Abstract
This manuscript provides expert recommendations on the role of allogeneic hematopoietic cell transplantation (allo-HCT) for cutaneous T-cell lymphoma (CTCL), specifically Mycosis Fungoides (MF) and Sezary Syndrome (SS). Critical aspects such as patient selection, timing, and bridging therapy are addressed, as well as donor source, conditioning regimens and post-transplant management. These consensus guidelines are based on a thorough literature review and discussions among leading dermatologists and hematologists. These recommendations aim to harmonise clinical practice towards improving patient outcomes in these rare but aggressive lymphomas. It is of critical importance to consider allo-HCT early in the management of eligible patients with high-risk disease. Advanced stage, large-cell transformation, relapsed or refractory disease following systemic treatment, and N3-stage lymph node involvement are indicators that should trigger consultation with a transplant hematologist in parallel with a donor search. Early interaction between dermatologists and transplant hematologists is vital to avoiding delays, which can significantly impact post-transplant outcomes and survival.

This EBMT Practice Harmonisation & Guidelines Committee consensus provides practical recommendations for the selection, timing, and conduct of allogeneic transplantation in advanced-stage mycosis fungoides and Sézary syndrome, aiming to optimize outcomes through early multidisciplinary collaboration and evidence-based decision making.
Similar content being viewed by others
Introduction
Cutaneous T-cell lymphomas (CTCL) are rare cancers, with an estimated incidence of about 6 to 10 cases per million per year [1], although rates may vary based on geographical location [2] and ethnicity. The most prevalent subtype is mycosis fungoides (MF), accounting for approximately 50-60% of cases [3]. MF presents in early stage with cutaneous patches and/or plaques and may have low level blood involvement and lymph node enlargement. Advanced stages may present with tumors or erythroderma and may have neoplastic involvement of blood, lymph nodes or rarely internal organs. Around a third of early stage MF patients progress to advanced stages [4]. Sezary Syndrome (SS) is an aggressive leukemic variant of CTCL which is traditionally defined by the triad of erythroderma, generalized lymphadenopathy and the presence of neoplastic T-cells in skin, lymph nodes, and blood. The neoplastic cells, known as Sézary cells, exhibit distinctive cerebriform nuclei, expressing CD4 often with loss of CD7 and/or CD26. Of note, in HTLV-1 endemic areas, HTLV-1 serology is mandatory to rule out smoldering or aggressive cutaneous adult T cell leukemia/lymphoma, which may have a clinical presentation similar to MF [5].
Definitions of advanced-stage CTCL and high-risk advanced-stage CTCL are reported in Table 1.
While early stages of MF are treated by external (skin-directed) therapies as first-line treatment, later stages need systemic therapies including cytotoxic agents [6,7,8,9] and targeted therapies [10, 11].
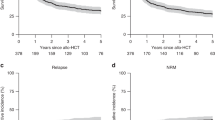
Advanced-stage MF and SS are associated with a poor prognosis [12] with most patients not surviving beyond 5 years from diagnosis and nowadays allo-HCT remains the only curative option [13,14,15,16,17,18]. Historical data from the EBMT registry show 5-year progression-free (PFS) and overall (OS) survival rates after allo-HCT of 26% and 38%, respectively, underscoring its curative potential but also the challenges associated with the high relapse incidence (RI) observed post-transplant [14,15,16,17].
A recent meta-analysis on allo-HCT reported 1-year and 3-year OS rates of 51% and 40%, respectively, with PFS rates of 42% at 1 year and 33% at 3 years [18].
In a recent, controlled, prospective study [14], 99 patients were stratified by disease severity and matched for confounding factors using propensity scores. Patients allocated to allo-HCT demonstrated a significantly improved PFS (9.0 months) compared to patients offered conventional therapies (3.0 months), with a hazard ratio of 0.38 (95% CI 0.21–0.69; p < 0.0001) reflecting a 62% relative reduction in the risk of disease progression or death, therefore favoring allo-HCT in intention-to-treat and per-protocol analyses. Subgroup analyses revealed that the benefit of allo-HCT was consistent across MF and SS subtypes.
Although allo-HCT is mentioned in European guidelines and position statements for MF/SS, there is little detailed guidance on indications, optimal timing and disease status for allo-HCT, as well as about the specific modalities on how to best perform transplantation [19, 20].
In this project, a multidisciplinary panel of expert hematologists and dermatologists was convened to elaborate joint guidelines for allo-HCT in patients with advanced CTCL. These guidelines aim to address key clinical questions, specifically identifying which CTCL patients are suitable candidates for allo-HCT, determining the optimal timing for transplantation, and establishing best practices for performing allo-HCT in this patient population. Additionally, the guidelines provide recommendations on the post-transplant management of CTCL patients, emphasizing the importance of a collaborative approach between dermatologists and transplant physicians to ensure seamless counseling and care throughout the disease trajectory.
While these recommendations were developed through a European collaborative process, the underlying principles may be adapted to different health care systems and transplant practices. Regional variations exist — for example, in the availability of donor registries, conditioning protocols, and access to novel therapies — particularly between Europe, North America, and Asia. Contextual adaptation is encouraged to enhance global applicability.
Methodology
The workshop preceding this report adhered to the methodology outlined by the EBMT Practice Harmonisation & Guidelines (PH&G) Committee [21]. The chairs (GD & NS) selected a list of European “CTCL and allo-HCT experts” based on their professional expertise and willingness to actively participate in elaborating these recommendations.
Key methodological elements and topics to be addressed were identified during a series of discussions prior to the meeting. Preparation for the workshop included a thorough literature review.
Two primary work packages (WP) were established. WP1 focused on the general treatment algorithm for CTCL entities, including the role of allo-HCT, and WP2 addressed the specifics of allo-HCT, from preparatory phases to transplantation, post-transplant maintenance, and follow-up. Insights from internationally renowned reviewers further enriched these recommendations.
