Abstract
Rheumatoid arthritis (RA) is a prevalent and debilitating inflammatory disease that significantly impairs functional capacity and quality of life. RA accelerates musculoskeletal aging, leading to complications such as muscle degeneration and sarcopenia. Recent research has identified myopenia as a condition of significant muscle loss associated with illness, distinct from the muscle wasting seen in other chronic diseases like cancer cachexia or heart failure. In RA, myopenia is characterized by muscle depletion without concurrent significant fat loss, and it can affect individuals of all ages. While inflammation plays a central role, it is not the sole factor contributing to the high incidence of muscle wasting in RA. In subsequent discussions, secondary sarcopenia will be considered alongside myopenia, as both involve muscle wasting decline primarily due to disease. This review summarizes recent findings on the impact of RA-related myopenia and secondary sarcopenia on functional capacity, explores its underlying mechanisms, and discusses contemporary strategies to mitigate the process of musculoskeletal aging in RA patients.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by erosive arthritis, accelerated musculoskeletal aging, and systemic organ involvement. It affects approximately 0.5% of adults globally.1 RA predominantly affects women between the ages of 25 and 45 but can occur in individuals of any age and gender, with the highest incidence typically observed in the sixth decade of life. RA manifestation prior to the age of 60 is classified as young-onset RA (YORA), while onset after 60 is termed elderly-onset RA (EORA).2 Current demographic trends reveal that the prevalence of EORA is significantly influenced by sex and ethnicity. In the United States, the prevalence ranges from 0.5% to 1%, increasing to 2% among those over 60 years of age.3 Conversely, in China, pooled prevalence estimates indicate that 0.37% of the population suffers from RA, with 25.7% classified as EORA.4 These trends underscore the importance of considering ethnic and geographic factors in managing of RA.
Within the RA context, muscle wasting, also known as myopenia, represents a critical area of concern. Recently introduced, myopenia describes substantial muscle loss due to illness at any age and is marked by reduced muscle function and increased rates of morbidity and mortality.5 Myopenia holds particular relevance in RA, where chronic inflammation and systemic involvement lead to significant muscle degradation. Although the exact mechanisms underlying myopenia in RA remain elusive, they are believed to involve a complex interplay of immunological and hormonal changes, coupled with cytokine activity, contributing to muscle wasting and premature muscular aging. Recent studies have highlighted additional factors such as Oxidative stress, intermuscular adipose tissue (IMAT) accumulation, and insulin resistance, all of which exacerbate muscle wasting and further complicate disease progression. Elevated levels of myostatin, a negative regulator of muscle growth, may contribute to muscle wasting or rheumatoid cachexia (RC).6,7 A deeper understanding of the pathophysiology of RA is essential for developing effective treatments that can mitigate musculoskeletal decline and enhance patient outcomes.
Current strategies for managing myopenia in RA focus on lifestyle enhancements and risk factor modification through adequate nutrition and regular physical activity.8 Furthermore, the. Early administration of disease-modifying antirheumatic drugs (DMARDs) is vital, as Treat-to-Target therapy has been shown to prevent radiological damage, reduce morbidity and mortality, and improve overall functional capacity.9 This review aims to highlight the clinical significance of myopenia in RA and explore various strategies to delay musculoskeletal aging. Key approaches include managing early inflammation through lifestyle modifications, pharmacological interventions and reducing Oxidative stress to aid muscle regeneration. By addressing these areas, this review seeks to provide valuable insights and propose comprehensive strategies for effectively managing muscle wasting in individuals with RA.
Musculoskeletal aging in healthy individuals
Muscle remodeling naturally occurs with age, with muscle mass declining by 1%–2% annually after age 50, and accelerating significantly after age 70, particularly in men, who experience greater absolute and relative muscle loss.10 This is accompanied by increased intramuscular fat, muscle atrophy (especially in type IIa fibers, which affect strength and explosive power), reduced motor unit numbers, and impaired satellite cell function.
Aging also decreases muscle capillarization, affecting metabolic health and cardiovascular fitness.11 Inflammaging, marked by increased pro-inflammatory markers, further accelerates muscle degradation by impairing the muscle’s adaptive response to exercise.12 This remodeled muscle architecture significantly leads to reductions in muscle strength and power commonly observed in elderly individuals.13,14 Frailty, which marks an individual’s transition to disability, is characterized by symptoms such as fatigue and weakness, closely associated with muscle loss.15
Research conducted by Mitchell and colleagues indicates that after the age of 70, muscle loss occurs at a rate of 0.5%–1.0% annually with peak muscle mass decreasing by 4.7% per decade in men and 3.7% in women.10 This phenomenon is closely linked to age-related sarcopenia, which is characterized by a progressive decline in skeletal muscle mass and function. Muscle strength decreases by 10%–15% per decade until age 70, after which it accelerates to 25%–40% per decade.15,16 This decline is driven by both muscle mass loss and neuromuscular aging, including motor neuron loss, motor unit reduction, and neuromuscular junction degeneration.17
Frailty, characterized by reduced grip strength and slower gait speed, increases vulnerability to stressors and is more pronounced in women, who start with lower lean body mass, making them more susceptible to sarcopenia and its consequences, such as falls, disability, and mortality.15
Chronic conditions like cancer, diabetes, COPD, and heart failure exacerbate muscle loss. For RA patients, chronic inflammation, limited physical activity due to joint pain, and medication side effects further accelerate muscle mass and strength decline, warranting further exploration into muscle wasting in this population.
Musculoskeletal aging in RA
Musculoskeletal aging in RA involves myopenia, sarcopenia, and RC. Myopenia is muscle loss due to illness at any age, while primary forms of sarcopenia are related to aging and secondary forms linked to diseases. RC differs by combining significant muscle loss with a minimal or mild increase in fat accumulation, maintaining stable body weight but increasing mortality. Factors like Oxidative stress are recognized as key contributors in accelerating muscle degradation alongside elements like ACPAs, hormonal shifts, and insulin resistance.
Myopenia & Sarcopenia
Myopenia refers to clinically significant muscle wasting caused by any illness, regardless of age. In contrast, sarcopenia specifically refers to muscle wasting leading to a significant reduction in muscle mass. Sarcopenia can be classified into two types: primary and secondary. Primary sarcopenia is linked to age-related decreases in muscle mass, strength, and physical performance, illustrating the natural aging process. Primary sarcopenia is mainly influenced by aging-related factors like motor unit loss, systemic inflammation, and hormonal changes. In contrast, secondary sarcopenia is associated with disease-related declines, such as those from cancer, diabetes, COPD, or heart failure, and involves more severe muscle deterioration due to the underlying disease and its treatments, like chemotherapy or extended bed rest, resulting in a significant decline in muscle function and overall health.16
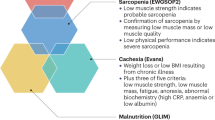
Myopenia and secondary sarcopenia exhibit a non-linear pattern of muscle deterioration due to disease, which is more severe than the linear decline associated with primary sarcopenia. Consequently, patients with RA experience significant reductions in muscle mass compared to their age- and sex-matched peers, making myopenia an indicator of early, disease-related muscular aging. This condition is particularly relevant to RA and other related diseases (Fig. 1).
Myopenia’s network: linking diseases and morbidity. This diagram illustrates the conditions contributing to musculoskeletal aging in RA, including myopenia, sarcopenia, and rheumatoid cachexia. These conditions, driven by systemic illnesses and inflammation, result in significant muscle loss and increased morbidity. Created in BioRender
RC and muscle wasting
RC is characterized by a substantial decrease in muscle mass and strength, often accompanied by a minimal or mild increase in fat accumulation.18 Unlike the forms of cachexia observed in conditions such as cancer and heart failure, RC typically does not lead to an overall decrease in body weight; the significant muscle mass loss is often offset by an increase in fat mass, resulting in a relatively stable overall body weight.19 Moreover, RC differs from primary sarcopenia as it tends to manifest at a younger age and involves a more severe loss of muscle mass. The severe loss of muscle mass heightens the risk of cardiovascular and contributes to higher mortality rates,20 significantly impairing patients’ quality of life. Importantly, RC also exhibits chronic inflammation, a trait it shares with muscle wasting observed in other chronic conditions.
Muscle wasting, in a broader context, refers to the reduction in muscle mass due to various factors. In earlier research, high-grade inflammation was recognized as a major catalyst for muscle wasting; however, recent findings suggest that inflammation alone does not fully account for the extent of muscle wasting observed in patients with RA. Although additional factors, such as physical activity and nutrition, are recognized to contribute, they also fail to fully explain the significant muscle loss observed. Consequently, researchers are now investigating other potential factors to better understand the mechanisms behind muscle wasting in RA patients.
One emerging factor that may help explain this discrepancy is Oxidative stress. Initially, Oxidative stress was recognized as a key contributor to muscle wasting in the general population, but its role in RA-related muscle wasting was less clear. Recent studies have confirmed that Oxidative stress plays a crucial role in RA-related muscle wasting, exacerbating disease progression by inducing muscle degradation. The mechanisms of how Oxidative stress induces muscle wasting will be elucidated in the following chapters. Additionally, other contributing factors, such as ACPAs, immunological and hormonal shifts, interleukin secretion, myostatin, intermuscular adipose tissue (IMAT), and insulin resistance, and their respective roles in muscle wasting, will also be discussed.
Genetics of RA and muscular aging
The genetic basis of RA plays a key role in disease onset and progression, as well as its impact on muscle loss. Variations in the HLA-DRB1 locus, such as specific alleles tied to the shared epitope (SE), are linked to increased RA risk. Additionally, other gene variants like those in ACTN3 and MSTN may also influence muscle loss in RA through complex polygenic interactions. While no single mutation has been directly linked to muscle wasting, the combined effect of multiple genes likely contributes to the variability in muscle degradation among RA patients. Further research is needed to better understand these genetic influences and their therapeutic implications.
Genetic factors of RA
The development of RA is heavily influenced by genetic factors, particularly the DRB1 locus within the HLA class II gene complex. Distinct amino acid sequences at positions 70 and 40 in certain DRB1 alleles are linked to RA, corroborating the ‘shared epitope’ hypothesis as a known RA risk factor.21 Research has shown that the presence of the DRB1 allele correlates with early disease onset, the occurrence of extra-articular symptoms, and increased radiographic damage.22 However, the genetic predisposition in EORA remains a subject of conflicting and inconsistent findings.23
Notable differences exist in the RA-associated DRB1 alleles between YORA and EORA, as well as among various ethnic groups. For instance, Gonzalez-Gay et al. conducted research within a Spanish cohort and identified associations between YORA and the DRB1/04 allele, and between EORA and the DRB1/01 allele.24 Their findings revealed that the shared epitope (SE, an amino acid sequence commonly found in certain alleles of the HLA-DRB1 gene), particularly associated with DRB1/04, was significantly more frequent in YORA patients, with a frequency of 77.8% compared to 37.1% in EORA. This genetic distinction suggests that DRB1/04 plays a stronger role in disease susceptibility and severity among younger RA patients. Furthermore, the study highlighted that EORA patients who are seronegative for rheumatoid factor (RF) exhibited a higher frequency of DRB1-13/14 alleles, indicating a potential shared genetic basis with polymyalgia rheumatica (PMR), which was also associated with these alleles. In a parallel study, Kim et al. explored the impact of the HLA-DRB1 and HLA-DQB1 genes on disease susceptibility and severity in both EORA and YORA patients.25 Their findings indicated a lower frequency of alleles encoding the common epitope in EORA compared to YORA, suggesting a diminished role in susceptibility to EORA. Conversely, Hellier et al. reported that HLA-DRB1/04-related alleles are unlikely to be significantly associated with EORA susceptibility,26 whereas Wu et al. documented a higher prevalence of the DRB1/04 allele in EORA patients.27 The observed discrepancies may be attributable to variations in the ethnic and racial composition of the study cohorts, potentially affecting the distribution of HLA alleles, necessitating further research to elucidate its role in disease onset and progression across diverse populations.
