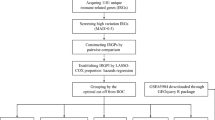
Abstract
Background
Immune checkpoint inhibitors (ICI) have revolutionized the treatment for multiple cancers. However, most of patients encounter resistance. Synthetic viability (SV) between genes could induce resistance. In this study, we established SV signature to predict the efficacy of ICI treatment for melanoma.
Methods
We collected features and predicted SV gene pairs by random forest classifier. This work prioritized SV gene pairs based on CRISPR/Cas9 screens. SV gene pairs signature were constructed to predict the response to ICI for melanoma patients.
Results
This study predicted robust SV gene pairs based on 14 features. Filtered by CRISPR/Cas9 screens, we identified 1,861 SV gene pairs, which were also related with prognosis across multiple cancer types. Next, we constructed the six SV pairs signature to predict resistance to ICI for melanoma patients. This study applied the six SV pairs signature to divide melanoma patients into high-risk and low-risk. High-risk melanoma patients were associated with worse response after ICI treatment. Immune landscape analysis revealed that high-risk melanoma patients had lower natural killer cells and CD8+ T cells infiltration.
Conclusions
In summary, the 14 features classifier accurately predicted robust SV gene pairs for cancer. The six SV pairs signature could predict resistance to ICI.
Similar content being viewed by others
Background
Immune checkpoint inhibitors (ICI) revolutionized the treatment of patients with solid tumors, such as advanced melanoma [1, 2]. ICI enhances T cell activity by inhibiting immunosuppressive checkpoint molecules, including cytotoxic T-lymphocyte associated antigen 4 (CTLA-4), programmed cell death 1 (PD-1), and programmed cell death protein ligand 1 (PD-L1), and so on. Nevertheless, despite the clinical success of ICI, only approximately a third of patients exhibit durable responses but will eventually experience a relapse [3]. Resistance to ICI was evident in clinical trials, where within the cohort of advanced melanoma patients, only 16% achieved a complete response (CR) [4]. Hence, exploring resistant biomarkers for ICI and screening patients who could benefit from ICI have become urgent problems in the field of immunotherapy [5]. Broadly, hot tumors with the characteristic of T cell infiltration, high expression of PD-L1 (encoded by CD274), and up-regulation of interferon gamma signatures respond to ICI treatment, while cold tumors not [6,7,8]. Indeed, tumor mutational burden (TMB) has been shown to be an efficient biomarker of the response to treatment with ICI in solid tumors [9]. However, ICI biomarkers for stratifying responders to immunotherapy differ considerably across various cancer types, the underlying resistant mechanism remained unknown.
Synthetic viability (SV), also known as synthetic rescue, one type of genetic interactions, mediates both primary or adaptive resistance in cancer therapy [10,11,12]. SV is defined as the combination of gene alterations that can rescue the lethal effects of a single gene alteration [11, 13]. Currently, genome-wide CRISPR/Cas9 screens are increasingly used to identify SV gene pairs and to determine mechanisms of drug resistance [14,15,16]. However, the roles of SV in cancer cell resistance to ICI are still elusive. Cancer driver genes are classified as oncogenes and tumor suppressor genes (TSGs) [17]. Recently, using CRISPR/Cas9, Parrish et al. identified SV gene pairs of TSGs, such as CDKN2A/CDK2NB and FBXO25/FBXO32 [18]. Zhao et al. systematically analyzed how combinations of inactivated TSGs altered the tumorigenic growth characteristics [19]. Furthermore, many of the genetic interactions have been identified in a context-specific cell line and may not be reproduced across multiple studies [20, 21]. Therefore, it is urgent to systematically identify robust TSGs and oncogenes related SV gene pairs, which may participate in ICI resistance.
In this study, we developed an ensemble classifier to predict robust cancer genes related SV gene pairs by integrating genome-wide CRISPR/Cas9 screens in cancer cell lines. Then, we investigated the impact of SV gene pairs alterations on prognosis in patients across various cancer types. Moreover, we explored the six SV pairs signature to predict the clinical benefit of ICI treatment for melanoma patients. This study also compared the six SV pairs signature with previously published signatures of response to immunotherapy. Finally, we dissected the role of the immune landscape in determining the benefit of immunotherapy.
