Abstract
Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) is a common and potentially severe complication of CD19 CAR-T therapy. While some clinical risk factors have been described, the contribution of cytokines, particularly in plasma and cerebrospinal fluid (CSF), remains limited. This study aimed to identify predictors and characterize cytokine patterns associated with ICANS to develop a multivariable risk model. We retrospectively analyzed 101 adult patients treated with axicabtagene ciloleucel (axi-cel) or tisagenlecleucel (tisa-cel) between 2019 and 2023. Cytokines (interleukin (IL)-1β, IL-6, IL-15, GM-CSF) were measured in plasma pre- and post-infusion, and in CSF during neurotoxicity. ICANS occurred in 36% of patients, more frequently with axi-cel (46% vs. 21%, p < 0.05). Autoimmune disease history and elevated IL-6 and IL-15 were associated with increased risk. CSF cytokines were also elevated during ICANS episodes. A multivariate model predicting any-grade ICANS included CAR-T product, time to cytokine release syndrome (CRS) onset, IL-6 at day 3, and pre-infusion D-dimer (AUC = 0.83). The model for grade 2–4 ICANS included number of prior therapies, grade ≥2 CRS, autoimmune disease, IL-15 at day 0, and GM-CSF (AUC = 0.80). Integrating cytokine profiles with clinical parameters may enable early ICANS risk stratification and improve personalized monitoring in CAR-T recipients.
Similar content being viewed by others
Introduction
Diffuse large B‑cell lymphoma (DLBCL) patients treated with first‑line R‑CHOP relapse or are refractory in 20–40% [1, 2]; of those who receive as a second line salvage high‑dose chemotherapy and autologous stem‑cell transplant (ASCT), 30–40% will relapse [3,4,5]. Relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL) likewise carries poor outcomes, with complete remission rates of 40–50% [6]. These unmet needs led to the development of CD19‑directed CAR‑T therapies, tisagenlecleucel (tisa-cel) and axicabtagene ciloleucel (axi-cel), which have shown durable remissions in third‑line R/R DLBCL and R/R ALL [7,8,9], and are currently being advanced into earlier lines [10,11,12,13].
A major concern with CAR-T therapies is the development of immune effector cell-associated neurotoxicity syndrome (ICANS), a potentially life-threatening complication reported in 15–69% of patients across clinical trials and real-world settings [7, 8, 14, 15]. ICANS encompasses a broad spectrum of neurological symptoms, ranging from mild cognitive deficits to severe manifestations such as encephalopathy, aphasia, and cerebral edema [16,17,18,19]. Its pathogenesis is closely tied to the proinflammatory milieu triggered by CAR-T cells – tumor often preceded by cytokine release syndrome (CRS). CRS is characterized by hyperpyrexia, hypotension, and hypoxemia, and is considered contributing factor to ICANS [16], as systemic inflammation can disrupt the blood-brain barrier, allowing cytokines and immune cells to infiltrate the central nervous system (CNS). Management of ICANS requires careful monitoring due to its varied presentation, in some cases fatal [20]. Although risk factors – such as previous inflammatory profiles – have been identified, patient heterogeneity continues to challenge the development of reliable predictive models [21, 22]. Importantly, the CAR-T co-stimulatory domain influences ICANS incidence, with CD28-based products (axi-cel) associated with higher risk than 4-1BB-based products (tisa-cel) [7, 8]. Additional factors such as CAR-T expansion dynamics and tumor burden further complicate understanding of ICANS pathophysiology. The use of corticosteroids and anti-interleukin therapies has significantly changed the clinical management of CRS and ICANS compared to earlier studies [23,24,25,26].
Pro-inflammatory cytokines are key mediators in the pathophysiology of ICANS, with elevated levels in serum and cerebrospinal fluid (CSF) linked to both the risk and severity of neurotoxicity. In plasma, increased concentrations of interleukin-15 (IL-15) have been consistently associated with ICANS across multiple studies, along with significant elevations in interleukin-6 (IL-6), interleukin-10 (IL-10), and granulocyte-macrophage colony-stimulating factor (GM-CSF) [19, 27]. In the CSF, although CAR-T cells are frequently detected in patients, their presence alone does not correlate directly with ICANS. However, elevated protein levels are consistently observed during clinical flares, likely reflecting local inflammatory responses [19, 27]. While measuring proinflammatory mediators in the CSF may offer prognostic insights, interpretation remains limited due to small numbers of studies and lumbar punctures performed in these patients [27, 28]. Further research is needed to clarify the specific roles and interactions of these cytokines within the CNS [19, 27].
Accurate prediction of ICANS onset could improve the use of preventive strategies and reduce unnecessary healthcare utilization. In this context, the primary objectives of our study were to develop a multivariate risk model for ICANS using real-world data from patients undergoing CD19-directed CAR-T therapy and to evaluate CSF cytokine profiles in a subset of patients to identify potential biomarkers of neurotoxicity onset and severity.