Gene variants and polygenic perspective
Numerous gene variants have been proposed as potential contributors to RA, yet evidence establishing strong associations between specific variants and premature muscular aging remains scarce. For instance, although the ACTN3 R577X polymorphism influences human muscle phenotypes, debates persist regarding the ‘favorable’ role of the R or X alleles in aging processes. Moreover, the MSTN K153R variation is recognized for its potential to explain differences in muscle phenotypes among older individuals, but further research with a significantly larger sample size is essential, given the rarity of the ‘unfavorable’ 153 R allele. Despite the current gaps in understanding, ongoing research continues to explore gene variants that may be linked to RA and other autoimmune disorders.
A study by Zhang et al. found associations between the ARNT rs11204735, AHRR rs2292596, and rs2672725 polymorphisms with increased susceptibility to RA and altered AHR methylation levels.28 Furthermore, research by Huang et al. has identified crucial genes and pathways potentially related to RA, including STAT4, PTPN22, and TRAF1/C5, which play significant roles in immune regulation and inflammation.29 These findings highlight the complex and interconnected nature of RA-related genetic variants, supporting the notion that RA likely results from multiple polygenic traits rather than a single polymorphism.30 Wen and Yu discovered significant genetic correlations by identifying shared loci between RA and other autoimmune diseases, including multiple sclerosis, type 1 diabetes, and inflammatory bowel disease, indicating a common genetic basis for these conditions.31
Potential genetic associations between RA and muscle wasting
Existing evidence does not definitively identify a genotype linked to accelerated age-related muscle wasting, recent studies improve our ability to predict such outcomes.30
Chronic inflammation, the central pathogenic factor in RA, highlights the importance of understanding IL-6 expression differences at the genetic level. IL-6 plays a crucial role in RA pathogenesis by mediating immune responses, driving chronic inflammation, and promoting bone destruction. Genetic variations, such as single nucleotide polymorphisms (SNPs) in the IL-6 gene promoter region (e.g., -174G/C and -572G/C), are associated with increased RA susceptibility and disease activity.32 In RA patients, IL-6 expression is not only elevated systemically but also markedly upregulated in synovial tissues compared to peripheral blood. This localized overexpression contributes to synovial hyperplasia (synovitis) and angiogenesis, which intensify joint damage and have systemic repercussions.33 The following section will explore in detail how IL-6 influences muscle wasting mechanisms in RA patient.
Pathogenesis of musculoskeletal aging in RA
The pathogenesis of musculoskeletal aging in RA involves six key mechanisms, among which oxidative stress—caused by excessive reactive oxygen species (ROS)—drives protein degradation and cellular damage.Anti-citrullinated protein antibodies (ACPAs) amplify inflammation, leading to muscle and bone deterioration. Immunological and hormonal alterations in RA patients, particularly immune dysregulation, further exacerbate muscle breakdown. Interleukin-6 (IL-6) activates the JAK-STAT pathway, promoting muscle protein degradation and impairing regeneration. Myostatin inhibits muscle growth by triggering proteolytic pathways, causing atrophy. Lastly, intermuscular adipose tissue (IMAT) accumulation and insulin resistance disrupt muscle metabolism, decrease protein synthesis, and accelerate muscle wasting.
Oxidative stress
When the production of free radicals, such as reactive oxygen species (ROS), exceeds the capacity of the antioxidant defense system to neutralize them, Oxidative stress occurs. Free radicals are highly reactive molecules that can attack lipids, proteins, and DNA, leading to cellular damage. Oxidative stress has been shown to play a role in muscle loss in normal aging and metabolic processes. In pathological states, such as RA, this effect is amplified, contributing to more pronounced muscle atrophy.
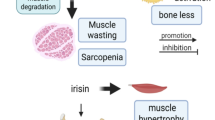
In RA, chronic inflammation is a key pathological feature. In RA patients, he immune system is overactive, leading to the production of numerous inflammatory cytokines like TNF-α, IL-1, and IL-6, which subsequently promote ROS generation (Fig. 2). RA patients exhibit higher ROS levels compared to healthy individuals, alongside a diminished antioxidant capacity. Research indicates that mitochondrial ROS production is elevated in the whole blood and mononuclear cells of RA patients compared to healthy individuals, highlighting Oxidative stress as a key factor in RA pathology.34
The mechanisms of ROS-induced muscle atrophy are complex. ROS disrupts redox homeostasis, upregulating apoptotic pathways such as the NF-κB and FoxO signaling cascades, which lead to significant protein degradation35 (Fig. 2). This oxidative imbalance may also activate proteolytic enzymes, including calpains and caspase-3, thereby further enhancing protein hydrolysis. Moreover, the oxidation of muscle proteins renders them more susceptible to proteolytic damage. In addition to promoting protein degradation, ROS accumulation can inhibit critical signaling pathways that control protein synthesis. Specifically, ROS has been shown to impair mRNA translation at early stages, reducing the ability of muscle satellite cells to activate and infiltrate muscle fibers, ultimately leading to impaired muscle regeneration (Fig. 2). These combined mechanisms contribute to the progressive muscle wasting observed in conditions such as RA.36
SIRT1 and HSP play crucial roles in counteracting muscle wasting caused by Oxidative stress. They work through different mechanisms to reduce inflammation and oxidative damage, thereby protecting muscle tissues. Firstly, SIRT1, a NAD + -dependent deacetylase, is essential in mitigating Oxidative stress. SIRT1 activates the cell’s antioxidant defense system by deacetylating antioxidant enzymes like FOXO1 and FOXO3, which helps reduce the harmful effects of ROS on muscle and joint tissues. Additionally, SIRT1 inhibits pro-inflammatory pathways such as NF-κB, lowering the production of pro-inflammatory cytokines like TNF-α and IL-1β, thus decreasing inflammation-induced muscle degradation.37 Secondly, heat shock proteins (HSP), such as HSP-70, have been identified as inhibitors of inflammatory cytokine production, serving as protective factor against Oxidative stress to muscle cells.38 In contrast, aberrant glycosylation patterns of IgG antibodies are believed to enhance pro-inflammatory activity, thus exacerbating muscle degradation.39 A deeper understanding of these mechanisms is crucial for comprehending the full scope of muscle wasting in RA.
Anti-citrullinated protein antibodies (ACPAs)
Two primary subtypes of RA are distinguishable based on the presence or absence of anti-citrullinated protein antibodies (ACPAs). ACPAs are autoantibodies that target peptides and proteins undergoing citrullination, a post-translational modification that occurs under inflammatory conditions. These autoantibodies are highly specific for RA and are detectable years before the clinical onset of the disease, making them a valuable tool for early diagnosis. Approximately 67% of RA patients are ACPA-positive, establishing ACPAs as a crucial diagnostic marker for early, undifferentiated arthritis.40,41 The enzyme peptidyl arginine deiminase (PAD) catalyzes citrullination, a calcium-dependent process that converts positively charged arginine into neutral, polar citrulline. The ACPA-positive subtype generally correlates with a more severe clinical course than the ACPA-negative form.42,43 This severity is marked by greater bone destruction, driven by increased osteoclast activity. ACPA-positive patients show enhanced osteoclast differentiation and activation, leading to more bone erosion and joint damage, even without obvious inflammation. Although ACPA-negative patients have a milder clinical course, standard therapies such as methotrexate (MTX) and rituximab tend to be less effective in ACPA-negative patients,44,45,46 underscoring the need for further research into the distinct pathophysiological mechanisms of these subtypes. This review will focus particularly on ACPA-positive RA, examining its onset and progression in the contexts of EORA and YORA.
Immunological and hormonal shifts
Current insights into the clinical and laboratory differences between EORA and YORA remain incomplete. However, prevailing theories suggest that the pathogenesis of EORA is influenced by immunological and hormonal shifts. As individuals age, there is a notable decrease in protective immune responses alongside an increase in reactivity to autoantigens (Fig. 2). This decline stems from changes in T-cell phenotypes, impaired apoptosis, inadequate antigen presentation, and cytokine imbalances. Age-related thymic involution exacerbates these issues by altering T-cell composition and reducing T-cell proliferation, cytokine production, and antibody synthesis post-vaccination. The decline in protective immune responses, particularly in the ability to clear pathogens and maintain immune tolerance, leads to a loss of self-tolerance. This allows autoreactive T cells to persist and target self-antigens, promoting autoimmune processes such as those seen in EORA. The weakened immune surveillance results in an increased reactivity to autoantigens, which is a hallmark of EORA compared to YORA, contributing to the more aggressive disease progression in elderly patients. Further impairments in age-related self-tolerance mechanisms also weaken the immune response in elderly patients with RA.
Interleukin secretion and myopenia
A study by Straub et al. suggests that serum levels of DHEA (Dehydroepiandrosterone, one of the key hormones secreted by the adrenal glands, belonging to the third major category of adrenal steroids) are negatively correlated with IL-6. In healthy individuals, higher levels of DHEA are associated with relatively lower levels of IL-6. As age increases, DHEA levels gradually decline while IL-6 levels rise, potentially contributing to increased inflammatory responses. The study also indicates that serum DHEA levels in RA patients are significantly lower than in healthy individuals. This reduction in DHEA levels in RA patients may be linked to certain immunoregulatory dysfunctions associated with chronic inflammatory conditions.47 It is hypothesized that increased IL-6 levels contribute to the rapid onset and pronounced acute phase response characteristic of EORA. Corroborating this, Punzi et al. found significantly elevated IL-6 levels and acute phase responses in the synovial fluid of EORA patients compared to those with YORA, highlighting the critical role of IL-6.33 Interestingly, no statistical differences were observed in the levels of IL-1 and IL-8 between EORA and YORA patients. Moreover, Bennett et al. further elaborated that IL-6 plays a pivotal role in the pathophysiology of muscle wasting, particularly in conditions like RA-associated sarcopenia.48 Chronic elevation of IL-6, along with other pro-inflammatory cytokines, accelerates muscle protein breakdown by upregulating the ubiquitin-proteasome system (UPS), which leads to muscle atrophy48,49 (Fig. 2). Additionally, IL-6 disrupts the self-renewal capacity of muscle stem cells (MuSCs), hindering muscle regeneration (Fig. 2). This process is mediated through the JAK-STAT pathway, where chronic activation of STAT3 promotes catabolic processes and muscle loss (Fig. 2). IL-6-induced mitochondrial dysfunction also contributes to reduced muscle contractility and increased Oxidative stress, exacerbating muscle fatigue and weakness commonly seen in rheumatoid sarcopenia.