Methods
Multi-omics data of cancer cell lines and bulk pan-cancer samples
We obtained CRISPR/Cas9 genetic perturbation data, expression, somatic mutation, and gene level copy number data from DepMap Portal (version 21Q4) (https://depmap.org/portal/), which consists of 859 cancer cell lines [22]. Gene expression and copy number alterations (CNA) data of 32 cancer types of The Cancer Genome Atlas (TCGA, https://portal.gdc.cancer.gov/) were downloaded from the UCSC Xena (https://xenabrowser.net/datapsges/). CNA were determined by Genomic Identification of Significant Targets in Cancer (GISTIC) [23]. We also acquired mutation profiles of 32 cancer types from cBioPortal (https://www.cbioportal.org).
Melanoma cohorts with immune checkpoint inhibitors treatment
This study constructed SV signature to predict immunotherapy response for melanoma patients. The Liu2019 cohort, including 121 melanoma patients, was used as a training cohort. Other datasets were validation cohorts. Expression, mutation, CNA data, and clinical information of immunotherapy melanoma patients were obtained from the cBioPortal (https://www.cbioportal.org) and the literature (Supplementary Table S1). The clinical survival outcomes included overall survival (OS), progression-free survival, and disease-free survival. To define the response and non-response patients, we used the criteria defined in the original clinical trials as much as possible. For the Liu2019 cohort where response evaluation criteria in solid tumors (RECIST) information was available, we used CR or partial response (PR) as response and progressive disease (PD) as non-response [7]. In Gide2019, melanoma patients with CR or PR or stable disease (SD) > six months were classified as responders, while melanoma patients with PD or SD < six months were classified as non-responders based on RECIST [24]. For Riaz2017 cohort, the TMB was obtained from the original study.
Feature score and classifier construction
Paralog gene pairs were obtained from Kegel et al. [25]. Protein complex membership information were downloaded from the comprehensive resource of mammalian protein complexes database (CORUM, https://doi.org/10.1093/nar/gky973) [26]. Shared protein-protein interactors (PPI), a quantitative feature, estimated the shared PPI of gene1 and gene2 by applying a hypergeometric test. The “Igraph” R package was used to calculate the average shortest distance. The essentiality of shared PPI was defined as the mean essentiality of shared PPI of gene1 and gene2. The similarity of biological process score, estimated from “GOSemSim” R package, was defined as the similarity of biological process annotation of gene1 and gene2 in Gene Ontology. The details information of 14 features was presented in Supplementary Table S2.
Next, we used the random forest to assemble the classifier implementation in “randomForest” R package. To prevent over-fitting, we selected low maximum tree depth and relatively high minimum leaf node weight. The parameters used in the final model were as follows: ntree = 500 (i.e., number of trees), mtry = 3, importance = TRUE, proximity = TRUE. All parameters not listed were left as the default.
TSGs and oncogenes were downloaded from the TSGene and ONGene databases, respectively [27, 28]. We focused on four types of SV gene pairs (Supplementary Fig. S1A): (i) TSGL-TSGL interaction, where the loss function of TSG1 was rescued by the loss function of TSG2 [18]; (ii) OGL-OGG interaction, where the loss function of oncogene caused by drug inhibition was rescued by the gain function of another oncogene; (iii) OGL-TSGL interaction, where the loss function of oncogene caused by drug inhibition was rescued by the loss function of TSG [11]; (vi) TSGL-OGG interaction, where the loss function of TSG was rescued by the gain function of oncogene [29]. Loss or gain function of gene was estimated from gene expression, mutation, and copy number data (see details in Supplementary methods).
Filtered SV by CRISPR/Cas9 screens
To identify the significant association between gene2 alteration (loss or gain of function) and gene1 dependency, we used a multiple linear regression model with the form: gene1_dependency ~ gene2_status (loss or gain of function) + C (cell_line_lineage) (Supplementary Fig. S1C, left). Cell lines dependency score was downloaded from the DepMap Portal (version 21Q4). Parental cell lines information, expression, mutation, and copy number data were downloaded from the Cancer Cell Line Encyclopedia project (CCLE, https://portals.broadinstitute.org/ccle) [22]. The number of cell lines for each cancer type was presented in Supplementary Fig. S2. We restricted gene2 status (loss or gain of function) alteration in more than ten cell lines. We applied false discovery rate (FDR) multiple testing correction on the P value. Gene pairs with a negative coefficient and FDR-adjusted P value less than 0.05 were considered as candidate SV.