Methods
Study population and data collection
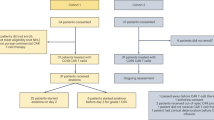
This retrospective study was conducted at a tertiary care center in Spain between 1 June 2019 and 30 June 2023. It included all adult patients who received commercial CD19-directed CAR-T therapy, either axi-cel or tisa-cel, for hematologic malignancies in a third-line or later setting. Baseline clinical, neurological, and laboratory data—including CAR-T product type, comorbidities, treatment-related toxicities, and laboratory parameters before and after infusion—were collected. The study was approved by the Research Ethics Committee for Medicines at Hospital General Universitario Gregorio Marañón, Madrid, in accordance with the Declaration of Helsinki (protocol number 148.22), as authorized by the Biomedical Research Foundation, and informed consent was obtained.
CAR-T procedures and management
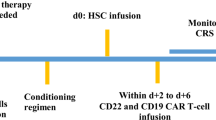
Patients received CAR-T therapy per institutional protocols. Pre-treatment evaluations included clinical and laboratory assessments, with cytokine levels measured on day 0 and metabolic tumor volume (MTV) assessed via pre-lymphodepletion PET-CT. Following lymphodepletion and infusion, patients were monitored during hospitalization and follow-up. Cytokines were reassessed on day 3, and pro-inflammatory markers were monitored daily. Neurological function was evaluated daily using the ICE score (Immune Effector Cell-Associated Encephalopathy Score).
CRS and ICANS were managed per institutional guidelines. Neurological assessments (CT/MRI, EEG, transcranial Doppler) were performed in patients with findings or ICANS grade ≥2. Treatments included corticosteroids, IL-6 antagonists (tocilizumab, siltuximab), and the IL-1 antagonist anakinra, based on severity and response. Initially, patients with CRS could receive up to three doses of tocilizumab [23]; however, by late 2021, institutional protocols were revised to restrict tocilizumab administration to a maximum of two doses, in alignment with emerging safety data. If ICANS co-occurred with CRS, tocilizumab was given, followed by steroids if needed. Refractory cases received siltuximab or anakinra. In severe, treatment-resistant cases, cytokine adsorption or intrathecal therapy was used. Antiepileptic prophylaxis was reserved for ICANS grade 2–4. Management varied slightly over time due to evolving protocols.
Study variables and endpoints
The primary objective of the study was to identify clinical and laboratory predictors—including serum and CSF cytokine profiles—associated with ICANS, to construct a multivariate risk model. ICANS was analyzed both as any grade and as “relevant” (grades 2–4), to better capture clinically significant events. ICANS and CRS were graded per American Society for Blood and Marrow Transplantation and Cellular Therapy (ASTCT) [23]; although tremors and myoclonus are not included in ASTCT, they were recorded, and all patients met ICANS diagnostic criteria.
As a secondary objective, ICANS was descriptively analyzed regarding clinical presentation, labs, treatments, and outcomes. Serum inflammatory markers and cytokines (IL-1β, IL-6, IL-15, GM-CSF) were measured using the Ella ProteinSimple platform on day 0 and day 3 post-infusion. CSF cytokines were assessed at peak of ICANS. Elevated plasma cytokine levels were described relative to reference ranges from Kim et al. [29]: IL-1β, 2.04 ± 4.93 pg/mL (range: 0.17–24 pg/mL); IL-6, 2.91 ± 6.45 pg/mL (range: 0.16–37.7 pg/mL); IL-15, 3.04 ± 2.17 pg/mL (range: 1.25–13.1 pg/mL); and GM-CSF, 40.9 ± 108.6 pg/mL (range: 0.5–728.1 pg/mL) [29]. While no CSF thresholds are standardized, serum-derived values were used for comparison. Importantly, all cytokine measurements were entered into our univariate and multivariate models as continuous variables to preserve the full range of observed values; the cutoff values described above were used only for descriptive summaries and did not drive model selection. For context, control subjects with non-inflammatory disorders have shown median CSF levels of IL-1β at 0.3 pg/mL (range: 0–0.8 pg/mL) and IL-6 at 3.6 pg/mL (range: 1.7–7.2 pg/mL) [30], although one study reported that IL-6 was undetectable in healthy CSF [31]. Additional variables included CRS severity/duration, timing of CRS and ICANS onset, and other biomarkers such as LDH, D-dimer, and CRP.
