Abstract
Pretransplant renal dysfunction has historically been associated with increased non-relapse mortality (NRM) and inferior overall survival. Novel approaches in conditioning and GVHD prophylaxis have reduced the toxicity of transplant over time, however, the impact of pre-transplant eGFR in the contemporary era is unknown. The aim of this study was to identify a pre-transplant eGFR value associated with increased transplant-related mortality. This retrospective study was performed using data from 724 adult patients who underwent first allogeneic hematopoietic cell transplant (alloHCT) from January 2012 through December 2021. The optimal pre-transplant eGFR value for risk of NRM was identified using Cox-restricted cubic spline plot analysis. Those with an eGFR <70 ml/min had the highest risk for NRM (p < 0.0001). Multivariate analysis confirmed that the risk of NRM remained significantly higher for eGFR <70 ml/min compared to the other higher eGFR categories, while there were no significant differences between the higher eGFR categories. Pre-transplant renal dysfunction is associated with poor outcomes after alloHCT and remains an important criterion when considering patients for transplant. Efforts to preserve renal function prior to transplant by limiting nephrotoxic exposures may have implications for optimizing outcomes after transplant, particularly in patients with other comorbidities.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic cell transplantation (alloHCT) is a curative therapy for many hematologic diseases [1]. Although outcomes after alloHCT have improved over time [2], pre-transplant renal dysfunction remains an independent risk factor for non-relapse mortality after transplant [3]. As such, pretransplant kidney function continues to be a standard criterion for selection of patients for alloHCT [4, 5].
The Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) is a validated composite risk score based on pre-transplant comorbidities that is used to predict non-relapse mortality (NRM) in patients undergoing alloHCT [5, 6]. Within the HCT-CI, patients are considered to have renal dysfunction if they have a serum creatinine that is >2 mg/dL. More recently, estimated glomerular filtration rate (eGFR) has emerged as a better estimate of renal function in the alloHCT population, given that serum creatinine can vary widely based on age, sex, and race [3, 7,8,9]. Previous studies evaluating pretransplant renal dysfunction have shown that an eGFR <60 mL/min in such patients is independently associated with increased NRM and inferior overall survival (OS) [3, 7]. More recent approaches have further refined the performance of alloHCT with reduction in toxicities. In particular, the emergence of post-transplant cyclophosphamide (PTCy) as GvHD prophylaxis has enhanced outcomes [10, 11]. It is unknown whether outcomes with or without PTCy differ in relation to pre-transplant eGFR.
Despite refinements in alloHCT over time [12], we hypothesized that baseline renal function is still independently associated with differences in outcomes in the contemporary era. The aim of this study was to identify an optimal pre-transplant eGFR cut point for determining those at increased risk of HCT-related mortality. An exploratory analysis was performed to assess the effect of PTCy on outcomes in relation to pre-transplant eGFR.
Methods
Study design and patient population
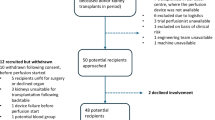
This retrospective observational study was performed using data from adult patients who underwent first alloHCT at the Cleveland Clinic from January 2012 through December 2021. Patients ≥18 years old who underwent their first alloHCT were included. Those receiving syngeneic or umbilical cord blood grafts were excluded.
The haploidentical transplant recipients routinely received PTCy, and thus outcomes could not be compared to a group without PTCy. Therefore, a cohort of 8/8 HLA matched related and unrelated donor transplants for AML, MDS and ALL who received Busulfan (100 mg/m2 daily × 4 days without pharmacokinetic direction) and Fludarabine (40 mg/m2 daily × 4 days) conditioning was used to compare outcomes between those with and without PTCy in relation to pre-transplant eGFR.
Study outcomes
NRM was defined as death from any cause other than disease progression or relapse. OS was estimated from the time of transplant until death from any cause. The first relapse was determined as the time of initial evidence of disease relapse after alloHCT. NRM, OS and relapse were censored at 5 years in analyses. Standard criteria were used for classifying acute and chronic GvHD [13, 14]. The first acute GVHD and first chronic GVHD after alloHCT were examined. Acute GVHD was censored at 6 months and chronic GVHD was censored at 12 months in analysis.