Survival analysis in TCGA
The patients were divided into two groups according to the status of SV gene pairs as follows: patients with simultaneous alteration (loss or gain of function) of gene1 and gene2 and others (Supplementary Fig. S1C, right). The OS time of the two groups for specific cancer types was tested using log-rank test, P < 0.05 was considered statistically significant and the results were represented by Kaplan-Meier plots. We defined SV-score as the number of co-occurrence alteration (loss or gain of function) in multi-omics of SV gene pairs in a cancer sample. We defined drug-score for chemotherapy as the number of alteration (loss or gain of function) in multi-omics of SV partners in a cancer sample. The cutoff for high or low SV-score was the median across all samples. We applied multivariate Cox regression analysis to estimate the prognosis of SV-score and drug-score.
Construction and validation of the SV signature
First, we applied univariate Cox regression analysis to select the six SV gene pairs in immunotherapy melanoma cohorts based on clinical survival-related SV in TCGA skin cutaneous melanoma (SKCM) (Supplementary Fig. S1D). Second, we quantified a risk score for each melanoma patient based on the six SV pairs signature through multivariate Cox regression analysis. The formula of the RiskScore was as follows:
where exp denoted exponential, the SV gene pairs alteration status equaled 1, and the non-alteration status equaled 0. ‘L’ or ‘G’ represented loss or gain of function for gene. “SurvivalROC” R package was used to determine the cutoff for classifying patients into low-risk and high-risk melanoma patients. Finally, the same formula and cutoff were applied to the other validation cohorts and the TCGA SKCM cohort.
Evaluation of immune infiltration in melanoma samples
A total of 29 immune process signature gene sets were obtained from the study of He et al. and Bagaev et al. [30, 31]. Enrichment scores of the signature gene sets in each sample were determined by applying the single-sample gene set enrichment analysis (ssGSEA) approach of the “GSVA” R package [32]. In the TCGA SKCM cohort, the levels of tumor-infiltrating lymphocytes were evaluated by analyzing the information from Thorsson et al. [33]. The proportions of 22 types of infiltrating immune cells were estimated via the CIBERSORT method based on gene expression data [34].
Statistical analysis
Functional enrichment analysis and clustering of immune processes were conducted using the “clusterProfler” R package. Associations between the SV gene pairs and OS were presented via the Kaplan-Meier method, survival curves were compared via the log-rank test. C-index was determined to compare the accuracy of the six SV pairs signature with other existing signatures of ICI response [35]. Spearman’s rank correlation analysis was applied to estimate the associations between the six SV pairs signature and other transcriptome signatures. Statistical analysis of comparisons between two groups was conducted using the one-sided Wilcoxon rank sum test. Associations between the six SV pairs signature and immune infiltration, copy number deleted, and gene mutation were analyzed via Fisher’s exact test. The “pROC” R package was used to estimate the area under the curve (AUC) values. R software (https://www.r-project.org/) was applied to perform all statistical analyses. P value < 0.05 was considered to indicate significance.
Results
Identification of features to predict synthetic viable interactions
We obtained 220 high-confident gene pairs from CRISPR/Cas9 genetic perturbation screens as true-positive SV gene pairs and randomly selected the same number of gene pairs as non-SV. We collected 14 features for SV gene pairs (Supplementary Table S2), including comparative properties and boolean (true/false) properties. Comparing with non-SV, SV gene pairs had higher essentiality of shared PPI, biological process similarity score, essentiality of shared protein complex membership, co-expression coefficient, average shortest distance score, pathway number, subcellular co-localization score, and conservation score (P = 8.2E-22, P = 6.2E-6, P = 1.8E-4, P = 2.1E-9, P = 1.2E-14, P = 3.2E-26, P = 1.2E-3, P = 4.9E-8, one-sided Wilcoxon rank-sum test, Fig. 1a–h). Comparing features between SV and non-SV gene pairs, a high proportion of SV gene pairs had features of co-occurring alteration, paralog gene, protein complex membership and shared PPI (Fig. 1i). We assessed the predictive performance of each feature for SV gene pairs by analyzing receiver-operating characteristic (ROC) and computing AUC. Paralog gene and shared PPI had better performance in predicting SV gene pairs (Fig. 1j). Both paralog gene and shared PPI had an AUC greater than 0.7. Overall, SV gene pairs in cancer cells tended to be functionally similar.
a–h Significant differences of multiple features between SV and non-SV gene pairs. P values were computed by one-sided Wilcoxon rank-sum test and P < 0.05 was considered as statistically significant. i The percentage of multiple features for SV and non-SV gene pairs. j The AUC for each feature as an individual classifier.
