time to reach a platelet count above 20 × 109 cells/L without platelet transfusion was also 11 days (range: 7–14 days). Graft rejection occurred in 3 out of 312 evaluable patients (0.96%). Two of these patients immediately underwent a second transplant with a different donor on day 28 after the first transplant, Their white blood cell engraftment occurred on days 11 and 15, while the platelet engraftment occurred on days 16 and 28, respectively. The remaining patient experienced recovery of autologous hematopoiesis. All but three patients were confirmed to achieve full donor chimerism by day +30 after transplantation.
aGVHD and cGVHD
The incidence of grades II–IV and III–IV aGVHD by day 200 was 35.3% and 8.9%, respectively (Fig. 2a). There was no statistically significant difference in the probability of aGVHD between patients who received high-dose total nucleated cell (TNC) versus low-dose (Fig. 2b). Figure 2c presents the incidences of grade III–IV aGVHD stratified by donor-recipient relationship. The incidence of severe aGVHD was significantly higher in sibling donor-recipient pairs compared to child-to-parent pairs (p = 0.0002). Similarly, the incidence was also higher in sibling versus parent-to-child pairs (p = 0.034). However, the difference between child-to-patient and patient-to-child pairs did not reach statistical significance (p = 0.051). The probabilities of total chronic GVHD (cGVHD) and extensive cGVHD at 2 years were 40.7% and 14.7%, respectively (Fig. 2d).
a Cumulative incidence of aGVHD grades II–IV and III–IV. b Comparison of the incidence of aGVHD among recipients of high and low doses of total nucleated cell (TNC). c The incidences of grade III–IV aGVHD stratified by donor-recipient relationship. d Cumulative incidence of extensive cGVHD for patients in our conditioning regimen.
Infections and transfusions
CMV reactivation is a major concern after haplo-HSCT, as patients with CMV viremia are at high risk of progressing to fatal CMV disease. In this study, CMV viremia was observed in 111 of 312 (35.5%) patients, with a median time to reactivation of 26 days within the first 100 days. However, the 100-day incidence of CMV-associated pneumonia was only 1.9% (3/312), and there was no CMV-associated mortality.
Despite antifungal prophylaxis, invasive fungal infections (IFIs). Proven or probable IFIs were observed in 81 of 312 patients (25.6%) within 6 months post-transplant. Voriconazole was effective in treating 24.7% of these cases, likely due to Aspergillus infections. Amphotericin B was effective in 18 cases (5.8%), where the etiology was Mucorales, with 15 of these cases also having concomitant aspergillosis. The incidence of Pneumocystis jirovecii infection at 6 months after transplantation was 4.2% (13/312).
Regarding transfusions, the median number of red blood cell and platelet transfusions per patient was 4 units (range: 0–20) and 3 (range: 0–31), respectively. Twenty and 5 patients did not require red blood cell or platelet transfusions, respectively.
NRM and relapse
The cumulative incidence of NRM at 100 days, 1 year, and 2 years post-transplantation was 2.9%, 4.4%, and 6.6%, respectively (Fig. 3a). The main causes of NRM were infections (10 patients), grade IV aGVHD (5 patients), diffuse alveolar hemorrhage (1 patient), and thrombotic microangiopathy (1 patient). The cumulative incidence of relapse(CIR) at 100 days, 1 year, and 2 years after transplantation was 7.1%, 20.2%, and 24.2%, respectively (Fig. 3a). Patients with acute lymphoblastic leukemia (ALL) had a significantly higher risk of relapse compared to patients with acute myeloid leukemia (AML) (hazard ratio, 0.58; 95% confidence interval, 0.35–0.96; p = 0.036) (Fig. 3b).
OS, RFS and GRFS
The median follow-up among survivors was 25.5 months (range: 6–93 months). The last patient in this cohort was followed for 6 months. The 4-year OS and RFS rates were 78.9% and 70.7%, respectively, as estimated by the Kaplan–Meier method (Fig. 3c). The 4-year GvHD relapse-free survival (GRFS) rate was 47.3% (Fig. 3c). Patients with AML had significantly improved RFS compared to those with ALL (hazard ratio, 0.52; 95% confidence interval, 0.31–0.88; p = 0.013) (Fig. 3d). However, OS was not statistically different between the AML and ALL groups. Patients with parent-donors had significantly improved OS compared to those with sibling-donors (hazard ratio, 0.29; 95% confidence interval, 0.12–0.71; p = 0.006) (Fig. 3e). Although other particular donor-recipient pair relationships appeared to influence OS and RFS, this trend did not achieve statistical significance (Fig. 3e, f).