Furthermore, Gamerith et al. noted a substantially higher anti-IgG-Fab/free aFab ratio in YORA patients than in EORA patients. This elevated ratio suggests a greater presence of rheumatoid factor (RF), indicating divergent immunoregulatory mechanisms across different age groups.50 Despite this, the differences in RF between EORA and YORA imply that RF may not play a significant role in the development of myopenia in either group. Instead, these findings further support the hypothesis that elevated IL-6 level is the primary driver of muscle wasting in both EORA and YORA, potentially leading to premature muscle aging. Conversely, the acute onset in EORA could lead to sustained muscle damage, thus mimicking premature muscle aging. Comprehensive research is essential to unravel the complex mechanisms contributing to premature muscular aging in RA.
Myostatin regulation and myopenia
Myostatin, scientifically known as Growth Differentiation Factor-8 (GDF-8), is a member of the TGF-β superfamily, specifically belonging to the Growth Differentiation Factor (GDF) subfamily. Furthermore, as a pivotal regulator of muscle growth, myostatin is primarily produced in skeletal muscle and is also present in cardiac muscle and adipose tissue.51 Its mechanism of action involves interactions with type II activin receptors, initiating signaling cascades that impede muscle growth and differentiation. Myostatin frequently engages with the Smad-mediated signaling pathway, potentially inducing the expression of atrophy-related ubiquitin ligases such as Atrogin1 and MURF1. Moreover, myostatin inhibits the transcription of genes that promote myogenesis, further restraining muscle growth.51 A study by Gonzalez-Ponce et al. revealed that RA patients exhibited significantly higher serum myostatin levels compared to controls, suggesting that elevated myostatin levels (≥ 17 ng/mL) are effective markers for identifying RA complications, myopenia and reduced skeletal muscle mass.6 Additionally, Lin et al. discovered that high myostatin levels correlated with increased rates of radiographic progression in RA patients. Collectively, RA patients with myopenia and elevated myostatin levels exhibited the most substantial radiographic progression, the lowest likelihood of remission, and the poorest long-term outcomes52 (Fig. 2). Itoh et al. concluded that Smad3-STAT3 crosstalk plays a significant role in various pathophysiological contexts, such as tumorigenesis, fibrosis, and T cell differentiation.53 However, there is currently no relevant research investigating this crosstalk in RC. Given that both STAT3 and Smad3 are downstream mediators in the mechanisms through which IL-6 and myostatin contribute to muscle atrophy, exploring this crosstalk could hold potential research value. Understanding how these two pathways interact might provide new insights into the progression of RC and possibly open avenues for therapeutic interventions.
Intermuscular adipose tissue (IMAT) and insulin resistance
The accumulation of intermuscular adipose tissue (IMAT) is a common feature in RA patients and is associated with increased fat mass and decreased muscle mass. IMAT refers to metabolically active fat located within and between skeletal muscle fibers, distinct from subcutaneous and visceral fat. Its accumulation disrupts muscle metabolism by promoting local inflammation and metabolic dysregulation. Unlike subcutaneous and visceral fat, IMAT is embedded within muscle structures and has a direct impact on muscle metabolism and function. IMAT accumulation is accompanied by abnormal release of free fatty acids that enter muscle cells and interfere with insulin signaling by reducing IRS-1(Insulin Receptor Substrate 1) phosphorylation and inhibiting the Akt pathway. The inhibition of IRS-1 phosphorylation disrupts the activation of the Akt pathway, a central component in glucose uptake and metabolism. This impairs the regulation of glucose and amino acid uptake, further reducing muscle protein synthesis and accelerating muscle atrophy.54
IMAT also contributes to chronic muscle inflammation by releasing local inflammatory mediators such as MCP-1. This ectopic fat deposition worsens energy and protein metabolism in muscle, contributing to the progression of muscle loss in RA patients54 (Fig. 2).
Unique musculoskeletal aging mechanisms in RA versus other chronic diseases
In chronic diseases, muscle atrophy is a prevalent pathological feature driven by mechanisms such as chronic inflammation, metabolic dysregulation, and impaired signaling pathways. For instance, in diabetes, chronic low-grade systemic inflammation and insulin resistance play central roles, where pro-inflammatory cytokines (e.g., TNF-α, IL-6) suppress muscle protein synthesis and enhance proteolysis. Hyperglycemia and the accumulation of advanced glycation end products (AGEs) further exacerbate oxidative stress and damage to muscle cells.55 Similarly, in cancer, muscle atrophy is closely associated with cancer cachexia, a multifactorial syndrome characterized by rapid loss of lean body mass. Pro-inflammatory cytokines within the tumor microenvironment (e.g., IL-6, TNF-α) activate NF-κB and STAT3 signaling pathways, resulting in increased protein degradation, metabolic reprogramming, and mitochondrial dysfunction.56,57 These processes underscore the shared yet distinct inflammatory and metabolic drivers of muscle loss in chronic diseases.
In contrast, the mechanisms underlying muscle atrophy in RA are distinct due to the disease’s autoimmune nature and the dual impact of localized joint inflammation and systemic inflammatory effects. RA is characterized by aberrant activation of the immune system, leading to chronic synovitis and systemic inflammation. Elevated levels of inflammatory cytokines such as TNF-α, IL-1, and IL-6 not only mediate joint destruction but also directly contribute to skeletal muscle atrophy through systemic circulation.58 These cytokines activate the ubiquitin-proteasome system (UPS) and autophagy-lysosome pathways, promoting proteolysis while concurrently inhibiting anabolic pathways critical for muscle protein synthesis.48,49 Furthermore, RA-associated joint pain and functional limitations reduce physical activity and mechanical loading on muscles, exacerbating disuse-induced muscle atrophy. Unlike the metabolic dysregulation driving muscle loss in diabetes or the cachexia-induced systemic catabolism in cancer, muscle atrophy in RA is uniquely characterized by the interplay of local joint inflammation and systemic immune-mediated effects. This multifactorial mechanism highlights the distinct pathological complexity of RA-related muscle loss.
Clinical features of myopenia in RA
Myopenia encompasses a spectrum of clinical features, presenting distinct characteristics between EORA and YORA patients.
Three distinct clinical patterns of EORA
EORA can be categorized into three clinical profiles.59 The most prevalent profile, observed in 70% of cases, is characterized by joint erosions, RF positivity, and a significantly poorer prognosis compared to YORA patients. The second profile, evident in 25% of cases, resembles PMR and is marked by an abrupt onset, inflammation in the proximal limb joints, absence of RF, and generally a better prognosis than that observed in YORA patients. Notably, approximately 25% of PMR cases display non-erosive polyarthritis, frequently included in the differential diagnosis.60 The third profile is indicative of seronegative EORA, characterized by arthritis affecting the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints, along with involvement of proximal limb joints. This profile mirrors remitting seronegative symmetrical synovitis with pitting edema (RS3PE) syndrome. It is distinguished by an abrupt onset, spontaneous remission within 3 to 18 months, hand pitting edema, HLA-B27 positivity, and wrist tenosynovitis.61
EORA vs YORA
EORA extends beyond PMR, encompassing conditions such as sarcoidosis, crystal arthritis, and hepatitis C.62 EORA usually presents acutely with symptoms similar to PMR but has fewer rheumatoid nodules and a lower rate of RF positivity than YORA. Patients with EORA typically have lower joint scores but show more significant functional impairment, as reflected by elevated Health Assessment Questionnaire disability index (HAQ-DI) scores.
Clinical research63,64 has recorded the involvement of both large and small joints at the initial diagnosis of EORA. The prevalence of RF and ACPAs in EORA patients is comparable to or slightly lower than that observed in YORA patients. A study focusing on Turkish patients noted a predominant involvement of the shoulder joint in EORA, whereas joints such as the PIP joints, elbows, MCP joints, and ankles were more frequently affected in YORA.65
EORA is associated with a lower incidence of RA deformities, less pulmonary involvement, and fewer cases of secondary Sjögren’s syndrome (SjS). Conversely, symptoms such as lymphadenopathy, muscle pain, weight loss, and PMR-like features are more prevalent in EORA. The detection rates of serological markers, including RF, anti-Ro, antinuclear antibody, and anti-La, are lower in EORA patients. However, van der Heijde et al. identified an increased risk of anemia, elevated erythrocyte sedimentation rate (ESR), and higher C-reactive protein (CRP) levels in this group.66
Chen et al. conducted a comparative study revealing that EORA patients showed increased IL-6 levels and reduced TNFα levels.67 Multivariate analyses indicated that higher IL-1 levels were associated with ACPAs, while increased TNFα levels correlated with constitutional symptoms in EORA patients. Additionally, EORA patients typically presented with an acute onset, constitutional symptoms, and multiple comorbidities, distinguishing them from YORA patients.
Lance et al. identified a particularly severe and erosive form of EORA, characterized by PMR-like symptoms, acute onset, involvement of both small and large joints, and joint space narrowing.68 This aggressive phenotype features rapid disease progression, polyarticular involvement of small joints, joint erosions in the hands and wrists, and early loss of hand function. Remarkably, secondary SjS was noted in 63% of these EORA patients, compared to 25% in those diagnosed with YORA. (Fig. 3)
Although there are no specific studies currently exploring the differences in myopenia between EORA and YORA, we can still infer some distinctions based on the characteristics of the disease and the differences in the affected populations. EORA patients typically have lower baseline muscle mass and strength due to age-related muscle wasting, which is further exacerbated by RA-associated inflammation, leading to accelerated muscle protein degradation. Additionally, EORA patients are more affected by neuromuscular aging, which significantly contribute to muscle weakness. Reduced physical activity, driven by age-related decline, comorbidities, and joint pain, often resulting in faster progression of myopenia. In contrast, YORA patients typically begin with higher muscle mass and are less affected by neuromuscular aging during the early stages of the disease. However, prolonged chronic inflammation leads to gradual muscle loss, with functional limitations and activity reductions becoming more pronounced over time as the disease progresses. Recognizing these differences is essential for developing strategies.
Myopenia in Chinese RA patients
Previous discussions have highlighted the growing concern of myopenia and muscle wasting in RA patients globally. In the context of RA in China, myopenia has emerged as a significant concern, correlated with physical impairments and joint deterioration.69 It is also characterized as a condition linked to body composition, prevalent among elderly Chinese individuals with RA.4 Mediation analysis indicated that approximately half of EORA patients exhibit myopenia, which exacerbates physical dysfunction, highlighting the importance of recognizing cultural and gender-specific nuances within the RA context.4
Laboratory findings of myopenia in RA
Research comparing EORA with YORA consistently demonstrates that EORA is characterized by elevated levels of CRP, decreased hemoglobin levels, and an increased ESR.70 However, findings regarding the role of ACPAs and RF in distinguishing EORA from YORA are inconclusive. While some studies report a lower frequency of these antibodies in EORA, others find similar levels in both groups.64,66,71 Serhal et al. observed significantly higher levels of RF and ACPAs in YORA patients compared to those with EORA.72 Additionally, Pan et al. reported a positive association between the disease activity score (DAS28-CRP) and myopenia, with an adjusted odds ratio (AOR) of 1.56, and found myopenia to be independently associated with physical dysfunction, exhibiting an AOR of 2.98 in patients with early RA.73 These findings underscore the importance of early and aggressive management of disease activity to prevent myopenia and subsequent physical dysfunction in patients with early RA.