An ensemble classifier to predict synthetic viable interactions
This study combined 14 features into a random forest ensemble classifier to predict SV gene pairs. Fig. 2a showed the weights of 14 features in random forest classifier. Random forest classifier achieved an AUC of 0.87 (Fig. 2b). We also investigated classifier performance in predicting the published drug resistant genes (Supplementary Fig. S3). Classifier identified resistant genes with AUCs of 0.75 ~ 1.00 and precision of about 93% (Fig. 2c, d). This work focused on four types of TSGs and oncogenes related SV gene pairs (Supplementary Fig. S1A). We next predicted SV for TSGs and oncogenes-related gene pairs based on 14 features from ensemble random forest classifier (Supplementary Fig. S1B). Then, our study identified 1,861 SV gene pairs using CRISPR/Cas9 genetic perturbation screen, and SV gene pairs with the regression coefficients less than -0.3 were shown in Fig. 2e (FDR-adjusted P < 0.05, multiple linear regression). Further, we revealed cancer cell lines with gain function of FGFR1 or PDGFRA had lower dependency scores compared to other cancer cell lines, after EFGR knockout (P = 7.1E-4, Fig. 2f; P = 1.0E-3, Fig. 2g, multiple linear regression). In accordance with previous studies, FGFR1 and PDGFRA have been associated with resistance to EGFR inhibitors [36, 37].
a The weights of 14 features from random forest classifier. b ROC curve for ensemble classifier. c, d The AUC and precision for ensemble classifier’s performance in predicting published resistant genes. e The regression coefficients of SV gene pairs were estimated by multiple linear regression model (FDR-adjusted P < 0.05). Significant differential dependency scores of EGFR in cancer cell lines where FGFR1 (f) or PDGFRA (g) was gain of function versus where FGFR1 or PDGFRA was wild type. P values were calculated from the multiple linear regression model and FDR-adjusted P < 0.05 was considered statistically significant.
Prognosis-related SV gene pairs across multiple cancers
We extracted prognosis-related SV gene pairs in the datasets from TCGA (P < 0.05, log-rank test, Fig. 3a). In breast carcinoma (BRCA), brain lower grade glioma (LGG), head and neck squamous cell carcinoma (HNSC), and SKCM, patients with high SV-score (defined by median of scores) showed significantly worse OS (P = 1.2E-6, Fig. 3b; P = 3.3E-12, Fig. 3c; P = 1.6E-10, Fig. 3d; P = 9.5E-12, Fig. 3e, log-rank test). In the cancer types of BRCA, LGG, HNSC, and SKCM, multivariate Cox regression analysis showed that SV-score was an independent predictive risk factor for prognosis (P < 0.05, Fig. 3f–i). Besides, we found that AUCs of predicting response to drug by SV gene pairs were greater than 0.6 for multiple treatments (Fig. 3j). In brief, this work indicated that SV gene pairs were associated with prognosis of patients by multiple treatments (P < 0.05, multivariate Cox regression analysis, Fig. 3k).
a The overlapping of survival related SV gene pairs across various cancer types. Kaplan-Meier plots of patients with low and high SV-score (cutoff was median of scores) in BRCA (b), LGG (c), HNSC (d), and SKCM (e) cohorts. The P values were computed by log-rank test. Multivariate Cox regression analysis of the SV-score, age, and stage in BRCA (f), LGG (g), HNSC (h), and SKCM (i) cohorts. j AUCs for SV gene pairs performance in predicting drug response. k Prediction of SV gene pairs in terms of survival: The x-axis displays the hazard ratio of patients result from multivariate Cox regression analysis of SV gene pairs. For P value, * denotes P < 0.05, ** denotes P < 0.01, and *** denotes P < 0.001.
Construction of SV signature to predict immunotherapy response for melanoma
This study identified the six SV pairs signature that were significantly related to the prognosis of melanoma with ICI treatment (P < 0.05, univariate Cox regression analysis). Next, we quantified a risk score for each melanoma patient based on the six SV pairs signature through multivariate Cox regression model.