Statistical analysis
Statistical analyses were performed using Stata® for Windows (version 16). A significance threshold of p < 0.05 was applied to all tests. Univariate analyses employed logistic regression, two-tailed t-tests for continuous variables, and Pearson’s chi-squared or Fisher’s exact tests for categorical variables. Ordinal data were analyzed with nonparametric trend tests and the Cochran-Armitage test. Univariate logistic regression models were used to examine the association of various predictors with ICANS occurrence; cytokine concentrations were analyzed as continuous variables to preserve data variability and subsequently compared against pre-specified thresholds (based on established reference values) to define “elevated” levels. Multivariate analysis was conducted via a stepwise model selection approach, incorporating variables significant in univariate analyses (entry p < 0.05, retention p < 0.10), with model fit assessed using the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC), and the final model chosen based on the lowest values for optimal fit.
Results
Patient characteristics, neurological assessments and therapeutical interventions
A total of 101 adult patients were included, with baseline characteristics summarized in Table 1. Neurological comorbidities were present in 72% of patients, primarily peripheral neuropathy (51.5%), basal tremor (16.8%), and ischemic stroke (4%). Pre–CAR-T brain MRI was performed in 83 patients, and CSF analysis was available in 13. CNS involvement was confirmed in 4 cases—three with CSF infiltration by lymphoma and one with a parenchymal mass. Most patients received axi-cel (58%) and 71% required bridging therapy prior to CAR-T infusion. CRS occurred in 91% of patients (92/101), with severe cases in 5% (5/101). ICANS was documented in 36 patients (35.6%) with 15.8% experiencing grade 1 (16/101) and 19.7% (20/101) grade 2–4, with a median time from CAR-T infusion to ICANS onset of 6 days (Table 2). Clinical manifestations of ICANS included altered mental status (69%), fluctuating aphasia (39%), dysgraphia (28%), and myoclonus (22%). Notably, 75% developed new-onset tremor, which resolved after ICANS. Focal neurological deficits were less common, including cranial nerve palsy (8%), limb paresis (5%), and sensory deficits (3%). Prophylactic antiepileptic treatment was given to 39% of ICANS patients (14/36), primarily levetiracetam (95%). Seizures occurred in 8% (3/36), with two resolving and one progressing to fatal status epilepticus. EEG was performed in 47% (17/36), revealing epileptiform discharges in 18% and slow rhythm/encephalopathy patterns in 59%. Neuroimaging was conducted in 75% of ICANS cases (27/36), identifying new lesions in 22%. Findings were compatible with Posterior Reversible Encephalopathy Syndrome (PRES) (n = 2) and one fatal case of diffuse malignant cerebral edema on day 4. In severe or refractory neurotoxicity, cytokine absorption filtration was used in 17% (6/36) and intrathecal therapy in 11% (4/36), involving hydrocortisone alone or combined with cytarabine and methotrexate. Overall, 83% (30/36) of ICANS cases resolved completely.
Univariate and multivariate analysis
Univariate analysis (Table 3) showed that axi-cel was associated with a higher incidence of any-grade ICANS compared to tisa-cel (45.7% vs. 21.4%, p = 0.02), and a non-significant trend toward higher grade 2–4 ICANS (25.4% vs. 11.9%, p = 0.09). CRS severity was strongly associated with ICANS: any history of CRS was linked to higher rates of any-grade (57.5% vs. 21.3%, p < 0.01) and grade 2–4 ICANS (35% vs. 9.8%, p < 0.01), while severe CRS (grades 3–4) was also correlated with increased neurotoxicity (any-grade: 80.0% vs. 33.0%, p = 0.03; grade 2–4: 60.0% vs. 17.7%, p = 0.02). ICANS risk was further associated with longer CRS duration (6.7 vs. 4.4 days for any-grade; 7.3 vs. 4.7 days for grade 2–4; p = 0.01 and p = 0.02) and earlier CRS onset (1.4 and 1.2 days vs. 2.7 days, respectively; p < 0.001). Higher numbers of tocilizumab doses after CRS were also linked to increased risk (p < 0.001 for any-grade, p = 0.04 for grade 2–4). Patients with autoimmune diseases (AID) were significantly more likely to develop ICANS (OR 4.99 for any-grade; OR 5.07 for grade 2–4) (Supplementary Table 1).
A greater disease burden, reflected in elevated pre-infusion MTV, was observed in patients with ICANS (574 mL for any-grade, 678.9 mL for grade 2–4, vs. 231 mL in non-ICANS; p = 0.02). LDH levels at day 0 were significantly higher in ICANS cases (415.8 vs. 278.3 U/L, p < 0.01), along with elevated pre-infusion D-dimer levels (1241.4 vs. 545.1 ng/mL, p < 0.01). Post-infusion (day 3) D-dimer remained elevated in both ICANS groups (p < 0.01). Day 0 CRP was significantly associated with ICANS (105.6 vs. 39.7 mg/dL, p < 0.01), though this difference was not seen on day 3.