State of the art
First-line therapy
First-line treatments for advanced-stage CTCL are outlined in recent EORTC consensus guidelines by Latzka et al., which recommend tailoring therapy based on the disease type (MF or SS), presence of large-cell transformation, disease burden, progression rate, and patient-related factors such as comorbidities and performance status [19, 22].
Treatment options include extracorporeal photopheresis, systemic therapies such as interferon-alpha, bexarotene and other oral retinoids, or low-dose methotrexate. In patients with limited disease and slow evolution, skin directed therapies may also be useful, alone or in combination, such as phototherapy (UVB/PUVA) or low dose RT. Total-skin electron-beam therapy is also an option in patients with widespread skin disease [23] but may be best followed by a systemic treatment such as maintenance or allo-HCT. Patients with widespread disease may benefit from brentuximab-vedotin (BV) [11] or mogamulizumab [10], (both off-label in first-line treatment in Europe), romidepsin (off-label in Europe) [24] or chemotherapy [6,7,8,9]. Allo-HCT may be considered in first remission for younger patients less than 65 year-old with SS or selected high-risk patients with MF who are eligible. [13,14,15, 17, 20, 25].
Treatment of refractory/relapsed patients
Second-line treatment of advanced MF includes brentuximab vedotin (BV) and mogamulizumab, which are preferred due to their favorable efficacy and safety profiles. Chemotherapy (mostly single agent) remains an option, and multi-agent chemotherapy may be used as a bridge to allo-HCT in patients with refractory and/or bulky disease. In Sézary syndrome, first-line systemic treatment frequently consists of combination approaches such as extracorporeal photopheresis (ECP) plus pegylated interferon-alpha, with early switching to mogamulizumab in the case of insufficient response. Indeed, the extent of blood involvement, particularly B2, is considered a surrogate marker for response to mogamulizumab. Recently, Bozonnat et al. published real-world data confirming the efficacy of mogamulizumab in reducing leukemic disease burden for the first time with mogamulizumab, thereby supporting its early integration into the treatment algorithm [26].
Although not approved in Europe, the histone deacetylase (HDAC) inhibitor romidepsin, has shown some efficacy in the treatment of advanced-stage CTCL and is a therapeutic option [24]. Mogamulizumab is approved and can be used in patients with advanced-stage MF as second-line treatment [10]. Because the drug target, CCR4, is also expressed on normal regulatory T-cells, its use before allo-HCT has been associated with an increased risk of acute graft-versus-host disease (GVHD), as observed in studies on adult T-cell leukemia/lymphoma. Therefore a 6-week wash-out period between the last administration of mogamulizumab and allo-HCT is recommended [27, 28].
Workshop recommendations
Role of allo-HCT in CTCL
The prognosis of patients with advanced-stage CTCL remains poor with a median OS often below 40 months [12]. For these patients, immunotherapy with allo-HCT, based on its inherent graft-versus-lymphoma (GvL) effect, is the only curative option.
Evidence from retrospective and matched controlled trials demonstrated that allo-HCT significantly improved PFS compared to non-HCT therapies in patients with advanced-stage CTCL [14] (Table 2). However, this potential benefit must be carefully weighed against the substantial morbidity associated with allo-HCT, which carries a not negligible risk of treatment-related mortality, along with a high risk of relapse. Moreover, the long-term survival outcomes remain modest and, in some reports, comparable to those achieved with conventional therapies [12, 29, 30] raising important questions about the optimal timing and indication for allo-HCT. In this context, careful patient selection is paramount. High-risk advanced-stage disease includes patients with stage IIB to IVB MF or SS who present with at least one poor-prognostic factor among the following: large-cell transformation [31, 32], significant nodal (N3), or visceral (M1) involvement [22], previous relapse or refractoriness after first-line systemic treatment (excluding methotrexate, retinoids, and interferon) [14]. Notably, the subgroup of patients with LCT-MF appears to derive the greatest benefit from allo-HCT, with the CUTALLO trial showing more favorable outcomes in this population, supporting its prioritization for transplant evaluation. For rare entities, such as primary subcutaneous gamma-delta T-cell lymphoma, or CD8+ aggressive epidermotropic primary CTCL, only case reports and small series have been published to date. Some of these publications reported long-term remissions after allo-HCT, which strongly suggest a clinically relevant GvL effect also in these entities [33,34,35].
Although not included as “poor-prognosis factor” in the CUTALLO trial, elevated serum lactate dehydrogenase (LDH) level at diagnosis has been identified as an independent pejorative criterion for patients with advanced-stage CTCL in the Cutaneous Lymphoma International Consortium (CLIC) analysis [12], and such patients should also be considered for allo-HCT. In the CLIC analysis, 3 groups of advanced-stage MF/SS patients could be defined, respectively low (0-1 risk factor), intermediate (2 risk factors) and high risk (3-4 factors), based on the 4 following risk factors: stage IV disease, age greater than 60 years, large-cell transformation in the skin and elevated serum LDH. High-risk patients as well as younger individuals in the intermediate-risk group according to CLIC should be considered for allo-HCT [12].
Regarding Sézary syndrome, the emergence of novel therapies—particularly mogamulizumab, which has shown the ability to prolong progression-free survival while maintaining quality of life (QoL) and demonstrating a favorable toxicity profile—may allow deferral of allo-HCT in patients with risk factors for transplant-related mortality (TRM), particularly in those achieving durable responses.
In general, early transplant referral remains advisable for high-risk CTCL patients; however, the decision should be individualized, taking into account disease trajectory, comorbidities, and patient preference, as preservation of quality of life with less intensive therapies may be preferable in selected cases.
Recommendations
-
For patients with early-stage MF (IA–IIA), allo-HCT is generally not indicated.
-
For patients with stage IB/IIA MF who have relapsed or are refractory after multiple lines of systemic therapy, allo-HCT may be considered on an individual basis.
-
Patients with large cell transformation, stage IV MF and patients with SS, should be considered for allo-HCT regardless of the treatment line.