We determined a cutoff value (cutoff = 1) to divide melanoma patients into high-risk and low-risk groups. High-risk melanoma patients had shorter OS than low-risk patients in the training cohort (P = 8.0E-9, log-rank test, Fig. 4a). Importantly, melanoma patients with high-risk scores were significantly associated with unfavorable response of ICI treatment in the training cohort and validation cohort (P = 1.0E-4, Fig. 4b; P = 2.1E-3, Fig. 4c, Fisher’s exact test). Patients with ICI non-response exhibited significantly increased risk scores (P = 2.6E-5, Fig. 4d; P = 2.1E-3, Fig. 4e, one-sided Wilcoxon rank-sum test; P = 0.03, Supplementary Fig. S4A; P = 0.02, Supplementary Fig. S4B, Kruskal-Wallis test). Low-risk melanoma patients tended to be associated with higher TMB compared with high-risk patients (P = 0.03, Fisher’s exact test, Fig. 4f; P = 0.02, one-sided Wilcoxon rank-sum test, Supplementary Fig. S4C). Low-risk melanoma patients showed higher neoantigen load and cytolytic score compared with high-risk patients in Riaz2017 cohort (P = 0.03, Fig. 4g; P = 0.01, Fig. 4h, one-sided Wilcoxon rank-sum test). In addition, this study systematically compared the performance of the six SV pairs signature with the existing signatures of ICI response, such as mutation and expression-associated signatures (Supplementary Table S3). The predictive power of the six SV pairs signature was greater than some existing signatures in Liu2019, Riaz2017, VanAllen2015, and Snyder2014 cohorts (Fig. 4i–m). The six SV pairs signature also presented significant association with some other signatures in multiple cohorts (P < 0.05, Spearman’s rank correlation test, Supplementary Fig. S4D).
a Kaplan-Meier plot of melanoma patients with high-risk versus low-risk in training cohort. The P values were computed by log-rank test. The proportion of melanoma patients who responded to ICI treatment in the high-risk and low-risk in Liu2019 (b) and Gide2019 cohort (c). The P values were computed by Fisher’s exact test. A significant differential risk score of melanoma patients between response and non-response in Liu2019 (d) and Gide2019 (e) cohort. The P values were computed from one-sided Wilcoxon rank-sum test. f The proportion of melanoma with high TMB in the high-risk and low-risk patients in the Riaz2017 cohort. The P values were computed by Fisher’s exact test. Significant differential neoantigen load (g) and cytolytic score (h) of melanoma patients between high-risk and low-risk in Riaz2017 cohort. The P values were computed by one-sided Wilcoxon rank-sum test. Comparison of C-indexes between the six SV pairs signature and expression (i, j) and mutation (k, l, m) associated signatures from published literature across multiple datasets.
Immune landscape of the high-risk and low-risk melanoma patients
Using the risk model obtained from the training cohort (see Methods), we classified patients into high-risk and low-risk groups in the immunotherapy melanoma datasets and TCGA SKCM dataset. The low-risk melanoma patients were characterized by greater abundance of natural killer (NK) cells and major histocompatibility complex (MHC) molecules (FDR-adjusted P < 0.05, one-sided Wilcoxon rank-sum test, Fig. 5a, b, Supplementary Fig. S4E). Meanwhile, we clustered melanoma patients into low-immune and high-immune infiltration groups based on immune signature scores estimated by ssGSEA for 29 immune gene sets from He et al. Interestingly, the high immune cell infiltration samples were significantly enriched in cases from the low-risk samples in TCGA SKCM patients (P = 1.0E-13, Fisher’s exact test, Fig. 5c). Additionally, the correlation among immune cell activities in the low-risk patients was significantly higher than that in high-risk patients (P < 0.05, Spearman’s rank correlation test, Supplementary Fig. S4F; P = 3.2E-5, one-sided Wilcoxon rank-sum test, Fig. 5d). Analyzing the immune landscape score according to Thorsson et al., we found augmented levels of B cells, NK cells, and CD8+ T cells in the low-risk patients than high-risk patients (FDR-adjusted P < 0.05, one-sided Wilcoxon rank-sum test, Fig. 5e). Furthermore, expression of chemokines and MHC genes were higher in the low-risk melanoma patients than that in the high-risk melanoma patients. (FDR-adjusted P < 0.05, one-sided Wilcoxon rank-sum test, Fig. 5f, g). Immune checkpoint molecules (such as PDCD1, CD274, and CTLA4) and co-stimulatory molecules were also highly expressed in low-risk melanoma group (FDR-adjusted P < 0.05, one-sided Wilcoxon rank-sum test, Fig. 5h, i). Thus, immune checkpoint molecules may mediate response to ICI treatment in low-risk melanoma patients.