Measurement and assessment of Myopenia and muscle wasting in RA
Longitudinal observational studies show that the loss of appendicular skeletal muscle mass due to aging is gradual, with men experiencing approximately a 5% decline after reaching peak muscle mass and women experiencing a slightly lower decline.74,75 Muscle loss is often accompanied by an increase in fat mass, leading to a condition known as sarcopenic obesity, which is characterized by the coexistence of sarcopenia and obesity, and results in a detrimental body composition76 that adversely affects cardio-metabolic health, deteriorates muscle quality, and increases the risk of obstructive sleep apnea, disability, and mortality.76,77 Lin et al. found that myopenia overlapping with excessive fat presented the worst radiographic scores and highest rates of previous glucocorticoid treatment and hypertension in RA patients with a normal BMI.78
The role of BMI in assessing myopenia in RA patients remains ambiguous.5 Due to the distinct pathophysiological mechanisms involved, RA-associated myopenia differs from primary sarcopenia in terms of body composition. Unlike primary sarcopenia, which is typically characterized by a proportional decrease in both muscle mass and overall body weight, RA patients may present with a normal or even reduced BMI, yet they still experience significant muscle wasting alongside increased fat mass, a condition often termed RC, highlighting the limitations of using BMI as a sole indicator for muscle mass evaluation in RA.79 Furthermore, the combined effects of muscle mass reduction, increased fat accumulation and RA-induced joint damage significantly impair patients’ mobility, further exacerbating the progression of myopenia and muscle wasting in RA.
Given these differences, traditional measures like BMI may not accurately capture the extent of body composition changes in RA-associated myopenia. Instead, more sophisticated methods such as bioelectrical impedance analysis (BIA) or dual-energy X-ray absorptiometry (DXA) are recommended to assess muscle mass and fat distribution.80 BIA, while portable and cost-effective, is susceptible to inaccuracies caused by hydration status and posture, making it more suitable for outpatient and community settings.81 In contrast, DXA is considered the gold standard due to its high precision and capability to concurrently evaluate bone density and fat distribution. However, it is limited by factors such as interstitial fluid accumulation during acute inflammation, strict patient positioning requirements, and radiation exposure.82 Understanding these limitations is crucial to ensure appropriate application and interpretation of these tools in clinical settings.
Prognosis of Myopenia in RA
Currently, the direct link between myopenia and the prognosis of RA remains elusive. Lin et al. identified baseline myopenia as an independent risk factor for one-year radiographic progression in RA patients, with an AOR of 2.5.83 Research regarding the onset age of RA presents varied outcomes; some studies suggest that EORA may have a more favorable prognosis compared to YORA, while others report no significant differences or even poorer outcomes. These discrepancies may arise from variations in disease duration, patient selection biases, and differences in seropositivity rates between younger and older patients.
A prospective study by Pease et al. noted that persistent arthritis was observed in 39% of seropositive patients, compared to only 6% of seronegative patients.84 Further supporting these findings, Calvo-Alén et al. reported a higher prevalence of swollen joints, greater radiological damage, and increased mortality risks in seropositive patients.85 This suggests that RF and ACPAs may not serve as reliable prognostic markers for EORA. Krams et al. found that EORA patients in the ESPOIR cohort exhibited higher erosion rates and HAQ scores, along with lower one-year remission rates compared to YORA patients.86 However, by the third year, YORA patients showed significantly less radiographic progression, lower HAQ scores, and improved remission rates relative to those with EORA. In terms of mortality associated with myopenia in EORA, seropositive patients exhibited significantly higher mortality rates, whereas this trend was not observed in seronegative patients.70
Population-based factors such as race and gender should be carefully considered when we discuss the prognosis of myopenia in RA. In a clinical study conducted by Baker et al., muscle wasting in RA patients of different races and genders was analyzed.80 Their findings revealed that African Americans are at a higher risk of muscle loss, likely due to genetic factors and healthcare disparities. Additionally, males with RA tend to experience more significant muscle wasting and functional decline compared to females. Muscle wasting, or myopenia, weakens muscle strength, impairing patients’ physical capacity. As body weight decreases, the mechanical load on bones is also reduced. This leads to a decline in trabecular BMD, cortical bone thickness, and Appendicular Lean Mass Index (ALMI), indicating a deterioration of bone structure, also indirectly indicateing the potential risk of osteoporosis in RA patients.
Exercise and RA
Studies indicate that individuals with RA exhibit reduced and impaired exercise capacity compared to healthy counterparts. This limitation is attributable to RA-related symptoms such as pain, joint damage, decreased bone density, stiffness, and muscle weakness.87,88,89 Nonetheless, regular physical activity offers numerous benefits for RA patients, including reduced inflammation and enhanced joint function; thus, tailored exercise routines and regimens are recommended.90
Benefits of aerobics and resistance training in RA patients
Structured physical exercise programs are widely recognized for providing significant, lasting benefits to RA patients,91 without exacerbating disease activity or causing additional joint damage.92 Recent investigations have explored various exercise modalities, including resistance training and aerobic exercises, as well as their combination.93 Aerobic activities, such as cycling, swimming, dancing, walking, and running, have been shown to effectively mitigate joint damage in RA patients, provided that the exercise programs adhere to RA-specific guidelines designed to minimize cardiovascular disease risk, maximize quality of life, and maintain daily functional capabilities (Fig. 4).
Effects of Exercise Therapy on RA. The diagram illustrates how the reasonable combination of different exercise modalities, guided by the FITT model (Frequency, Intensity, Time, and Type), could restore functions in RA patients. Aerobic training, resistance training, Tai Chi, yoga, and adjunctive therapies like cryotherapy and hydrotherapy work together to improve exercise capacity and alleviate symptoms of RA. Created in BioRender
Cooney et al. explored the impact of exercise interventions on myopenia and cachexia, revealing that intensive progressive resistance training (PRT) can increase lean body mass, reduce fat mass, enhance strength, and boost overall functionality.94 PRT is recognized as an effective method for increasing the size and strength of skeletal muscles, and it is deemed safe for RA patients, even at higher intensity levels. Furthermore, PRT has been shown to effectively increase mechanical loading on bones, thereby promoting bone mineral density (BMD) enhancement and maintenance. This increased mechanical stimulus plays a critical role in preventing further deterioration of bone structure and mitigating the risk of osteoporosis. For RA patients who are at a heightened risk of osteoporosis due to the prolonged use of corticosteroids, such interventions can delay bone loss and improve skeletal integrity. Resistance training also improves tendon rigidity and strengthens connective tissues, while cyclic loading activities promote cartilage health and enhance joint lubrication. Additionally, when combined with mobility exercises, PRT can increase the range of motion for RA patients. Peres et al. also demonstrated that integrating PRT exercise programs with cold-water immersion during recovery periods can further improve muscle function, alleviate pain, and enhance overall well-being in RA patients.95
According to research by Pedersen et al, skeletal muscle functions as an endocrine organ, releasing myokines such as IL-6 during contraction.96 Unlike its previously discussed role as a catalyst in muscle aging, IL-6 released during exercise appears to exhibit anti-inflammatory properties. This is primarily achieved by suppressing TGF-β activity, thus alleviating inflammation and reducing muscle soreness, a process potentially linked to the STAT3-SMAD3 crosstalk. However, the specific effects of muscle-derived IL-6 in RA patients during exercise remain unclear. Further investigation could provide valuable insights into the development of exercise-based therapies for addressing muscle wasting in RA patients.
Tai Chi and Yoga for RA
The evidence concerning the efficacy of Tai Chi for RA remains ambiguous. A recent Cochrane review concluded that the benefits of Tai Chi on clinical outcomes such as joint pain, activity limitation, and function in RA are uncertain, and neither clear benefits nor adverse effects could be confirmed due to the very low quality of evidence.97 Further analysis suggested that while some clinical improvement was noted, it was not statistically significant in terms of pain reduction and disease pattern as assessed using the ACR20 measurement. Improvements in disability and quality of life were observed. Given the low level of evidence in the studies reviewed, a cautious approach to data analysis is advised. The three studies included were found to lack reliability in providing an accurate and comprehensive summary of the effects of Tai Chi on individuals diagnosed with RA.98 However, Tai Chi has been shown to significantly improve lower extremity range of motion, particularly in the ankle joint, among individuals with RA.99
The evidence for Yoga in treating RA was less convincing than anticipated. A recent case-based review highlighted the potential of Yoga as a supplementary intervention for RA, suggesting it may enhance patients’ quality of life and alleviate disease symptoms.100 However, this study suffered from a small sample size, and further research is necessary to substantiate the purported benefits.100 More recent studies have shown promising outcomes with Yoga, demonstrating benefits in reducing pain, morning stiffness, and overall disease activity101 (Fig. 4).
Although there is no strong evidence to suggest that Tai Chi and yoga have a significant positive impact on the long-term prognosis of RA patients, they do contribute to improving overall function and quality of life. While Tai Chi and yoga may not directly enhance muscle quality as much as strength training or aerobic exercise, they help by improving relaxation, coordination, flexibility, and muscle endurance. Through these mechanisms, they indirectly enhance the efficiency and strength of muscle use, thereby reducing pain, stiffness, and joint dysfunction.
Symptom control, hydrotherapy, cryotherapy and further research
Exercise has been shown to alleviate RA-associated pain and stiffness, reduce fatigue, and enhance functional capacity and well-being without exacerbating the disease. A study by Al-Qubaeissy et al. indicated that hydrotherapy significantly reduces pain and improves the health status of RA patients.102 It is important to note that the pain relief experienced by RA patients from hydrotherapy has only been demonstrated in short-term studies, and its long-term efficacy remains uncertain, requiring further validation. According to the study by Peres et al., cryotherapy, the use of extreme cold to freeze whole-body at –110 °C or local cooling with cold packs or air to reduce inflammation, has shown efficacy in alleviating joint pain and swelling in patients with RA, particularly in those not receiving glucocorticoid therapy.95 It is hypothesized that the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS) was active via cold-induced stress during cryotherapy. Notably, patients subjected to cryotherapy exhibited an increase in plasma IL-6 levels, yet their pain scores significantly decreased, suggesting a dissociation between inflammatory markers and clinical symptom improvement.