-
Eligible patients with relapsed or refractory stage IIB and III should be considered for allo-HCT, as soon as response is achieved.
-
For rare entities such as primary subcutaneous gamma-delta T-cell lymphoma, or CD8 aggressive epidermotropic primary CTCL:
-
○
A donor search should be initiated upon diagnosis.
-
○
Allo-HCT can be considered as consolidation in patients achieving complete response (CR), or partial response (PR) following first-line treatment.
-
○
Timing and disease status at allo-HCT
CR, PR, very good PR (VGPR), stable disease (SD) and progressive disease (PD) are defined according to ISCL/USCLC/EORTC response criteria [22].
Disease status at allo-HCT is a critical prognostic factor significantly influencing outcomes. Patients undergoing allo-HCT in CR or VGPR before disease became refractory to conventional therapies and/ or organ involvement have better PFS and OS, with much lower relapse rates as compared to patients with advanced, uncontrolled skin and organ involvement [15, 17, 18, 25, 36]. In a study by the London group on 41 patients [37], those who achieved CR before allo-HCT experienced significantly lower rates of progression or relapse compared to patients not in CR (20.8% compared to 70.6%, p = 0.006). The median OS and PFS were 4.88 vs 1.53 years and 4.88 vs. 0.62 years, respectively. Furthermore, patients with measurable residual disease (mRD) in blood or skin had a higher probability of disease progression or relapse than patients who achieved molecular remission in both blood and skin, with incidences of progression or relapse of 87% and 9%, respectively [36, 38].
Salvage therapy or response induction before allo-HCT
Salvage therapy aims not only at reducing tumor burden in order to create the most favorable conditions for the subsequent allo-HCT, but also at managing symptoms and improving quality of life.
Patients with significant residual disease (i.e., mSWAT [modified Severity Weighted Assessment Tool] >10 or persistent extracutaneous disease) should not directly undergo transplantation if there remains a realistic option to achieve CR or VGPR. The mSWAT is an internationally recognized skin scoring system that evaluates the tumor burden in skin by multiplying the percentage of body surface area involved by a weighting factor of 1 (for patches), 2 (for plaques) or 4 (for tumors) [22]. Beyond chemotherapy, mogamulizumab for SS without large-cell transformation [10], total-skin electron-beam therapy [23], BV in CD30-positive disease, alone or in combination with chemotherapy [39, 40] may be effective. For patients with transformed MF, multi-agent chemotherapy should be considered as a reasonable option [41].
Total skin electron beam therapy (TSEB) targets cutaneous manifestations of CTCL and can be used to reduce tumor load and possibly achieve remission prior to allo-HCT. TSEB is generally well-tolerated and effective in reducing the cutaneous tumor burden, thereby helping to bring the disease into remission prior to transplant [23, 38, 42]. The total dose varies between 12 and 36 Gy depending on disease extent, type of lesions and degree of skin infiltration [23, 43].
Although randomized trials are lacking, expert consensus suggests tailoring pre-transplant therapy based on disease characteristics and treatment goals.
• In Stage IIB disease with large-cell transformation (LCT), aggressive behavior justifies an attempt to achieve the deepest possible remission before allo-HCT. CHOP or CHOEP-based regimens are reasonable options, and brentuximab vedotin, particularly in CD30+ disease, may be preferred due to its favorable toxicity profile and efficacy in inducing remission. When CD30 expression is present, BV alone or in combination with chemotherapy may be used as a bridge to transplant.
• In Stage III/IV disease, treatment selection should balance the urgency of disease control, patient fitness, and toxicity risk. In frail patients or slow-progressing SS, ECP alone or with low-dose interferon may be suitable, particularly when rapid disease control is not essential. However, when the goal is to achieve CR or VGPR before transplant, more intensive strategies like mogamulizumab followed by total-skin electron beam irradiation (TSEBI) should be considered.
In the absence of validated prospective algorithms, multidisciplinary discussions between dermatologists and transplant physicians remain essential to adapt the treatment approach to the individual patient.
Recommendations
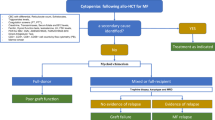
Figure 1 illustrates treatment strategies and the transplant algorithm.
-
Patients with high-risk advanced CTCL (both MF and SS) meeting the indication for allo-HCT, should undergo transplantation as soon as they reach CR.
-
For patients not reaching CR after salvage therapy, PR or VGPR or SD with limited residual disease (i.e. mSWAT <10) are also considered acceptable conditions.
-
There are no specific recommendations regarding the optimal number and modalities of prior therapies before allo-HCT.
-
For refractory cases, including those post-TSEB, allo-HCT is not recommended.
MF mycosis fungoides, SS Sezary syndrome, allo-HCT allogeneic hematopoietic cell transplantation, CR complete remission, PR partial remission, VGPR very good PR, SD stable disease, PD progressive disease, RIC reduced intensity conditioning. α: patients with SS, rare entities or large cell transformation should be considered to allo-HCT regardless on the line of treatment, in CR or PR; β: according to age, PS, comorbidities; γ: According to center preference; δ: GVHD prophylaxis should follow general guidelines for allo-HCT.
Prerequisites for allo-HCT in CTCL
Age, comorbidities, and performance status
While allo-HCT is a potentially curative option, its success is also influenced by age, performance status, and comorbidities. Advanced age is generally associated with a higher risk of transplant-related complications and non-relapse mortality (NRM), although reduced-intensity conditioning (RIC) has made allo-HCT feasible in older patients. Patients with a good performance status and a low comorbidity index can anticipate more favorable outcomes (HCT-comorbidity index [CI], 0-2).
Recommendations
-
There are no data supporting specific limits for age, comorbidities, and performance status of patients with MF and SS who are considered candidates for allo-HCT.
-
Age, HCT-CI, performance status and comorbidities must be weighed against disease status to determine the overall risks of allo-HCT.
-
General principles for allo-HCT in lymphomas should be followed [41].