Cytokine profiling showed significantly elevated IL-6 and IL-15 levels in patients with ICANS. On day 0, IL-6 and IL-15 levels were 34.7 vs. 12.5 pg/mL (p = 0.02) and 38.5 vs. 34.0 pg/mL (p = 0.01), respectively. These differences were more pronounced on day 3: IL-6 levels reached 3168.5 vs. 355.7 pg/mL (p < 0.01), and IL-15 levels were 79.4 vs. 35.7 pg/mL (p < 0.01). GM-CSF levels were significantly higher on day 3 in patients with grade 2–4 ICANS (3.2 vs. 2.2 pg/mL, p < 0.01), but not significantly different for any-grade ICANS.
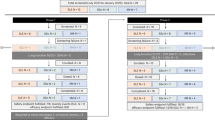
Multivariate analysis identified key predictors for ICANS (Table 4). For any-grade ICANS, the best-fit model included CAR-T product (axi-cel), time to CRS onset, day 3 IL-6, and baseline D-dimer (AUC = 0.84; sensitivity = 61.8%; specificity = 90.2%) (Fig. 1a). For grade 2–4 ICANS, the model included number of prior therapy lines, CRS grade ≥2, AID, and day 0 levels of GM-CSF and IL-15 (AUC = 0.81; sensitivity = 47.4%; specificity = 97.1%) (Fig. 1b).
Variables not significantly associated with ICANS included age, sex, prior bridging therapy, neurological comorbidities, fibrinogen, and pre-infusion MRI findings.
CSF cytokine profiles
Cytokine analysis in the CSF of eight patients with significant ICANS revealed elevated levels of IL-6, IL-15, GM-CSF, and IL-1β at the peak of symptoms. All patients had high CSF protein levels (range: 54–224 mg/dL), with or without mild lymphocytic pleocytosis (Fig. 2). Serial CSF evaluations were performed in six cases, showing a persistent elevation of these cytokines until symptom resolution. Five of these patients received axi-cel and one received tisa-cel. IL-6 and IL-15 levels exhibited strong positive correlation trends with grade 2–4 ICANS (Spearman’s rho = 0.85, p = 0.07; rho = 0.78, p = 0.09, respectively). Similarly, GM-CSF (rho = 0.65, p = 0.15) and IL-1β (rho = 0.60, p = 0.20) followed the same pattern, further supporting a potential association between elevated CSF cytokines and increased ICANS severity. CAR-T cell expansion in the CSF was detected in six patients via flow cytometry, with a median count of 867.2 cells/mL (range: 226.5–2071.5), representing a median of 39% (range: 6–43%) of total CSF leukocytes. Two additional samples showed no detectable CAR-T cells.
Heat-map of CSF concentrations of IL-6, IL-15, GM-CSF and IL-1β (pg/mL) measured at the time of maximum neurotoxicity (grade 2–4; n = 8). Each row represents an individual patient; columns correspond to cytokine levels. Color intensity reflects the measured concentration (scale bar); numeric values are overlaid in each cell. A side bar encodes the ICANS grade (2, 3 or 4) for each patient. CSF cerebrospinal fluid, GM-CSF granulocyte–macrophage colony-stimulating factor, IL interleukin, ICANS immune effector cell-associated neurotoxicity syndrome.
A particularly illustrative case involved a 49-year-old patient with DLBCL who developed late biphasic ICANS grade 3, with a second neurotoxicity episode occurring 40 days post-infusion, following initial resolution. This relapse coincided with a new CAR-T cell expansion in both serum (>100,000 cells/mL) and CSF, alongside elevated CSF cytokines (IL-6: 126 pg/mL; IL-1β: 1.65 pg/mL; IL-15: 21.1 pg/mL; GM-CSF: 5 pg/mL). The late-phase ICANS was successfully treated with corticosteroids. Disease progression was later confirmed by bone marrow infiltration (28% morphologically abnormal cells, 15% by immunophenotyping), despite a negative PET-CT, with notable intracellular CD19 negativity.
Survival and hospitalization outcomes
Median overall survival (OS) was 10 months and median progression-free survival (PFS) was 6 months, with no significant differences for these outcomes between patients who developed ICANS and those who did not. Therapeutic strategies for immune-mediated complications of CAR-T therapy are detailed in Table 5. ICANS resolved in 83% of cases following therapeutic interventions. Among the 101 infusion patients, 36 (36%) developed ICANS; of these, seven (7% of all CAR-T infusion) did not survive. Notably, four of these deaths were directly attributed to ICANS (4% of all CAR-T infusions), while the remaining three resulted from multisystem inflammatory syndrome (MAS), sepsis, or hematologic disease progression. Median hospital stay was similar between groups (16.5 days, range 5–86), but ICU admissions were significantly higher in ICANS patients (42% vs. 9%, p < 0.05), with a median ICU stay of 5 days (range 1–74).