Statistical analysis
Patients’ demographics and clinical characteristics before alloHCT were described descriptively using the median and interquartile range (IQR) for numerical variables and frequency. eGFR was calculated using the 2021 CKD-EPI Creatinine Equation from the Chronic Kidney Disease Epidemiology Collaboration (www.kidney.org/content/ckd-epi-creatinine-equation-2021) [15] using patients’ stable creatinine prior to hospitalization for transplant. The distribution of pre-transplant eGFR was described using median, IQR and range. The association between eGFR and risk of NRM was examined using Cox restricted cubic spline plot with competing risk of death from disease progression or relapse, and the optimal pre-transplant eGFR cut point for high-risk of NRM after alloHCT was identified at the point where both low and upper 95% CI were above 1 [16]. Patients were categorized into the following eGFR groups: <70, 70–89, 90–119, and ≥120 ml/min based upon the observed association in the spline plot along with an established cut point of 90 ml/min/1.73 m² for abnormal kidney function, and the frequency (%) of each category was reported [17]. Associations of eGFR, demographics, prognostic factors, and HCT characteristics with NRM, relapse and OS were evaluated using Cox proportional hazard model, variables with p < 0.05 in univariate analysis were included in multivariate survival analysis. The impact of renal function on both acute and chronic GVHD was also explored using survival analysis, and the cumulative incidence between eGFR <90 and ≥90 was compared due to the small sample size in eGFR <70 ml/min and GVHD cases. Non-transplant-related death was considered as a competing risk in the NRM analysis, and death of any cause was considered as a competing risk in both relapse analysis and GVHD analysis. A sub-analysis was performed in AML, MDS and ALL patients who received BuFlu conditioning. NRM was compared between eGFR <70 and ≥70 ml/min in patients who received PTCy and those who did not have PTCy using the Gray’s test. Due to the modest sample size, this analysis is exploratory. All analyses were performed using SAS version 9.4, two-sided p values are presented, p < 0.05 is considered statistically significant.
Results
Baseline characteristics
Baseline characteristics of the 724 patients included in the study are summarized in Table 1. The median (IQR) age of patients at time of transplantation was 58 (47, 65) years, with 57% males and 43% females. The median pre-transplant eGFR (IQR) was 96.7 (79.5, 107.5) ml/min, range between 14.3 to 156.9, and nearly 40% of patients had a pre-transplant eGFR <90 ml/min. A modified HCT-CI that excluded renal dysfunction was used to quantify the baseline comorbidity burden. Patients were grouped into low-risk (score of 0), intermediate-risk (score of 1-2), and high-risk (score of 3+) according to the validated index [5]. Low, intermediate, and high-risk scores corresponded with 17%, 31%, and 52% of the study population, respectively. The validated Disease Risk Index [18] was used to classify patients’ underlying disease risk, which included 17% low-risk, 56% intermediate-risk, 24% high-risk patients, and 3% the DRI was not applicable for those without hematologic malignancies. Myeloablative conditioning was used in 43% of cases and donor source included 17% haploidentical, 28% matched related, and 55% unrelated donors.
Effect of pre-transplantation renal dysfunction on non-relapse mortality and overall survival
Association between pre-transplant eGFR level and NRM is illustrated in Fig. 1. The Cox restricted cubical spline plot shows that the risk of NRM increased at eGFR <90 ml/min, and the risk became significantly higher with an eGFR <70 ml/min (HR and 95% CI above 1.57, 1.01–2.45), and reduced risk was observed with an eGFR ≥120, although not statistically significant. Based on the observed association, patients were grouped into eGFR <70, 70-89, 90-119 and ≥120 ml/min which represented a distribution of 15.8% (N = 114), 22.4% (N = 162), 52.4% (N = 379) and 9.4% (N = 68) of the study population, respectively.
The Cox-restricted cubical spline plot shows that the risk of NRM was significantly higher with an eGRF below 70. The left vertical line shows the eGFR 70 ml/min cutoff, while the bold horizontal down sloping line and the dotted lines above and below show the hazard ratio and both low and upper 95% confidence intervals are above 1.
Figure 2a shows that the cumulative incidence of NRM was significantly different among eGFR groups (p < 0.0001). Patients with eGFR <70 ml/min had the highest NRM cumulative incidence among eGFR categories. The estimated 24-month cumulative incidence was 39.0% in the eGFR <70 ml/min category compared to 19.6% in the 70–89, 21.4% in the 90–119 and 12.0% in the ≥120 ml/min cohorts. The risk of NRM in the eGFR <70 ml/min group was significantly higher than the other higher eGFR categories, while differences among the higher eGFR categories were not statistically significant.