Donor choice
In the study by the London group [37] the median OS for 41 patients with MF (n = 34) or SS (n = 7) was 4.84 years for matched unrelated donors (MUD), 3.55 years for matched related donors (MRD), and only 0.66 years for haploidentical transplantation. However, it is important to note that only 3 patients underwent haploidentical transplantation in this series, limiting the interpretation and practical consequences of these results. In general, published studies on haploidentical transplants in CTCL remain scarce [44].
A recent meta-analysis included data on only 12 patients who underwent haploidentical transplantation [45]; this small sample size makes it difficult to draw definitive conclusions.
A study by the Milan group [46] analyzed 63 patients with MF (n = 40) or SS (n = 23) transplanted using HLA-identical sibling donors (n = 22), unrelated donors (n = 28; 15 HLA-matched and 13 HLA-mismatched), and haploidentical donors (n = 13). MRD demonstrated better survival outcomes and lower transplant-related mortality (TRM) compared to both unrelated and haploidentical donors. While survival outcomes for haploidentical transplants were slightly inferior to MRD, they were comparable to MUD. TRM for haploidentical donors was relatively low, but the cumulative incidence of relapse or progression was notably higher than in MRD, indicating a need for enhanced disease control strategies in this cohort. Larger studies on haploidentical transplantation in T-cell lymphomas have reported outcomes comparable to those of MRD and MUD [47,48,49].
In most diseases, MRD are the preferred choice. However, transplantation from an HLA-MUD has been shown to achieve comparable outcomes [50]. Therefore, MRD are preferred over mismatched MUD and haploidentical donors. The use of unrelated cord blood transplantation is declining and should be reserved for specific cases where no living donor is available [51].
Recommendations
-
The selection of a suitable donor should align with established guidelines for all lymphomas, including T-cell lymphomas, following a hierarchical preference: MRD, MUD, haploidentical donor, or 1-antigen-MMUD (Fig. 1).
What is the recommended conditioning regimen and the strategy for GVHD prevention?
Available literature supports the use of RIC as the preferred approach for most patients, especially older individuals or those with significant comorbidities [17, 20, 52].
A recent systematic review and meta-analysis comparing outcomes of myeloablative conditioning (MAC) and RIC for MF and SS revealed that cumulative OS was significantly better after RIC (58% [95% CI, 47%–68%]) compared to MAC (30% [95% CI, 7%–42%]; p < 0.001) [45]. These findings underscore the role of RIC in optimizing outcomes by balancing efficacy and toxicity, considering patient’s age, overall health, disease burden, and risk of relapse. Low-dose total body irradiation (LdTBI) has also been used for conditioning; however, there is no evidence to suggest that LdTBI should be preferred over non-LdTBI regimens.
Weng et al. from Stanford University School of Medicine reported on chemo-free reduced-intensity regimen in a series of 35 patients, the majority of whom had Sézary syndrome (n = 22), while 13 had MF [36]. Conditioning regimen included TSEB, total nodal irradiation and anti-thymocyte globulin. The 1- and 2-year NRM were of 3% and 14%, respectively and the 2-, 3-, and 5-year OS rates were 68%, 62%, and 56%, with a significantly lower incidence of disease progression or relapse in patients achieving a molecular remission (9% vs 87%; P = 0.02). The same conditioning regimen was initially analyzed in a small cohort of 17 patients from the UK. The 2-year OS was 79% and TRM was low, suggesting that chemo-free conditioning could be a good and effective alternative before allo-HCT [13]. Long-term outcomes of Stanford chemo-free regimen was recently reported by Morris et al. [37], with a cumulative incidence of NRM of 12.6% at 2 years and a 5-year OS rate of 37.7% (MF 36.7%, SS 57.1%).
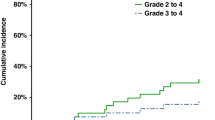
Outside of haploidentical transplantation, GVHD prophylaxis in allo-HCT typically includes a combination of a calcineurin inhibitor (CNI) with either methotrexate (MTX) or mycophenolate mofetil (MMF) [53]. Data on GVHD prophylaxis, specifically in PTCL patients, remain limited. The addition of anti-thymoglobulin to the conditioning regimen has been shown to reduce GVHD incidence in patients with acute lymphoblastic lymphoma or T-cell lymphoma [54]. T-cell depletion by alemtuzumab is not generally recommended because high relapse rates after allo-HCT were repeatedly observed [55]. While post-transplant cyclophosphamide is primarily used in haploidentical settings [56], its application is expanding to other donor-recipient scenarios.
Recommendations
-
RIC should be the preferred regimen.
-
With no data supporting a specific RIC regimen, centres should stay with the locally established RIC regimens.
-
GVHD prophylaxis should follow general guidelines for allo-HCT [57].
What is the role of post-transplant measurable residual disease monitoring, maintenance therapy and immunomodulation strategies?
Although the achievement of post-transplantation molecular remission has been reported to associate with significantly better survival outcomes [36, 37], there are no available data supporting the recommendation of post-allo HCT mRD monitoring nor the use of maintenance strategies for CTCL (e.g., bexarotene, interferon alfa, or extracorporeal photopheresis) [55, 58, 59], even in mRD-positive patients.
While no standardized approach currently exists for post-allo-HCT MRD monitoring in CTCL, future clinical trials should incorporate sensitive and disease-specific tools. Flow cytometry (FACS) can be used for monitoring residual circulating Sézary cells in the blood. In parallel, T-cell receptor (TCR) rearrangement studies — including clonality assessment and quantification — may offer a more precise evaluation of minimal disease burden and should be considered for inclusion in prospective protocols.
Recommendations
-
There is no evidence supporting mRD monitoring in routine practice for CTCL.
-
Post-transplant maintenance or preemptive therapies in mRD-positive patients are not standard of care and should be restricted to clinical trials.
How should post-transplant relapse in CTCL be managed?