Discussion
Incidence of ICANS and comparison with real world evidence (RWE)
In our cohort of 101 adult patients treated with CD19 CAR-T therapy, ICANS occurred in 36%, with 33% of cases classified as severe (grade 3–4). Any-grade ICANS was significantly more frequent in the axi-cel group (46%) than in the tisa-cel group (21%) (p < 0.05), consistent with the higher neurotoxicity risk linked to CD28 co-stimulatory domains [7, 8]. While our overall ICANS rate is slightly lower than some U.S. cohorts (45–50%), it aligns with findings from the DESCAR-T registry, the US Lymphoma Consortium, and the GETH-GELTAMO studies [14, 15, 32, 33]. The greater severity of ICANS in our population may reflect a higher-risk group (47.5% with primary refractory disease suggesting an elevated tumor load) and greater use of axi-cel. Temporal shifts in clinical practice and evolving management strategies during the study period may also have contributed to outcome variability.
CRS Features and ICANS Risk
In our cohort, CRS features were strongly linked to ICANS risk. Consistent with prior research, we found that early onset and greater severity of CRS are directly related to an increased risk of developing ICANS [27, 34]. However, our study also identifies the duration of CRS as a significant predictor of ICANS in univariate analysis, suggesting that prolonged inflammatory responses may further exacerbate neurotoxicity. This addition enriches the current understanding of CRS-ICANS interplay and emphasizes the need to consider not just the intensity but also the persistence of CRS symptoms in risk assessments.
Serum and CSF cytokine profiles related to ICANS
Our investigation of cytokine profiles may provide further insight into the pathophysiology of ICANS. Elevated plasma levels of IL-6 and IL-15 on both day 0 (pre-infusion) and day 3 post-infusion were strongly associated with the development of any-grade and grade 2–4 neurotoxicity. In addition, increased pre-infusion levels of GM-CSF were particularly associated with grade 2–4 ICANS, consistent with findings from the ZUMA-1 trial, which linked elevated GM-CSF levels to grade ≥3 neurotoxicity [35]. Supporting evidence from prior studies, including those by Gust et al. [19] and Santomasso et al. [27], demonstrated significant increases in plasma cytokines such as IL-6, interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α) from day 3 post-infusion in patients who developed ICANS [27]. Gust et al. reported that these elevations were accompanied by evidence of blood–brain barrier (BBB) disruption and endothelial activation occurring within the first 36 hours following CAR-T infusion [19]. The detection of elevated cytokines as early as day 0 in our cohort suggests that baseline systemic inflammation may predispose to neurotoxicity. These findings imply that the inflammatory cascade leading to ICANS likely begins prior to infusion, offering a potential window for early risk assessment and intervention.
Consistent with this systemic cytokine profile, our CSF data showed that patients experiencing significant neurotoxicity also exhibited elevated IL-6, IL-15, and GM-CSF levels at the peak of symptoms, along with increased protein concentrations and, in some cases, mild lymphocytic pleocytosis. Serial assessments confirmed sustained cytokine elevation in the CSF until symptom resolution. Notably, we observed a trend toward positive correlations between CSF IL-6 and IL-15 levels and ICANS severity (p < 0.1) early after CAR-T infusion, reinforcing their potential pathogenic role. In line with our findings, Gust et al. described early BBB disruption in patients with severe ICANS, facilitating the leakage of systemic cytokines such as IL-6, IFN-γ, and TNF-α into the CSF and contributing to neurovascular dysfunction [18]. Additionally, other studies have demonstrated that IL-6, IL-8, MCP-1, and IP-10 are significantly elevated in the CSF of patients with severe neurotoxicity—especially at day 3 post-infusion—suggesting a combination of local CNS production and systemic inflammatory infiltration [27].
Multivariate analysis and predictive models
Therefore, CRS and cytokines early after CAR-T cell infusion, were identified as risks factors for ICANS appearance. When integrated into multivariate models, several clinical and laboratory parameters emerged as determinant predictors of ICANS. For any-grade ICANS, the optimal model incorporated CAR-T therapy type, time from infusion to CRS onset, day 3 IL-6 levels, and pre-infusion D-dimer, achieving an AUC of 0.83. For grade 2–4 ICANS, the best model included the number of prior therapy lines, the occurrence of grade ≥2 CRS, and pre-infusion levels of IL-15 and GM-CSF, along with AID (AUC = 0.80). These models underscore that routinely measured clinical and analytical variables can be combined to effectively stratify ICANS risk and potentially guide early, targeted interventions.
