Abstract
Importance
Intra-arterial therapies(IATs) are promising options for unresectable hepatocellular carcinoma(HCC). Stratifying the prognostic risk before administering IAT is important for clinical decision-making and for designing future clinical trials.
Objective
To develop and validate a machine learning(ML)-based decision support model(MLDSM) for recommending IAT modalities for unresectable HCC.
Design, setting, and participants
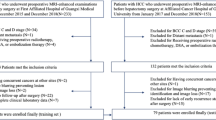
Between October 2014 and October 2022, a total of 2,959 patients with HCC who underwent initial IATs were enroled retrospectively from 13 tertiary hospitals. These patients were divided into the training cohort (n = 1700), validation cohort (n = 428), and test cohort (n = 200).
Main outcomes and measures
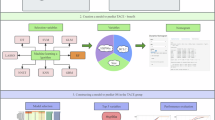
Thirty-two clinical variables were input, and five supervised ML algorithms, including eXtreme Gradient Boosting (XGBoost), Categorical Gradient Boosting (CatBoost), Gradient Boosting Decision Tree (GBDT), Light Gradient Boosting Machine (LGBM) and Random Forest (RF), were compared using the areas under the receiver operating characteristic curve (AUC) with the DeLong test.
Results
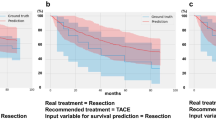
A total of 1856 patients were assigned to the IAT alone Group(I-A), and 1103 patients were assigned to the IAT combination Group(I-C). The 12-month death rates were 31.9% (352/1103) in the I-A group and 50.4% (936/1856) in the I-C group. For the test cohort, in the I-C group, the CatBoost model achieved the best discrimination when 30 variables were input, with an AUC of 0.776 (95% confidence intervals [CI], 0.833–0.868). In the I-A group, the LGBM model achieved the best discrimination when 24 variables were input, with an AUC of 0.776 (95% CI, 0.833–0.868). According to the decision trees, BCLC grade, local therapy, and diameter as top three variables were used to guide clinical decisions between IAT modalities.
Conclusions and relevance
The MLDSM can accurately stratify prognostic risk for HCC patients who received IATs, thus helping physicians to make decisions about IAT and providing guidance for surveillance strategies in clinical practice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The in-house developed medical database of this study is publicly accessible at: http://www.yunedc.cn/#/login. In addition, we also provided the codes of development of the ML based model are available in opensource repositories (https://github.com/tyewu/DiseasePredict) for the convenience of public use.
Code availability
In addition, we also provided the codes of development of the ML based model are available in opensource repositories (https://github.com/tyewu/DiseasePredict) for the convenience of public use.
Change history
22 January 2025
The original online version of this article was revised: “In this article the author Jianjun Han has been affiliated with a wrong institution. This has been corrected”.
03 February 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41416-025-02940-7
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram L, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–50. https://doi.org/10.1002/hep.29913.
Villanueva A. Hepatocellular Carcinoma. N. Engl J Med. 2019;380:1450–62. https://doi.org/10.1056/NEJMra1713263.
Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155–66. https://doi.org/10.1111/liv.12818.
Ghanaati H, Mohammadifard M, Mohammadifard M. A review of applying transarterial chemoembolization (TACE) method for management of hepatocellular carcinoma. J Fam Med Prim Care. 2021;10:3553–60. https://doi.org/10.4103/jfmpc.jfmpc_2347_20.
Sidaway P. HAIC-FO improves outcomes in HCC. Nat Rev Clin Oncol. 2022;19:150. https://doi.org/10.1038/s41571-022-00599-0.
He M, Li Q, Zou R, Shen JX, Fang WQ, Tan GS, et al. Sorafenib plus hepatic arterial infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5:953–60. https://doi.org/10.1001/jamaoncol.2019.0250.
Zhang Z, Li C, Liao W, Huang Y, Wang Z A Combination of Sorafenib, an Immune Checkpoint Inhibitor, TACE and Stereotactic Body Radiation Therapy versus Sorafenib and TACE in Advanced Hepatocellular Carcinoma Accompanied by Portal Vein Tumor Thrombus. Cancers. 2022;14. https://doi.org/10.3390/cancers14153619.