Nevertheless, further research is essential to determine the optimal frequency, intensity, duration, and type of exercise (FITT model) and to assess the effectiveness of combining different types of exercise. For example, FITT model optimization, tailored to age, gender, and disease stage, could guide individualized exercise prescriptions. Additionally, more studies are required to explore the best strategies for integrating exercise into patients’ daily routines with varying degrees of disease severity. Different types of exercise, such as low-impact aerobics and resistance training, may provide distinct benefits, and their combination has the potential to enhance therapeutic outcomes. Further research should focus on optimizing exercise therapy by investigating the efficacy of combining these approaches. (Fig. 4)
Dietary interventions to alleviate muscle wasting in RA patients
It is hypothesized that factors such as dietary habits, inflammation, and physical activity may influence Oxidative stress mechanisms, impacting both pro- and anti-oxidative processes to intervene the synthesis of muscle protein. For example, compared to the typical Western diet, which is high in red meat, saturated fats, and refined sugars and is associated with increased inflammation and Oxidative stress, the Mediterranean Diet (MD) offers significant benefits. Rich in anti-inflammatory nutrients like fruits, vegetables, whole grains, and fatty fish, the MD helps reduce both inflammation and Oxidative stress. Its positive effects are largely due to its high content of antioxidants and omega-3 fatty acids, which balance oxidative and inflammatory processes, potentially alleviating RA symptoms. Additionally, the MD promotes beneficial gut bacteria through its fiber content, further reducing systemic inflammation and Oxidative stress.103
Management of frailty and prefrailty in RA myopenia
Frailty, associated with decreased muscle strength and elevated inflammatory markers,104 contributes to its persistence.105,106 Recent findings indicate that frailty and prefrailty occur more frequently in patients with YORA than previously recognized.107 As frailty becomes more prevalent with age, timely management of frailty is critical for addressing myopenia. Future research could focus on assessing frailty in a large cohort to determine whether RA patients who achieve sustained remission with early treatment and avoid joint damage exhibit lower frailty scores compared to those who receive less intensive treatment.
Myopenia, heart failure and premature myocardial aging in RA
RA, like many chronic autoimmune diseases, is associated with significantly elevated cardiovascular morbidity and mortality.108,109,110,111,112 Notably, the cardiovascular risk for RA patients is comparable to that of individuals with type 2 diabetes mellitus.113,114 This risk is influenced by common factors such as hypertension, obesity, metabolic dysfunction-associated fatty liver disease (MAFLD), dyslipidemia, and hyperglycemia, as well as RA-specific factors including elevated systemic inflammation.111,115,116,117
Additionally, the association between RA and metabolic syndrome further complicates the cardiovascular risk profile. Kerekes et al. highlighted that RA patients often exhibit components of metabolic syndrome, including central obesity, dyslipidemia, insulin resistance, and hypertension, which all contribute to an increased cardiovascular disease (CVD) risk.79 Unlike in the general population, RA patients present a “lipid paradox,” where lower levels of total cholesterol and LDL cholesterol are paradoxically associated with higher cardiovascular risk due to the pro-inflammatory state that drives atherosclerosis. Furthermore, RA-specific alterations in adipokine profiles, such as elevated levels of pro-inflammatory leptin and resistin, exacerbate metabolic dysregulation and contribute to both metabolic syndrome and cardiovascular complications.
Heart failure (HF), sometimes termed myopenia in cardiac muscle, is linked to increased inflammation and prevalent cardiovascular risks.118,119 Pro-inflammatory cytokines in HF contribute to myocardial damage, cardiac muscle myopenia, as well as other pathological outcomes via mechanisms such as arterial stiffness and endothelial dysfunction.120,121,122,123,124,125,126 The systemic inflammation observed in RA exacerbates these processes, accelerating the development of both systolic and diastolic dysfunction as well as left ventricular concentric remodeling. This chronic inflammatory burden not only directly contributes to myocardial aging but also interacts with metabolic syndrome components, such as insulin resistance and dyslipidemia, worsening cardiovascular outcomes.5
Numerous studies have assessed the likelihood of HF in RA patients, revealing that HF can develop independently of traditional risk factors among this demographic.120,124,127,128 Ischemic heart disease, a common cause of HF, does not fully account for the elevated HF risk observed in RA. Research indicates that non-ischemic HF risks in RA are closely associated with the severity of the disease and can manifest early.124 A recent Danish cohort study129 identified that RA patients exhibit a 30% greater rate of hospitalization for HF compared to the general population. These findings suggest that RA may significantly contribute to the risk of HF and accelerate cardiac aging. RA patients frequently exhibit elevated levels of cardiac biomarkers, such as troponins and pro-B-type natriuretic peptides, which are critical indicators of heart disease and HF prognosis.130 Thus, the systemic inflammation associated with RA may independently elevate the risk of HF and accelerate myocardial aging, irrespective of traditional risk factors. It is likely that the increased risk of HF in RA patients involves systolic and diastolic dysfunction, as well as left ventricular concentric remodeling.120,121,124,128
Given the complex interaction between RA, metabolic syndrome, and cardiovascular health, research indicates that the increased risk of HF in RA patients involves not only systolic and diastolic dysfunction but also the synergistic effects of systemic inflammation and metabolic dysregulation. The interplay between these factors leads to a higher incidence of atherosclerosis, myocardial fibrosis, and ultimately heart failure.
Ongoing and future research should investigate the potential benefits of comprehensive heart failure screening in this population. Are there specific biomarkers that could serve as early warning signals for cardiovascular diseases associated with RA? Does the detailed relationship between systemic inflammation and myocardial remodeling need to be confirmed through long-term follow-up studies? Should routine screening for heart failure be considered in RA patients, and what would be the cost-effectiveness of such an approach?
Myopenia and pharmacological intervention in RA
The age of onset and disease progression in RA are crucial in determining the optimal strategies to delay and treat myopenia. Although limited data exist on the effectiveness of pharmacological treatments such as synthetic DMARDs and biologics in managing myopenia, the European League Against Rheumatism supports and advocates for their use. This approach utilizes the anti-inflammatory properties of DMARDs to mitigate myopenia, delay muscle aging, and reduce cardiovascular morbidity and mortality associated with RA.112,113 Additionally, DMARDs are postulated to improve joint health and function, potentially allowing for higher levels of physical activity, which may subsequently decrease additional risk factors such as hypertension and diabetes.131,132
Studies analysing DMARDs
A comprehensive review of 12 studies revealed that patients who received DMARD treatments following more severe disease progression exhibited an increased likelihood of radiographic joint space narrowing and bone erosions.133 These findings strongly correlate with the presentation of myopenia in RA, as corroborated by a recent cross-sectional study.69 Moreover, a study by Masoud et al. highlighted that synthetic DMARDs and biologics may reduce the risk of sudden cardiac mortality among RA patients.131 Similarly, Wu et al. discovered that RA patients treated with a combination of at least one DMARD and statins experienced a reduction in cardiovascular-related mortality and significant decreases in disease activity.134 Given the efficacy of DMARDs as a treatment and the adverse outcomes associated with suboptimal RA management—primarily premature bone erosions and poorer functional outcomes—prompt intervention is imperative.135 Consequently, a rheumatologist’s assessment to confirm an RA diagnosis and initiate DMARD-based treatment promptly is crucial for any patient presenting with unexplained new-onset polyarthritis.
Oral corticosteroids and pharmacological therapies
Oral corticosteroids are anti-inflammatory medications that can modify the progression of RA disease,136 potentially delaying the onset of myopenia and promoting healthy musculoskeletal aging. However, these benefits must be weighed against the significant risk of osteoporosis. The primary treatment focuses on managing symptoms with practical measures to alleviate joint stiffness, pain, and fatigue by reducing systemic inflammation. Based on the articles by Guo et al. and Ben et al. and in conjunction with 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis, we summarized the available options for contemporary pharmacological therapies for RA137,138,139(Fig. 5).
Tailored treatment approaches for RA
The primary objectives of RA treatment are to manage RA disease progression, reduce radiographic and structural damage progression, and preserve functional capacity.139,140 As previously mentioned, the treatment approach for EORA should closely align with that for YORA. Thus, the ultimate goal should be achieving either remission or low disease activity, adhering to treat-to-target principles.
Pharmacological considerations and treatment disparities
While DMARDs used for YORA are generally applicable to EORA, it is crucial to consider the altered pharmacokinetics and pharmacodynamics in older patients, necessitating vigilant monitoring for side effects.141 Patients with EORA often contend with a higher prevalence of comorbidities and engage in polypharmacy, thereby increasing the risk of drug-drug interactions and potential adverse effects.142 Treatment strategies for EORA tend to be less aggressive and more variable compared to those for YORA. Data from the CORONA database indicate that EORA patients receive less intensive treatment than their age- and sex-matched YORA counterparts, despite similar levels of disease activity and severity.141
According to the same database, MTX usage was slightly higher among EORA patients (63.9%) compared to YORA patients (59.6%), although the average MTX dosage was higher among YORA patients.141,143 Notably, EORA patients were less likely to use multiple conventional or biological DMARDs compared to YORA patients. The incidence of drug-related toxicity was comparable in both groups, though MTX toxicity occurred more frequently in YORA patients. These findings suggest that age may influence the severity of the disease and the range of treatment options, with EORA patients often receiving less aggressive therapy despite having similar disease durations, activities, and severities as YORA patients.
Swiss registry data revealed that first-line corticosteroid use was significantly higher among EORA patients, along with a lower follow-up rate for biological drug use.143 In a study by Genevay et al. involving 1 571 RA patients treated with anti-TNFα drugs, withdrawal rates and changes in Disease Activity Score (DAS28) were consistent across groups after two years.144 However, despite clinical improvements, EORA patients exhibited significantly less enhancement in HAQ scores. Moreover, TNF inhibitors were shown to be less effective in EORA patients compared to YORA patients, as evidenced by lesser improvements in HAQ scores.145
Conclusive analysis of DMARDs in EORA and YORA
Tocilizumab, abatacept, rituximab, and tofacitinib exhibit limited evidence of effectiveness in treating EORA. Specifically, tocilizumab demonstrates lower efficacy in EORA compared to YORA, although drug retention rates and discontinuation due to side effects are comparable in both groups.146 Additionally, no data are available on the effectiveness of abatacept in EORA, but tofacitinib has been shown to be equally effective in both EORA and YORA according to randomized controlled trials.147
A recent study on myopenia in EORA and YORA has revealed several distinct characteristics of EORA: a more balanced sex distribution, a higher incidence of acute onset with systemic symptoms, increased involvement of large joints, and reduced RF positivity.148 EORA patients are generally diagnosed earlier, exhibit less erosive disease, and use DMARDs less frequently than YORA patients. Further research is imperative to explore the impact of myopenia on the prognosis of RA, particularly focusing on secondary sarcopenia, cachexia, and frailty in older adults. Understanding these factors is crucial for assessing their influence on RA outcomes, while also considering the side effects of DMARDs in both EORA and YORA.
Therapeutic approaches to mitigate muscle loss in RA
Besides traditional pharmacological intervention and their tailored treatment approaches to RA, several drug classes may mitigate muscle loss through various mechanisms.