Moreover, various predictive scoring systems and models have been explored to predict ICANS, each with inherent strengths and limitations. Simple tools such as m-EASIX (based on CRP and platelet counts alone) offer pragmatic clinical applicability due to their simplicity but may lack specificity in capturing dynamic risk changes [36]. Point-based scoring systems like Rubin’s provide clear thresholds predicting ICANS risk, but their binary approach limits their ability to reflect temporal evolution [21]. In contrast, machine-learning algorithms, particularly XGBoost, demonstrate a strong ability to handle complex interactions among multiple variables, though their clinical implementation necessitates rigorous calibration and may be less transparent due to their complexity [37]. Our study’s identification of cytokine and clinical parameters aligns with variables highlighted by XGBoost methodologies, reinforcing the potential utility of such advanced analytical approaches. Forecasting models such as those proposed by Amidi et al. integrate dynamic patient data, offering real-time, day-by-day risk assessments that may significantly enhance clinical decision-making concerning ICU transfer, corticosteroid initiation, or safe patient discharge [38]. Selecting appropriate predictive tools thus involves balancing simplicity, interpretability, and temporal resolution to optimize clinical outcomes.
Importantly, the univariate analysis in this study was exploratory and aimed not only to guide multivariate model construction but also to identify a focused set of potentially relevant predictors. These findings may serve as a basis for future confirmatory studies with larger populations, allowing a more efficient design that limits the number of variables tested and reduces the risk of type I error.
Clinical and therapeutic considerations
Our findings highlight several important considerations for the clinical management and prevention of ICANS. Patient-specific features emerged as key contributors to neurotoxicity risk. In particular, a history of autoimmune disease (AID) was a strong predictor, associated with a nearly fivefold increase in both any-grade and severe ICANS. This suggests that underlying immune dysregulation may predispose patients to ICANS and underscores the importance of closely monitoring this high-risk subgroup.
Additionally, markers of tumor burden—including elevated metabolic tumor volume (MTV) on pre-lymphodepletion PET-CT and increased serum LDH—were significantly associated with ICANS in univariate analysis, supporting previous findings [39]. Although these associations did not persist in multivariate models, they nonetheless point to the role of baseline disease burden in shaping inflammatory risk. Furthermore, elevated post-infusion D-dimer levels were strongly associated with the subsequent development of ICANS [16], reinforcing its potential as a useful biomarker for early risk stratification.
From a therapeutic standpoint, corticosteroids achieved an 83% resolution rate in our cohort, confirming their efficacy as first-line treatment for neurotoxicity. However, our results also raise caution regarding cumulative tocilizumab exposure. Although effective for cytokine release syndrome (CRS), the inability of tocilizumab to cross the blood–brain barrier may permit systemic inflammation to persist and propagate neurotoxicity [18, 19]. In our study, higher cumulative doses of tocilizumab were associated with increased ICANS incidence, particularly in patients with concurrent risk factors. This observation aligns with several reports identifying tocilizumab as a potential risk modifier for ICANS, though data across studies remain heterogeneous. Some analyses have found no statistically significant association association [19, 21, 23, 27, 38, 39], while others—including ours—highlight a concerning trend [18, 19, 28]. These inconsistencies emphasize the need for individualized treatment strategies, particularly when repeated tocilizumab dosing is being considered in the setting of unresolved CRS and pre-existing ICANS risk factors.
Our findings support integrating these predictive models into clinical practice as a potential framework for personalized risk assessment using routine CAR-T parameters. If validated in larger, prospective cohorts, these models could be instrumental to design a risk index that guides prophylactic measures and optimizes therapeutic interventions, ultimately enhancing patient outcomes.
Limitations
Nonetheless, several limitations must be acknowledged. As a retrospective study, our analysis is inherently subject to selection bias and the limitations of available data. The relatively small sample size, especially of the CSF cytokine analysis subgroup, and the fact that CSF cytokines were measured only in patients who developed ICANS, constrain our ability to distinguish neurotoxicity‑specific signatures from systemic inflammation. Furthermore, cytokine measurements were limited to two time‑points (day 0 and day 3 post‑infusion), which may not fully capture the dynamic inflammatory milieu preceding ICANS; however, given a median ICANS onset of day 6, these early assays were chosen to reflect pre‑symptomatic risk and offer a parsimonious, cost‑sensitive approach for broader clinical implementation. Notably, CSF analysis was an exploratory, proof-of-concept effort aimed at deepening the understanding of ICANS pathophysiology and was not intended for model development, as samples were collected post-onset. Moreover, the absence of an independent validation cohort restricts the immediate clinical applicability of our predictive models. Notably, the combined analysis of the products may introduce confounding effects. Additionally, modifications to management protocols implemented over the study period may have influenced the outcomes. Future prospective studies with larger, more diverse populations will be essential to confirm these associations and to refine our models further.
Conclusion
In conclusion, this real-world study elucidates critical risk factors and cytokine signatures associated with ICANS in patients undergoing CD19 CAR-T therapy. The integration of clinical parameters such as CAR-T product type, CRS characteristics and AID history with laboratory markers including IL-6, IL-15, D-dimer, CRP, ferritin and GM-CSF provides a promising strategy for early risk stratification. These insights not only deepen our understanding of the pathophysiological underpinnings of ICANS but also pave the way for more personalized and preemptive approaches to its management. Ultimately, our study supports the development of targeted interventions that could mitigate neurotoxicity and enhance the overall safety and efficacy of CAR-T therapy.