Management of post-transplant relapse requires a multidisciplinary approach, emphasizing close collaboration with dermatologists and radiotherapists. Relapses with early stage MF lesions may be managed with skin-directed therapy or possibly donor-lymphocyte infusion (DLI) and must therefore be differentiated from GVHD or skin infections [14, 37].
Dermatologists and dermato-pathologists play a crucial role in accurately evaluating and diagnosing any skin disease occurring after allo-HCT. Biopsies of affected skin are often necessary.
Unfortunately, post-transplant relapses, predominantly in the skin, are common but may be low grade and frequently managed with skin-directed treatment. Relapse in other compartments such as blood, lymph nodes or internal organs may also occur. A significant number of patients achieve a second remission post-transplant with prolonged disease control after tapering of immunosuppressive drugs, local radiotherapy (RT) or TSEB, and disease-specific treatments [14]. Management of post-transplant relapse typically involves tapering of immunosuppressive therapy and/or DLI to elicit or enhance a GVL effect. DLI have shown efficacy in inducing durable remissions, especially in cases where measurable residual disease or early signs of relapse are detected [14]. As with other disorders, DLI requires careful monitoring due to the significant risk of GVHD.
These immunological approaches should be combined with disease-specific treatments, tailored to the patient’s condition and disease stage. Localized skin lesions may be managed with radiation therapy, while patch-stage disease could be treated with skin-directed topical therapies. Systemic or widespread skin relapses may require systemic treatment, such as BV or IFN-α, particularly in case of relapse with large-cell transformation [39].
Recommendations
-
Conventional therapy combined with immune tapering is recommended.
-
DLI is recommended as the primary immunomodulatory strategy for relapse after allo-HCT.
-
Localized RT or skin-directed therapy are suitable options in patients with limited skin relapse.
-
When feasible, TSEB is a recommended option for patients with diffuse skin relapse.
-
There is no recommendation for a second allo-HCT, but it may be considered if appropriate, possibly from a different donor.
Areas for investigation in allo-HCT for CTCL
-
Given the rarity of the disease and the heterogeneity of its clinical presentations, much of the current evidence on allo-HCT is derived from retrospective analyses, registry data, and small single-center studies. Prospective, randomized trials remain exceedingly difficult to conduct in this setting. Limitations such as small sample sizes, lack of standardized treatment protocols, and selection bias are inherent to most available studies, particularly those evaluating donor source, optimal timing, the number of lines of salvage treatment prior to allo-HCT, conditioning regimens, and post-transplant interventions. In particular, the impact of modern GVHD prophylaxis, including post-transplantation cyclophosphamide (PTCy), on outcomes remains unclear at this time. These data limitations must be acknowledged when interpreting existing literature and applying it to clinical decision-making.
-
As with other details of the transplant procedure, there is insufficient evidence regarding the role and methodologies for mRD monitoring, as well as the utility of maintenance therapy following allo-HCT in CTCL. Ongoing research into CAR T-cell therapy [60] as a potential alternative to allo-HCT in CTCL, or as a bridge to transplant, highlights a promising avenue for future investigation.
Conclusions
This expert consensus involving dermatologists and hematologists taking care of CTCL patients to receive allo-HCT underscores the critical importance of careful patient selection, optimal disease control before transplant, and post-transplant management. Most importantly, early consultation and discussions between the treating dermatologist/hematologist and the transplant team is crucial to allow for timely transplantation for patients in whom allo-HCT is indicated.
Further studies are necessary to refine patient selection, evaluate maintenance and relapse therapies, and explore innovative approaches such as immune intervention including monoclonal and bispecific antibodies, antibody-drug conjugates, checkpoint inhibitors and CAR T-cell therapy. Although these novel therapies are still under investigation in CTCL, early-phase trials suggest promising activity, particularly for relapsed or refractory disease. CAR-T cell constructs targeting CD4 or CD30, as well as bispecific antibodies (e.g., CD30 × CD16 or CD3 × CD4), are currently in development, though clinical availability remains limited outside of trials. These strategies may eventually serve as adjuncts or alternatives to allo-HCT in selected patients.
References
Dobos G, de Masson A, Ram-Wolff C, Beylot-Barry M, Pham-Ledard A, Ortonne N, et al. Epidemiological changes in cutaneous lymphomas: an analysis of 8593 patients from the French Cutaneous Lymphoma Registry. Br J Dermatol. 2021;184:1059–67.
Dobos G, Pohrt A, Ram-Wolff C, Lebbé C, Bouaziz JD, Battistella M, et al. Epidemiology of cutaneous T-cell lymphomas: a systematic review and meta-analysis of 16,953 patients. Cancers. 2020;12:2921.
Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, Swerdlow SH, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703–14.
Scarisbrick JJ, Quaglino P, Prince HM, Papadavid E, Hodak E, Bagot M, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol. 2019;181:350–7.
Marchetti MA, Pulitzer MP, Myskowski PL, Dusza SW, Lunning MA, Horwitz SM, et al. Cutaneous manifestations of human T-cell lymphotrophic virus type-1-associated adult T-cell leukemia/lymphoma: a single-center, retrospective study. J Am Acad Dermatol. 2015;72:293–301.
Dummer R, Quaglino P, Becker JC, Hasan B, Karrasch M, Whittaker S, et al. Prospective international multicenter phase II trial of intravenous pegylated liposomal doxorubicin monochemotherapy in patients with stage IIB, IVA, or IVB advanced mycosis fungoides: final results from EORTC 21012. J Clin Oncol. 2012;30:4091–7.
Straus DJ, Duvic M, Horwitz SM, Hymes K, Goy A, Hernandez-Ilizaliturri FJ, et al. Final results of phase II trial of doxorubicin HCl liposome injection followed by bexarotene in advanced cutaneous T-cell lymphoma. Ann Oncol. 2014;25:206–10.
Marchi E, Alinari L, Tani M, Stefoni V, Pimpinelli N, Berti E, et al. Gemcitabine as frontline treatment for cutaneous T-cell lymphoma: phase II study of 32 patients. Cancer. 2005;104:2437–41.