Significant differential of 29 immune signatures scores from He et al. (a) and Bagaev et al. (b) estimated by the ssGSEA method between low-risk and high-risk patients across melanoma cohorts. c The proportions of high immune and low immune infiltration estimated by 29 immune signatures from He et al. in the high-risk and low-risk melanoma patients. The P values were computed by Fisher’s exact test. d Significant differential of correlation coefficients among 29 immune signatures from He et al. between high-risk and low-risk patients in TCGA SKCM dataset. e Significant differential immune signatures score from Thorsson et al. between high-risk and low-risk melanoma patients. Significant differential expression of chemokines (f), MHC (g), co-inhibitors (h), and co-stimulators (i) genes between low-risk and high-risk patients across melanoma cohorts. The P values were calculated from one-sided Wilcoxon rank-sum test with FDR-adjusted. Fold change (FC) values were calculated by ratio of mean gene expression from low-risk and high-risk patients. For P value, * denotes P < 0.05, ** denotes P < 0.01, and *** denotes P < 0.001.
Genome features between the high-risk and low-risk melanoma patients
This work detected significant differences in mutated genes between the high-risk and low-risk melanoma patients and found that mutated genes were significantly enriched in low-risk patients compared with high-risk patients (P < 0.05, Fisher’s exact test, Supplementary Fig. S5A). This result was consistent with the previous discovery that low-risk patients were related to high TMB and neoantigen load (Fig. 4f, g). Copy number deleted genes were significantly enriched in interleukin receptor and MHC class I receptor activity related molecular function in Liu2019, VanAllen2015, and TCGA SKCM cohorts (P < 0.05, hypergeometric test, Fig. 6a–c). And, high-risk patients had significant copy number deletion compared with low-risk patients (P = 0.02, Fisher’s exact test, Fig. 6d–g). Notably, copy number deleted genes that participating in multiple metabolic-related biological process were significantly enriched in high-risk melanoma patients (P < 0.05, hypergeometric test, Supplementary Fig. S5B; P = 1.0E-3, P = 1.0E-3, P = 7.0E-3, P = 0.02, Fisher’s exact test, Supplementary Fig. S5C-F). Thus, the results indicated that inactivation of MHC class I and interleukin receptor contributed to resistance to ICI in high-risk melanoma patients.
Discussion
In this work, we established 14 features ensemble classifier to comprehensively predict robust SV gene pairs for cancer. As excepted, SV gene pairs were related to poor survival across various cancer types in the TCGA. Moreover, this work revealed a signature consisting of the six SV pairs to predict resistance to ICI treatment for melanoma patients. Compared with the low-risk patients, high-risk patients of melanoma showed lower immune cell infiltration of NK cells and CD8+ T cell. To the best of our knowledge, this was the first study to investigate SV effect across melanoma cohorts with ICI treatment.
Paralog gene was considered to measure functional similarity for SV gene pairs. Average shortest distance in PPI could also be used to predict SV gene pairs. Genes that have closer distance were more likely to encode similarity functionality, which could explain why gene pairs with closer distance were more likely to be SV. The predictive power of individual feature may be affected by limitations of the underlying data sources, particularly those features derived from PPI and protein localization. The Human Protein Atlas contained high-confidence antibody-derived localization for only part of TSGs and oncogenes. Despite the above-noted limitations, we showed that features were sufficient for learning the general trends that distinguish SV from non-SV gene pairs (Fig. 2b–d).
After constructing the six SV pairs signature to predict ICI response, we calculated overall immune cell infiltration levels for melanoma patients. The immune score was significantly higher in the low-risk patients than in the high-risk patients, which confirmed the stronger anti-tumor immune activity. Many studies have shown that the density of tumor-infiltrating lymphocytes is positively associated with the immune response in kinds of cancers [38]. In addition to a high level of NK cells, B cells, and CD8+ T cells, the low-risk patients were characterized by over-expression of immune checkpoint genes, such as PDCD1, CD274, and CTLA-4, compared with the high-risk patients. Therefore, activated anti-tumor immunity, high PDCD1, CD274, and CTLA-4 expression, and enhanced tumor immunogenicity might explain why the low-risk melanoma patients were found to be more likely to benefit from ICI treatment than the high-risk patients. Furthermore, we also identified four SV signature that was associated with resistance to ICI treatment in non-small cell lung cancer (Supplementary method, Supplementary Fig. S6).