Anti-inflammatory agents: Chronic inflammation significantly contributes to muscle wasting in RA. TNF-α inhibitors reduce systemic inflammation by blocking TNF-α, which activates the NF-κB pathway and upregulates the ubiquitin-proteasome system, leading to muscle protein degradation. By inhibiting TNF-α, these agents help preserve muscle mass. Similarly, IL-6 inhibitors target IL-6, which activates the JAK/STAT pathway, promoting muscle catabolism.149 JAK inhibitors also block this pathway, may further mitigating muscle degradation and supporting muscle health.
Anabolic therapies: In addition to controlling inflammation, anabolic agents such as selective androgen receptor modulators (SARMs) and myostatin inhibitors have emerged as promising treatments for muscle loss.150 SARMs stimulate muscle protein synthesis and prevent muscle atrophy by selectively activating androgen receptors, while myostatin inhibitors reduce muscle wasting by blocking myostatin, a negative regulator of muscle growth.
Nutritional and metabolic modulators: Nutritional supplements like vitamin D, omega-3 fatty acids, and branched-chain amino acids (BCAAs) play supportive roles in preserving muscle mass. Vitamin D enhances muscle function and reduces inflammation, improve muscle protein synthesis, and BCAAs help to decrease muscle breakdown and support recovery.151,152,153,154
Together, these therapeutic strategies represent promising avenues for addressing muscle loss in RA. They not only aim to control the underlying inflammation but also target the anabolic and metabolic processes critical for maintaining muscle mass and function.
Integrated management of RA: combining pharmacologic and non-pharmacologic approaches
Pharmacologic intervention remains the cornerstone of RA management, aiming to control inflammation, slow disease progression, and prevent joint damage while preserving functional capacity and quality of life. DMARDs are the foundation, effectively suppressing systemic inflammation, improving joint function, and mitigating RA-associated complications.131,134 Additionally, the previously mentioned drugs with potential to mitigate muscle loss should also be considered. However, these treatments carry risks such as gastrointestinal issues, liver dysfunction, infection susceptibility, and osteoporosis, necessitating regular monitoring and individualized adjustments to balance efficacy and safety.
Non-pharmacologic interventions complement pharmacologic therapy by addressing broader health aspects. Personalized exercise programs based on the FITT principle (Frequency, Intensity, Time, Type) enhance joint mobility, muscle strength, and bone health while reducing inflammation. Dietary interventions, such as the Mediterranean diet rich in omega-3 fatty acids and antioxidants, support joint health and reduce systemic inflammation.103 Psychological support, through cognitive behavioral therapy (CBT) and patient groups, addresses depression and anxiety, improving adherence and overall well-being.155
Due to variations in patients’ age, disease duration, and comorbidities, traditional fixed-dose pharmacological treatments often fail to achieve optimal efficacy. Personalized treatment plans tailored to the individual patient’s condition can effectively improve outcomes. By combining pharmacologic and non-pharmacologic strategies, RA management achieves comprehensive disease control, improving both clinical outcomes and patient quality of life. Pharmacologic treatments provide foundational inflammation control, while non-pharmacologic approaches enhance holistic, long-term care.
Conclusion
The intersection of myopenia and accelerated musculoskeletal aging in RA represents a multifaceted area of research, highlighting both the urgency and potential for optimizing patient outcomes through targeted care. This review has examined the pathophysiological mechanisms of muscle wasting in RA, underscoring inflammation, Oxidative stress, cytokine activity and hormonal and genetic factors as central contributors. These insights offer a robust foundation for developing targeted interventions that address both muscle degradation and the overall disease trajectory, thereby improving physical function and quality of life for RA patients.
To address the specific complexities of RA-related muscle wasting, a multidisciplinary approach involving pharmacological treatment, personalized exercise interventions, nutritional support, and psychological therapy has been shown to effectively mitigate muscle wasting and improve functional outcomes and quality of life in patients with RA. Early detection of myopenia combined with a tailored management strategy encompassing pharmacological treatments, individualized exercise regimens designed by physical therapists, psychological support from mental health professionals and dietary modifications may alleviate muscle loss, reduce frailty, and mitigate risks associated with sarcopenic obesity. Specific pharmacological treatments such as cytokine inhibitors, muscle-enhancing agents, and emerging biologic therapies should be considered for their potential to improve muscle mass and function. The integration of these strategies in clinical practice could significantly delay the progression of muscle wasting and enhance patient resilience, fostering improved physical functionality and lower mortality risks associated with RA.
Future research directions should focus on expanding the understanding of RA-specific muscle loss mechanisms within a collaborative framework. This includes further investigation of pharmacological approaches such as DMARDs and biologics, which show potential in mitigating inflammation and slowing muscle decline. Additionally, personalized treatment options, taking into account individual inflammation levels, disease severity, and comorbidities (e.g., age, obesity, metabolic conditions), are likely to optimize care and improve outcomes for RA-associated muscle deterioration.
This review aims to inspire ongoing exploration into RA-related myopenia and its implications across diverse RA populations. By advancing research in this critical area, we can contribute to a holistic and patient-centered approach in managing chronic inflammatory diseases, fostering joint health, muscular resilience, and overall physical well-being.
References
Xu, D.F., Xu, J. & Dai, L. Myopenia and musculoskeletal aging in rheumatoid arthritis. Rheumatoid Arthritis - Other Perspectives towards a Better Practice (2020).
El-Labban, A. S., Omar, H. A., El-Shereif, R. R., Ali, F. & El-Mansoury, T. M. Pattern of young and old onset rheumatoid arthritis (YORA and EORA) among a group of egyptian patients with rheumatoid arthritis. Clin. Med. Insights Arthritis Musculoskelet. Disord. 3, 25–31 (2010).
Rasch, E. K., Hirsch, R., Paulose-Ram, R. & Hochberg, M. C. Prevalence of rheumatoid arthritis in persons 60 years of age and older in the United States: effect of different methods of case classification. Arthritis Rheum. 48, 917–926 (2003).
Ma, J. D. et al. Muscle wasting, a neglected complication associated with physical dysfunction in elderly patients with rheumatoid arthritis: a cross-sectional observational study. Scand. J. Rheumatol. 50, 280–289 (2021).
Fearon, K., Evans, W. J. & Anker, S. D. Myopenia-a new universal term for muscle wasting. J. Cachexia Sarcopenia Muscle 2, 1–3 (2011).
Gonzalez-Ponce, F. et al. Myostatin levels and the risk of myopenia and rheumatoid cachexia in women with rheumatoid arthritis. J. Immunol. Res 2022, 7258152 (2022).
Carnac, G., Vernus, B. & Bonnieu, A. Myostatin in the pathophysiology of skeletal muscle. Curr. Genomics 8, 415–422 (2007).
Wabe, N. & Wiese, M. D. Treating rheumatoid arthritis to target: physician and patient adherence issues in contemporary rheumatoid arthritis therapy. J. Eval. Clin. Pr. 23, 486–493 (2017).
Aletaha, D. & Smolen, J. S. Diagnosis and management of rheumatoid arthritis: A review. JAMA 320, 1360–1372 (2018).
Mitchell, W. K. et al. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 3, 260 (2012).
Landers-Ramos, R. Q. & Prior, S. J. The microvasculature and skeletal muscle health in aging. Exerc Sport Sci. Rev. 46, 172–179 (2018).
Kunz, H. E. & Lanza, I. R. Age-associated inflammation and implications for skeletal muscle responses to exercise. Exp. Gerontol. 177, 112177 (2023).
Narici, M. V. & Maffulli, N. Sarcopenia: characteristics, mechanisms and functional significance. Br. Med Bull. 95, 139–159 (2010).
Thomas, D. R. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin. Nutr. 26, 389–399 (2007).
Fried, L. P. et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med Sci. 56, M146–M156 (2001).
Bauer, J. et al. Sarcopenia: A time for action. An SCWD position paper. J. Cachexia Sarcopenia Muscle 10, 956–961 (2019).
Larsson, L. et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol. Rev. 99, 427–511 (2019).
Roubenoff, R. et al. Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J. Clin. Invest 93, 2379–2386 (1994).
Rajbhandary, R., Khezri, A. & Panush, R. S. Rheumatoid cachexia: What is it and why is it important? J. Rheumatol. 38, 406–408 (2011).
Roubenoff, R. Rheumatoid cachexia: a complication of rheumatoid arthritis moves into the 21st century. Arthritis Res Ther. 11, 108 (2009).
Gregersen, P. K., Silver, J. & Winchester, R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 30, 1205–1213 (1987).
Mattey, D. L. et al. Independent association of rheumatoid factor and the HLA-DRB1 shared epitope with radiographic outcome in rheumatoid arthritis. Arthritis Rheum. 44, 1529–1533 (2001).
Weyand, C. M., Hicok, K. C., Conn, D. L. & Goronzy, J. J. The influence of HLA-DRB1 genes on disease severity in rheumatoid arthritis. Ann. Intern Med 117, 801–806 (1992).
Gonzalez-Gay, M. A. et al. Seronegative rheumatoid arthritis in elderly and polymyalgia rheumatica have similar patterns of HLA association. J. Rheumatol. 28, 122–125 (2001).
Kim, E. J. et al. Shared epitope and radiologic progression are less prominent in elderly onset RA than young onset RA. Rheumatol. Int 33, 2135–2140 (2013).
Hellier, J. P., Eliaou, J. F., Daurès, J. P., Sany, J. & Combe, B. HLA-DRB1 genes and patients with late onset rheumatoid arthritis. Ann. Rheum. Dis. 60, 531–533 (2001).
Wu, H. et al. Interaction between RANKL and HLA-DRB1 genotypes may contribute to younger age at onset of seropositive rheumatoid arthritis in an inception cohort. Arthritis Rheum. 50, 3093–3103 (2004).
Zhang, T. P. et al. The contribution of genetic variation and aberrant methylation of aryl hydrocarbon receptor signaling pathway genes to rheumatoid arthritis. Front Immunol. 13, 823863 (2022).
Huang, H. et al. Identification of key candidate genes and pathways in rheumatoid arthritis and osteoarthritis by integrated bioinformatical analysis. Front Genet 14, 1083615 (2023).
Garatachea, N. & Lucía, A. Genes and the ageing muscle: A review on genetic association studies. Age (Dordr.) 35, 207–233 (2013).
Wen, Y. P. & Yu, Z. G. Identifying shared genetic loci and common risk genes of rheumatoid arthritis associated with three autoimmune diseases based on large-scale cross-trait genome-wide association studies. Front Immunol. 14, 1160397 (2023).
Shao, M. et al. Association of interleukin-6 promoter polymorphism with rheumatoid arthritis: a meta-analysis with trial sequential analysis. Clin. Rheumatol. 41, 411–419 (2022).
Punzi, L. et al. Synovial fluid levels of proinflammatory interleukins and their inter-relationships in elderly vs younger onset rheumatoid arthritis. Aging (Milano) 8, 277–281 (1996).
Miesel, R., Murphy, M. P. & Kröger, H. Enhanced mitochondrial radical production in patients with rheumatoid arthritis correlates with elevated levels of tumor necrosis factor alpha in plasma. Free Radic. Res. 25, 161–169 (1996).
Dodd, S. L., Gagnon, B. J., Senf, S. M., Hain, B. A. & Judge, A. R. ROS-mediated activation of NF-κB and Foxo during muscle disuse. Muscle Nerve 41, 110–113 (2010).