Pellegrini C, Stefoni V, Casadei B, Maglie R, Argnani L, Zinzani PL. Long-term outcome of patients with advanced-stage cutaneous T cell lymphoma treated with gemcitabine. Ann Hematol. 2014;93:1853–7.
Kim YH, Bagot M, Pinter-Brown L, Rook AH, Porcu P, Horwitz SM, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19:1192–204.
Prince HM, Kim YH, Horwitz SM, Dummer R, Scarisbrick J, Quaglino P, et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet. 2017;390:555–66.
Scarisbrick JJ, Prince HM, Vermeer MH, Quaglino P, Horwitz S, Porcu P, et al. Cutaneous Lymphoma International Consortium study of outcome in advanced stages of mycosis fungoides and Sézary syndrome: effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol. 2015;33:3766–73.
Ritchie S, Qureshi I, Molloy K, Yoo J, Shah F, Stevens A, et al. Evaluation of haematopoietic stem cell transplantation in patients diagnosed with cutaneous T-cell lymphoma at a tertiary care centre: should we avoid chemotherapy in conditioning regimes? Br J Dermatol. 2020;182:807–9.
de Masson A, Beylot-Barry M, Ram-Wolff C, Mear JB, Dalle S, d'Incan M, et al. Allogeneic transplantation in advanced cutaneous T-cell lymphomas (CUTALLO): a propensity score matched controlled prospective study. Lancet. 2023;401:1941–50.
Domingo-Domenech E, Duarte RF, Boumedil A, Onida F, Gabriel I, Finel H, et al. Allogeneic hematopoietic stem cell transplantation for advanced mycosis fungoides and Sézary syndrome. An updated experience of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2021;56:1391–401.
Duarte RF, Canals C, Onida F, Gabriel IH, Arranz R, Arcese W, et al. Allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sézary syndrome: a retrospective analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2010;28:4492–9.
Iqbal M, Reljic T, Ayala E, Sher T, Murthy H, Roy V, et al. Efficacy of allogeneic hematopoietic cell transplantation in cutaneous T cell lymphoma: results of a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2020;26:76–82.
Goyal A, Foss F. Allogeneic transplantation and cellular therapies in cutaneous T-cell lymphoma. Expert Rev Anticancer Ther. 2024;24:41–58.
Latzka J, Assaf C, Bagot M, Cozzio A, Dummer R, Guenova E, et al. EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—update 2023. Eur J Cancer. 2023;195:113343.
Goyal A, O'leary D, Dabaja B, Weng WK, Zain J, Cutler C, et al. ASTCT and USCLC clinical practice recommendations for allogeneic stem cell transplant in mycosis fungoides and Sézary syndrome. Transplant Cell Ther. 2024;30:1047–60.
Yakoub-Agha I, Greco R, Onida F, de la Cámara R, Ciceri F, Corbacioglu S, et al. Practice harmonization workshops of EBMT: an expert-based approach to generate practical and contemporary guidelines within the arena of hematopoietic cell transplantation and cellular therapy. Bone Marrow Transplant. 2023;58:696–700.
Olsen EA, Whittaker S, Willemze R, Pinter-Brown L, Foss F, Geskin L, et al. Primary cutaneous lymphoma: recommendations for clinical trial design and staging update from the ISCL, USCLC, and EORTC. Blood. 2022;140:419–37.
Hoppe RT, Harrison C, Tavallaee M, Bashey S, Sundram U, Li S, et al. Low-dose total skin electron beam therapy as an effective modality to reduce disease burden in patients with mycosis fungoides: results of a pooled analysis from 3 phase-II clinical trials. J Am Acad Dermatol. 2015;72:286–92.
Whittaker SJ, Demierre M-F, Kim EJ, Rook AH, Lerner A, Duvic M, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28:4485–91.
Duarte RF, Boumendil A, Onida F, Gabriel I, Arranz R, Arcese W, et al. Long-term outcome of allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sézary syndrome: a European Society for Blood and Marrow Transplantation Lymphoma Working Party extended analysis. J Clin Oncol. 2014;32:3347–8.
Bozonnat A, Beylot-Barry M, Dereure O, D'Incan M, Quereux G, Guenova E, et al. Real-life efficacy of immunotherapy for Sézary syndrome: a multicenter observational cohort study. EClinicalMedicine. 2024;73:102679.
Lechowicz MJ, Smith C, Ristuccia R, Dwyer K. Allogeneic hematopoietic stem cell transplantation after mogamulizumab in T-cell lymphoma patients: a retrospective analysis. Int J Hematol. 2024;119:736–44.
Fuji S, Inoue Y, Utsunomiya A, Moriuchi Y, Uchimaru K, Choi I, et al. Pretransplantation anti-CCR4 antibody mogamulizumab against adult T-cell leukemia/lymphoma is associated with significantly increased risks of severe and corticosteroid-refractory graft-versus-host disease, nonrelapse mortality, and overall mortality. J Clin Oncol. 2016;34:3426–33.
Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713–22.
Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730–9.
Pulitzer M, Myskowski PL, Horwitz SM, Querfeld C, Connolly B, Li J, et al. Mycosis fungoides with large cell transformation: clinicopathological features and prognostic factors. Pathology. 2014;46:610–6.
Kadin ME, Hughey LC, Wood GS. Large-cell transformation of mycosis fungoides-differential diagnosis with implications for clinical management: a consensus statement of the US Cutaneous Lymphoma Consortium. J Am Acad Dermatol. 2014;70:374–6.
Cyrenne BM, Gibson JF, Subtil A, Girardi M, Isufi I, Seropian S, et al. Transplantation in the treatment of primary cutaneous aggressive epidermotropic cytotoxic CD8-positive T-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2018;18:e85–93.
Isufi I, Seropian S, Gowda L, Wilson LD, Roberts K, Girardi M, et al. Outcomes for allogeneic stem cell transplantation in refractory mycosis fungoides and primary cutaneous gamma Delta T cell lymphomas. Leuk Lymphoma. 2020;61:2955–61.