In summary, this study developed a classifier to predict robust SV gene pairs and systematically identified SV signature for assessing the effect of ICI treatment across multiple melanoma cohorts. Furthermore, our study revealed distinct immune landscapes between high-risk and low-risk melanoma patients. Overall, we proposed a new tumor classification system with the potential to guide ICI treatment decisions. Elucidating the molecular mechanism underlying the effect of each SV gene pairs on immunotherapy in vivo and vitro functional experiments warrant our future work.
Data availability
All data analyzed during this study can be downloaded from TCGA, CCLE, DepMap, and cBioPortal. Other information was provided in the Supplementary Information files.
Code availability
The code used for the analysis is available upon request. For more detailed information, please contact the corresponding author (YYG).
References
Malmberg R, Zietse M, Dumoulin DW, Hendrikx J, Aerts J, van der Veldt AAM, et al. Alternative dosing strategies for immune checkpoint inhibitors to improve cost-effectiveness: a special focus on nivolumab and pembrolizumab. Lancet Oncol. 2022;23:e552–e61.
Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021;184:5309–37.
Petroni G, Buque A, Coussens LM, Galluzzi L. Targeting oncogene and non-oncogene addiction to inflame the tumour microenvironment. Nat Rev Drug Discov. 2022;21:440–62.
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30:582–8.
Torrejon DY, Abril-Rodriguez G, Champhekar AS, Tsoi J, Campbell KM, Kalbasi A, et al. Overcoming genetically based resistance mechanisms to PD-1 blockade. Cancer Discov. 2020;10:1140–57.
Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018;362:eaar3593.
Liu D, Schilling B, Liu D, Sucker A, Livingstone E, Jerby-Arnon L, et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat Med. 2019;25:1916–27.
Liu YT, Sun ZJ. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 2021;11:5365–86.
Patterson A, Auslander N. Mutated processes predict immune checkpoint inhibitor therapy benefit in metastatic melanoma. Nat Commun. 2022;13:5151.
Lee JS, Nair NU, Dinstag G, Chapman L, Chung Y, Wang K, et al. Synthetic lethality-mediated precision oncology via the tumor transcriptome. Cell 2021;184:2487–502.e13.
Sahu AD, S Lee J, Wang Z, Zhang G, Iglesias-Bartolome R, Tian T, et al. Genome-wide prediction of synthetic rescue mediators of resistance to targeted and immunotherapy. Mol Syst Biol. 2019;15:e8323.
Han Y, Wang C, Dong Q, Chen T, Yang F, Liu Y, et al. Genetic interaction-based biomarkers identification for drug resistance and sensitivity in cancer cells. Mol Ther Nucleic Acids. 2019;17:688–700.
Gu Y, Wang R, Han Y, Zhou W, Zhao Z, Chen T, et al. A landscape of synthetic viable interactions in cancer. Brief Bioinform. 2018;19:644–55.
Wilson J, Loizou JI. Exploring the genetic space of the DNA damage response for cancer therapy through CRISPR-based screens. Mol Oncol. 2022;16:3778–91.
Llorca-Cardenosa MJ, Aronson LI, Krastev DB, Nieminuszczy J, Alexander J, Song F, et al. SMG8/SMG9 heterodimer loss modulates SMG1 kinase to drive ATR inhibitor resistance. Cancer Res. 2022;82:3962–73.
Dong Q, Liu M, Chen B, Zhao Z, Chen T, Wang C, et al. Revealing biomarkers associated with PARP inhibitors based on genetic interactions in cancer genome. Comput Struct Biotechnol J. 2021;19:4435–46.
Gerstung M, Jolly C, Leshchiner I, Dentro SC, Gonzalez S, Rosebrock D, et al. The evolutionary history of 2,658 cancers. Nature 2020;578:122–8.
Parrish PCR, Thomas JD, Gabel AM, Kamlapurkar S, Bradley RK, Berger AH. Discovery of synthetic lethal and tumor suppressor paralog pairs in the human genome. Cell Rep. 2021;36:109597.
Zhao X, Li J, Liu Z, Powers S. Combinatorial CRISPR/Cas9 screening reveals epistatic networks of interacting tumor suppressor genes and therapeutic targets in human breast cancer. Cancer Res. 2021;81:6090–105.