Stavropoulos-Kalinoglou, A. Muscle wasting in rheumatoid arthritis: The role of oxidative stress. World J. Rheumatol. 4, 44–53 (2014).
Poniewierska-Baran, A., Bochniak, O., Warias, P. & Pawlik, A. Role of sirtuins in the pathogenesis of rheumatoid arthritis. Int. J. Mol. Sci. 24, 1532 (2023).
Fouani, M. et al. Heat shock proteins alterations in rheumatoid arthritis. Int. J. Mol. Sci. 23, 5828 (2022).
Gyebrovszki, B. et al. The role of IgG Fc region N-glycosylation in the pathomechanism of rheumatoid arthritis. Int. J. Mol. Sci. 23, 5828 (2022).
Nishimura, K. et al. Meta-analysis: Diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann. Intern Med. 146, 797–808 (2007).
Bizzaro, N. et al. Anti-cyclic citrullinated peptide antibody titer predicts time to rheumatoid arthritis onset in patients with undifferentiated arthritis: Results from a 2-year prospective study. Arthritis Res Ther. 15, R16 (2013).
Malmström, V., Catrina, A. I. & Klareskog, L. The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting. Nat. Rev. Immunol. 17, 60–75 (2017).
Padyukov, L. et al. A genome-wide association study suggests contrasting associations in ACPA-positive versus ACPA-negative rheumatoid arthritis. Ann. Rheum. Dis. 70, 259–265 (2011).
Schuerwegh, A. J. et al. Evidence for a functional role of IgE anticitrullinated protein antibodies in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 107, 2586–2591 (2010).
van Dongen, H. et al. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: A double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 56, 1424–1432 (2007).
Sellam, J. et al. B cell activation biomarkers as predictive factors for the response to rituximab in rheumatoid arthritis: a six-month, national, multicenter, open-label study. Arthritis Rheum. 63, 933–938 (2011).
Straub, R. H., Lehle, K., Herfarth, H., Weber, M., Falk, W., Preuner, J. & Schölmerich, J. Dehydroepiandrosterone in relation to other adrenal hormones during an acute inflammatory stressful disease state compared with chronic inflammatory disease: role of interleukin-6 and tumour necrosis factor. Eur. J. Endocrinol. 146, 365–374 (2002).
Bennett, J. L., Pratt, A. G., Dodds, R., Sayer, A. A. & Isaacs, J. D. Rheumatoid sarcopenia: loss of skeletal muscle strength and mass in rheumatoid arthritis. Nat. Rev. Rheumatol. 19, 239–251 (2023).
Zanders, L. et al. Sepsis induces interleukin 6, gp130/JAK2/STAT3, and muscle wasting. J. Cachexia Sarcopenia Muscle 13, 713–727 (2022).
Gamerith, F. et al. Differences in anti-Fab antibodies in adult and late onset rheumatoid arthritis. Rheumatol. Int 13, 107–112 (1993).
Mitra, A., Qaisar, R., Bose, B. & Sudheer, S. P. The elusive role of myostatin signaling for muscle regeneration and maintenance of muscle and bone homeostasis. Osteoporos. Sarcopenia 9, 1–7 (2023).
Lin, J. Z. et al. Myokine myostatin is a novel predictor of one-year radiographic progression in patients with rheumatoid arthritis: A prospective cohort study. Front Immunol. 13, 1005161 (2022).
Itoh, Y., Saitoh, M. & Miyazawa, K. Smad3–STAT3 crosstalk in pathophysiological contexts. Acta Biochimica et. Biophysica Sin. 50, 82–90 (2018).
Shaw, C. S., Clark, J. & Wagenmakers, A. J. M. The effect of exercise and nutrition on intramuscular fat metabolism and insulin sensitivity. Annu. Rev. Nutr. 30, 13–34 (2010).
Du, H. et al. Advanced glycation end products induce skeletal muscle atrophy and insulin resistance via activating ROS-mediated ER stress PERK/FOXO1 signaling. Am. J. Physiol. Endocrinol. Metab. 324, E279–E287 (2023).
Johnson, D. E., O'Keefe, R. A. & Grandis, J. R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer.Nat. Rev. Clin. Oncol. 15, 234–248 (2018).
Eskiler, G. G. et al. IL-6 mediated JAK/STAT3 signaling pathway in cancer patients with cachexia. Bratisl. Lek. Listy 66, 819–826 (2019).
Huffman, K. M. et al. Molecular alterations in skeletal muscle in rheumatoid arthritis are related to disease activity, physical inactivity, and disability. Arthritis Res Ther. 19, 12 (2017).
Healey, L. A. Subsets of rheumatoid arthritis in the aged. Arthritis Rheum. 29, 149 (1986).
Healey, L. A. & Sheets, P. K. The relation of polymyalgia rheumatica to rheumatoid arthritis. J. Rheumatol. 15, 750–752 (1988).
McCarty, D. J., O’Duffy, J. D., Pearson, L. & Hunter, J. B. Remitting seronegative symmetrical synovitis with pitting edema. RS3PE syndrome. Jama 254, 2763–2767 (1985).
Inoue, K., Shichikawa, K., Nishioka, J. & Hirota, S. Older age onset rheumatoid arthritis with or without osteoarthritis. Ann. Rheum. Dis. 46, 908–911 (1987).
Goemaere, S. et al. Onset of symptoms of rheumatoid arthritis in relation to age, sex and menopausal transition. J. Rheumatol. 17, 1620–1622 (1990).
Ferraccioli, G. F. et al. Clinical features, scintiscan characteristics and X-ray progression of late onset rheumatoid arthritis. Clin. Exp. Rheumatol. 2, 157–161 (1984).
Turkcapar, N. et al. Late onset rheumatoid arthritis: clinical and laboratory comparisons with younger onset patients. Arch. Gerontol. Geriatr. 42, 225–231 (2006).
van der Heijde, D. M. et al. Older versus younger onset rheumatoid arthritis: results at onset and after 2 years of a prospective followup study of early rheumatoid arthritis. J. Rheumatol. 18, 1285–1289 (1991).
Chen, D. Y. et al. Proinflammatory cytokine profiles of patients with elderly-onset rheumatoid arthritis: a comparison with younger-onset disease. Gerontology 55, 250–258 (2009).
Lance, N. J. & Curran, J. J. Late-onset, seropositive, erosive rheumatoid arthritis. Semin Arthritis Rheum. 23, 177–182 (1993).
Lin, J. Z. et al. Myopenia is associated with joint damage in rheumatoid arthritis: a cross-sectional study. J. Cachexia Sarcopenia Muscle 10, 355–367 (2019).
van Schaardenburg, D. et al. Outcome of rheumatoid arthritis in relation to age and rheumatoid factor at diagnosis. J. Rheumatol. 20, 45–52 (1993).
Cho, S. K. et al. Do patients with elderly-onset rheumatoid arthritis have severe functional disability? Semin Arthritis Rheum. 42, 23–31 (2012).
Serhal, L., Lwin, M. N., Holroyd, C. & Edwards, C. J. Rheumatoid arthritis in the elderly: Characteristics and treatment considerations. Autoimmun. Rev. 19, 102528 (2020).
Pan, J. et al. Muscle mass loss is associated with physical dysfunction in patients with early rheumatoid arthritis. Front Nutr. 9, 1007184 (2022).
Siparsky, P. N., Kirkendall, D. T. & Garrett, W. E. Jr. Muscle changes in aging: understanding sarcopenia. Sports Health 6, 36–40 (2014).
Keller, K. & Engelhardt, M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J. 3, 346–350 (2013).
Molino, S., Dossena, M., Buonocore, D. & Verri, M. Sarcopenic obesity: An appraisal of the current status of knowledge and management in elderly people. J. Nutr. Health Aging 20, 780–788 (2016).
Zamboni, M., Mazzali, G., Fantin, F., Rossi, A. & Di Francesco, V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr. Metab. Cardiovasc Dis. 18, 388–395 (2008).
Lin, J. Z. et al. Neglected extra-articular manifestations in rheumatoid arthritis patients with normal body mass index: reduced skeletal muscle overlapping overfat. Ther. Adv. Chronic Dis. 11, 2040622320975241 (2020).
Kerekes, G. et al. Rheumatoid arthritis and metabolic syndrome. Nat. Rev. Rheumatol. 10, 691–696 (2014).
Baker, J. F. et al. Muscle deficits in rheumatoid arthritis contribute to inferior cortical bone structure and trabecular bone mineral density. J. Rheumatol. 44, 1777–1785 (2017).
Cheng, K. Y. et al. Diagnosis of sarcopenia by evaluating skeletal muscle mass by adjusted bioimpedance analysis validated with dual-energy X-ray absorptiometry. J. Cachexia Sarcopenia Muscle 12, 2163–2173 (2021).
Li, J., She, B., He, M., Yuan, C. & Li, N. Advances in imaging examination of bone density and bone quality. Endokrynol. Pol. 76, 29–39 (2025).
Lin, J.Z. et al. Reduced skeletal muscle independently predicts 1-year aggravated joint destruction in patients with rheumatoid arthritis. Ther. Adv. Musculoskelet Dis. 12, 1759720×20946220 (2020).
Pease, C. T., Bhakta, B. B., Devlin, J. & Emery, P. Does the age of onset of rheumatoid arthritis influence phenotype?: a prospective study of outcome and prognostic factors. Rheumatol. (Oxf.) 38, 228–234 (1999).
Calvo-Alén, J. et al. Outcome of late-onset rheumatoid arthritis. Clin. Rheumatol. 24, 485–489 (2005).
Krams, T. et al. Effect of age at rheumatoid arthritis onset on clinical, radiographic, and functional outcomes: The ESPOIR cohort. Jt. Bone Spine 83, 511–515 (2016).
Metsios, G. S. et al. Rheumatoid arthritis, cardiovascular disease and physical exercise: a systematic review. Rheumatol. (Oxf.) 47, 239–248 (2008).
Lee, D. M. & Weinblatt, M. E. Rheumatoid arthritis. Lancet 358, 903–911 (2001).
Ekdahl, C. & Broman, G. Muscle strength, endurance, and aerobic capacity in rheumatoid arthritis: a comparative study with healthy subjects. Ann. Rheum. Dis. 51, 35–40 (1992).
Li, Z. & Wang, X. Q. Clinical effect and biological mechanism of exercise for rheumatoid arthritis: A mini review. Front Immunol. 13, 1089621 (2022).
de Jong, Z. et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? Results of a randomized controlled trial. Arthritis Rheum. 48, 2415–2424 (2003).
Häkkinen, A. Effectiveness and safety of strength training in rheumatoid arthritis. Curr. Opin. Rheumatol. 16, 132–137 (2004).
Stavropoulos-Kalinoglou, A. et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann. Rheum. Dis. 72, 1819–1825 (2013).
Cooney, J. K. et al. Benefits of exercise in rheumatoid arthritis. J. Aging Res. 2011, 681640 (2011).