Univariate and multivariate analyses for associations of eGFR and other factors with NRM are presented in Table 2. The results remained the same after adjusting for age, Karnofsky performance status (KPS), intensity of conditioning, graft type, donor source, busulfan/fludarabine (BuFlu) conditioning and HCT-CI without renal function [Adjusted HR (95% CI) compared to the higher eGFR categories: 1.7 (1.1–2.6) to eGFR 70-89, 1.9 (1.3–2.7) to eGFR 90–119, and 2.7 (1.3–5.7) to eGFR ≥120]. There were no significant differences in NRM among the other higher pre-transplant eGFR groups.
Similar patterns were observed in the OS analysis. OS was also significantly different among eGFR groups (Fig. 2b, p = 0.0008). The estimated 24-month OS was 45.9% in the eGFR <70 ml/min category compared to 59.5% in the 70–89 ml/min, 57.5% in the 90–119 ml/min and 68.4% in the ≥120 ml/min cohorts.
In both univariate and multivariate analyses (Table 3), OS for patients with an eGFR <70 was significantly worse than all three higher eGFR categories. Early death in the eGFR <70 ml/min cohort was nearly 3 times higher compared to those with an eGFR ≥120, and nearly 2 times higher compared to those with eGFRs of 70–89 and 90–119 ml/min after adjusting for age, KPS, intensity of conditioning, graft type, busulfan/fludarabine use, HCT-CI without renal function, and DRI that potentially associated with OS in univariate analysis.
Pre-transplant eGFR was not significantly associated with relapse (data not shown). It also was not associated with acute GVHD and chronic GVHD, and the cumulative incidences were similar between eGFR <90 and ≥90 ml/min (data not shown). The most common causes of death within 5 years after alloHCT were relapse (52.9%), infection (17.0%) and organ failure (7.7%). Relapse was less common (38.4% vs 56.5%) while the infection was more common (21.9% vs 15.8%) in the eGFR<70 ml/min cohort compared to the ≥70 ml/min group, while organ failure was similar (8.2% vs 7.5%).
Effect of using PTCy-based GVHD prophylaxis on alloHCT outcomes in patients with underlying renal dysfunction
An exploratory analysis was performed to compare NRM between those with a baseline eGFR <70 and ≥70 ml/min in relation to the use of PTCy. This analysis included 257 patients with AML, MDS, and ALL who underwent matched related or unrelated alloHCT from 2019 to 2021 with BuFlu conditioning. Among the 169 who received BuFlu without PTCy, 35 had an eGFR <70 ml/min. There were 88 patients who received BuFlu with PTCy, of which 24 had an eGFR <70 ml/min. No differences in NRM were observed when comparing PTCy vs. no PTCy for those with an eGFR <70 ml/min. Similarly, when considering those with an eGFR >70 ml/min there were no differences in NRM between the PTCy and no PTCy cohorts. When considering those who received PTCy, the difference in NRM between eGFR <70 and ≥70 ml/min was 49.2% vs. 17.3% at 24 months (p = 0.008) while for those who did not receive PTCy this was 37.1% vs 23.5%, respectively (p = 0.05), as shown in Fig. 3.
Discussion
The current study found that a pre-transplant eGFR <70 ml/min was the optimal eGFR cut point for predicting high risk of HCT-related mortality. However, relapse was not associated with pre-existing renal dysfunction.
Assessment of pre-existing kidney function is an important criterion when considering patients for transplant. The Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) is a tool used to determine the risk of NRM after transplant based on pre-existing comorbidities. Within the HCT-CI, renal dysfunction is defined as a serum creatinine >2, history of a kidney transplant, or the need for dialysis [5]. A serum creatinine >2 is a very limited definition of kidney dysfunction, as serum creatinine can vary widely in individuals depending on several factors, including race, sex, age, and muscle mass. Baseline eGFR, rather than creatinine, has been shown to be a better indicator of pre-transplant renal function in the alloHCT population [3, 7].
Assessment of kidney function prior to transplant should include a careful assessment of GFR. Creatinine-based estimating equations are the most readily available means of assessing GFR and remain the initial step. In select patients where non-GFR creatinine determinants may be important to consider (e.g., extremes of either increased or decreased muscle mass), cystatin C-based equations need to be utilized for a more accurate GFR estimation. Importantly, the gold standard for GFR assessment remains iothalamate or iohexol clearance-based measurements. In select patients, particularly those where the estimated GFR may fall close to the cutoff values, iothalamate/iohexol-based GFR assessment might be needed. This may allow for better patient selection and counseling prior to alloHCT.