Univariate and multivariate analyses
Factors associated with event occurrence are summarized in Table 3. In the univariate analysis, several factors were associated with poor outcomes after haplo-SCT. Older age of recipients was associated with lower RFS [HR 1.018, 95% CI 1.001–1.035; p = 0.035]. Positive minimal residual disease (MRD) before HSCT predicted worse OS [p = 0.0007], RFS [p = 0.0002], and higher CIR [p = 0.0006]. The development of grade II–IV aGVHD and cGVHD was associated with inferior OS, RFS, higher CIR, and increased NRM. Furthermore, grade III–IV aGVHD was specifically associated with worse OS and NRM.
In the multivariable analysis, older age of recipients was associated with higher NRM [HR 1.047, 95% CI 1.000–1.096; p = 0.049]. Positive MRD before HSCT remained a significant predictor of poor outcomes, including worse OS [HR 4.114, 95% CI 2.274–7.444; p = 0.0003], RFS [HR 7.535, 95% CI 4.406–12.885; p = 0.0002], and higher CIR [HR 6.619, 95% CI 4.174–11.461; p = 0.0006]. Other factors, such as donor-recipient relationships, TNC count in the graft, CD34+ cell count in the graft, grade II–IV aGVHD, grade III–IV aGVHD, and cGVHD, were not significantly associated with OS, RFS, CIR, or NRM in this analysis.
Discussion
Allogeneic hematopoietic stem cell transplantation is a complex and multifaceted procedure, particularly in the case of haplo-HSCT. The design of the conditioning regimen, including the prevention of aGVHD and infections, is a crucial component of the entire transplantation system and has a significant impact on the development of post-transplant complications and immune reconstitution [20, 21]. The two mainstream transplantation strategies for haplo-HSCT, the Beijing protocol and the Baltimore protocol, have both demonstrated unique advantages and clinical efficacy. However, these protocols still require adjustments and optimization to address the problems encountered in clinical practice and to mitigate their respective disadvantages [22, 23]. Our center’s haplo-HSCT program has made appropriate adjustments to address the issues identified in the previous process of adopting the Beijing protocol. These adjustments have been designed to optimize the conditioning regimen, enhance infection prevention, and improve the safety and efficacy of haplo-HSCT.
In China, the ATG-based Beijing protocol is widely employed for allo-HSCT [24]. Although ATG, a polyclonal immunoglobulin G derived from rabbits immunized with human thymocytes, effectively reduces the risk of aGVHD, it can adversely affect various B cell subsets and increase the risk of infections, especially viral infections [7]. To mitigate these risks, some centers have reduced ATG dosages [8, 25]. Alternatively, our center has adopted the use of Anti-T-Lymphocyte Globulin, sourced from rabbits immunized with Jurkat T lymphoblastoid cells. This change aims to target and deplete alloreactive T cells more selectively, showing lower infection rates and comparable efficacy in preventing aGVHD compared to the Beijing protocol [26, 27]. Although 35.5% of our patients developed CMV infections, these were generally well-managed with oral ganciclovir, leading to minimal CMV disease progression [28]. These preliminary findings suggest that ATLG may support better immune reconstitution post-transplant than the Beijing protocol. However, rigorous comparative trials are essential to verify these results and further explore the immunological impacts of these regimens.
Haploidentical transplantation regimens require a delicate balance between effectively clearing mature lymphocytes to prevent engraftment failure and minimizing associated toxicity. the differences in the manufacturing processes of ATLG and ATG lead to distinct product profiles, with ATLG exhibiting a relatively higher focus on anti-T cell antibodies, studies have shown that the anti-T cell antibody titer of ATLG is significantly lower than that of ATG [13, 29]. This suggests that ATLG may not fully replace the T cell-depleting function of ATG in clinical applications. To address this limitation, we designed our transplantation conditioning by combining ATLG with fludarabine and basiliximab, a chimeric anti-CD25 monoclonal nondepleting antibody, administered before and after hematopoietic stem cell infusion to enhance T lymphocyte depletion. This approach is based on two key factors. First, fludarabine, a purine analog, possesses potent immunosuppressive properties and can effectively eliminate mature T cells [30, 31]. Second, basiliximab specifically blocks the alpha-subunit of the IL-2 receptor (IL-2R) on activated T cells, thereby preventing T cell proliferation and activation of the effector arms of the immune system. Basiliximab has been identified as a potential therapeutic agent for the prevention of GVHD [32, 33].