Peres, D. et al. Effects of an exercise program and cold-water immersion recovery in patients with rheumatoid arthritis (RA): feasibility study. Int. J. Environ. Res. Public Health 20, 6128 (2023).
Pedersen, B.K. Muscle as a secretory organ. in Comprehensive Physiology 1337-1362 (2013).
Mudano, A. S., Tugwell, P., Wells, G. A. & Singh, J. A. Tai Chi for rheumatoid arthritis. Cochrane Database Syst. Rev. 9, Cd004849 (2019).
Imoto, A. M. et al. Evidence for the efficacy of Tai Chi for treating rheumatoid arthritis: an overview of systematic reviews. Sao Paulo Med J. 139, 91–97 (2021).
Han, A. et al. Tai chi for treating rheumatoid arthritis. Cochrane Database Syst. Rev. 3, CD004849 (2004).
Sagtaganov, Z., Yessirkepov, M. & Bekaryssova, D. Yoga as a complementary therapy for rheumatoid arthritis: A case-based review. Rheumatol. Int 44, 1575–1579 (2024).
Sagtaganov, Z., Yessirkepov, M., Bekaryssova, D. & Suigenbayev, D. Managing rheumatoid arthritis and cardiovascular disease: the role of physical medicine and rehabilitation. Rheumatol. Int. 44, 1749–1756 (2024).
Al-Qubaeissy, K. Y., Fatoye, F. A., Goodwin, P. C. & Yohannes, A. M. The effectiveness of hydrotherapy in the management of rheumatoid arthritis: a systematic review. Musculoskelet. Care 11, 3–18 (2013).
Gioia, C., Lucchino, B., Tarsitano, M. G., Iannuccelli, C. & Di Franco, M. Dietary habits and nutrition in rheumatoid arthritis: can diet influence disease development and clinical manifestations? Nutrients 12, 1456 (2020).
Yao, X., Li, H. & Leng, S. X. Inflammation and immune system alterations in frailty. Clin. Geriatr. Med 27, 79–87 (2011).
Dent, E., Kowal, P. & Hoogendijk, E. O. Frailty measurement in research and clinical practice: A review. Eur. J. Intern Med 31, 3–10 (2016).
de Vries, N. M. et al. Outcome instruments to measure frailty: A systematic review. Ageing Res. Rev. 10, 104–114 (2011).
Haider, S. et al. Frailty in seropositive rheumatoid arthritis patients of working age: A cross-sectional study. Clin. Exp. Rheumatol. 37, 585–592 (2019).
Armstrong, E. J., Harskamp, C. T. & Armstrong, A. W. Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. J. Am. Heart Assoc. 2, e000062 (2013).
Avina-Zubieta, J. A., Thomas, J., Sadatsafavi, M., Lehman, A. J. & Lacaille, D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann. Rheum. Dis. 71, 1524–1529 (2012).
Filimon, A. M. et al. Cardiovascular involvement in inflammatory bowel disease: Dangerous liaisons. World J. Gastroenterol. 21, 9688–9692 (2015).
Soubrier, M. et al. Cardiovascular risk in rheumatoid arthritis. Jt. Bone Spine 81, 298–302 (2014).
Ahlehoff, O. et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J. Intern Med 270, 147–157 (2011).
Brady, S. R. et al. The role of traditional cardiovascular risk factors among patients with rheumatoid arthritis. J. Rheumatol. 36, 34–40 (2009).
Lindhardsen, J. et al. The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann. Rheum. Dis. 70, 929–934 (2011).
del Rincón, I. et al. Systemic inflammation and cardiovascular risk factors predict rapid progression of atherosclerosis in rheumatoid arthritis. Ann. Rheum. Dis. 74, 1118–1123 (2015).
Zou, Y. W. et al. Association between metabolic dysfunction-associated fatty liver disease and cardiovascular risk in patients with rheumatoid arthritis: A cross-sectional study of Chinese cohort. Front Cardiovasc Med 9, 884636 (2022).
Zou, Y. W. et al. Prevalence and influence of hypouricemia on cardiovascular diseases in patients with rheumatoid arthritis. Eur. J. Med Res 27, 260 (2022).
Go, A. S. et al. Heart disease and stroke statistics-2014 update: A report from the American Heart Association. Circulation 129, e28–e292 (2014).
Kalogeropoulos, A. et al. Inflammatory markers and incident heart failure risk in older adults: The Health ABC (Health, Aging, and Body Composition) study. J. Am. Coll. Cardiol. 55, 2129–2137 (2010).
Aslam, F., Bandeali, S. J., Khan, N. A. & Alam, M. Diastolic dysfunction in rheumatoid arthritis: a meta-analysis and systematic review. Arthritis Care Res. (Hoboken) 65, 534–543 (2013).
Cioffi, G. et al. Prognostic role of subclinical left ventricular systolic dysfunction evaluated by speckle-tracking echocardiography in rheumatoid arthritis. J. Am. Soc. Echocardiogr. 30, 602–611 (2017).
Foster, W., Carruthers, D., Lip, G. Y. & Blann, A. D. Inflammation and microvascular and macrovascular endothelial dysfunction in rheumatoid arthritis: effect of treatment. J. Rheumatol. 37, 711–716 (2010).
Marti, C. N. et al. Endothelial dysfunction, arterial stiffness, and heart failure. J. Am. Coll. Cardiol. 60, 1455–1469 (2012).
Midtbø, H., Semb, A. G., Matre, K., Kvien, T. K. & Gerdts, E. Disease activity is associated with reduced left ventricular systolic myocardial function in patients with rheumatoid arthritis. Ann. Rheum. Dis. 76, 371–376 (2017).
Paulus, W. J. & Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 62, 263–271 (2013).
Fan, F. et al. Comparison of inflammation, arterial stiffness and traditional cardiovascular risk factors between rheumatoid arthritis and inflammatory bowel disease. J. Inflamm. (Lond.) 11, 29 (2014).
Mantel, Ä., Holmqvist, M., Andersson, D. C., Lund, L. H. & Askling, J. Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J. Am. Coll. Cardiol. 69, 1275–1285 (2017).
Nicola, P. J. et al. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum. 52, 412–420 (2005).
Khalid, U. et al. Incident heart failure in patients with rheumatoid arthritis: a nationwide cohort study. J. Am. Heart Assoc. 7, e007227 (2018).
Avouac, J. et al. Inflammation and disease activity are associated with high circulating cardiac markers in rheumatoid arthritis independently of traditional cardiovascular risk factors. J. Rheumatol. 41, 248–255 (2014).
Masoud, S., Lim, P. B., Kitas, G. D. & Panoulas, V. Sudden cardiac death in patients with rheumatoid arthritis. World J. Cardiol. 9, 562–573 (2017).
Peters, M. J. et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann. Rheum. Dis. 69, 325–331 (2010).
Finckh, A., Liang, M. H., van Herckenrode, C. M. & de Pablo, P. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: A meta-analysis. Arthritis Rheum. 55, 864–872 (2006).
Wu, D. et al. Systemic complications of rheumatoid arthritis: Focus on pathogenesis and treatment. Front Immunol. 13, 1051082 (2022).
Fuchs, H. A., Kaye, J. J., Callahan, L. F., Nance, E. P. & Pincus, T. Evidence of significant radiographic damage in rheumatoid arthritis within the first 2 years of disease. J. Rheumatol. 16, 585–591 (1989).
Cohen, S. & Emery, P. The American college of rheumatology/european league against rheumatism criteria for the classification of rheumatoid arthritis: a game changer. Ann. Rheum. Dis. 69, 1575–1576 (2010).
Guo, Q. et al. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 6, 15 (2018).
Ben Mrid, R. et al. Anti-rheumatoid drugs advancements: New insights into the molecular treatment of rheumatoid arthritis. Biomed. Pharmacother. 151, 113126 (2022).
Fraenkel, L. et al. American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. (Hoboken) 73, 924–939 (2021).
Smolen, J. S. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 82, 3–18 (2023).
Tutuncu, Z., Reed, G., Kremer, J. & Kavanaugh, A. Do patients with older-onset rheumatoid arthritis receive less aggressive treatment? Ann. Rheum. Dis. 65, 1226–1229 (2006).
Walker, J. & Wynne, H. Review: the frequency and severity of adverse drug reactions in elderly people. Age Ageing 23, 255–259 (1994).
Mueller, R. B. et al. Does addition of glucocorticoids to the initial therapy influence the later course of the disease in patients with early RA? Results from the Swiss prospective observational registry (SCQM). Clin. Rheumatol. 36, 59–66 (2017).
Genevay, S. et al. Tolerance and effectiveness of anti-tumor necrosis factor alpha therapies in elderly patients with rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 57, 679–685 (2007).
Bathon, J. M. et al. Safety and efficacy of etanercept treatment in elderly subjects with rheumatoid arthritis. J. Rheumatol. 33, 234–243 (2006).
Pers, Y. M. et al. Efficacy and safety of tocilizumab in elderly patients with rheumatoid arthritis. Jt. Bone Spine 82, 25–30 (2015).
Curtis, J. R. et al. Efficacy and safety of tofacitinib in older and younger patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 35, 390–400 (2017).
Kobak, S. & Bes, C. An autumn tale: geriatric rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 10, 3–11 (2018).
Bermejo-Álvarez, I. et al. Effects of tofacitinib on muscle remodeling in experimental rheumatoid sarcopenia. Int. J. Mol. Sci. 24, 13181 (2023).
Rolland, Y., Dray, C., Vellas, B. & Barreto, P. S. Current and investigational medications for the treatment of sarcopenia. Metabolism 149, 155597 (2023).
Liu, H. et al. Associations between sarcopenia and circulating branched-chain amino acids: a cross-sectional study over 100,000 participants. BMC Geriatr. 24, 541 (2024).
Minamino, H. et al. Serum vitamin D status inversely associates with a prevalence of severe sarcopenia among female patients with rheumatoid arthritis. Sci. Rep. 11, 20485 (2021).
Gwinnutt, J. M. et al. Effects of diet on the outcomes of rheumatic and musculoskeletal diseases (RMDs): systematic review and meta-analyses informing the 2021 EULAR recommendations for lifestyle improvements in people with RMDs. RMD Open 8, e002167 (2022).
Lanchais, K., Capel, F. & Tournadre, A. Could omega-3 fatty acids preserve muscle health in rheumatoid arthritis? Nutrients 12, 223 (2020).
Pope, J. E. Management of fatigue in rheumatoid arthritis. RMD Open 6, e001084 (2020).
Acknowledgements
This study was supported in part by the National Natural Science Foundation of China (Grant No. 82350710800, 82374470, 82202757), and Shenzhen Medical Research Fund B2302005, and NHMRC, APP1163933.
Author information
Authors and Affiliations
Contributions
Conceptualization, preparation, and revision of the manuscript, J.X., S.Z.; Preparation of the manuscript, H.J., G.W., Q.L., J.R., J.C., S.K., O.C., D.X., and L.D. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jin, H., Wang, G., Lu, Q. et al. Pathophysiology of Myopenia in rheumatoid arthritis. Bone Res 13, 64 (2025). https://doi.org/10.1038/s41413-025-00438-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41413-025-00438-9