An eGFR <60 ml/min is commonly defined in clinical practice as moderate kidney dysfunction, and is the threshold for stage 3 chronic kidney disease [17]. In the general population, an eGFR <60 ml/min has been shown to be an independent predictor of mortality risk [19]. In previous studies evaluating the effect of renal dysfunction on outcomes after alloHCT, a pretransplant eGFR <60 ml/min was used to categorize patients with moderate kidney dysfunction [3, 7, 20, 21]. Using data from an alloHCT population and not from the general population alone, our study found that a pre-transplant eGFR <70 ml/min (using the most contemporary 2021 CKD-EPI Creatinine Equation, which excludes race) was the optimal cut point for identifying those at increased risk of HCT-related mortality.
Our study corroborates prior evidence that pre-existing renal dysfunction as measured by eGFR is a prognostic factor for mortality after alloHCT. A retrospective, registry study by Farhardfar et al. with over 13,000 patients from an older transplant time interval found that a pre-transplant eGFR <60 ml/min was associated with increased risk of NRM and post-transplant renal failure requiring dialysis [3]. Shouval et al. reported more than 1200 cases in which an eGFR <60 ml/min was also associated with increased NRM and overall mortality [7]. In contrast, some smaller studies found that an eGFR <60 ml/min was not associated with worse NRM and OS in the reduced intensity conditioning setting with fludarabine/melphalan or 200 cGy total body irradiation with or without fludarabine [20, 21]. While these results may be valuable for select populations, they are not likely applicable to a broader population and different conditioning regimens.
To our knowledge, our series is the first study to compare outcomes between those who were treated with and without PTCy in relation to pre-transplant eGFR. Our analysis observed that in the setting of matched related or unrelated donor alloHCT with BuFlu conditioning, a pre-transplant eGFR <70 ml/min had significantly worse NRM with or without PTCy. As compared to those who did not receive PTCy, the PTCy group had a greater difference in NRM between those with an eGFR <70 and ≥70 ml/min. This may be related to better tolerance of PTCy in those with higher baseline eGFRs, which may be associated with less GvHD-related mortality. Although our results suggest there is no mortality detriment to using PTCy-based GVHD prophylaxis in patients with reduced renal function, assessments of larger populations are necessary to better define the impact in disease-specific groups.
It is important to note that although these patients received PTCy as part of their regimen for GVHD prophylaxis, most of these patients also received tacrolimus and mycophenolate mofetil (MMF). Given the renal toxicity of calcineurin inhibitors (CNIs), exposure to tacrolimus may have precipitated further renal dysfunction in these patients [22, 23]. In attempts to avoid the negative effects of CNIs, there have been multiple studies that have evaluated CNI-free GVHD prophylaxis [24,25,26,27,28,29]. One recent phase III trial published by Luznik et al. evaluated outcomes of 327 alloHCT patients who received single-agent cyclophosphamide or a T-cell-depleted peripheral blood stem cell graft as compared to tacrolimus + methotrexate for GvHD prophylaxis and found that PTCy alone was associated with more acute GVHD. However, the rates of chronic GvHD, relapse-free survival, and overall survival were similar. The authors concluded that the single-agent PTCy regimen could potentially be offered to patients with contraindications to CNIs [24]. CD3+TCRαβ/CD19+ depletion has also been an effective alternative approach to avoid CNIs with HLA mismatched family or unrelated donor alloHCT [30, 31]. Evidence is lacking regarding CNI-free GVHD prophylaxis in patients with underlying renal dysfunction, and this presents an important opportunity for future research. Currently, if such patients are otherwise felt to be appropriate candidates for alloHCT then the use of a reduced-intensity conditioning approach with limiting nephrotoxic agents and CNI-free GVHD prophylaxis may be most appropriate.
This retrospective, single-center study had some limitations. Data on the underlying cause and duration of many patients’ pre-transplant renal dysfunction was not known. The sample size for some of the disease groups included in the analysis may have limited the generalizability of the outcome results for these populations. The exploratory analysis of patients receiving PTCy-based GVHD prophylaxis was limited to only AML, MDS and ALL patients who underwent HLA-matched related and unrelated donor transplants with busulfan and fludarabine (BuFlu) conditioning. The finding of no difference in NRM between those treated with or without PTCy may not be generalizable for other disease groups, HLA mismatched grafts, reduced intensity conditioning, other myeloablative conditioning regimens or older patients.