Dede M, McLaughlin M, Kim E, Hart T. Multiplex enCas12a screens detect functional buffering among paralogs otherwise masked in monogenic Cas9 knockout screens. Genome Biol. 2020;21:262.
Sun S, Baryshnikova A, Brandt N, Gresham D. Genetic interaction profiles of regulatory kinases differ between environmental conditions and cellular states. Mol Syst Biol. 2020;16:e9167.
Ghandi M, Huang FW, Jane-Valbuena J, Kryukov GV, Lo CC, McDonald ER 3rd, et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 2019;569:503–8.
Beroukhim R, Getz G, Nghiemphu L, Barretina J, Hsueh T, Linhart D, et al. Assessing the significance of chromosomal aberrations in cancer: methodology and application to glioma. Proc Natl Acad Sci USA. 2007;104:20007–12.
Gide TN, Quek C, Menzies AM, Tasker AT, Shang P, Holst J, et al. Distinct immune cell populations define response to anti-PD-1 monotherapy and anti-PD-1/Anti-CTLA-4 combined therapy. Cancer Cell. 2019;35:238–55.e6.
De Kegel B, Quinn N, Thompson NA, Adams DJ, Ryan CJ. Comprehensive prediction of robust synthetic lethality between paralog pairs in cancer cell lines. Cell Syst. 2021;12:1144–59.e6.
Ruepp A, Waegele B, Lechner M, Brauner B, Dunger-Kaltenbach I, Fobo G, et al. CORUM: the comprehensive resource of mammalian protein complexes–2009. Nucleic Acids Res 2010;38:D497–501.
Zhao M, Kim P, Mitra R, Zhao J, Zhao Z. TSGene 2.0: an updated literature-based knowledgebase for tumor suppressor genes. Nucleic Acids Res. 2016;44:D1023–31.
Liu Y, Sun J, Zhao M. ONGene: A literature-based database for human oncogenes. J Genet Genomics. 2017;44:119–21.
Magen A, Das Sahu A, Lee JS, Sharmin M, Lugo A, Gutkind JS, et al. Beyond synthetic lethality: charting the landscape of pairwise gene expression states associated with survival in cancer. Cell Rep. 2019;28:938–48.e6.
He Y, Jiang Z, Chen C, Wang X. Classification of triple-negative breast cancers based on Immunogenomic profiling. J Exp Clin Cancer Res. 2018;37:327.
Bagaev A, Kotlov N, Nomie K, Svekolkin V, Gafurov A, Isaeva O, et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell. 2021;39:845–65.e7.
Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinforma. 2013;14:7.
Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The Immune Landscape of Cancer. Immunity 2018;48:812–30.e14.
Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–7.
Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. discussion 207-12
Lu Y, Liu Y, Oeck S, Zhang GJ, Schramm A, Glazer PM. Hypoxia induces resistance to EGFR inhibitors in lung cancer cells via upregulation of FGFR1 and the MAPK Pathway. Cancer Res. 2020;80:4655–67.
Yeo AT, Jun HJ, Appleman VA, Zhang P, Varma H, Sarkaria JN, et al. EGFRvIII tumorigenicity requires PDGFRA co-signaling and reveals therapeutic vulnerabilities in glioblastoma. Oncogene 2021;40:2682–96.
Rohaan MW, Borch TH, van den Berg JH, Met O, Kessels R, Geukes Foppen MH, et al. Tumor-infiltrating lymphocyte therapy or ipilimumab in advanced melanoma. N. Engl J Med. 2022;387:2113–25.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 32270710 to Yunyan Gu; No. 82271208 to Yang Hui), the Outstanding Youth Foundation of Heilongjiang Province of China (No. YQ2021H005 to Yunyan Gu) and the HMU Marshal Initiative Funding (No. HMUMIF-21023 to Yunyan Gu).
Author information
Authors and Affiliations
Contributions
Y.Y.G. and M.Y.L. conceived and designed the study. M.Y.L., Q.D., and B.C. completed data processing, analysis, and a draft of the paper. K.D.L., Z.X.Z., Y.Q.W., and S.P.Z. extracted data and visualized the results. Y.H., H.M.H., X.Y.S., and Z.X.J. provided valuable suggestions for the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, M., Dong, Q., Chen, B. et al. Synthetic viability induces resistance to immune checkpoint inhibitors in cancer cells. Br J Cancer 129, 1339–1349 (2023). https://doi.org/10.1038/s41416-023-02404-w
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41416-023-02404-w