Brüggen M-C, Kerl K, Haralambieva E, Schanz U, Chang YT, Ignatova D, et al. Aggressive rare T-cell lymphomas with manifestation in the skin: a monocentric cross-sectional case study. Acta Derm Venereol. 2018;98:835–41.
Weng W-K, Arai S, Rezvani A, Johnston L, Lowsky R, Miklos D, et al. Nonmyeloablative allogeneic transplantation achieves clinical and molecular remission in cutaneous T-cell lymphoma. Blood Adv. 2020;4:4474–82.
Morris SL, Thomas BR, Palanicawandar R, Whittaker S, Child F, Wain M, et al. Long term outcomes of nonmyeloablative allogeneic stem cell transplantation with TSEB TLI and ATG for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2024;59:874–9.
Weng W-K, Armstrong R, Arai S, Desmarais C, Hoppe R, Kim YH. Minimal residual disease monitoring with high-throughput sequencing of T cell receptors in cutaneous T cell lymphoma. Sci Transl Med. 2013;5:214ra171.
André R, Ram-Wolff C, Battistella M, Peffault de Latour R, Petit A, Bouaziz JD, et al. Effectiveness of brentuximab vedotin before and after allogeneic stem-cell transplantation in the management of transformed mycosis fungoides. Br J Dermatol. 2020;182:1503–4.
Dumont M, Ram-Wolff C, Roelens M, Brice P, Peffault de Latour R, Battistella M, et al. Efficacy and safety of brentuximab vedotin plus bendamustine in advanced-stage primary cutaneous T-cell lymphomas. Br J Dermatol. 2019;181:1315–7.
Dangien A, Ram-Wolff C, Brice P, Battistella M, Roelens M, Moins-Teisserenc H, et al. Ifosfamide and etoposide in advanced-stage, relapsed or refractory primary cutaneous T-cell lymphomas. Br J Dermatol. 2020;183:961–2.
Duvic M, Donato M, Dabaja B, Richmond H, Singh L, Wei W, et al. Total skin electron beam and non-myeloablative allogeneic hematopoietic stem-cell transplantation in advanced mycosis fungoides and Sezary syndrome. J Clin Oncol. 2010;28:2365–72.
Elsayad K, Guenova E, Fournier B, Fernandes C, Clementel E, Papadavid E, et al. Real-world pattern-of-care analysis of primary cutaneous lymphomas radiation therapy among European organisation for research and treatment of cancer members. Int J Radiat Oncol Biol Phys. 2024;S0360-3016:03581–8.
Baron MK, Osborn JD, Tao R, Lee CJ. Haploidentical hematopoietic cell transplantation for mycosis fungoides/Sezary syndrome. Leuk Lymphoma. 2020;61:231–3.
Goyal A, O’Leary D, Foss F. Allogeneic stem cell transplant for treatment of mycosis fungoides and Sezary syndrome: a systematic review and meta-analysis. Bone Marrow Transplant. 2024;59:41–51.
Onida F, Cavallaro F, Alberti-Violetti S, Goldaniga M, Barbullushi K, Serpenti F, et al. Allogeneic hematopoietic cell transplantation in patients with advanced mycosis fungoides and Sézary syndrome: a retrospective analysis including 63 consecutive patients with long-term median follow-up. Bone Marrow Transplant 2024;59:20–139; 103-O112.
Hamadani M, Ngoya M, Sureda A, Bashir Q, Litovich CA, Finel H, et al. Outcome of allogeneic transplantation for mature T-cell lymphomas: impact of donor source and disease characteristics. Blood Adv. 2022;6:920–30.
Mussetti A, Kanate AS, Wang T, He M, Hamadani M, Finel SR, et al. Haploidentical versus matched unrelated donor transplants using post-transplantation cyclophosphamide for lymphomas. Transplant Cell Ther. 2023;29:184.e1–e9.
Ayuk F, Beelen DW, Bornhäuser M, Stelljes M, Zabelina T, Finke J, et al. Relative impact of HLA matching and non-HLA donor characteristics on outcomes of allogeneic stem cell transplantation for acute myeloid leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2018;24:2558–67.
Yakoub-Agha I, Mesnil F, Kuentz M, Boiron JM, Ifrah N, Milpied N, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24:5695–702.
Fatobene G, Rocha V, St Martin A, Hamadani M, Robinson S, Bashey A, et al. Nonmyeloablative alternative donor transplantation for Hodgkin and non-Hodgkin lymphoma: from the LWP-EBMT, Eurocord, and CIBMTR. J Clin Oncol. 2020;38:1518–26.
Stamouli M, Gkirkas K, Karagiannidi A, Iliakis T, Chondropoulos S, Thomopoulos T, et al. Allogeneic stem cell transplantation with a novel reduced intensity conditioning regimen for the treatment of patients with primary cutaneous T-cell lymphomas. Clin Hematol Int. 2021;3:72–6.
Luft T, Gras L, Koster L, Kröger N, Schröder T, Platzbecker U, et al. Methotrexate versus mycophenolate mofetil prophylaxis in allogeneic hematopoietic cell transplantation for chronic myeloid malignancies: a retrospective analysis on Behalf of the Chronic Malignancies Working Party of the EBMT. Am J Hematol. 2025;100:38–51.
Gu Z, Li F, Li M, Zhang L, Xu S, Wang L, et al. Similar survival but less chronic GVHD in antithymocyte globulin-based myeloablative haploidentical transplant compared with matched sibling transplant for adult T-cell acute lymphoblastic leukemia/lymphoma. Cell Transplant. 2024;33:9636897241270401.
Gordon ER, Trager MH, Kwinta BD, Stonesifer CJ, Lee KJ, Adeuyan O, et al. Maintenance therapy for CTCL: importance for prevention of disease progression. Leuk Lymphoma. 2024;65:1883–90.