In our transplantation system, we retained cyclophosphamide before transplantation but adjusted its usage and dosage to minimize associated toxicities, such as hemorrhagic cystitis and cardiac complications, which are common concerns with higher doses [33]. The dose of cyclophosphamide before hematopoietic stem cell infusion was reduced from 1.8 g to 1.2–1.4 g. Additionally, to avoid the pain caused by the aggravation of oral mucosal ulcers associated with low-dose methotrexate after transplantation in the “Beijing protocol,” we drew inspiration from the Baltimore protocol and administered low-dose cyclophosphamide (0.6 mg/m2, administered intravenously on day +1, and 0.4 mg/m2 intravenously on days +3, +5, and +11) after transplantation, instead of using methotrexate [34]. This modification also appropriately compensates for the reduction of cyclophosphamide before transplantation. Our study and Wang et al. indicate that the revised protocol was able to maintain the prevention of acute graft-versus-host disease (aGVHD) while significantly reducing the incidence of severe oral mucosal ulcers and improved patient comfort [35]. Our results regarding cGVHD are suboptimal. We can explore optimizing the dosage of post-transplant cyclophosphamide to reduce the incidence and severity of cGVHD without significantly increasing systemic toxicity.
Furthermore, our revised regimen has removed the two-day cytarabine component and oral simvastatin from the original Beijing protocol, shortening the conditioning regimen period from 10 to 6 days. This optimization has reduced the risk of severe infections during the period of prolonged neutropenia and the central recurrence rate did not significantly increase after transplantation.
The results from our patient cohort have been promising, with all cases successfully achieving engraftment, and the observed toxicity being manageable. These findings demonstrate that our optimized conditioning regimen has effectively balanced the need for robust lymphocyte clearance and minimized the associated toxicity, ultimately meeting the preset goals for haploidentical transplantation. In conclusion, our novel protocol for haplo-SCT is associated with low infection rates and acceptable risks of graft failure, severe GVHD, and mortality. This approach represents a safe and effective haploidentical transplantation strategy.
Data availability
The datasets generated during and/or analyzed during this study are available from the corresponding author on reasonable request.
Change history
11 November 2024
The original online version of this article was revised: Due to a typesetting error, Figures e and f were duplicated, while Figures a and b were omitted.
06 January 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41409-024-02470-5
References
Montoro J, Balaguer RA, Sanz J. Recent advances in allogeneic transplantation for acute myeloid leukemia. Curr Opin Oncol. 2023;35:564–73.
Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical vs identical-siblingm transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125:3956–62.
Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31:1310–6.
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant. 2006;38:291–7.
Chang YJ, Zhao XY, Huang XJ. Haploidentical stem cell transplantation for acute myeloid leukemia: current therapies, challenges and future prospective. Front Oncol. 2021;11:758512.
Bosch M, Dhadda M, Hoegh-Petersen M, Liu YP, Hagel LM, Podgorny P, et al. Immune reconstitution after antithymocyte globulin-conditioned hematopoietic cell transplantation. Cytotherapy. 2012;14:1258–75.
Wang Y, Liu DH, Liu KY, Xu LP, Zhang XH, Han W, et al. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer. 2013;119:978–85.
Zhou X, Lu X, Tang L, Yan H, Chen W, Shi W, et al. Optimization of ATG dose in haploid hematopoietic stem cell transplantation for hematologic malignancies. Chin J Hematol. 2020;41:557–63.
Oostenbrink LVE, Zijde CMJ, Kielsen K, Hoogendijk AMJ, Ifversen M, Müller KG, et al. Differential elimination of anti-thymocyte globulin of fresenius and genzyme impacts T-cell reconstitution after hematopoietic stem cell transplantation. Front Immunol. 2019;10:315.
Duftner C, Dejaco C, Hengster P, Bijuklic K, Joannidis M, Margreiter R, et al. Apoptotic effects of antilymphocyte globulins on human pro-inflammatory CD4+CD28- T-cells. PLoS ONE. 2012;7:e33939.
Leitner J, Pfistershammer KG, Majdic O, Zlabinger G, Steinberger P. Interaction of antithymocyte globulins with dendritic cell antigens. Am J Transplant. 2011;11:138–45.
Gérard S, Claudia S, Wolfgang AB, Hellmut DO, Matthias S, Axel RZ, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117:6375–82.
Popow I, Leitner J, Grabmeier KP, Majdic O, Zlabinger GJ, Kundi M, et al. A comprehensive and quantitative analysis of the major specificities in rabbit antithymocyte globulin preparations. Am J Transplant. 2013;13:3103–13.
Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–238.
Martinelli G, Trabetti E, Farabegoli P, Testoni N, Bandini G, Motta MR, et al. Early detection of bone marrow engraftment by amplification of hypervariable DNA regions. Haematologica. 1997;82:156–60.
Thiede C, Bornhäuser M, Oelschlägel U, Brendel C, Leo R, Daxberger H, et al. Sequential monitoring of chimerism and detection of minimal residual disease after allogeneic blood stem cell transplantation (BSCT) using multiplex PCR amplification of short tandem repeat-markers. Leukemia. 2001;15:293–302.