Data availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–5.
Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–8.
Van Den Neste E, Schmitz N, Mounier N, Gill D, Linch D, Trneny M, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transpl. 2016;51:51–7.
Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–90.
Berning P, Fekon M, Ngoya M, Goldstone AH, Dreger P, Montoto S, et al. Hematopoietic stem cell transplantation for DLBCL: a report from the European Society for Blood and Marrow Transplantation on more than 40,000 patients over 32 years. Blood Cancer J. 2024;14:106.
Malard F, Mohty M. Acute lymphoblastic leukaemia. Lancet. 2020;395:1146–62.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378:439–48.
Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386:640–54.
Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399:2294–308.
Houot R, Bachy E, Cartron G, Gros FX, Morschhauser F, Oberic L, et al. Author Correction: Axicabtagene ciloleucel as second-line therapy in large B cell lymphoma ineligible for autologous stem cell transplantation: a phase 2 trial. Nat Med. 2024;30:2089.
Sehgal A, Hoda D, Riedell PA, Ghosh N, Hamadani M, Hildebrandt GC, et al. Lisocabtagene maraleucel as second-line therapy in adults with relapsed or refractory large B-cell lymphoma who were not intended for haematopoietic stem cell transplantation (PILOT): an open-label, phase 2 study. Lancet Oncol. 2022;23:1066–77.
Bachy E, Le Gouill S, Di Blasi R, Sesques P, Manson G, Cartron G, et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat Med. 2022;28:2145–54.
Jacobson CA, Locke FL, Ma L, Asubonteng J, Hu ZH, Siddiqi T, et al. Real-World Evidence of Axicabtagene Ciloleucel for the Treatment of Large B Cell Lymphoma in the United States. Transplant Cell Ther. 2022;28:581.e1–e8.
Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. 2022;22:85–96.
Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Muller-Tidow C, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32:34–48.
Gu T, Hu K, Si X, Hu Y, Huang H. Mechanisms of immune effector cell-associated neurotoxicity syndrome after CAR-T treatment. WIREs Mech Dis. 2022;14:e1576.
Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017;7:1404–19.
Rubin DB, Danish HH, Ali AB, Li K, LaRose S, Monk AD, et al. Neurological toxicities associated with chimeric antigen receptor T-cell therapy. Brain. 2019;142:1334–48.
Rubin DB, Al Jarrah A, Li K, LaRose S, Monk AD, Ali AB, et al. Clinical Predictors of Neurotoxicity After Chimeric Antigen Receptor T-Cell Therapy. JAMA Neurol. 2020;77:1536–42.
Greenbaum U, Strati P, Saliba RM, Torres J, Rondon G, Nieto Y, et al. CRP and ferritin in addition to the EASIX score predict CAR-T-related toxicity. Blood Adv. 2021;5:2799–806.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transpl. 2019;25:625–38.
Gazeau N, Liang EC, Wu QV, Voutsinas JM, Barba P, Iacoboni G, et al. Anakinra for Refractory Cytokine Release Syndrome or Immune Effector Cell-Associated Neurotoxicity Syndrome after Chimeric Antigen Receptor T Cell Therapy. Transplant Cell Ther. 2023;29:430–7.
Wehrli M, Gallagher K, Chen YB, Leick MB, McAfee SL, El-Jawahri AR, et al. Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS). J Immunother Cancer. 2022;10:e003847.
Locke FL, Neelapu SS, Bartlett NL, Lekakis LJ, Jacobson CA, Braunschweig I, et al. Tocilizumab Prophylaxis Following Axicabtagene Ciloleucel in Relapsed or Refractory Large B-Cell Lymphoma. Transplant Cell Ther. 2024;30:1065–79.
Santomasso BD, Park JH, Salloum D, Riviere I, Flynn J, Mead E, et al. Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia. Cancer Discov. 2018;8:958–71.
Gust J, Ponce R, Liles WC, Garden GA, Turtle CJ. Cytokines in CAR T Cell–Associated Neurotoxicity. Front Immunol. 2020;11:577027.
Kim HO, Kim HS, Youn JC, Shin EC, Park S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J Transl Med. 2011;9:113.
Mangioris G, Pittock SJ, Yang B, Fryer JP, Harmsen WS, Dubey D, et al. Cerebrospinal Fluid Cytokine and Chemokine Profiles in Central Nervous System Sarcoidosis: Diagnostic and Immunopathologic Insights. Ann Neurol. 2024;96:704–14.