Yoshimitsu M, Utsunomiya A, Fuji S, Fujiwara H, Fukuda T, Ogawa H, et al. A retrospective analysis of haplo-identical HLA-mismatch hematopoietic transplantation without posttransplantation cyclophosphamide for GVHD prophylaxis in patients with adult T-cell leukemia-lymphoma. Bone Marrow Transplant. 2019;54:1266–74.
Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7:e157–67.
Pileri A, Fava P, Fuligni F, Gunnella S, Guglielmo A, Astrua C, et al. Bexarotene as maintenance treatment after therapies other than skin-directed therapy in advanced-stage mycosis fungoides: a pilot study. J Eur Acad Dermatol Venereol. 2019;33:e367–9.
Kudelka MR, Switchenko JM, Lechowicz MJ, Esiashvili N, Flowers CR, Khan MK, et al. Maintenance therapy for cutaneous T-cell lymphoma after total skin electron irradiation: evidence for improved overall survival with ultraviolet therapy. Clin Lymphoma Myeloma Leuk. 2020;20:757–67.e3.
Iyer SP, Sica RA, Ho PJ, Prica A, Zain J, Foss FM, et al. Safety and activity of CTX130, a CD70-targeted allogeneic CRISPR-Cas9-engineered CAR T-cell therapy, in patients with relapsed or refractory T-cell malignancies (COBALT-LYM): a single-arm, open-label, phase 1, dose-escalation study. Lancet Oncol. 2024;S1470-2045:00508–4.
Author information
Authors and Affiliations
Contributions
GD, PD and NS conceived and designed the manuscript topics to be covered. GD and NS were the leaders of the workshop. IYA, FO, ALR and ISO proposed the methodology. GD, AdM, PB, AB, SF, RD, CPF, EG, OH, CK, OT, WJ, EG, POR, EP, JS, IEK, SNQ, YS, GW, AS, EP, PLOR, ALR, ISO, JS, IYA, FO and NS undertook literature research, interpretation of data and formulated the recommendations. All co-authors played an important role in interpreting results, revised the manuscript, approved the final version, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Competing interests
GD: Honoraria: Takeda, Amgen. Travel support: Takeda. Research Support: Takeda, Ideogen. ADM: Honoraria: Takeda, Kyowa Kirin, Helsinn, Recordati Rare Diseases, Almirall, Novartis. Travel support: Kyowa Kirin, Recordati Rare Diseases, Novartis. Research Support: Kyowa Kirin. PD: none reported. AB: Honoraria: Takeda, Amgen, Janssen, Roche, Maat Pharma Research Support: Takeda, Roche, Novartis, Janssen. PB: none reported. SF: none reported. RFD: none reported. CPF: Consultancy: AbbVie, Arvinas, Atarabio, BMS, GenMab, Gilead/Kite, Incyte, Lilly, Morphosys, Ono, Roche, SERB, SOBI, Takeda. Research funding: BeiGene, AbbVie/GenMab, Incyte. EG: honoraria and/or grant support from Mallinckrodt, Helsinn, Takeda Pharmaceuticals, Recordati Rare Diseases, Novartis, Sanofi, Stemline Therapeutics, and Kyowa Kirin. OH: Research fund, BMS, Bluueprint, Abbvie, Roche. CK: Research support BMS, Travel support Takeda. AT: Honoraria: Genesis Pharma, SOBI, Johnson & Johnson, Abbvie. OT: Honoraria: Takeda, BeiGene. Travel support: Takeda, Abbvie, Astra-Zeneca, BeiGene. Research Support: Takeda. WJ: Honoraria: Takeda, AbbVie. IEK: is supported by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—493624047 (Clinician Scientist CareerS Muenster), Honoraria: Kite/Gilead, Travel grants: BeiGene. SNQ: Honoraria: Jazz Pharma, Novartis, Abbvie, Gilead; Travel support: Abbvie, Gilead, Sanofi, Neovii. YS: none reported. GW: Honoraria: Takeda, Gilead Scienes, Novartis, Clinigen; Travel support: Medac, Janssen. AS: Honoraria: Takeda, BMS/Celgene, MSD, Janssen, Amgen, Novartis, Gilead Kite, Sanofi, Roche, GenMab, Abbvie, Jazz Pharmaceuticals, Therakos, Menarini; Consultancy: Takeda, BMS/Celgene, Novartis, Janssen, Gilead, Sanofi, GenMab, Abbvie; Speaker’s Bureau: Takeda; Research Support: Takeda; Non-profit organizations: Presidency of the EBMT. EP: Takeda, Recordati, Helsinn, Kyowa Kirin, Novartis, UCB, Leo, Abbvie, Jansen. PLOR: Honoraria: Takeda, Kyowa Kirin, Helsinn, Recordati Rare Diseases. Travel support: UCB, Kyowa Kirin, Recordati Rare Diseases, Novartis. ALR: none reported. ISO: none reported. JS: Honoraria & Consultancy Helsinn, Recordati, Takeda, Therakos, Kyowa Kirin. IYA: Honoraria: Novartis, BMS, Kite/Gilead, Janssen and Pfizer. FO: Honoraria: Takeda, MEDAC Pharma, Kyowa Kirin; Travel support: Takeda, Jazz Pharma, Kyowa Kirin; NS: Travel support: Beigene, Roche, Stocks: BMS.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is co-published in the journals Bone Marrow Transplantation [https://doi.org/10.1038/s41409-025-02696-x] and the Journal of the European Academy of Dermatology and Venereology [https://doi.org/10.1111/jdv.70042]. Either citation can be used when citing this article. The articles are identical except for minor stylistic and spelling differences in keeping with each journal’s style.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Damaj, G., de Masson, A., Dreger, P. et al. Allogeneic hematopoietic cell transplant in cutaneous T-cell lymphomas: recommendations from the EBMT PH&G Committee. Bone Marrow Transplant 60, 1565–1573 (2025). https://doi.org/10.1038/s41409-025-02696-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02696-x