Lencioni R, Llovet JM, Han G, Tak WY, Yang JM, Alfredo G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090–8. https://doi.org/10.1016/j.jhep.2016.01.012.
McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73:4–13. https://doi.org/10.1002/hep.31288.
An C, Zuo M, Li W, Chen Q, Wu P. Infiltrative Hepatocellular Carcinoma: Transcatheter arterial chemoembolization versus hepatic arterial infusion chemotherapy. Front Oncol. 2021;11:747496. https://doi.org/10.3389/fonc.2021.747496.
An C, Yao W, Zuo M, Li W, Chen Q, Wu P Pseudo-capsulated hepatocellular carcinoma: hepatic arterial infusion chemotherapy versus Transcatheter Arterial Chemoembolization. Acad Radiol. 2023. https://doi.org/10.1016/j.acra.2023.06.021.
Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV, Fotiadis DI. Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J. 2015;13:8–17. https://doi.org/10.1016/j.csbj.2014.11.005.
An C, Yang H, Yu X, Han Z, Cheng Z, Liu F, et al. A machine learning model based on health records for predicting recurrence after microwave ablation of hepatocellular carcinoma. J Hepatocell Carcinoma. 2022;9:671–84. https://doi.org/10.2147/JHC.S358197.
Uche-Anya E, Anyane-Yeboa A, Berzin TM, Ghassemi M, May FP. Artificial intelligence in gastroenterology and hepatology: how to advance clinical practice while ensuring health equity. Gut. 2022;71:1909–15. https://doi.org/10.1136/gutjnl-2021-326271.
Moons KGM, Altman DG, Reitsma JB, Ioannidis JPA, Macaskill P, Steyerberg EW. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–73. https://doi.org/10.7326/M14-0698. Jan 6
EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. https://doi.org/10.1016/j.jhep.2018.03.019.
Cardella JF, Kundu S, Miller DL, Millward SF, Sacks D. Society of Interventional Radiology Clinical Practice Guidelines. J Vasc Inter Radio. 2009;20:S189–191. https://doi.org/10.1016/j.jvir.2009.04.035.
Liu W, Wei R, Chen J, Li Y, Pang H, Zhang W, et al. Prognosis prediction and risk stratification of transarterial chemoembolization or intraarterial chemotherapy for unresectable hepatocellular carcinoma based on machine learning. Eur Radiol. 2024 Jan 30. https://doi.org/10.1007/s00330-024-10581-2.
Wang K, Tian J, Zheng C, Yang H, Ren J, Liu Y, et al. Interpretable prediction of 3-year all-cause mortality in patients with heart failure caused by coronary heart disease based on machine learning and SHAP. Comput Biol Med. 2021;137:104813. https://doi.org/10.1016/j.compbiomed.2021.104813.
Ma M, Liu R, Wen C, Xu W, Xu Z, Wang S, et al. Predicting the molecular subtype of breast cancer and identifying interpretable imaging features using machine learning algorithms. Eur Radiol. 2022;32:1652–62. https://doi.org/10.1007/s00330-021-08271-4.
Li QJ, He MK, Chen HW, Fang WQ, Zhou WM, Liu X, et al. Hepatic arterial infusion of Oxaliplatin, Fluorouracil, and Leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized Phase III trial. J Clin Oncol. 2022;40:150–60. https://doi.org/10.1200/JCO.21.00608.
Jin ZC, Zhong BY, Chen JJ, Zhu HD, Sun JH, Yin GW, et al. Real-world efficacy and safety of TACE plus camrelizumab and apatinib in patients with HCC (CHANCE2211): a propensity score matching study. Eur Radiol. 2023. https://doi.org/10.1007/s00330-023-09754-2.
Johnson PJ, Berhane S, Kagebayashi C, Shinji S, Mabel T, Helen LR, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–8. https://doi.org/10.1200/JCO.2014.57.9151.
Reig M, Forner A, Rimola J, Joana F, Marta B, Ángeles G, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681–93. https://doi.org/10.1016/j.jhep.2021.11.018.
Song S, Bai M, Li X, Guo S, Yang W, Li C, et al. Early predictive value of circulating biomarkers for sorafenib in advanced hepatocellular carcinoma. Expert Rev Mol Diagn. 2022;22:361–78. https://doi.org/10.1080/14737159.2022.2049248.