Wullschleger A, Kapina V, Molnarfi N, Courvoisier DS, Seebach JD, Santiago-Raber ML, et al. Cerebrospinal fluid interleukin-6 in central nervous system inflammatory diseases. PLoS One. 2013;8:e72399.
Kwon M, Iacoboni G, Reguera JL, Corral LL, Morales RH, Ortiz-Maldonado V, et al. Axicabtagene ciloleucel compared to tisagenlecleucel for the treatment of aggressive B-cell lymphoma. Haematologica. 2023;108:110–21.
Bastos-Oreiro M, Gutierrez A, Reguera JL, Iacoboni G, Lopez-Corral L, Terol MJ, et al. Best Treatment Option for Patients With Refractory Aggressive B-Cell Lymphoma in the CAR-T Cell Era: Real-World Evidence From GELTAMO/GETH Spanish Groups. Front Immunol. 2022;13:855730.
Holtzman NG, Xie H, Bentzen S, Kesari V, Bukhari A, El Chaer F, et al. Immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T-cell therapy for lymphoma: predictive biomarkers and clinical outcomes. Neuro Oncol. 2021;23:112–21.
Neelapu SS, Jacobson CA, Ghobadi A, Miklos DB, Lekakis LJ, Oluwole OO, et al. Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood. 2023;141:2307–15.
Pennisi M, Sanchez-Escamilla M, Flynn JR, Shouval R, Alarcon Tomas A, Silverberg ML, et al. Modified EASIX predicts severe cytokine release syndrome and neurotoxicity after chimeric antigen receptor T cells. Blood Adv. 2021;5:3397–406.
Huang JJ, Liang EC, Albittar A, Portuguese AJ, Wuliji N, Torkelson A, et al. Early prediction of severe ICANS after standard-of-care CD19 CAR T-cell therapy using gradient-boosted classification trees [abstract]. J Clin Oncol. 2024;42:7034.
Amidi Y, Eckhardt CA, Quadri SA, Malik P, Firme MS, Jones DK, et al. Forecasting immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor t-cell therapy. J Immunother Cancer. 2022;10:e005459.
Ababneh HS, Ng AK, Abramson JS, Soumerai JD, Takvorian RW, Frigault MJ, et al. Metabolic parameters predict survival and toxicity in chimeric antigen receptor T-cell therapy-treated relapsed/refractory large B-cell lymphoma. Hematol Oncol. 2024;42:e3231.
Acknowledgements
The authors express their profound appreciation to the dedicated teams and supporting departments. Their exceptional professionalism and persistent efforts were critical in advancing this project.
Funding
This research was conducted without the need for specific funding. Funding for MGL was supported by the SEHH-CRIS. Funding for SG-P was obtained from Instituto de Salud Carlos III (JR20/00033), and through the following grant: PI23/01037 (ISCIII) co-financed by the European Union.
Author information
Authors and Affiliations
Contributions
MGL, CSS and DGC designed the study, collected the data, performed the analyses and drafted the manuscript. MK, IGC, RB, MBO, JMGD, SGP, MP, VEV, DC and RGS reviewed and edited the manuscript. The remaining authors (PF-C, YFB, VAPF, RCB, JLRH, MLMG, CV-B) contributed to patient care and/or data acquisition. All authors (MGL, CSS, DGC, MK, IGC, RB, MBO, JMGD, SGP, MP, VEV, DC, RGS, PF-C, YFB, VAPF, RCB, JLRH, MLMG and CV-B) provided critical feedback and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
MK reports compensation for consultancy services from AbbVie, Incyte, and Sanofi, as well as speaker’s bureau fees from Novartis, Pfizer, and Takeda. MBO reports compensation that includes honoraria and board fees from Roche, Novartis, BMS, AbbVie, Kite, and Incyte, as well as research funding from Kite, AbbVie, and Incyte. MLMG reports compensation for consulting services and speaking fees from Merck, Biogen, Novartis, Sanofi, Almirall, BMS, J&J, ROCHE, Amgen, Horizon, AstraZeneca, Neuraxpharm and Viatri. SG-P has received speaker honoraria from Sanofi, Roche, Merck, and Biogen and has participated on Merck and Bristol Myers Squibb advisory boards.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41409_2025_2679_MOESM1_ESM.docx
Supplementary Table S1. History of Autoimmune Disease, clinical features and ICANS outcome in patients treated with CD19 CAR-T therapy
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gomez-Llobell, M., Serra Smith, C., Gómez-Costas, D. et al. ICANS risk model in CD19 CAR-T therapy: insights from serum and CSF cytokine profiling. Bone Marrow Transplant 60, 1351–1360 (2025). https://doi.org/10.1038/s41409-025-02679-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02679-y
This article is cited by
-
Immune effector cell-associated neurotoxicity syndrome following CAR T-cell therapy: a review of recent advances
Journal of Translational Medicine (2025)