In conclusion, our study found that an eGFR <70 ml/min is an independent prognostic factor for mortality after alloHCT, which may have important implications when selecting patients for transplant. Future investigation with larger subgroup analyses regarding specific diagnoses, conditioning regimens, and transplant modalities may further identify which patients are more appropriate for transplant and those who may benefit from alternative treatment approaches. Our results also suggest that additional efforts to preserve renal function prior to transplant by limiting nephrotoxic exposures may have implications for optimizing outcomes after transplant, particularly in patients with other comorbidities.
Data availability
All data were obtained from the Cleveland Clinic’s Unified Transplant Database.
References
Snowden JA, Sánchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant. 2022;57:1217–39. https://www.nature.com/articles/s41409-022-01691-w
McDonald GB, Sandmaier BM, Mielcarek M, Sorror M, Pergam SA, Cheng GS, et al. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: comparing 2003–2007 versus 2013–2017 cohorts. Ann Intern Med. 2020;172:229. https://annals.org/aim/fullarticle/2759352/survival-nonrelapse-mortality-relapse-related-mortality-after-allogeneic-hematopoietic-cell
Farhadfar N, Dias A, Wang T, Fretham C, Chhabra S, Murthy HS, et al. Impact of pretransplantation renal dysfunction on outcomes after allogeneic hematopoietic cell transplantation. Transplant Cell Ther. 2021;27:410–22. https://linkinghub.elsevier.com/retrieve/pii/S266663672100717X
Renaghan AD, Jaimes EA, Malyszko J, Perazella MA, Sprangers B, Rosner MH. Acute kidney injury and CKD associated with hematopoietic stem cell transplantation. Clin J Am Soc Nephrol. 2020;15:289–97. https://journals.lww.com/10.2215/CJN.08580719
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. https://ashpublications.org/blood/article/106/8/2912/21830/Hematopoietic-cell-transplantation-HCTspecific
ElSawy M, Storer BE, Pulsipher MA, Maziarz RT, Bhatia S, Maris MB, et al. Multi‐centre validation of the prognostic value of the haematopoietic cell transplantation‐ specific comorbidity index among recipient of allogeneic haematopoietic cell transplantation. Br J Haematol. 2015;170:574–83. https://onlinelibrary.wiley.com/doi/10.1111/bjh.13476
Shouval R, De Jong CN, Fein J, Broers AEC, Danylesko I, Shimoni A, et al. Baseline renal function and albumin are powerful predictors for allogeneic transplantation-related mortality. Biol Blood Marrow Transplant. 2018;24:1685–91. https://linkinghub.elsevier.com/retrieve/pii/S108387911830257X
Levey AS, Stevens LA, Schmid CH, Zhang Y(Lucy), Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604. http://annals.org/article.aspx?doi=10.7326/0003-4819-150-9-200905050-00006
Swedko PJ, Clark HD, Paramsothy K, Akbari A. Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med. 2003;163:356. http://archinte.jamanetwork.com/article.aspx?doi=10.1001/archinte.163.3.356
Bolaños-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N Engl J Med. 2023;388:2338–48. http://www.nejm.org/doi/10.1056/NEJMoa2215943
Galli E, Metafuni E, Giammarco S, Limongiello MA, Innocenti I, Autore F, et al. Triple Post-Transplant Cyclophosphamide (PTCY) based GVHD prophylaxis: HLA matched versus HLA haploidentical transplants. Bone Marrow Transplant. 2022;57:532–7. https://www.nature.com/articles/s41409-022-01574-0
Miyata M, Ichikawa K, Matsuki E, Watanabe M, Peltier D, Toubai T. Recent advances of acute kidney injury in hematopoietic cell transplantation. Front Immunol. 2022;12:779881. https://www.frontiersin.org/articles/10.3389/fimmu.2021.779881/full
Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57:267–76
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–49. http://www.nejm.org/doi/10.1056/NEJMoa2102953
Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. https://onlinelibrary.wiley.com/doi/10.1002/sim.4780080504
Stevens PE, Levin A.Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–30.