Hiraoka A, Ishimaru Y, Kawasaki H, Aibiki T, Okudaira T, Toshimori A, et al. Tumor Markers AFP, AFP-L3, and DCP in Hepatocellular Carcinoma Refractory to Transcatheter Arterial Chemoembolization. Oncology. 2015;89:167–74. https://doi.org/10.1159/000381808.
Zhou H, Song T. Conversion therapy and maintenance therapy for primary hepatocellular carcinoma. Biosci Trends. 2021;15:155–60. https://doi.org/10.5582/bst.2021.01091.
Fan J, Tang ZY, Yu YQ, Wu ZQ, Ma ZC, Zhou XD, et al. Improved survival with resection after transcatheter arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma. Dig Surg. 1998;15:674–8. https://doi.org/10.1159/000018676.
Shi F, Lian S, Mai Q, Mo ZQ, Zhuang WH, Cui W, et al. Microwave ablation after downstaging of hepatocellular carcinoma: outcome was similar to tumor within Milan criteria. Eur Radiol. 2020;30:2454–62. https://doi.org/10.1007/s00330-019-06604-y.
Binnewies M, Roberts EW, Kersten K, Vincent C, Douglas FF, Miriam M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–50. https://doi.org/10.1038/s41591-018-0014-x.
Cao J, Su B, Peng R, Tang H, Tu DY, Tang YH, et al. Bioinformatics analysis of immune infiltrates and tripartite motif (TRIM) family genes in hepatocellular carcinoma. J Gastrointest Oncol. 2022;13:1942–58. https://doi.org/10.21037/jgo-22-619.
Liu F, Liu D, Wang K, Xie XH, Su LY, Kuang M, et al. Deep learning radiomics based on contrast-enhanced ultrasound might optimize curative treatments for very-early or early-stage hepatocellular carcinoma patients. Liver Cancer. 2020;9:397–413. https://doi.org/10.1159/000505694.
Ding W, Wang Z, Liu FY, Cheng ZG, Yu XL, Han ZY, et al. A hybrid machine learning model based on semantic information can optimize treatment decision for naïve single 3-5-cm HCC patients. Liver Cancer. 2022;11:256–67. https://doi.org/10.1159/000522123.
Funding
Beijing Municipal Education Commission Science and Technology Project KM202010025005; The capital health research and development of special (2022- 2-7083); Beijing Municipal Natural Science Foundation7222100; Tongzhou District Science and Technology Commission Project KJ2022CX021; Beijing Xisike Clinical Oncology Research Foundation Y-Young2024-0090. This article was supported by the National Natural Science Foundation of China (NSFC No. 82272101) and the Natural Science Foundation of Shandong Province (No. ZR2021MH060).
Author information
Authors and Affiliations
Contributions
Conception and design: Wendao Liu, Chao An. Development of methodology: Ran Wei,Wang Li. Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): Yan Fu, Xiaolong Gong,Wang Yao, Chengzhi Li. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): Yansheng Li, Fatian Wu, Kejia Liu. Writing, review, and/or revision of the manuscript: Chao An, Mengxuan Zuo. Administrative, technical, or material support (i.e., reporting or organising data, constructing databases): Chengzhi Li, Dong Yan, Jianjun Han. Study supervision: Peihong Wu.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This retrospective study was approved from the Institutional Review Board of National Cancer Centre (NCC-010298) and was conducted following the principles of the Declaration of Helsinki. The requirement for written informed consent was waived because of the retrospective nature of the study.
Consent to participate
Yes.
Consent to publication
Yes.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: “In this article the author Jianjun Han has been affiliated with a wrong institution. This has been corrected”.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
An, C., Wei, R., Liu, W. et al. Machine learning-based decision support model for selecting intra-arterial therapies for unresectable hepatocellular carcinoma: A national real-world evidence-based study. Br J Cancer 131, 832–842 (2024). https://doi.org/10.1038/s41416-024-02784-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-024-02784-7
This article is cited by
-
Machine learning mortality prediction model for cyclosporine therapy in pediatric aplastic anemia
Annals of Hematology (2026)