Jordan RC, Stuart DS, Andrea LJ, Helena L, Joanne M, Geoffrey IC, et al. Monitoring of chimerism following allogeneic hematopoietic stem cell transplantation (HSCT): technical recommendations for the use of short tandem repeat (STR) based techniques, on behalf of the United Kingdom National External Quality Assessment Service for Leucocyte Immunophenotyping Chimerism Working Group. Br J Haematol. 2015;168:26–37.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401.
Ephraim JF. Haploidentical transplantation for hematologic malignancies: where do we stand? Hematol Am Soc Hematol Educ Program. 2012;2012:230–6.
Reisner Y, Martelli MF, Gabutti V. Haploidentical hematopoietic stem cell transplantation: state of the art. Bone Marrow Transplant. 2011;46:159–66.
Lv M, Chang YJ, Huang XJ. Update of the “Beijing Protocol” haplo-identical hematopoietic stem cell transplantation. Bone Marrow Transplant. 2019;54:703–7.
Luznik L, O’Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. 2012;39:683–93.
Xu LP, Lu PH, Wu DP, Sun ZM, Liu QF, Han MZ, et al. Hematopoietic stem cell transplantation activity in China 2019: a report from the Chinese Blood and Marrow Transplantation Registry Group. Bone Marrow Transplant. 2021;56:2940–47.
Li XY, Yang J, Cai Y, Huang CM, Xu XW, Qiu HY, et al. Low-dose anti-thymocyte globulin plus low-dose post-transplant cyclophosphamide-based regimen for prevention of graft-versus-host disease after haploidentical peripheral blood stem cell transplants: a large sample, long-term follow-up retrospective study. Front Immunol. 2023;14:1252879.
Chang YJ, Huang XJ. Haploidentical stem cell transplantation: anti-thymocyte globulin-based experience. Semin Hematol. 2016;53:82–9.
Huo MR, Pei XY, Li D, Chang YJ, Xu LP, Zhang XH, et al. Impact of HLA allele mismatch at HLA-A, -B, -C, -DRB1, and -DQB1 on outcomes in haploidentical stem cell transplantation. Bone Marrow Transplant. 2018;53:600–8.
Einsele H, Ljungman P, Boeckh M. How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation. Blood. 2020;135:1619–29.
Huang W, Yu L, Cao T, Li Y, Liu Z, Li H, et al. The efficacy and safety of rabbit anti-thymocyte globulin vs rabbit anti-T-lymphocyte globulin in peripheral blood stem cell transplantation from unrelated donors. Leuk Lymphoma. 2016;57:355–63.
Bergmann L, Fenchel K, Jahn B, Mitrou PS, Hoelzer D. Immunosuppressive effects and clinical response of fludarabine in refractory chronic lymphocytic leukemia. Ann Oncol. 1993;4:371–5.
Bredeson CN, Zhang MJ, Agovi MA, Bacigalupo A, Bahlis NJ, Ballen K, et al. Outcomes following HSCT using fludarabine, busulfan, and thymoglobulin: a matched comparison to allogeneic transplants conditioned with busulfan and cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:993–1003.
Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055–66.
Huang R, Tu SF, Deng L, Kang Q, Song CY, Li YH. Myeloablative haploidentical hematopoietic stem cell transplantation using basiliximab for graft-versus-host disease prophylaxis. Hematology. 2015;20:313–9.
Goldberg MA, Antin JH, Guinan EC, Rappeport JM. Cyclophosphamide cardiotoxicity: an analysis of dosing as a risk factor. Blood. 1986;68:1114–8.
Wang Y, Wu DP, Liu QF, Xu LP, Liu KY, Zhang XH, et al. Low-dose post-transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients. J Hematol Oncol. 2019;12:88–94.
Acknowledgements
We appreciate the suggestions and assistance provided by Professor Duo-Nan Yu from the Institute of Hematology, Sichuan Academy of Medical Sciences, for this study. We thank all patients, the data collection team who participated in this study, and the families who provided us access to their facilities to complete this research.
Funding
This work was supported by the Fundamental Research Funds for Sichuan Provincial Department of Science and Technology (2023YFS0207), Sichuan Provincial Youth Science and Technology Fund (2023NSFSC1627).
Author information
Authors and Affiliations
Contributions
X-BH designed the study, interpreted the data, and wrote the manuscript. XY and C-LL performed the research and the statistical analysis. RZ, WW, X-MY, YW, YM, and W-QP provided clinical patient data. J-WH and YZ helped with data analysis. All the authors critically reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Due to a typesetting error, Figures e and f were duplicated, while Figures a and b were omitted.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, XB., Yang, X., Li, CL. et al. Haploidentical hematopoietic stem cell transplantation for hematologic malignancies: a novel conditioning regimen with anti-T lymphocyte immunoglobulin instead of anti-thymocyte globulin for in vivo T cell depletion. Bone Marrow Transplant 60, 39–46 (2025). https://doi.org/10.1038/s41409-024-02433-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-024-02433-w