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71. https://ashpublications.org/blood/article/123/23/3664/33201/Validation-and-refinement-of-the-Disease-Risk
Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–81. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673610606745
De Souza JA, Saliba RM, Patah P, Rondon G, Ribeiro R, De Padua Silva L, et al. Moderate renal function impairment does not affect outcomes of reduced-intensity conditioning with fludarabine and melphalan for allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:1094–9. https://linkinghub.elsevier.com/retrieve/pii/S1083879109002341
Kersting S, Verdonck LF. Successful outcome after nonmyeloablative allogeneic hematopoietic stem cell transplantation in patients with renal dysfunction. Biol Blood Marrow Transplant. 2008;14:1312–6. https://linkinghub.elsevier.com/retrieve/pii/S1083879108003856
Randhawa PS, Starzl TE, Demetris AJ. Tacrolimus (FK506)-associated renal pathology. Adv Anat Pathol. 1997;4:265. http://journals.lww.com/00125480-199707000-00032
Abramson MH, Gutgarts V, Zheng J, Maloy MA, Ruiz JD, Scordo M, et al. Acute kidney injury in the modern era of allogeneic hematopoietic stem cell transplantation. Clin J Am Soc Nephrol. 2021;16:1318–27.
Luznik L, Pasquini MC, Logan B, Soiffer RJ, Wu J, Devine SM, et al. Randomized phase III BMT CTN trial of calcineurin inhibitor-free chronic graft-versus-host disease interventions in myeloablative hematopoietic cell transplantation for hematologic malignancies. J Clin Oncol. 2022;40:356–68.
Devine SM, Carter S, Soiffer RJ, Pasquini MC, Hari PN, Stein A, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17:1343–51.
Solomon SR, Sanacore M, Zhang X, Brown S, Holland K, Morris LE, et al. Calcineurin inhibitor–free graft-versus-host disease prophylaxis with post-transplantation cyclophosphamide and brief-course sirolimus following reduced-intensity peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:1828–34. https://linkinghub.elsevier.com/retrieve/pii/S1083879114004467
Iqbal M, Nieto FAM, Brannick KM, Li Z, Murthy H, Foran J, et al. A calcineurin inhibitor free graft versus host disease prophylaxis for patients undergoing matched related and matched unrelated donor allogeneic hematopoietic cell transplant. Transplant Cell Ther. 2023;29:327.e1–327.e9. https://linkinghub.elsevier.com/retrieve/pii/S2666636723010680
Lazzari L, Balaguer-Roselló A, Montoro J, Greco R, Hernani R, Lupo-Stanghellini MT, et al. Post-transplant cyclophosphamide and sirolimus-based graft-versus-host disease prophylaxis after allogeneic stem cell transplantation for acute myeloid leukemia. Bone Marrow Transplant. 2022;57:1389–98. https://www.nature.com/articles/s41409-022-01725-3
Bejanyan N, Pidala JA, Wang X, Thapa R, Nishihori T, Elmariah H. et al.A phase 2 trial of GVHD prophylaxis with PTCy, sirolimus, and MMF after peripheral blood haploidentical transplantation.Blood Adv. 2021;5:1154–63. https://ashpublications.org/bloodadvances/article/5/5/1154/475291/A-phase-2-trial-of-GVHD-prophylaxis-with-PTCy
Leahy AB, Li Y, Talano JA, Elgarten CW, Seif AE, Wang Y, et al. Unrelated donor α/β T cell- and B cell-depleted HSCT for the treatment of pediatric acute leukemia. Blood Adv. 2022;6:1175–85.
Ramanathan S, Lum SH, Nademi Z, Carruthers K, Watson H, Flood T, et al. CD3+TCRαβ/CD19+-depleted mismatched family or unrelated donor salvage stem cell transplantation for graft dysfunction in inborn errors of immunity. Transplant Cell Ther. 2023;29:513.e1. https://linkinghub.elsevier.com/retrieve/pii/S2666636723013210
Author information
Authors and Affiliations
Contributions
RMS, DPN, HL—designed the study, performed the data analyses and writing the manuscript. DC, SP, DD, JE, AM, RH, SJR, DJ, ATG, RMD, BP, BKH, CB, MK and CSS. Assisted in data analysis interpretation and writing the manuscript. SF and AB—data analysis for manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval and Consent to Participate
All methods were performed in accordance with the relevant guidelines and regulations of the Cleveland Clinic’s Institutional Review Board which approved the study (IRB 4927; IRB Organization Registration Information, IORG0000301). The Cleveland Clinic Foundation’s Federalwide Assurance, FWA00005367, had also been approved by the Office for Human Research Protections (OHRP). All patients provided informed consent prior to transplant.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nurse, D., Li, H., Cenin, D. et al. Influence of pre-transplant estimated glomerular filtration rate (eGFR) on clinical outcomes after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 60, 787–794 (2025). https://doi.org/10.1038/s41409-025-02556-8
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02556-8